Abstract
Schizophrenia is a major mental disorder that affects approximately 1% of the population worldwide. Cognitive deficits are a key feature of schizophrenia and a primary cause of long-term disability. Current neurophysiological models of schizophrenia focus on distributed brain dysfunction with bottom-up as well as top-down components. Bottom-up deficits in cognitive processing are driven by impairments in basic perceptual processes that localize to primary sensory brain regions. Within the auditory system, deficits are apparent in elemental sensory processing, such as tone matching following brief delay. Such deficits lead to impairments in higher-order processes such as phonological processing and auditory emotion recognition. Within the visual system, deficits are apparent in functioning of the magnocellular visual pathway, leading to higher-order deficits in processes such as perceptual closure, object recognition, and reading. In both auditory and visual systems, patterns of deficit are consistent with underlying impairment of brain N-methyl-d-aspartate receptor systems.
Keywords: auditory, visual, NMDA receptors, event-related potentials, glutamate
INTRODUCTION
When schizophrenia was first described by Emil Kraeplin in the late 1800s, cognitive dysfunction was viewed as the core of the disorder, as embodied by the term “dementia praecox.” This conceptualization has been abundantly confirmed since that time. Even as adolescents, individuals with schizophrenia show reductions in IQ of approximately one-half standard deviation, corresponding to standardized IQ scores in the range of 90–95. At first presentation, the deficit increases to slightly over one standard deviation across a range of cognitive domains, corresponding to standardized IQ scores of 80–85. Whether the decline occurs gradually during the premorbid period, as suggested by some birth cohort and follow-back studies, or rapidly at the time of initial psychotic decompensation (Woodberry et al. 2008) remains a subject of ongoing debate. Nevertheless, much of the long-term morbidity of schizophrenia may be simply due to the cognitive dysfunction that represents the core of the disorder. It is sobering that more than 100 years after the syndrome was first described, the underlying pathophysiologies both of cognitive dysfunction and of schizophrenia remain to be determined.
Where In the Brain Is Schizophrenia?
One of the most critical issues in schizophrenia research is the identification of the brain regions that are most involved in the schizophrenic process. Although there is rarely explicit debate on this topic, it is one where the majority of researchers vote with their publications, there being far more literature on dysfunction of regions such as prefrontal cortex or hippocampus in schizophrenia than of other brain regions, and particularly of sensory cortices. Nevertheless, it is worth noting that when widespread neuropsychological batteries are employed, such as the recently developed National Institute of Mental Health (NIMH)-funded MATRICS consensus cognitive battery (MCCB), patients show no greater impairment in domains that are thought to localize to pre-frontal brain regions (e.g., working memory, executive processing) than in those that localize to more posterior brain regions, such as visual memory, arguing against specific localization of brain dysfunction.
Debates about integrity of sensory processing in schizophrenia go back at least to the time of Kraeplin and Bleuler. For example, Kraeplin specifically postulated that key features of schizophrenia might reflect excitation of sensory centers and cited other evidence of perceptual-level dysfunction. In contrast, Bleuler explicitly denied seeing evidence of sensory deficit. As Bleuler (1950) stated in a famous passage:
Sensory response to external stimulus is quite normal. To be sure, the patients will complain that everything appears to be different and frequently we can observe the absence of the “feeling of familiarity” with known things. However, this strangeness is usually attributable to a deficit in customary association and particularly to an alteration of emotional emphasis, not to disturbances of sensation.
Bleuler’s views, and particularly his propensity to attribute evident and self-reported sensory-level disturbances to vague top-down processes, proved influential to most subsequent theoreticians. The first counterarguments to Bleuler did not begin to emerge until the refocus on self-reported perceptual disturbances by McGhie & Chapman (1961) and others in the early 1960s and with the advent of psychophysiological techniques such as eye-movement recording and event-related potentials (ERPs) in the early 1970s (Holzman 1972). As always, modern conceptualizations of bottom-up dysfunction depend upon the seminal contributions of earlier generations of schizophrenia researchers.
Cognitive Models in Schizophrenia
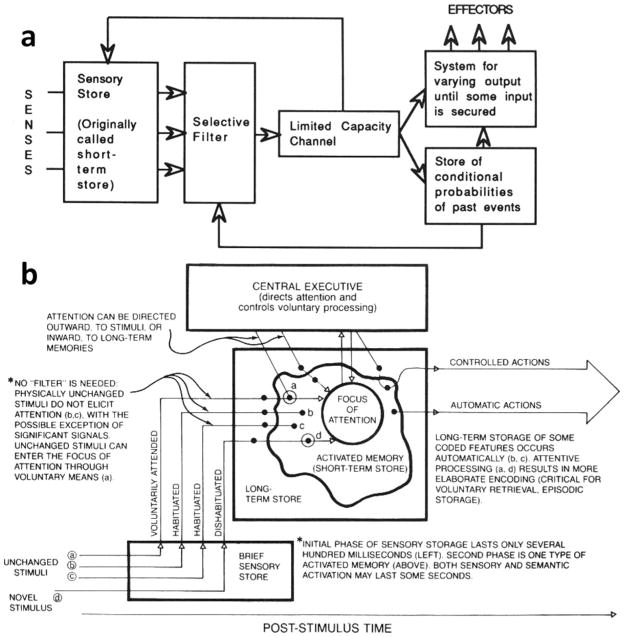
Another factor contributing greatly to progress over recent years has been a shift in general cognitive models that are available for schizophrenia research. Schizophrenia research first came of age in the era of top-down models such as those proposed by Broadbent in the 1950s and 1960s. In such models, sensory brain regions were considered to play a passive, almost hardwired role in transmitting minimally processed sensory information to higher cortical regions, where “cognitive” processing then occurred. Furthermore, virtually all processing of information, even at the level of primary sensory processing, was considered to be under control of a limited-capacity attentional filter (Figure 1a). (Cowan 1995).
Figure 1.
Evolving conceptual models of the interactions between sensory and attentional systems in brain. (a) Linear model proposed by Broadbent in 1958, postulating direct effects of attention on sensory stores. (b) Modified model proposed by Cowan et al. in 1988, illustrating both pre- and postattentional stages of sensory/perceptual processing. (From Cowan 1995, pp. 9 and 31.)
In contrast to these “Broadbentian” approaches, more recent models acknowledge a much more distributed pattern of cognitive processing throughout the brain, with an increasing role being assigned to primary sensory regions. For example, in the 1970s, Alan Baddeley turned his focus to the actions of a central executive memory system, but nevertheless needed to postulate the existence of additional “slave” memory systems that store sensory-specific information outside the control of the central executive. Similarly, Cowan and others in the 1980s and 1990s emphasized that not only significant information storage but also information processing such as novelty detection occurs outside the “attentional spotlight” (Cowan 1995) (Figure 1b). These models provide a broader framework for interpretation of cognitive deficits in schizophrenia than those available to earlier generations of schizophrenia researchers.
NEUROCHEMICAL MODELS OF SCHIZOPHRENIA
Finally, it is impossible to consider functional models of schizophrenia without considering underlying brain neurochemistry. Top-down conceptualizations of schizophrenia are strongly embedded in dopaminergic theories of schizophrenia, and particularly on actions of dopamine within prefrontal cortex. In contrast, distributed models of schizophrenia, which incorporate both bottom-up and top-down aspects of impaired neurocognition, are more strongly related to more recently developed glutamatergic and gamma-aminobutyric acid (GABA)ergic models ( Javitt 2007).
Dopamine Theory of Schizophrenia
The dopamine theory is based upon the observations that psychostimulants such as amphetamine induce clinical symptoms closely resembling those of schizophrenia, and, second, that antipsychotics, such as chlorpromazine, can reverse such deficits. Psychostimulants induce their psychotomimetic effects by stimulating dopamine release, whereas antipsychotics function primarily by blocking dopamine (D2) receptors, supporting dopaminergic involvement in schizophrenia. In rodents, dopaminergic pathways in the brain project most extensively to basal ganglia via the nigrostriatal system and to frontal cortex and limbic brain regions via the mesocortical and mesolimbic systems, respectively. In primates, dopaminergic projections have a somewhat more widespread distribution, but nevertheless little innervation of primary sensory cortices is observed (Lewis et al. 1987). The prominent dopaminergic projection to prefrontal cortex in both rodents and humans has traditionally been one of the main bases for models that focus primarily on prefrontal and top-down dysfunction in schizophrenia.
Although the dopamine model is very effective for conceptualization of positive symptoms, its explanatory value for other features of schizophrenia has, over the years, proven disappointing. Dopaminergic psychotomimetic agents, such as amphetamine, do not cause and may, in fact, ameliorate cognitive dysfunction in schizophrenia. Furthermore, classic antipsychotics such as chlorpromazine or haloperidol have proven to have limited effects on cognition. Although some beneficial effects may be observed for atypical antipsychotics such as clozapine, a hallmark of such agents is their lack of specificity for dopamine over a wide variety of other neurotransmitter systems. A preferential effect of atypical antipsychotics on cognitive functioning would thus argue against, rather than for, primary involvement of dopaminergic systems in neurocognitive dysfunction in schizophrenia (Javitt 2007).
PCP/NMDA Model
An alternative neurochemical hypothesis of schizophrenia was first developed starting in the late 1950s and early 1960s based upon the psychotomimetic actions of the novel compounds phencyclidine (PCP; “angel dust”) and the closely related compound ketamine. Although these drugs were originally developed as potential anesthetics, in early clinical trials they were found to induce clinical symptoms that closely resembled both the positive and negative symptoms of schizophrenia. Moreover, as opposed to either amphetamine or lysergic acid diethylamide (LSD), these compounds induced not only positive but also negative symptoms of schizophrenia in normal volunteers and induced re-exacerbation of initial presenting symptoms in remitted schizophrenic subjects. To researchers at the time, therefore, PCP-induced psychosis appeared to induce schizophrenia-like symptoms to a far greater degree than did amphetamine or other psycho-stimulants (Javitt 1987).
PCP and ketamine induce their unique neurobehavioral effects by blocking neurotransmission at N-methyl-d-aspartate (NMDA)-type glutamate receptors. The ability of NMDA antagonists to induce schizophrenia-like disturbances in cognition suggests that endogenous NMDA dysfunction may be critical to underlying pathogenetic mechanisms (Javitt & Zukin 1991).
NMDA channels are blocked in a voltage-dependent fashion by Mg2+. When NMDA-containing cells are at rest, the voltage-dependent blockade prevents ions from flowing through the open NMDA channel despite the presence of glutamate. Thus, the majority of stimulus-related activity, at least in adults, is carried by non-NMDA glutamate receptors, which mediate brief depolarizations lasting tens of milliseconds at most. In contrast, if neurons are already partially depolarized, glutamate-induced activation of NMDA receptors leads to significant current flow lasting hundreds of milliseconds and thus producing robust postsynaptic response. NMDA receptor responses are also nonlinear, with small changes in input leading to increasingly large changes in output until saturation is reached. Many brain processes, including both sensory response and activation/deactivation of neural ensembles, depend upon nonlinear processes and thus on underlying NMDA function.
NMDA receptors also differ from other glutamate receptors in that they permit inflow of calcium, as well as sodium, ions. After entering the cell through open, unblocked NMDA receptors, calcium ions activate a second messenger cascade that leads to alteration of the connections between the pre- and post-synaptic neuron via long-term potentiation (LTP) and long-term depression (LTD). LTP and LTD are the primary process by which the brain encodes new information. Deficits in NMDA function would thus lead to deficits in the ability to acquire new information, as has been observed in schizophrenia.
Neuroscientist Donald Hebb, writing in the 1950s, proposed that the ability to learn new information (for example, the relationship between the unconditioned and conditioned stimulus in classical conditioning) depends upon the existence of discrete neural elements that could integrate information from two or more separate neural pathways (Cotman & Monaghan 1988). NMDA receptors possess this property in that they are sensitive both to membrane potential, which may be controlled by one input pathway, and to the presence or absence of presynaptic glutamate release, which may be controlled by another. As a result, NMDA receptors function in a Hebbian fashion and thus serve a unique role in brain information processing (Cotman & Monaghan 1988, Javitt 2007).
BOTTOM-UP CONTRIBUTIONS TO IMPAIRED PROCESSING IN SCHIZOPHRENIA
Given the wide distribution of NMDA receptors in schizophrenia, glutamatergic models suggest that deficits should be observed even within primary sensory cortices, but only involving processes directly mediated by NMDA, as opposed to non-NMDA, glutamate receptors. The role of NMDA receptors has been most extensively studied within the early auditory and visual systems, permitting stage-of-processing deficits to be tracked from early to late processes within and across these sensory systems. Nevertheless, NMDA receptors are abundant throughout other brain systems as well, including somatosensory and olfactory brain regions. Documented patterns of dysfunction across cortical domains ( Javitt et al. 1999) thus fit well with predictions of glutamatergic/NMDA models of schizophrenia.
