Abstract
Objective
The ability to read passages of information fluently and with comprehension is a basic component of socioeconomic success. Reading ability depends upon the integrity of underlying visual and auditory (phonological) systems. This study investigates the integrity of reading ability in schizophrenia relative to the integrity of underlying visual and auditory function.
Methods
Participants were 45 schizophrenia patients, 19 clinical high risk patients, and 65 controls. Reading was assessed using tests sensitive to visual vs. phonological reading dysfunction. Sensory, neuropsychological and functional outcome measures were also obtained.
Results
Schizophrenia patients showed reading deficits that were far more severe (effect size>2.0) than would be predicted based upon general neurocognitive impairments (effect size 1.0–1.4). The deficits correlated highly with both visual and auditory sensory measures, including impaired mismatch negativity (MMN) generation (r=.62, n=51, p=.0002). Patients with established schizophrenia showed both visual and phonological impairments, whereas high-risk patients showed isolated visual impairments. >70% of schizophrenia patients met criteria for acquired dyslexia, with 50% reading below 8th grade level despite intact premorbid reading ability. Reading deficits also correlated significantly (rp=.4, n=30, p=.03) with failure to match parental socioeconomic achievement, over and above contributions of more general cognitive impairment.
Conclusions
Patients with schizophrenia show severe deficits in reading ability that represent a potentially remediable cause of impaired socioeconomic function. Such deficits are not presently captured during routine clinical assessment. Deficits most likely develop during the years immediately surrounding illness onset and may contribute to the reduced educational and occupational achievement associated with schizophrenia.
Keywords: Specific reading disability (dyslexia), auditory processing, magnocellular processing, contrast sensitivity, ERP, mismatch negativity, schizophrenia
Introduction
We live in a literate world. In developed countries, such as the US, reading is taught starting in early school years, and reading ability increases progressively from grade school to high school to university. In developed countries, reading ability even as early as 3rd grade is substantially related to future achievement in school, work, family and societal roles (1), while adult literacy levels are strongly related to income and occupational achievement (2). Reading ability is also closely tied to the integrity of sensory brain systems, with strong visual and auditory (phonological) abilities needed for fluent reading of written information (3–5).
Over recent years, deficits