The Auditory System
Deficits in auditory ERP generation in schizophrenia have been documented since at least the early 1970s, with the demonstration of impaired generation of the auditory P300 potential (Roth & Cannon 1972). P300 is generated in response to attended, task-relevant stimuli and reflects late (>300 ms latency) activity in brain regions such as temporo-parietal and prefrontal cortices. Deficits in P300 in schizophrenia thus reflect impaired attention-dependent processing in higher cortical regions. Relatively little attention was paid to earlier stages of processing until the early 1980s, with the demonstration of mismatch negativity (MMN). MMN serves as an objective index of preattentive processing of stimulus deviance within primary auditory cortex (reviewed in Javitt 2000). Over recent years, deficits in early stages of auditory processing have been extensively documented and shown to relate to higher-order auditory disturbances using both neurophysiological and behavioral measures.
Neurophysiological measures of impaired early auditory processing
Functioning of the early auditory system can be assessed using ERPs, which trace sequential activation of the auditory system from brainstem through auditory thalamus (medial geniculate nucleus, or MGN) and primary auditory cortex (A1) to auditory association regions. Two sets of measures have proven particularly informative: MMN, which indexes function of the auditory echoic memory system, and auditory P1/N1, which index obligatory auditory responses to repetitive auditory stimulation. Deficits in both MMN and auditory P1/N1 generation may be traced to underlying dysfunction of NMDA activity at the level of primary auditory cortex.
Mismatch negativity
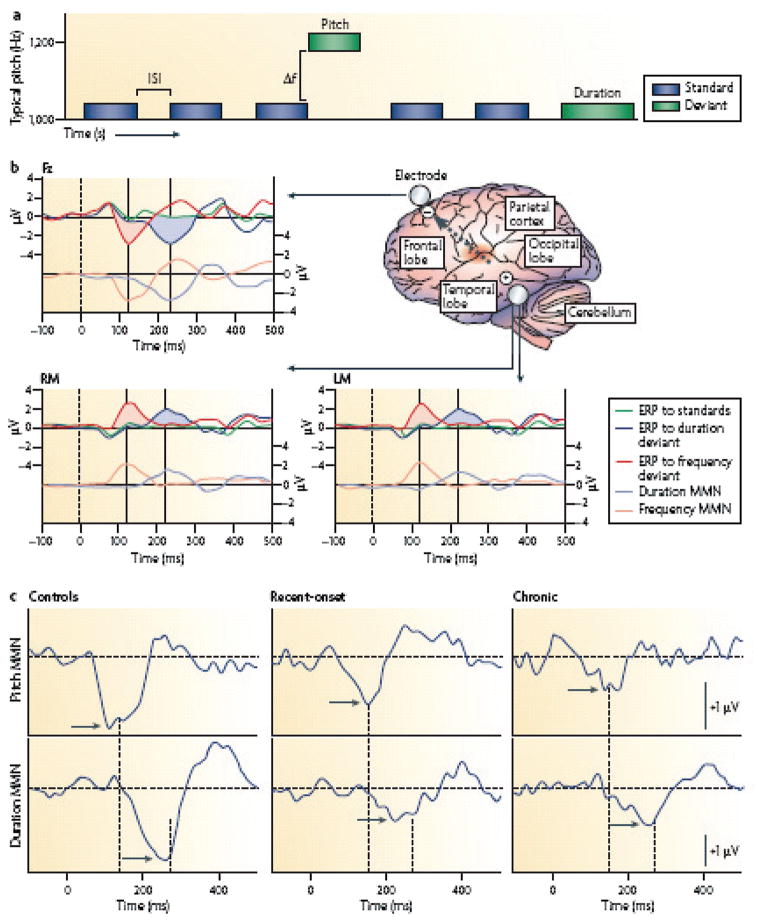
MMN reflects response of auditory cortex to infrequent changes in a repetitive pattern of auditory stimulation. Primary generators for MMN have been localized to auditory cortex based upon both ERP and magnetoencephalography (MEG) dipole mapping studies as well as upon direct intracranial recording in both human and primate models ( Javitt 2000). Deficits in MMN generation were first demonstrated in the early 1990s in response to duration and frequency (pitch) deviants. Since that time, these findings have been replicated more than 40 times, with minimal failures to replicate (Umbricht & Krljes 2005), making MMN one of the most strongly replicated biomarkers of neurocognitive dysfunction in schizophrenia ( Javitt et al. 2008) (Figure 2).
Figure 2.
Schematic diagram of mismatch negativity (MMN) generators in schizophrenia. (a) MMN is elicited in an auditory oddball paradigm in which a sequence of repetitive standard stimuli (blue boxes) is interrupted by stimuli that differ in a physical stimulus dimension such as pitch or duration ( green boxes). The deviant probability equals the number of deviants divided by the total number of stimuli. MMN reflects N-methyl-D-aspartate (NMDA)-dependent processing of stimulus deviance within the auditory sensory cortex. (b) Schematic diagram of MMN generators within the auditory cortex (located in the superior temporal lobe, shown in red ). Because of the orientation of MMN generators, the MMN reverses in polarity between the frontal midline electrode (Fz) and the left (LM) and right (RM) mastoids. Because pitch deviance can be detected at stimulus onset, but duration deviance can only be detected at the time of standard stimulus offset, duration MMN ( pale blue line) is delayed relative to pitch (frequency) MMN ( pink line). The dashed arrow indicates the orientation of the electrical field originating from the auditory cortex. Activity from auditory cortex characteristically inverts between the central midline electrode (Fz) and the mastoids (RM, LM) relative to a nose reference (not shown). (c) Characteristic waveforms at Fz from patients with recent onset or chronic schizophrenia versus controls. Peak MMN responses (arrows) are significantly reduced in patients with schizophrenia relative to controls, for both pitch (top line) and duration (bottom line). Dashed lines illustrate the latency shift in response to pitch versus duration to deviant stimuli. (From Javitt et al. 2008, p. 73.)
In primates, generators of MMN have been localized to superficial layers of auditory cortex and may be inhibited by direct application of NMDA antagonists within auditory cortex ( Javitt 2000, Javitt et al. 1996). The role of NMDA receptors in MMN generation has also been confirmed in ketamine challenge studies in humans using both ERP (Umbricht et al. 2000) and MEG (Kreitschmann-Andermahr et al. 2001) recordings, as well as in rodent studies ( Javitt et al. 2008, Tikhonravov et al. 2008). As opposed to NMDA antagonists, deficits in MMN generation are not induced by either D1 or D2 antagonists, or by psilocybin, a psychotomimetic that targets primarily serotonin 5-HT2A receptors ( Javitt et al. 2008).
In addition to the well-characterized temporal MMN generators, some researchers have also postulated frontal-MMN generators, although their existence remains a matter of controversy. Deficits in MMN generation cannot be attributed specifically to failure of frontal MMN generation, since deficits in MMN generation are observed both in MEG, which is insensitive to the putative frontal generator (Kreitschmann-Andermahr et al. 1999, Pekkonen et al. 2002, Thonnessen et al. 2008), and in fMRI, which shows activation deficits specifically within auditory cortex in schizophrenia (Mathiak et al. 2002). Whether there is an independent frontal MMN generator and whether it shows disturbances independent of the auditory cortical generator in schizophrenia thus remains an area of activate investigation.
Auditory P1/N1
In addition to MMN that is elicited by infrequent deviant stimuli, function of auditory cortex is also indexed by a series of components that are elicited by repetitive standards. Key potentials include auditory P100 (P1), which reflects activity primarily of primary cortex, and N100 (N1), which has generators primarily in both primary cortex and secondary auditory regions of planum temporale. Deficits in generation of N1 have been reported in schizophrenia since the early 1980s (Ford et al. 2001a), although the component remains relatively understudied.
More recently, it has been suggested that N1 amplitude is reduced when individuals are talking, rather than listening, suggesting that N1 may be modulated by top-down “collateral discharge” from speech areas and that such modulation may be impaired in schizophrenia (Ford et al. 2001b). Nevertheless, N1 deficits are observed whether schizophrenia patients are talking or listening, suggesting that the top-down control deficits are not the primary determinants of the N1 abnormalities in schizophrenia. In intracranial studies, N1 generators are localized primarily to infragranular layers of auditory cortex ( Javitt 2000). As with MMN, deficits in N1 generation similar to those observed in schizophrenia may be observed in monkeys treated with NMDA antagonists ( Javitt et al. 2000a), suggesting that, as with MMN, deficits in N1 generation may serve as indices of NMDA dysfunction within sensory brain regions and thus support bottom-up models.
Behavioral correlates of auditory cortex dysfunction
Generation of both MMN and N1 are linked to functioning of the auditory echoic memory system (Cowan 1995, Javitt 2000) and thus predict behavioral-level disturbances in early auditory function as well. Deficits in tone matching in schizophrenia had been reported, in fact, in schizophrenia in the early 1970s by Jonsson & Sjostedt (1973), who used the pitch intonation test. However, these findings were rarely cited and did not penetrate into general conceptualizations of schizophrenia at the time. Based upon the findings of impaired MMN generation in schizophrenia, a second series of studies was conducted beginning in the mid-1990s. These studies assessed not only the integrity of tone matching, but also the relationship of such deficits to the underlying physiology of the echoic memory system.
From animal studies, it was known that there is a double dissociation between effects of auditory and frontal cortical lesions on tone matching. Thus, lesions to auditory cortex elevate tone-matching thresholds even in the absence of distraction but do not affect susceptibility to distraction, whereas lesions of pre-frontal cortex do not affect thresholds in the absence of distracters but do increase susceptibility to distraction. This dissociation has been confirmed as well in humans with either auditory or prefrontal damage (Rabinowicz et al. 2000).
In studies conducted starting in the mid-1990s, patients were found to have elevated discrimination thresholds for tone matching even in the absence of distraction (Strous et al. 1995), with no increase in susceptibility to either visual ( Javitt et al. 1997) or auditory (Rabinowicz et al. 2000) distraction. Furthermore, patients showed normal sensory memory duration ( Javitt et al. 1997), with no increased susceptibility to auditory backward masking (March et al. 1999), suggesting that, as with other forms of working memory, deficits occurred primarily at the encoding stage. In chronic patients, deficits are most severe in patients with poor global outcome (Rabinowicz et al. 2000, Wexler et al. 1998). In first-episode patients, a subgroup of patients show elevated tone-matching thresholds (Rabinowicz et al. 2000), suggesting that tone-matching performance may be used to detect etiologically relevant subgroups.
Although the majority of studies have focused on pitch discrimination, deficits have also been observed in other relatively simple auditory tasks such as detection of abnormal tunes using the Distorted Tunes Task, a task specifically designed to evaluate genetic determinants of musical ability (Leitman et al. 2005) as well as of auditory memory (Robles et al. 2008). Deficits in both pitch ( Javitt et al. 2000b) and duration (Todd et al. 2003) discrimination in schizophrenia correlate with impaired generation of auditory MMN and N1 (Rojas et al. 2007) potentials, confirming the relationship between behavioral and neurophysiological indices.
Upward Consequences of Primary Auditory Impairments
Given the deficits in the ability of patients with schizophrenia to recognize differences between tones, one would expect deficits in processes dependent upon tone-matching ability. In Western languages, variations in pitch are used to convey different types of information. Rapid changes, for example, form the basis for individual speech elements (phonemes), whereas slower changes permit information to be conveyed by “tone of voice” (prosody). Finally, changes in the acoustic environment are a critical trigger for capture of attention.
Auditory deficits in schizophrenia, such as impairments in auditory emotion detection, have traditionally been considered to reflect impaired function within higher-level regions such as limbic cortices. More recent studies, however, find subjective experience of emotion to be quite normal (Kring et al. 2003). Bottom-up models of auditory dysfunction thus provide an alternative framework for interpretation of conflicting conceptualizations of neurocognitive dysfunction in schizophrenia.
Upward consequences 1: phonetic processing
One of the simplest processes that may be affected by impaired tone-matching ability is that of auditory comprehension. In Western languages, elemental speech sounds (phonemes) reflect complex tonal modulations, with different phonemes conveyed by different combinations of pitches, intensities, and intervals between successive energy bursts. Thus, disturbances in processing of the elemental sensory features might lead to impairments even in a process as simple as speech recognition.
As with other aspects of sensory processing dysfunction in schizophrenia, deficits in speech perception are embedded within older schizophrenia literature but are not emphasized in current conceptualizations of the disorders. For example, Lawson et al. (1964) documented deficits in speech comprehension in schizophrenia that reflected inability to take advantage of speech regularities, whereas Kugler & Caudrey (1983) showed deficits specifically in processing of phonemes (e.g., /ba/ versus /da/). Nevertheless, these early findings were not strongly pursued until recent years.
A more recent study (Cienfuegos et al. 1999) evaluated phonological processing in schizophrenia more formally using a psychoacoustically validated /da/-/ba/ continuum. Although patients performed as well as controls in differentiating extreme /ba/ and /da/ exemplars, they were significantly more variable than were controls in response to stimuli toward the middle of the continuum. Subsequent studies used MMN to further probe the system and demonstrated significantly impaired MMN generation to categorical changes in speech sounds in schizophrenia using both high-resolution electroencephalography (Bruder et al. 2001, Kasai et al. 2002) and MEG (Kasai et al. 2003) techniques.
Deficits in phonological processing may also lead to reading impairments in schizophrenia. A recent study has demonstrated that patients show significantly impaired performance on tests of reading, with particular deficits on tests of phonological awareness (Revheim et al. 2006). Findings that tone-matching deficits in patients with schizophrenia are accompanied by phonological-processing impairments are consistent as well with findings of impaired MMN to frequency (Kujala et al. 2006) and frequency-modulated-deviant (Stoodley et al. 2006) tones in developmental dyslexia. Over recent years, deficits in phonetic MMN generation have been found to correlate with impairments in both verbal memory and social skills acquisition in schizophrenia (Kawakubo et al. 2007), emphasizing the importance of early-stage auditory dysfunction.
Upward consequences 2: prosodic processing and social cognition
A second major process that relies upon intact tonal discrimination ability is interpretation of prosody. Prosody is a general term that refers to the “intonation pattern (pitch contour) of speech, word stress (a complex subjective variable based on timing, pitch, and loudness), pauses that sometimes occur at the ends of major syntactic elements of sentences, and the lengthening of final rounds in words immediately prior to close boundaries” (Edwards et al. 2002). Such modulations operate across specific speech segments (e.g., syllables, words) to convey additional meaning from that conveyed by the words themselves.
As opposed to the millisecond-range pitch transitions that contribute to phonetic processing, prosodic modulations occur over hundreds of milliseconds to seconds. Prosody is studied most intensively with regard to emotion (affective prosody), with certain features such as pitch or intensity modulation contributing largely to the perception of happiness or anger, respectively ( Juslin & Laukka 2003). As with speech sounds, emotions may be perceived categorically, with discrimination between sentences being easier across, rather than within, emotional boundaries (Laukka 2005).
Other types of suprasegmental information are also conveyed by pitch modulations. For example, whether an utterance is a statement or question is conveyed largely by whether the pitch increases or decreases at the end of a sentence (interrogative prosody). Similarly, word meaning may be significantly altered by location of stress across syllables (semantic prosody), whereas meaning of a sentence may be conveyed by location of stress across words. The common attribute of sarcasm, for example, may be conveyed simply by changing stress in a sentence from the final word to an earlier word (attitudinal prosody). Deficits in basic auditory perceptual abilities would thus be expected to compromise ability to decode suprasegmental auditory information, leading to deficits in decoding specific types of prosodies.
Deficits in the ability of patients with schizophrenia to identify the intended emotion of auditory stimuli have been well recognized since at least the early 1970s. Early studies stressed that emotion deficits were most likely part of a more generalized sensory-processing deficit. In contrast, subsequent studies proposed more specific involvement of limbic circuitry or global right hemisphere dysfunction (reviewed in Edwards et al. 2001). A deficit in the ability to identify emotion based upon tone of voice and facial expression is the primary contributor to the construct of “social cognition,” which refers to the ability of subjects to utilize cognitive processes to engage in social interaction. A deficit in social cognition, in turn, is considered one of the primary determinants of functional outcome in schizophrenia (Brekke et al. 2005). Consequently, deficits in auditory emotion detection, to the extent that they occur in schizophrenia, may be considered primary determinants of impaired functional outcome in schizophrenia.
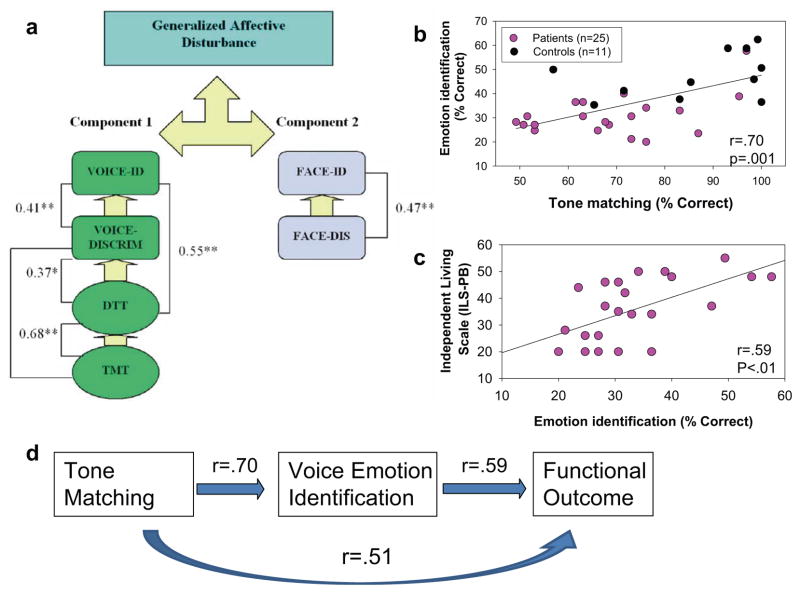
A series of studies over the past several years have examined the relationship between impaired prosodic ability in schizophrenia and underlying deficits in auditory sensory processing. An initial study evaluated relative dysfunction of auditory and visual emotion-detection ability in schizophrenia relative to impaired sensory processing as measured using both the Tone Matching and Distorted Tunes Tasks. As expected, deficits in auditory emotion recognition were observed in schizophrenia and were correlated with impaired auditory sensory ability. Deficits in visual emotion recognition ability were observed as well. However, visual emotion-processing deficits were not correlated with either auditory sensory or auditory emotion measures, suggesting that emotion-processing deficits in schizophrenia cluster within, rather than across, modalities (Leitman et al. 2005) (Figure 3a).
Figure 3.
Relationship between tone matching and voice emotion discrimination in schizophrenia. (a) Factor analysis showing separate clustering of auditory versus visual affective discrimination tests, and interrelationship among impairments in a tone matching task (TMT), distorted tunes task (DTT), voice emotion discrimination (VOICE-DISCRIM), and voice emotion identification (VOICE-ID) in schizophrenia, with separate clustering of face emotion discrimination (FACE-DIS) and face emotion identification (FACE-ID) tasks. Values are correlation coefficients (r); * = p < 0.05, ** p < 0.01. (From Leitman et al. 2005.) Scatter plots show relationship between (b) tone matching and voice emotion identification and (c) voice emotion identification and global outcome, as reflected by the Independent Living Scale. (From Leitman et al. 2008.) (d ) Schematic diagram of bottom-up relationships between impaired auditory sensory processing.
A second study evaluated auditory emotion processing relative to other forms of prosodic impairments as well as structural integrity of auditory cortex as measured using diffusion tensor imaging (DTI) of auditory projection systems. Deficits in emotion recognition ability were again observed and were accompanied by deficits in other forms of prosody, including semantic and interrogative prosody. Furthermore, deficits in affective prosodic ability correlated with impaired integrity of primary auditory radiations, supporting bottom-up models (Leitman et al. 2008). In a parallel study, deficits in attitudinal prosodic detection were observed as well, and the deficits correlated with underlying impairments in tone-matching ability (Leitman et al. 2006).
A final study evaluated the degree to which patients could utilize specific types of acoustic information in making emotional discriminations using a prosodic stimulus set with well-characterized psychoacoustic properties (Juslin & Laukka 2001). Several potentially salient acoustic features were analyzed. These included several pitch-based measures including degree of pitch variability of the fundamental frequency of each utterance, the tonal contour, and the mean pitch of each utterance, as well as two intensity-based measures—absolute voice intensity and variability of intensity.
For normal volunteers, increased levels of pitch variability increased the degree to which they were able to identify intended happy stimuli as portraying happiness, whereas decreased levels increased the degree to which they were able to identify fearful stimuli as portraying fear. Similarly, pitch contour assisted in distinguishing fearful from sad stimuli, whereas reduced mean pitch gave rise to the percept of disgust. Patients showed significantly less ability to take advantage of these features and so showed similar degrees of correct identification across levels of pitch cue. In contrast, patients were able to take advantage of intensity cues, which contributed primarily to the percept of anger, suggesting a relatively specific deficit in utilization of pitch-related information (Leitman et al. 2008).
As in prior studies, deficits in tone matching correlated significantly with deficits in voice emotion identification (Figure 3b), which in turn correlated with functional outcome (Figure 3c). Significant direct correlations with functional outcome were observed as well, suggesting additional paths from basic sensory deficits to poor outcome in schizophrenia (Figure 3d).
Upward consequences 3: attention
A final domain in which deficits in early auditory processing affect more global aspects of function in schizophrenia is in the realm of attention. Although top-down aspects of attention have been extensively studied in schizophrenia, bottom-up aspects have received much less investigation. In top-down attention, also known as “endogenous” attention or attentional allocation, the central executive acts on sensory/perceptual filters to help select which aspects of the sensory surround receive allocated, capacity-limited processing resources. In contrast, in bottom-up attention, also known as “exogenous attention” or “attentional capture,” nonattended features of the environment become salient and capture attention, producing an automatic alerting response. These attentional systems produce complementary but often opposing influences on allocation of limited-capacity information-processing resources (Cowan 1993).
In change detection/attentional alerting paradigms, preattentive transient/change detection is indexed by the N1 and MMN, which are elicited preattentively in auditory cortex. Subsequent ERP components, such as novelty P3 (P3a), index the degree to which the N1/MMN processes lead to effective attentional capture. Increasing distraction, for example by increasing load of a simultaneous visual working memory task, reduces P3a amplitude but does not alter generation of N1 or MMN, suggesting that top-down attentional mechanisms do not gate input at the level of primary sensory cortex, but rather mostly prevent attention generated in auditory cortex from influencing higher-order brain regions (San Miguel et al. 2008). Thus, whereas deficits in MMN may lead to impairments in bottom-up, attentional capture, it is unlikely that they are caused by failures of top-down attentional allocation.
In normal volunteers, both MMN and P3a amplitudes during oddball tasks correlate with level of psychosocial functioning, as measured using a modified version of the Global Assessment of Function scale (Light & Braff 2005). P3a amplitude is also highly associated with immediate and delayed recall of verbal information (Light et al. 2007), suggesting that bottom-up attentional capture assists in the engagement of voluntary attentional mechanisms to the task at hand. Failure of automatic attentional capture in schizophrenia, therefore, may lead to disconnection of patients from sources of potential stimulus salience and ultimately to impaired ability to focus on appropriate aspects of the surrounding acoustic environment.
The Visual System
Early writers on schizophrenia assumed that individuals with schizophrenia were not blind and that early stages of sensory processing must be intact. However, even more than in the auditory system, patients do self-report perceptual distortions very early in the course of the illness. For example, McGhie & Chapman (1961) provide the following quotations from individuals at early stages of their illness:
Everything is in bits. You put the picture up bit by bit into your head. It’s like a photograph that’s torn in bits and put together again.
I don’t like moving fast…. Everything would be a jumbled mass. I have found that I can stop this from happening by going completely still and motionless.
Scales for detection of visual disturbances were subsequently operationalized by Klosterkotter et al. (2001), who reported that alterations in visual perception predicted progression to schizophrenia among prodromal individuals more strongly than did more traditional cognitive measures such as ideas of reference or language disturbance. Specific visual symptoms found to be discriminative include “changed perception of face or body of others,” “hypersensitivity to light or certain optic stimuli,” “changed perception of patient’s own face,” and “captivation of attention by details in the visual field” (Schultze-Lutter et al. 2007). Although they are nonspecific, these subjective perceptions point to sensory-level disturbances in visual processing.
Additional information regarding visual dysfunction came from visual backward masking studies in the early 1980s. Deficits were found particularly within the 60–500 ms interval following stimulus presentation (Braff & Saccuzzo 1985). Their findings, therefore, point to bottom-up deficits involving very early stages of visual information processing. At the time that visual processing deficits in schizophrenia were first described, visual systems were divided into discrete “transient” and “sustained” channels based upon psychophysiological studies in humans. The backward masking deficits were interpreted as showing hyperactive transient system response (Green et al. 1994), although underlying neural mechanisms were not identified.
Magnocellular and parvocellular visual systems
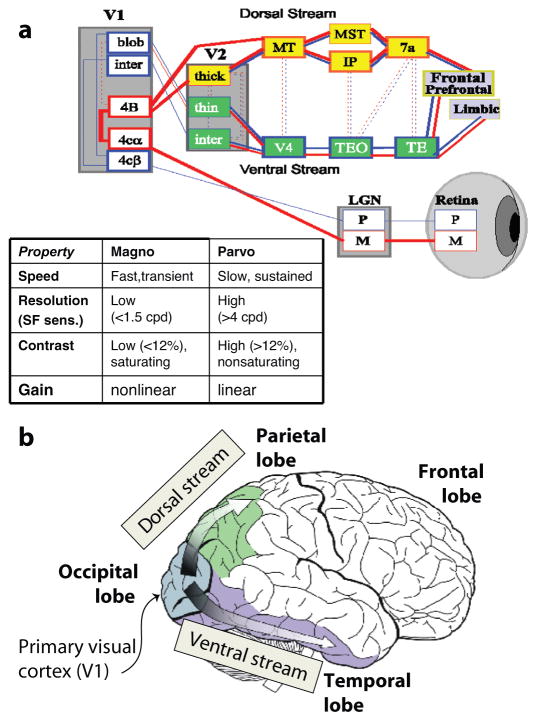
The early visual system is divided into two main components, the magnocellular and the parvocellular visual systems, that project from retina to primary visual cortex (striate cortex; V1) via the lateral geniculate nucleus (LGN). The magnocellular pathway consists of relatively few large neurons that transmit rapid, low-resolution information primarily to the dorsal visual stream, which is critical for orienting attention in space and is thus frequently referred to as the “where” system. In contrast, the parvocellular system transmits more fine-grained information more slowly and projects preferentially to the ventral visual stream, which is critical for object identification and is thus frequently referred to as the “what” system (Figure 4).
Figure 4.
(a) Schematic model of visual system showing projections of the magnocellular (M) and parvocellular (P) pathways, which project preferentially through lateral geniculate nucleus (LGN) to primary visual cortex (V1) and then to dorsal (“where”) and ventral (“what”) streams. (b) Illustrations of anatomic arrangements of visual regions (cartoon source: Google commons). (inset) Relative properties of magnocellular and parvocellular neurons.
Although the magnocellular and parvocellular systems are largely segregated at the subcortical level (retina and LGN), there is considerable cross-talk between systems at the cortical level. In particular, the systems appear to operate in a “frame and fill” manner, in which rapid transfer of information through the magnocellular and dorsal stream pathway sets up a low-resolution information template in the ventral visual stream pathway that is then filled in by the much slower-to-arrive parvocellular information (Chen et al. 2007, Kveraga et al. 2007). Activity within the ventral stream can also be divided into feed-forward, which reflects response to the parvocellular input, and recursive, which reflects interaction among early visual regions (Lamme & Roelfsema 2000).
The role of NMDA receptors has also been studied to a significant degree in animal models. In animals, magnocellular neurons function in a nonlinear gain mode, in which they show rapid increase in firing at low contrast levels but saturating response at higher contrast levels. This response profile is also frequently described as gain control, in that the degree of gain decreases with increasing contrast. NMDA receptor antagonists infused into V1 or LGN produce a characteristic effect in which they reduce the nonlinearity of the response. Infusion of NMDA receptors into LGN also preferentially affects activation of lagged cells, which may serve as the initial neuronal mediators of motion detection (Heggelund & Hartveit 1990, Kwon et al. 1991) while also preventing refinement of directional sensitivity within primary visual cortex (Rivadulla et al. 2001).
Thus, although NMDA receptors are not confined to either the magnocellular or parvocellular subsystems, processes that are selectively mediated by NMDA receptors such as nonlinear gain and motion detection may be more critical to magnocellular than to parvocellular processing. NMDA dysfunction would thus be expected to produce a greater effect on magnocellular than on parvocellular function based upon functional, rather than anatomic, considerations.
Neurophysiological approaches to early visual processing
As in the auditory system, functioning of the early visual system is most effectively studied using electrophysiological techniques, which can isolate activity both temporally and spatially. Although the magnocellular and parvocellular pathways cannot be disentangled anatomically, they can be functionally differentiated using preferred stimuli. For example, low-contrast, low-spatial-frequency stimuli are relatively selective for the magnocellular pathway, whereas high-contrast, high-spatial-frequency stimuli are relatively selective for the parvocellular pathway.
Two complementary neurophysiological techniques are used to probe function of the early visual pathway. In the steady-state visual-evoked potential (ssVEP) approach, stimuli are modulated at a set frequency (generally 4–12 Hz), and electrophysiological responses are analyzed in the frequency domain at the fundamental frequency (i.e., the frequency at which stimuli are presented) or harmonics thereof. In the transient ERP approach, discrete stimuli are presented repetitively at intervals long enough to permit the electrophysiological response to each individual stimulus to resolve before the next one is presented (generally >250 ms). Responses are then averaged across trials to differentiate stimulus-related from background electrical activity. Finally, fMRI can be used along with magno- and parvocellular-biased stimuli to isolate deficits at the level of primary visual cortex (V1).
ssVEP
ssVEPs have been studied in schizophrenia using a variety of stimulus types aimed at differentiating magnocellular and parvocellular responses. In general, low-contrast, low-spatial-frequency, moving, and rapidly flickering stimuli favor the magnocellular system, whereas high-spatial-frequency, chromatic contrast (i.e., color), and static stimuli favor the parvocellular system. High-contrast stimuli activate both systems simultaneously. However, if a pedestal is used to produce a standing sustained level of contrast, additional increments of contrast activate primarily the parvocellular system because responses within the magnocellular system are already saturated (Butler et al. 2008a).
Butler and coworkers performed an initial series of studies beginning in 2005 (Butler & Javitt 2005). These researchers assessed ssVEP as a function of luminance contrast, spatial frequency, and chromatic contrast. Patients showed greater deficits when stimuli were presented without, rather than with, a contrast pedestal, consistent with preferential dysfunction of the magnocellular system. Deficits were also seen to lower, but not higher, spatial-frequency stimuli. In contrast, responses to isoluminant chromatic stimuli did not differ significantly between groups, consistent with relative preservation of parvocellular system function. A subsequent study supported this finding and demonstrated a significant correlation between impaired ssVEP generation and reduced integrity of optic radiations from LGN to V1, also consistent with dysfunction of early visual-processing pathways in schizophrenia (Butler et al. 2005).
Other ssVEP paradigms have also been shown to be sensitive to visual impairment in schizophrenia. Kim et al. (2005a) used a windmill-dartboard-type stimulus, which gives a motion percept that occurs at even harmonics of the stimulus frequency. Deficits were seen at even harmonics but not at base frequency, consistent with a deficit in motion perception. ssVEPs have also been analyzed as a function of stimulation rate to high-contrast stimuli. Deficits in schizophrenia were particularly severe at more rapid stimulation rates (>17 Hz) (Krishnan et al. 2005), which favor the magnocellular system, also supporting greater dysfunction in magnocellular versus parvocellular circuits.
Deficits in magnocellular ssVEP generation similar to those observed in schizophrenia are seen in animal models following local infusion of NMDA antagonists into LGN or V1 (Figure 5). Deficits thus do not appear to reflect loss of magnocellular neurons per se (Selemon & Begovic 2007), but rather reflect deficits in neuronal function. Furthermore, although the parvocellular system is less affected than is the magnocellular system, consistent with its lesser dependency on nonlinear amplification processes, some reduction in parvocellular function nonetheless may be observed.
Figure 5.
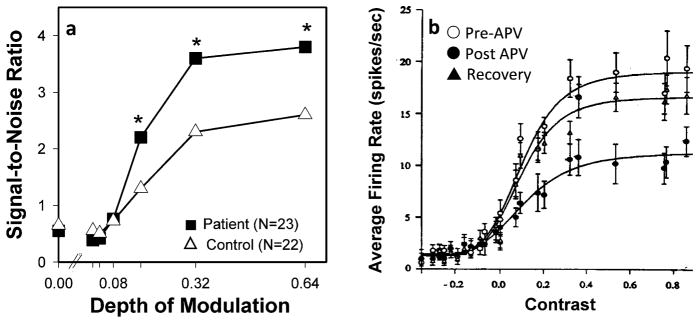
Comparative deficits in visual activation in schizophrenia patients versus cats treated with the NMDA antagonist 2-amino-5-phosphonovaleric acid (APV). (a) ssVEP amplitude (signal-to-noise ratio) in schizophrenic patients in response to magnocellular-biased stimuli. (From Butler et al. 2005.) (b) Effects of APV infusion into cat lateral geniculate nucleus (LGN) in response to stimulation. (From Kwon et al. 1991.)
Transient VEP
As compared to ssVEP, transient visual ERP approaches afford less opportunity for psychophysical manipulation of stimuli but offer greater opportunity to trace sequential activation through cortex. Disturbances in generation of transient visual ERP in schizophrenia have been reported since at least the late 1960s. However, only in recent years have the generators of the transient visual ERP been sufficiently characterized to permit assessment of underlying brain function (Butler et al. 2008a).
The major components of the visual ERP consist of an initial C1 component (peak latency ~90 ms) that C1 localizes to primary visual cortex, followed by P1 (90–120 ms), which has dual sources in dorsal and ventral visual streams, and N1 (~170 ms), which has a predominant component in the lateral occipital cortex (LOC) (Martinez et al. 2007). The C1 and N1 components are larger to stimuli that favor the parvocellular visual stream, consistent with predominant parvocellular input to primary visual cortex and ventral stream structures such as LOC. In contrast, P1 is larger to stimuli that favor the magnocellular visual system, consistent with a predominant magnocellular/dorsal stream component (Schechter et al. 2005).
As with ssVEP, the integrity of magnocellular and parvocellular systems has been evaluated using stimuli that favor the two systems. Deficits in dorsal stream P1 generation in schizophrenia were first reported in response to fragmented stimuli designed to address object recognition processes and subsequently to low-luminance-contrast, but not chromatic-contrast, stimuli (Schechter et al. 2005). Deficits in C1 and N1 generation were observed but did not survive covariation for between-group differences in visual acuity.
Most recently, findings of differential deficit to magnocellular-versus parvocellular-biased stimuli have been confirmed with high-density recording. Analyses focused primarily on dorsal and ventral P1 generators, which reflect initial activation of dorsal and ventral visual streams, respectively, and N1, which reflects subsequent stage processing primarily within the LOC, a ventral stream region. Patients showed intact responses of both P1 and N1 in response to high-spatial-frequency stimuli (parvo biased), but impaired response to low-spatial-frequency and low-contrast stimuli (magno biased). Thus, whereas deficits are expressed over dorsal versus ventral stream, as with ssVEP, the deficits appear to be driven by impairments arising subcortically within the magno-versus parvocellular pathways (Butler et al. 2007).
Since their initial demonstration in schizophrenia, deficits in P1 generation, particularly over dorsal stream, have been demonstrated in individuals at increased familial risk for schizophrenia (Yeap et al. 2006) as well as in individuals expressing a dysbindin risk haplotype for schizophrenia (Donohoe et al. 2008). Deficits have also been observed in response to abstract shape stimuli in a visual working-memory task (Haenschel et al. 2007) and to both attended and unattended stimuli in a spatial attention task (Luck et al. 2006). Other studies, however, have failed to find deficits in P1 generation in response to various stimulus types (e.g., van der Stelt et al. 2004) and have thus posited that early visual processes are normal. Critical issues, however, include types of stimuli used (magno biased versus parvocellular biased), location of recording electrodes (dorsal versus ventral stream), and latency range (early versus late).
fMRI
Finally, integrity of early visual processing in schizophrenia may be evaluated using fMRI. Early visual fMRI studies in schizophrenia used nonspecific checkerboard- or flash-type stimuli and reported normal or even increased response within primary visual cortex despite evidence of reduced activation within the dorsal visual stream (Braus et al. 2002).
A more recent study (Martinez et al. 2008), however, evaluated primary visual activation to low-versus high-spatial-frequency stimuli presented at low and high contrast. This study demonstrated significant, large effect-size deficits, particularly to stimuli biased toward magnocellular activation. Reduced activation was also observed in secondary visual regions, including middle occipital, middle temporal, and lingual gyrus, even to passively presented stimuli. In all cases, reductions reflected predominant loss of the low-spatial-frequency response, with compensatory increase in response to high-spatial-frequency response (Martinez et al. 2008).
Other fMRI studies have documented deficits in early-stage visual activation during a working memory task in adolescents with schizophrenia (Haenschel et al. 2007) and during a language task in individuals at increased familial risk for schizophrenia (Li et al. 2007). Overall, as with ERP studies, fMRI findings therefore demonstrate deficits in early visual processing when appropriate stimuli are used and suggest that such deficits may lead to secondary impairments in activation of higher cortical regions.
Behavioral correlates of early visual dysfunction
Over recent years, function of the early visual system in schizophrenia has also been extensively evaluated using behavioral paradigms. Two main types of paradigms have been used. First, “gain control” paradigms have investigated the ability of subjects to detect simple visual stimuli, such as low-contrast sine wave gratings or flickering lights. Second, “integration” paradigms have evaluated the degree to which subjects extract a pattern from disconnected stimulus elements (Butler et al. 2008a).
Patients show deficits in both types of paradigms, a finding that suggests a dysfunction of early stages of visual processing. In gain control paradigms, patients show the need for higher levels of contrast or longer stimulus durations before they are able to detect visual stimuli, which suggests a relative inability to amplify weak signals within the early visual pathways. These deficits are most severe when conditions are optimized to bias processing toward the magnocellular system (Butler & Javitt 2005).
In integration paradigms, patients show decreased ability to derive contours from distributed stimulus elements, also reflecting a relative inability to utilize the lower-spatial-frequency components of the display (Butler et al. 2008a). In normal volunteers, “global” (i.e., low-spatial-frequency) information is processing preferentially to “local” (i.e., high-spatial-frequency) information, in part because of its more rapid access to cortex. Thus, when the two streams of information compete, normal subjects perform less well when they must inhibit a global task (e.g., identify a shape from distributed dots) to perform a local task (e.g., count the dots). As first noted by Place & Gilmore (1980), individuals with schizophrenia perform paradoxically better when they must perform the local task in the presence of competing global information, consistent also with relative reduced sensitivity to low-versus high-spatial-frequency information. Deficits in integration have been reported in first-episode subjects, but not in ultra-high-risk prodromal subjects (Uhlhaas et al. 2006).
Although effects of NMDA antagonists have not been studied, Uhlhaas and coworkers have observed deficits in perceptual grouping in ketamine abusers that are similar to those observed in schizophrenia. In ketamine abusers, deficits were seen during the night of abuse but not three days later, when abusers were drug free (Uhlhaas et al. 2007). Similarly, Kurylo and coworkers have demonstrated decreases in perceptual organization by proximity and grouping in schizophrenia (Kurylo et al. 2007) that are similar to those observed in ketamine-treated rodents (Kurylo & Gazes 2008), further suggesting a role of NMDA dysfunction in behavioral as well as neurophysiological manifestations of visual dysfunction in schizophrenia.
Upward Consequences of Primary Visual Impairments
As with auditory perception, deficits in low-level visual processing should lead to impaired performance on tasks that depend upon intact visual-processing ability. As in the auditory system as well, individuals with schizophrenia are not impaired on routine optometric testing (e.g., eye charts), nor are deficits necessarily obvious in routine interaction. The issue, however, is whether and how the deficits in lower-level visual processing that can be demonstrated with neurophysiological and psychophysiological investigation conspire to impair more complex processes necessary for everyday function.
Upward consequences 1: motion detection
One of the most basic functions of the magnocellular visual system is detection of motion. As noted above, magnocellular neurons give rise to the percept of motion, largely through the involvement of NMDA receptor-dependent mechanisms (Heggelund & Hartveit 1990, Kwon et al. 1991, Rivadulla et al. 2001). Decoding of motion is then dependent upon magnocellular projects from V1 to visual areas MT and MST, which are particularly involved in representation of general and coherent motion, respectively. Therefore, deficits in magnocellular function should by definition lead to impaired motion detection ability. In fact, significant deficits have been observed in both behavioral (Chen et al. 2006) and fMRI (Braus et al. 2002) studies.
Although most studies have evaluated coherent motion thresholds, one study evaluated relative thresholds for incoherent versus coherent motion detection. Similar degrees of deficit were observed in both conditions and found to correlate with contrast detection thresholds for magnocellular-biased stimuli, supporting bottom-up concepts of impaired motion-detection ability (Kim et al. 2006). Furthermore, basic deficits in motion-detection ability also lead to impairments in higher-order processing such as biological motion detection (Kim et al. 2005b) or theory-of-mind conceptualizations (Kelemen et al. 2005), which are often based upon fleeting motion signals in facial expression. Reduced ability of patients to cancel out self-induced sensory information during eye movement has also been linked to disorders of agency in delusion formation (Lindner et al. 2005). Finally, well-documented deficits in smooth-pursuit motion detection may reflect underlying deficits in motion processing rather than deficits specifically of eye movement control (Javitt et al. 2008).
As with other tests of visual processing, evaluation of motion detection may aid in early detection of schizophrenia. In one study, motion and form coherence thresholds were measured in offspring of mothers with schizophrenia or bipolar disorder versus normal controls. Even during childhood (<11 years of age), the rate of development in the motion task was less pronounced in children of mothers with schizophrenia than in the other groups, whereas development of form perception was spared, suggesting that the progressive developmental abnormality of motion-sensitive visual areas may be a characteristic feature of schizophrenia vulnerability (Keri et al. 2006).
Upward consequences 2: perceptual closure
A second process that depends heavily upon intact interaction between the magnocellular and parvocellular visual streams is the ability to identify objects based upon partial information, otherwise known as perceptual closure. In common experience, individuals are often confronted with only fragmentary information (e.g., “cat behind the Venetian blind”), yet the brain is able to reconstruct the whole from the disconnected parts. Neurophysiological mechanisms underlying the perceptual closure process have been extensively evaluated over recent years, permitting assessment of mechanisms underlying dysfunction in schizophrenia.
As with other sensory-level deficits in schizophrenia, the concept of impaired perceptual closure in schizophrenia is not new. An initial study (Snyder 1961) evaluated the degree to which schizophrenia patients unconsciously filled in gaps when asked to reproduce a series of dashed-outline figures. Despite being told to reproduce the figures exactly as shown, controls tended to fill in the gaps in the dashed lines, whereas patients did not. Thus, patients were paradoxically more accurate on this task, reflecting, however, impaired underlying perceptual closure processes.
More detailed study of the perceptual closure process was enabled starting in the mid-1980s by the construction of a standardized, normed set of fragmented images for clinical investigation. In the most common implementation of the paradigm, an ascending method of limits approach is used in which objects are presented at progressively more complete levels until subjects are able to correctly identify the object. Thresholds for closure are then compared across individuals. Identification level can also be manipulated by several factors including stimulus repetition and semantic priming (Doniger et al. 2001).
When a stimulus that had previously been successfully identified is repeated, subjects are able to identify it at a higher level of fragmentation on repeat than on initial presentation. Similarly, if subjects are given a word cue that might identify the object, they identify the object at a more fragmented level when the cue is correct than when it is either incorrect or neutral. These manipulations thus permit assessment of relative integrity of bottom-up versus top-down mechanisms involving perceptual closure in schizophrenia.
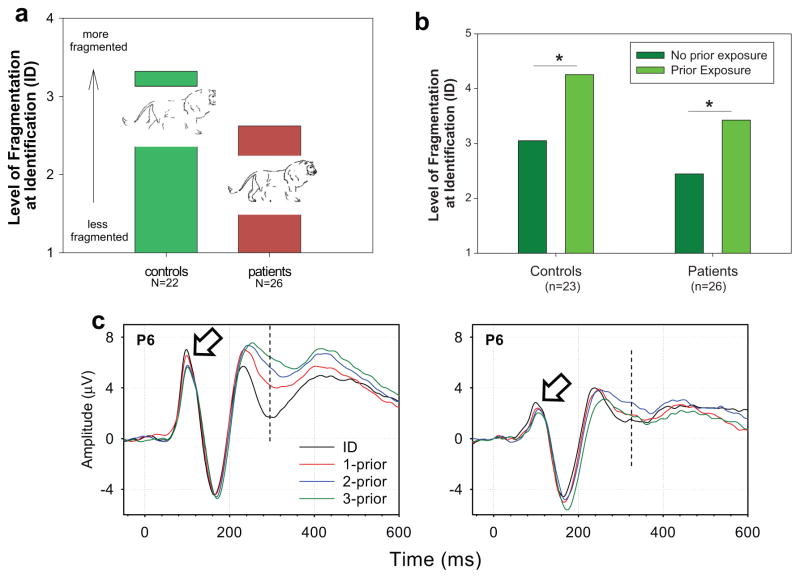
In an initial study, patients with schizophrenia were found to require significantly more complete (less fragmented) images than did controls in order to successfully identify objects (Figure 6a). However, patients showed gains in performance equivalent to those of controls to either stimulus repetition or semantic priming (Figure 6b). Although there are clearly both top-down and bottom-up elements to perceptual closure processes, the fact that patients showed deficits in the simplest condition (unprimed stimuli) while being able to take full advantage of both repetition and semantic-level priming suggests that deficits may particularly affect the bottom-up components of the system (Doniger et al. 2001).
Figure 6.
Perceptual closure performance in patients and controls. (a) Need for reduced level of fragmentation (more complete figure) for object identification in patients relative to controls. Insets show examples of stimuli at the corresponding levels of fragmentation. (From Doniger et al. 2001.) (b) Effects of stimulus repetition in patients and controls. (c) Closure negativity (Ncl) generation in schizophrenia patients (Sz) versus controls (Ctl) showing reduction in P1 amplitude (open arrow) and Ncl (dashed line). (From Doniger et al. 2002.)
In a subsequent series of studies, ERPs were used to assess underlying substrates for perceptual closure. Stimuli were again presented using an ascending method of limits approach and were averaged according to proximity relative to eventual level of identification for each stimulus. Stimuli elicited a large, early P1 potential, reflecting initial dorsal stream activation, followed by an N1 potential, reflecting subsequent activation within the ventral stream. Closure processes did not affect these initial components but were reflected in a subsequent ERP deflection (latency ~270 ms), termed closure negativity (Ncl) visual area LOC. Amplitude of Ncl increased slowly and progressively as stimuli neared the level of identification, with a discontinuous increase at the level of identification, suggesting that this ERP component reflects the closure process within ventral stream visual object-recognition regions (Doniger et al. 2000) (Figure 6c).
In the surface ERP waveforms, little activity was seen over either frontal or temporal brain regions. However, subsequent studies have further elaborated the critical nodes in the closure process. First, a high-density ERP/fMRI study demonstrated differential activation of LOC to closeable versus noncloseable stimuli, supporting the initial localization of Ncl generators within the ventral visual stream. Furthermore, the study demonstrated a frontal temporal generator that was active with slightly delayed time course to the posterior Ncl, as well as differential activation in the dorsal stream temporo-parietal-occipital region (Sehatpour et al. 2006). Second, an intracortical recording study in human epilepsy patients showed additional differential activity in hippocampus, with beta-band synchrony between hippocampus, frontal cortex, and LOC during the closure process (Sehatpour et al. 2008).
Overall, these studies suggest that a widespread network is activated during the closure process, involving not only ventral stream object-recognition regions but also inferior frontal and hippocampal regions, with virtually simultaneous activation of all three nodes. This network is thus compatible with frame-and-fill models of visual function, in which low-resolution information is projected rapidly to the frontal brain area via the dorsal stream, concurrent with slower projection of high-resolution information to the ventral stream. The rapid projection system permits initial “guesses” to be made, which then facilitate processing within ventral stream object-recognition regions (Chen et al. 2007, Kveraga et al. 2007). Deficits in magnocellular function would thus impair the feedback processing while leaving feedforward processing within the ventral stream relatively intact.
When Ncl was studied in schizophrenia, patients showed significantly reduced amplitude of both the early visual P1 component which reflects initial dorsal stream activation, and of the subsequent Ncl component, which reflects late processing within the ventral stream. In contrast, generation of the N1 was unimpaired, suggesting relatively intact initial ventral stream activation via the parvocellular system. These findings support a model in which direct input to the ventral visual stream via the parvocellular system is intact, but indirect input directed initially through the magnocellular visual system and subsequently through the dorsal visual stream is impaired (Doniger et al. 2002).
Upward consequence 3: face recognition/ reading
Finally, as in the auditory system, intact early visual function is critical for processes such as face emotion recognition. Deficits in the ability of patients to recognize facial emotion is well replicated and, along with impairment in auditory emotion recognition, forms the operationalized basis for impaired social cognition in schizophrenia. As with other aspects of impaired cognition in schizophrenia, the critical issue is whether face emotion recognition deficits in schizophrenia reflect high-order dysfunction in regions that process fear, such as amygdala, or instead reflect a simple inability of patients to decode the complex facial configurations that are used by people to convey specific emotions. Such distinctions are critical both to conceptualizations of schizophrenia and to potential remediation approaches.
One critical issue in the face emotion processing literature is whether deficits are confined to emotion identification, which would support a relatively high-order emotion deficit, or whether such deficits affect other types of facial processing as well, such as identity, age, or gender. A second issue is whether specific types of emotions are affected (e.g., positive versus negative) that would implicate specific emotional circuits or whether deficits are global across emotions as well as across visual discrimination features.
At present, although face emotion processing deficits are widely reported in schizophrenia, the specificity in comparison with other types of facial discriminations is still an area of ongoing investigation, in part because few studies have used psychometrically matched emotion and nonemotion tasks (Edwards et al. 2002). Among those that have used psychometrically matched tasks, most find results similar to those first reported by Kerr and Neale (Edwards et al. 2002), who concluded that emotion deficits reflected a generalized impairment in face processing rather than a specific emotion-recognition deficit. A more recent study has claimed differential deficits in emotion-processing tasks relative to other face-processing tasks (i.e., age, recognition) (Schneider et al. 2006). Even in such studies, however, performance on the control tasks was impaired as well, suggesting more general visual recognition deficits. Similarly, in fMRI studies of facial emotion identification, deficits are observed in activation of visual as well as frontal regions, suggesting sensory-level contributions to impaired emotional processing (Johnston et al. 2005).
As with other aspects of visual dysfunction, the deficit in face processing in schizophrenia seems most pronounced in the ability to utilize the types of information that are conveyed preferentially by the magnocellular system. For example, patients with schizophrenia require longer exposure times to discriminate accurately between two faces, a process dependent upon interaction between magnocellular and parvocellular projection systems. In contrast, patients show an intact face-inversion effect, reflecting relatively intact function of dedicated face-processing areas in fusiform gyrus, once correction is made for impairments in earlier stages of visual processing (Butler et al. 2008b). Similarly, patients with schizophrenia show relatively normal activation of fusiform gyrus, a ventral stream region when faces are presented for prolonged intervals (Yoon et al. 2006).
In contrast, patients who are studied using ERP show impaired responses to faces, including reduced N170 and P1 components (Campanella et al. 2006), which suggests an impaired early brain response to physical face features necessary for accurate face emotion identification. Finally, one study has demonstrated a direct, significant relationship between impaired social cognition and integrity of early visual processing assessed using backward masking (Sergi & Green 2003), again suggesting significant bottom-up contributions to the construct of impaired social cognition.
TOP-DOWN PARADIGMS
Although this review focuses mainly on bottom-up processes in schizophrenia, principles gleaned from the study of bottom-up mechanisms may be informative regarding the etiology of top-down mechanisms as well. Specifically, schizophrenia is associated with impairments in several domains, such as executive functioning, working memory, or long-term memory formation, for which localization to higher brain regions such as prefrontal cortex or hippocampus are well established. Such deficits are typically considered to reflect dysfunction of those specific brain regions. Findings from studies of bottom-up processing, however, suggest a potential alternative explanation—i.e., that deficits may be more parsimoniously considered to reflect dysfunction of NMDA receptors within specific brain regions rather than of the regions themselves.
In sensory brain regions, not all processes are impaired, which is why patients are neither deaf nor blind, but a subset of processes is implicated in the underlying dysfunction. For higher brain regions, the same questions can be asked. First, whether all processes mediated by specific brain regions such as prefrontal cortex or hippocampus are impaired, and second, whether the pattern of deficit that is observed corresponds to the pattern that would be expected based upon local NMDA dysfunction within higher brain regions. Although the role of NMDA receptors in many schizophrenia-related processes remains unknown, sufficient information is available to formulate initial hypotheses.
Executive Processing and Working Memory
Executive functioning refers to a set of processes involved in complex, goal-directed thought and behavior in multiple brain regions (e.g., prefrontal cortex, parietal cortex, basal ganglia) and multiple neurotransmitters (e.g., dopamine, glutamate, gamma-aminobutyric acid). Widely used measures of executive processing include the Wisconsin Card Sorting Test (WCST) and specific tests of set-shifting ability (Kerns et al. 2008). Working memory refers to the ability to maintain and manipulate information across brief delay and has also been linked to functioning of prefrontal cortex. Working memory is operationalized using the AX-version of the Continuous Performance Task (AX-CPT), among other paradigms (Barch & Smith 2008).
Although deficits in executive function and working memory are most often considered to reflect dysfunction of specific brain regions such as prefrontal cortex, tasks that are used to assess these constructs are reliably affected by NMDA antagonists such as ketamine or PCP in both human and animal models (e.g., Robbins & Murphy 2006, Umbricht et al. 2000). Furthermore, the pattern of dysfunction induced by NMDA antagonists closely resembles the pattern observed in schizophrenia (Krystal et al. 2005), suggesting that NMDA dysfunction may serve as a sufficient explanation for the types of executive processing and working memory impairments seen in schizophrenia.
Recent studies also suggest that not all paradigms that serve as tests of the executive processing/working memory domains show impairments in schizophrenia. For example, the task-switching paradigm is a widely used test of executive functioning in which subjects must alternate between two competing tasks. A characteristic feature of this task is that subjects are slower and less accurate on the first trial after a switch than on subsequent trials, particularly when the cue-to-trial interval is relatively short. Task-switching performance is reliably associated with frontal lobe function based upon both imaging and lesion studies and thus may serve as an objective test of prefrontal function in schizophrenia (Wylie et al. 2008).
Despite the widely postulated prefrontal involvement, patients with schizophrenia repeatedly have been found not to show increases in switch costs, even though they show impairments in other aspects of the task, such as increased costs of responding to incongruent versus congruent stimuli (Kieffaber et al. 2007, Meiran et al. 2000, Wylie et al. 2008). Furthermore, a similar pattern of results is seen in ketamine-challenged monkeys, with no increase in switch cost despite increased cost of stimulus incongruence (Stoet & Snyder 2006). Thus, just as not all bottom-up processes are intact in schizophrenia, not all top-down processes are impaired. The critical challenge to schizophrenia research is identification not only of those top-down tasks that show reliable deficits in schizophrenia, but also those tasks that do not. Only then can parsimonious explanations be proposed for the unique pattern of neurocognitive dysfunction observed in schizophrenia.
Verbal Learning and Memory
A final process that has been studied abundantly in schizophrenia is the process of learning and long-term memory formation (Ranganath et al. 2008). On a neuroanatomic level, learning is heavily dependent upon hippocampal function and is severely impaired following hippocampal lesion. On a neurochemical level, learning is critically dependent upon NMDA activation, which serves as the trigger for long-term potentiation (Morris 2006). The profiles of deficits produced by a neuroanatomical versus a neurochemical lesion, however, differ significantly.
Following hippocampal ablation, subjects develop an amnestic syndrome in which both the ability to learn new information and the ability to retain information that is learned are impaired. In contrast, following administration of ketamine or other NMDA antagonists, learning of new information is reduced, but retention remains relatively unaffected (Morris 2006). In schizophrenia, deficits are reliably observed in new learning (Ranganath et al. 2008). The classic amnestic syndrome, however, is not observed (Bilder et al. 2000), a finding that argues against neuroanatomical hypotheses but in favor of a local, NMDA-related pathogenesis.
Other processes, such as encoding strategies involving prefrontal cortex, may affect learning and memory in schizophrenia as well (Ranganath et al. 2008). Nevertheless, as in bottom-up processing, the critical issue may not be the particular brain regions involved but rather the specific underlying neurochemical mechanisms (Honey et al. 2005). Rather than being viewed as strictly bottom-up versus top-down processing, deficits within these regions may be viewed as side-in, with dysfunction within each region contributing to local aspects of information processing.
SUMMARY AND OVERVIEW
Neurocognitive dysfunction is a critical and enduring aspect of schizophrenia. The tendency in the schizophrenia literature has been to attribute cognitive dysfunction to deficits in higher-order processes such as working memory, attention, or executive processing, and to view more elemental forms of cognitive dysfunction as being driven top-down from higher cortical regions. Recent research argues against such constructs. Deficits may be observed within perceptual systems in schizophrenia that cannot be attributed to top-down dysregulation. Furthermore, deficits in lower-level perceptual processing may, of themselves, undermine the ability of the brain to perform more complex cognitive operations. In general, the pattern of deficit observed in both low- and high-level processing in schizophrenia resembles the pattern observed following treatment with NMDA receptor antagonists. NMDA dysfunction therefore may serve as a final common mechanism underlying the complex pattern of neurocognitive dysfunction associated with schizophrenia.
The poet William Blake observed in 1804:
“If Perceptive Organs vary, Objects of Perception seem to vary:
If the Perceptive Organs close, their Objects seem to close also.”
Further, “If the doors of perception were cleansed, every thing would appear to man as it is, infinite.” (Blake 2006)
Although it is not specifically related to schizophrenia, the quote clearly captures the link between perception and cognition. If perception is abnormal, cognition must be impaired as well. Optimal treatment of schizophrenia will ultimately require a cleansing of the doors, not only of cognition, but also of perception.
SUMMARY POINTS.
Schizophrenia is associated with cognitive deficits that represent a core component of the disorder.
Traditional models of schizophrenia focus on dysfunction of specific brain regions, such as prefrontal cortex, in the pathophysiology of schizophrenia.
More recent models focus on distributed dysfunction throughout cortex, with both bottom-up and top-down components.
In the auditory system, basic deficits in pitch processing lead to higher-level impairments such as prosody and auditory emotion recognition.
In the visual system, basic deficits in the function of the magnocellular system lead to impairments in perceptual closure, object recognition, and reading.
Within higher regions, although many processes are impaired, other processes, such as the ability to switch flexibly between tasks, remain paradoxically intact.
Findings are consistent with primary functional impairments within both sensory and higher-order association cortices as well as subcortical sensory pathways.
Overall, patterns of deficit are consistent with underlying dysfunction of N-methyl-d-aspartate (NMDA) receptor-mediated glutamatergic neurotransmission.
FUTURE ISSUES.
Deficits in early sensory processing are now well established in individuals with established schizophrenia and may be observed in unaffected family members as well. A critical issue is whether deficits are also apparent before schizophrenia onset and, if so, whether they may be effective in guiding early diagnosis and intervention.
Current medications for schizophrenia, including both typical and atypical antipsychotics, are relatively ineffective in treating neurocognitive deficits of schizophrenia, particularly deficits in early sensory processing. New treatments for schizophrenia are currently under development based upon glutamatergic and GABAergic models of the disorder. Research is needed to determine the effects of newer treatments on sensory deficits.
In schizophrenia, neurophysiological deficits resemble the pattern observed following administration of NMDA antagonists such as ketamine or PCP. Basic causes of NMDA dysfunction, however, need to be determined, including contributions of genetic polymorphisms (e.g., neuregulin, dysbindin, and d-amino acid oxidase) and neurochemical abnormalities (e.g., reduced brain d-serine and glutathione levels).
Up to 60% of chronic schizophrenia subjects show a dyslexia-like reading impairment related to underlying deficits in both phonological and orthographic processes. In general, illiteracy is a major contributor to adult functional impairment. Future research needs to address whether and how dyslexia-like deficits contribute to impaired vocational performance in schizophrenia and whether remediation approaches designed for dyslexia may be effective in reversing cognitive dysfunction in schizophrenia.
In animal models, deficits induced by NMDA antagonists can be reversed by agents such as d-serine or glycine transport inhibitors that stimulate NMDA function. Future research is needed to determine whether such agents are effective as well in the clinical treatment of schizophrenia.
Acknowledgments
I apologize in advance to all the investigators whose research could not be appropriately cited owing to space limitations. I would like to thank my coworkers at Nathan Kline Institute, including Drs. Pamela Butler, John Foxe, Nadine Revheim, Elisa Dias, John Smiley, and Charles Schroeder, whose work has been instrumental in developing these concepts. Preparation of this manuscript, as well as underlying research, was funded in part by NIH grants R37 MH49334, R01 DA03383, and R24 MH82790 to the author.
Glossary
- Event-related potentials (ERPs)
scalp-recorded electrical brain activity elicited in response to specific stimuli and task demands
- Phencyclidine (PCP)
PCP and the closely related compound ketamine are psychotomimetic agents that function by blocking neurotransmission at NMDA receptors
- N-methyl-D-aspartate (NMDA) receptors
a type of receptor for the neurotransmitter glutamate that are particularly involved in long-duration, neuromodulatory brain activity
- Long-term potentiation (LTP)
an NMDA-dependent process underlying learning and new memory formation
- Mismatch negativity (MMN)
an auditory event-related potential component that reflects preattentive stimulus change within auditory sensory cortex
- MGN
medial geniculate nucleus
- A1
primary auditory cortex
- Prosody
the intonation, stress, and timing patterns of speech that provide information beyond simple word meanings
- Magnocellular and parvocellular visual systems
subdivisions of the primate subcortical visual system that project from retina through lateral geniculate nucleus to primary visual cortex
- V1
primary visual cortex
- LGN
lateral geniculate nucleus
- ssVEP
steady state visual-evoked (event-related) potential
- Dorsal and ventral visual streams
subdivisions of the primate cortical visual system that constitute the “where” and “what” systems, respectively
- LOC
lateral occipital complex
- Perceptual closure
a process whereby the brain “fills in” a complete object based upon partial information
- Closure negativity (Ncl)
an ERP component that reflects the closure process
Footnotes
DISCLOSURE STATEMENT
The author holds intellectual property rights for the use of d-serine, glycine transport inhibitors, and other NMDA agonists in the treatment of schizophrenia.
LITERATURE CITED
- Barch DM, Smith E. The cognitive neuroscience of working memory: relevance to CNTRICS and schizophrenia. Biol Psychiatry. 2008;64:11–17. doi: 10.1016/j.biopsych.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, et al. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry. 2000;157:549–59. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- Blake W. The Prophetic Books of William Blake: Jerusalem. Whitefish, MT: Kessinger Publ; 2006. [Google Scholar]
- Bleuler E. Dementia Praecox of the Group of the Schizophrenias. New York: Intl. Univ. Press; 1950. [Google Scholar]
- Braff DL, Saccuzzo DP. The time course of information-processing deficits in schizophrenia. Am J Psychiatry. 1985;142:170–74. doi: 10.1176/ajp.142.2.170. [DOI] [PubMed] [Google Scholar]
- Braus DF, Weber-Fahr W, Tost H, Ruf M, Henn FA. Sensory information processing in neuroleptic-naive first-episode schizophrenic patients: a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2002;59:696–701. doi: 10.1001/archpsyc.59.8.696. [DOI] [PubMed] [Google Scholar]
- Brekke J, Kay DD, Lee KS, Green MF. Biosocial pathways to functional outcome in schizophrenia. Schizophr Res. 2005;80:213–25. doi: 10.1016/j.schres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Kayser J, Tenke CE, Friedman M, Malaspina D, Gorman JM. Event-related potentials in schizophrenia during tonal and phonetic oddball tasks: relations to diagnostic subtype, symptom features and verbal memory. Biol Psychiatry. 2001;50:447–52. doi: 10.1016/s0006-3223(01)01168-4. [DOI] [PubMed] [Google Scholar]
- Butler PD, Javitt DC. Early-stage visual processing deficits in schizophrenia. Curr Opin Psychiatry. 2005;18:151–57. doi: 10.1097/00001504-200503000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Martinez A, Foxe JJ, Kim D, Zemon V, et al. Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain. 2007;130:417–30. doi: 10.1093/brain/awl233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Silverstein SM, Dakin SC. Visual perception and its impairment in schizophrenia. Biol Psychiatry. 2008a;64:40–47. doi: 10.1016/j.biopsych.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Tambini A, Yovel G, Jalbrzikowski M, Ziwich R, et al. What’s in a face? Effects of stimulus duration and inversion on face processing in schizophrenia. Schizophr Res. 2008b;103:283–92. doi: 10.1016/j.schres.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, et al. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella S, Montedoro C, Streel E, Verbanck P, Rosier V. Early visual components (P100, N170) are disrupted in chronic schizophrenic patients: an event-related potentials study. Neurophysiol Clin. 2006;36:71–78. doi: 10.1016/j.neucli.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Chen CM, Lakatos P, Shah AS, Mehta AD, Givre SJ, et al. Functional anatomy and interaction of fast and slow visual pathways in macaque monkeys. Cereb Cortex. 2007;17:1561–69. doi: 10.1093/cercor/bhl067. [DOI] [PubMed] [Google Scholar]
- Chen Y, Levy DL, Sheremata S, Holzman PS. Bipolar and schizophrenic patients differ in patterns of visual motion discrimination. Schizophr Res. 2006;88:208–16. doi: 10.1016/j.schres.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cienfuegos A, March L, Shelley AM, Javitt DC. Impaired categorical perception of synthetic speech sounds in schizophrenia. Biol Psychiatry. 1999;45:82–88. doi: 10.1016/s0006-3223(98)00064-x. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Monaghan DT. Excitatory amino acids and neurotransmission: NMDA receptor and Hebb-type synaptic plasticity. Annu Rev Neurosci. 1988;11:61–80. doi: 10.1146/annurev.ne.11.030188.000425. [DOI] [PubMed] [Google Scholar]
- Cowan N. Activation, attention, and short-term memory. Mem Cogn. 1993;21:162–67. doi: 10.3758/bf03202728. [DOI] [PubMed] [Google Scholar]
- Cowan N. Attention and Memory: An Integrated Framework. New York: Oxford Univ. Press; 1995. [Google Scholar]
- Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59:1011–20. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- Doniger GM, Foxe JJ, Murray MA, Higgins BA, Schroeder CE, Javitt DC. Activation timecourse of ventral visual stream object-recognition areas: high-density electrical imaging of perceptual closure processes. J Cogn Neurosci. 2000;12:615–21. doi: 10.1162/089892900562372. [DOI] [PubMed] [Google Scholar]
- Doniger GM, Silipo G, Rabinowicz EF, Snodgrass JG, Javitt DC. Impaired sensory processing as a basis for object-recognition deficits in schizophrenia. Am J Psychiatry. 2001;158:1818–26. doi: 10.1176/appi.ajp.158.11.1818. [DOI] [PubMed] [Google Scholar]
- Donohoe G, Morris DW, De Sanctis P, Magno E, Montesi JL, et al. Early visual processing deficits in dysbindin-associated schizophrenia. Biol Psychiatry. 2008;63:484–89. doi: 10.1016/j.biopsych.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Edwards J, Jackson HJ, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clin Psychol Rev. 2002;22:789–832. doi: 10.1016/s0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- Edwards J, Pattison PE, Jackson HJ, Wales RJ. Facial affect and affective prosody recognition in first-episode schizophrenia. Schizophr Res. 2001;48:235–53. doi: 10.1016/s0920-9964(00)00099-2. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Kalba S, Marsh L, Pfefferbaum A. N1 and P300 abnormalities in patients with schizophrenia, epilepsy, and epilepsy with schizophrenia-like features. Biol Psychiatry. 2001a;49:848–60. doi: 10.1016/s0006-3223(00)01051-9. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Kalba S, Whitfield S, Faustman WO, Roth WT. Cortical responsiveness during talking and listening in schizophrenia: an event-related brain potential study. Biol Psychiatry. 2001b;50:540–49. doi: 10.1016/s0006-3223(01)01166-0. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. II Specifying the visual channels. Arch Gen Psychiatry. 1994;51:945–51. doi: 10.1001/archpsyc.1994.03950120017004. [DOI] [PubMed] [Google Scholar]
- Haenschel C, Bittner RA, Haertling F, Rotarska-Jagiela A, Maurer K, et al. Contribution of impaired early-stage visual processing to working memory dysfunction in adolescents with schizophrenia: a study with event-related potentials and functional magnetic resonance imaging. Arch Gen Psychiatry. 2007;64:1229–40. doi: 10.1001/archpsyc.64.11.1229. [DOI] [PubMed] [Google Scholar]
- Heggelund P, Hartveit E. Neurotransmitter receptors mediating excitatory input to cells in the cat lateral geniculate nucleus. I Lagged cells. J Neurophysiol. 1990;63:1347–60. doi: 10.1152/jn.1990.63.6.1347. [DOI] [PubMed] [Google Scholar]
- Holzman PS. Assessment of perceptual functioning in schizophrenia. Psychopharmacologia. 1972;24:29–41. doi: 10.1007/BF00402904. [DOI] [PubMed] [Google Scholar]
- Honey GD, Honey RA, O’Loughlin C, Sharar SR, Kumaran D, et al. Ketamine disrupts frontal and hippocampal contribution to encoding and retrieval of episodic memory: an fMRI study. Cereb Cortex. 2005;15:749–59. doi: 10.1093/cercor/bhh176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. Negative schizophrenic symptomatology and the PCP (phencyclidine) model of schizophrenia. Hillside J Clin Psychiatry. 1987;9:12–35. [PubMed] [Google Scholar]
- Javitt DC. Intracortical mechanisms of mismatch negativity dysfunction in schizophrenia. Audiol Neurootol. 2000;5:207–15. doi: 10.1159/000013882. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-d-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Jayachandra M, Lindsley RW, Specht CM, Schroeder CE. Schizophrenia-like deficits in auditory P1 and N1 refractoriness induced by the psychomimetic agent phencyclidine (PCP) Clin Neurophysiol. 2000a;111:833–36. doi: 10.1016/s1388-2457(99)00313-2. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Liederman E, Cienfuegos A, Shelley AM. Panmodal processing imprecision as a basis for dysfunction of transient memory storage systems in schizophrenia. Schizophr Bull. 1999;25:763–75. doi: 10.1093/oxfordjournals.schbul.a033417. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Shelley A, Ritter W. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin Neurophysiol. 2000b;111:1733–37. doi: 10.1016/s1388-2457(00)00377-1. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Spencer S, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7:1–17. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-d-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci USA. 1996;93:11962–67. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Strous RD, Grochowski S, Ritter W, Cowan N. Impaired precision, but normal retention, of auditory sensory (“echoic”) memory information in schizophrenia. J Abnorm Psychol. 1997;106:315–24. doi: 10.1037//0021-843x.106.2.315. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–8. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Johnston PJ, Stojanov W, Devir H, Schall U. Functional MRI of facial emotion recognition deficits in schizophrenia and their electrophysiological correlates. Eur J Neurosci. 2005;22:1221–32. doi: 10.1111/j.1460-9568.2005.04294.x. [DOI] [PubMed] [Google Scholar]
- Jonsson CO, Sjostedt A. Auditory perception in schizophrenia: a second study of the Intonation Test. Acta Psychiatr Scand. 1973;49:588–600. doi: 10.1111/j.1600-0447.1973.tb04450.x. [DOI] [PubMed] [Google Scholar]
- Juslin PN, Laukka P. Impact of intended emotion intensity on cue utilization and decoding accuracy in vocal expression of emotion. Emotion. 2001;1:381–412. doi: 10.1037/1528-3542.1.4.381. [DOI] [PubMed] [Google Scholar]
- Juslin PN, Laukka P. Communication of emotions in vocal expression and music performance: different channels, same code? Psychol Bull. 2003;129:770–814. doi: 10.1037/0033-2909.129.5.770. [DOI] [PubMed] [Google Scholar]
- Kasai K, Nakagome K, Itoh K, Koshida I, Hata A, et al. Impaired cortical network for preattentive detection of change in speech sounds in schizophrenia: a high-resolution event-related potential study. Am J Psychiatry. 2002;159:546–53. doi: 10.1176/appi.ajp.159.4.546. [DOI] [PubMed] [Google Scholar]
- Kasai K, Yamada H, Kamio S, Nakagome K, Iwanami A, et al. Neuromagnetic correlates of impaired automatic categorical perception of speech sounds in schizophrenia. Schizophr Res. 2003;59:159–72. doi: 10.1016/s0920-9964(01)00382-6. [DOI] [PubMed] [Google Scholar]
- Kawakubo Y, Kamio S, Nose T, Iwanami A, Nakagome K, et al. Phonetic mismatch negativity predicts social skills acquisition in schizophrenia. Psychiatry Res. 2007;152:261–65. doi: 10.1016/j.psychres.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Kelemen O, Erdelyi R, Pataki I, Benedek G, Janka Z, Keri S. Theory of mind and motion perception in schizophrenia. Neuropsychology. 2005;19:494–500. doi: 10.1037/0894-4105.19.4.494. [DOI] [PubMed] [Google Scholar]
- Keri S, Must A, Kelemen O, Benedek G, Janka Z. Development of visual motion perception in children of patients with schizophrenia and bipolar disorder: a follow-up study. Schizophr Res. 2006;82:9–14. doi: 10.1016/j.schres.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Nuechterlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biol Psychiatry. 2008;64:26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Kieffaber PD, O’Donnell BF, Shekhar A, Hetrick WP. Event-related brain potential evidence for preserved attentional set switching in schizophrenia. Schizophr Res. 2007;93:355–65. doi: 10.1016/j.schres.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Wylie G, Pasternak R, Butler PD, Javitt DC. Magnocellular contributions to impaired motion processing in schizophrenia. Schizophr Res. 2006;82:1–8. doi: 10.1016/j.schres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Zemon V, Saperstein A, Butler PD, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia: harmonic analysis. Schizophr Res. 2005a;76:55–65. doi: 10.1016/j.schres.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Kim J, Doop ML, Blake R, Park S. Impaired visual recognition of biological motion in schizophrenia. Schizophr Res. 2005b;77:299–307. doi: 10.1016/j.schres.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Klosterkotter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–64. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- Kreitschmann-Andermahr I, Rosburg T, Demme U, Gaser E, Nowak H, Sauer H. Effect of ketamine on the neuromagnetic mismatch field in healthy humans. Brain Res Cogn Brain Res. 2001;12:109–16. doi: 10.1016/s0926-6410(01)00043-x. [DOI] [PubMed] [Google Scholar]
- Kreitschmann-Andermahr I, Rosburg T, Meier T, Volz HP, Nowak H, Sauer H. Impaired sensory processing in male patients with schizophrenia: a magnetoencephalographic study of auditory mismatch detection. Schizophr Res. 1999;35:121–29. doi: 10.1016/s0920-9964(98)00115-7. [DOI] [PubMed] [Google Scholar]
- Kring AM, Barrett LF, Gard DE. On the broad applicability of the affective circumplex: representations of affective knowledge among schizophrenia patients. Psychol Sci. 2003;14:207–14. doi: 10.1111/1467-9280.02433. [DOI] [PubMed] [Google Scholar]
- Krishnan GP, Vohs JL, Hetrick WP, Carroll CA, Shekhar A, et al. Steady state visual evoked potential abnormalities in schizophrenia. Clin Neurophysiol. 2005;116:614–24. doi: 10.1016/j.clinph.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Perry EB, Jr, Gueorguieva R, Belger A, Madonick SH, et al. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch Gen Psychiatry. 2005;62:985–94. doi: 10.1001/archpsyc.62.9.985. [DOI] [PubMed] [Google Scholar]
- Kugler BT, Caudrey DJ. Phoneme discrimination in schizophrenia. Br J Psychiatry. 1983;142:53–59. doi: 10.1192/bjp.142.1.53. [DOI] [PubMed] [Google Scholar]
- Kujala T, Lovio R, Lepisto T, Laasonen M, Naatanen R. Evaluation of multi-attribute auditory discrimination in dyslexia with the mismatch negativity. Clin Neurophysiol. 2006;117:885–93. doi: 10.1016/j.clinph.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Kurylo DD, Gazes Y. Effects of ketamine on perceptual grouping in rats. Physiol Behav. 2008;95:152–56. doi: 10.1016/j.physbeh.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Kurylo DD, Pasternak R, Silipo G, Javitt DC, Butler PD. Perceptual organization by proximity and similarity in schizophrenia. Schizophr Res. 2007;95:205–14. doi: 10.1016/j.schres.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kveraga K, Boshyan J, Bar M. Magnocellular projections as the trigger of top-down facilitation in recognition. J Neurosci. 2007;27:13232–40. doi: 10.1523/JNEUROSCI.3481-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YH, Esguerra M, Sur M. NMDA and non-NMDA receptors mediate visual responses of neurons in the cat’s lateral geniculate nucleus. J Neurophysiol. 1991;66:414–28. doi: 10.1152/jn.1991.66.2.414. [DOI] [PubMed] [Google Scholar]
- Lamme VAF, Roelfsema PR. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 2000;23:571–79. doi: 10.1016/s0166-2236(00)01657-x. [DOI] [PubMed] [Google Scholar]
- Laukka P. Categorical perception of vocal emotion expressions. Emotion. 2005;5:277–95. doi: 10.1037/1528-3542.5.3.277. [DOI] [PubMed] [Google Scholar]
- Lawson JS, McGhie A, Chapman J. Perception of speech in schizophrenia. Br J Psychiatry. 1964;110:375–80. doi: 10.1192/bjp.110.466.375. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Foxe JJ, Butler PD, Saperstein A, Revheim N, Javitt DC. Sensory contributions to impaired prosodic processing in schizophrenia. Biol Psychiatry. 2005;58:56–61. doi: 10.1016/j.biopsych.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Laukka P, Juslin PN, Saccente E, Butler P, Javitt DC. Getting the cue: sensory contributions to auditory emotion recognition impairments in schizophrenia. Schizophr Bull. 2008 doi: 10.1093/schbul/sbn115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman DI, Ziwich R, Pasternak R, Javitt DC. Theory of Mind (ToM) and counterfactuality deficits in schizophrenia: misperception or misinterpretation? Psychol Med. 2006;36:1075–83. doi: 10.1017/S0033291706007653. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Foote SL, Goldstein M, Morrison JH. The distribution of tyrosine hydroxylase-immunoreaction fibers in primate neocortex is widespread but regionally specific. J Neurosci. 1987;7:279–90. doi: 10.1523/JNEUROSCI.07-01-00279.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Branch CA, Bertisch HC, Brown K, Szulc KU, et al. An fMRI study of language processing in people at high genetic risk for schizophrenia. Schizophr Res. 2007;91:62–72. doi: 10.1016/j.schres.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry. 2005;62:127–36. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR, Braff DL. Preattentive sensory processing as indexed by the MMN and P3a brain responses is associated with cognitive and psychosocial functioning in healthy adults. J Cogn Neurosci. 2007;19:1624–32. doi: 10.1162/jocn.2007.19.10.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner A, Thier P, Kircher TT, Haarmeier T, Leube DT. Disorders of agency in schizophrenia correlate with an inability to compensate for the sensory consequences of actions. Curr Biol. 2005;15:1119–24. doi: 10.1016/j.cub.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Fuller RL, Braun EL, Robinson B, Summerfelt A, Gold JM. The speed of visual attention in schizophrenia: electrophysiological and behavioral evidence. Schizophr Res. 2006;85:174–95. doi: 10.1016/j.schres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- March L, Cienfuegos A, Goldbloom L, Ritter W, Cowan N, Javitt DC. Normal time course of auditory recognition in schizophrenia, despite impaired precision of the auditory sensory (“echoic” ) memory code. J Abnorm Psychol. 1999;108:69–75. doi: 10.1037//0021-843x.108.1.69. [DOI] [PubMed] [Google Scholar]
- Martinez A, Hillyard SA, Dias EC, Hagler DJ, Jr, Butler PD, et al. Magnocellular pathway impairment in schizophrenia: evidence from functional magnetic resonance imaging. J Neurosci. 2008;28:7492–500. doi: 10.1523/JNEUROSCI.1852-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Ramanathan DS, Foxe JJ, Javitt DC, Hillyard SA. The role of spatial attention in the selection of real and illusory objects. J Neurosci. 2007;27:7963–73. doi: 10.1523/JNEUROSCI.0031-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiak K, Rapp A, Kircher TT, Grodd W, Hertrich I, et al. Mismatch responses to randomized gradient switching noise as reflected by fMRI and whole-head magnetoencephalography. Hum Brain Mapp. 2002;16:190–95. doi: 10.1002/hbm.10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34:103–16. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Meiran N, Levine J, Meiran N, Henik A. Task set switching in schizophrenia. Neuropsychology. 2000;14:471–82. doi: 10.1037//0894-4105.14.3.471. [DOI] [PubMed] [Google Scholar]
- Morris RG. Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. Eur J Neurosci. 2006;23:2829–46. doi: 10.1111/j.1460-9568.2006.04888.x. [DOI] [PubMed] [Google Scholar]
- Pekkonen E, Katila H, Ahveninen J, Karhu J, Huotilainen M, Tiihonen J. Impaired temporal lobe processing of preattentive auditory discrimination in schizophrenia. Schizophr Bull. 2002;28:467–74. doi: 10.1093/oxfordjournals.schbul.a006954. [DOI] [PubMed] [Google Scholar]
- Place EJS, Gilmore GC. Perceptual organization in schizophrenia. J Abnorm Psychol. 1980;89:409–18. doi: 10.1037//0021-843x.89.3.409. [DOI] [PubMed] [Google Scholar]
- Rabinowicz EF, Silipo G, Goldman R, Javitt DC. Auditory sensory dysfunction in schizophrenia: imprecision or distractibility? Arch Gen Psychiatry. 2000;57:1149–55. doi: 10.1001/archpsyc.57.12.1149. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Minzenberg MJ, Ragland JD. The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biol Psychiatry. 2008;64:18–25. doi: 10.1016/j.biopsych.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revheim N, Butler PD, Schechter I, Jalbrzikowski M, Silipo G, Javitt DC. Reading impairment and visual processing deficits in schizophrenia. Schizophr Res. 2006;87:238–45. doi: 10.1016/j.schres.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivadulla C, Sharma J, Sur M. Specific roles of NMDA and AMPA receptors in direction-selective and spatial phase-selective responses in visual cortex. J Neurosci. 2001;21:1710–19. doi: 10.1523/JNEUROSCI.21-05-01710.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Murphy ER. Behavioural pharmacology: 40+ years of progress, with a focus on glutamate receptors and cognition. Trends Pharmacol Sci. 2006;27:141–48. doi: 10.1016/j.tips.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles O, Blaxton T, Adami H, Arango C, Thaker G, Gold J. Nonverbal delayed recognition in the relatives of schizophrenia patients with or without schizophrenia spectrum. Biol Psychiatry. 2008;63:498–504. doi: 10.1016/j.biopsych.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Slason E, Teale PD, Reite ML. Neuromagnetic evidence of broader auditory cortical tuning in schizophrenia. Schizophr Res. 2007;97:206–14. doi: 10.1016/j.schres.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth W, Cannon E. Some features of the auditory evoked response in schizophrenics. Arch Gen Psychiatry. 1972;27:466–71. doi: 10.1001/archpsyc.1972.01750280034007. [DOI] [PubMed] [Google Scholar]
- San Miguel I, Corral MJ, Escera C. When loading working memory reduces distraction: behavioral and electrophysiological evidence from an auditory-visual distraction paradigm. J Cogn Neurosci. 2008;20:1131–45. doi: 10.1162/jocn.2008.20078. [DOI] [PubMed] [Google Scholar]
- Schechter I, Butler PD, Zemon VM, Revheim N, Saperstein AM, et al. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clin Neurophysiol. 2005;116(9):2204–15. doi: 10.1016/j.clinph.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Gur RC, Koch K, Backes V, Amunts K, et al. Impairment in the specificity of emotion processing in schizophrenia. Am J Psychiatry. 2006;163:442–47. doi: 10.1176/appi.ajp.163.3.442. [DOI] [PubMed] [Google Scholar]
- Schultze-Lutter F, Ruhrmann S, Picker H, von Reventlow HG, Brockhaus-Dumke A, Klosterkotter J. Basic symptoms in early psychotic and depressive disorders. Br J Psychiatry Suppl. 2007;51:s31–37. doi: 10.1192/bjp.191.51.s31. [DOI] [PubMed] [Google Scholar]
- Sehatpour P, Molholm S, Javitt DC, Foxe JJ. Spatiotemporal dynamics of human object recognition processing: an integrated high-density electrical mapping and functional imaging study of “closure” processes. Neuroimage. 2006;29:605–18. doi: 10.1016/j.neuroimage.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Sehatpour P, Molholm S, Schwartz TH, Mahoney JR, Mehta AD, et al. A human intracranial study of long-range oscillatory coherence across a frontal-occipital-hippocampal brain network during visual object processing. Proc Natl Acad Sci USA. 2008;105:4399–404. doi: 10.1073/pnas.0708418105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Begovic A. Stereologic analysis of the lateral geniculate nucleus of the thalamus in normal and schizophrenic subjects. Psychiatry Res. 2007;151:1–10. doi: 10.1016/j.psychres.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergi MJ, Green MF. Social perception and early visual processing in schizophrenia. Schizophr Res. 2003;59:233–41. doi: 10.1016/s0920-9964(01)00405-4. [DOI] [PubMed] [Google Scholar]
- Snyder S. Perceptual closure in acute paranoid schizophrenics. Arch Gen Psychiatry. 1961;5:406–10. doi: 10.1001/archpsyc.1961.01710160086010. [DOI] [PubMed] [Google Scholar]
- Stoet G, Snyder LH. Effects of the NMDA antagonist Ketamine on task-switching performance: evidence for specific impairments of executive control. Neuropsychopharmacology. 2006;31:1675–81. doi: 10.1038/sj.npp.1300930. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Hill PR, Stein JF, Bishop DV. Auditory event-related potentials differ in dyslexics even when auditory psychophysical performance is normal. Brain Res. 2006;1121:190–99. doi: 10.1016/j.brainres.2006.08.095. [DOI] [PubMed] [Google Scholar]
- Strous RD, Cowan N, Ritter W, Javitt DC. Auditory sensory (“echoic”) memory dysfunction in schizophrenia. Am J Psychiatry. 1995;152:1517–19. doi: 10.1176/ajp.152.10.1517. [DOI] [PubMed] [Google Scholar]
- Thonnessen H, Zvyagintsev M, Harke KC, Boers F, Dammers J, et al. Optimized mismatch negativity paradigm reflects deficits in schizophrenia patients. A combined EEG and MEG study. Biol Psychol. 2008;77:205–16. doi: 10.1016/j.biopsycho.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Tikhonravov D, Neuvonen T, Pertovaara A, Savioja K, Ruusuvirta T, et al. Effects of an NMDA-receptor antagonist MK-801 on an MMN-like response recorded in anesthetized rats. Brain Res. 2008;1203:97–102. doi: 10.1016/j.brainres.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Todd J, Michie PT, Jablensky AV. Association between reduced duration mismatch negativity (MMN) and raised temporal discrimination thresholds in schizophrenia. Clin Neurophysiol. 2003;114:2061–70. doi: 10.1016/s1388-2457(03)00246-3. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Millard I, Muetzelfeldt L, Curran HV, Morgan CJ. Perceptual organization in ketamine users: preliminary evidence of deficits on night of drug use but not 3 days later. J Psychopharmacol. 2007;21:347–52. doi: 10.1177/0269881107077739. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Phillips WA, Mitchell G, Silverstein SM. Perceptual grouping in disorganized schizophrenia. Psychiatry Res. 2006;145:105–17. doi: 10.1016/j.psychres.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57:1139–47. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- Van Der Stelt O, Frye J, Lieberman JA, Belger A. Impaired P3 generation reflects high-level and progressive neurocognitive dysfunction in schizophrenia. Arch Gen Psychiatry. 2004;61:237–48. doi: 10.1001/archpsyc.61.3.237. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Stevens AA, Bowers AA, Sernyak MJ, Goldman-Rakic PS. Word and tone working memory deficits in schizophrenia. Arch Gen Psychiatry. 1998;55:1093–96. doi: 10.1001/archpsyc.55.12.1093. [DOI] [PubMed] [Google Scholar]
- Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry. 2008;165:579–87. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- Wylie GR, Clark EA, Butler PD, Javitt DC. Schizophrenia patients show task switching deficits consistent with N-methyl-d-asparate (NMDA) system dysfunction, but not global executive deficits: implications for pathophysiology of executive dysfunction in schizophrenia. Schiz Bull. 2008 doi: 10.1093/schbul/sbn119. Epub Oct 3, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap S, Kelly SP, Sehatpour P, Magno E, Javitt DC, et al. Early visual sensory deficits as endophenotypes for schizophrenia: high-density electrical mapping in clinically unaffected first-degree relatives. Arch Gen Psychiatry. 2006;63:1180–88. doi: 10.1001/archpsyc.63.11.1180. [DOI] [PubMed] [Google Scholar]
- Yoon JH, D’Esposito M, Carter CS. Preserved function of the fusiform face area in schizophrenia as revealed by fMRI. Psychiatry Res. 2006;148:205–16. doi: 10.1016/j.pscychresns.2006.06.002. [DOI] [PubMed] [Google Scholar]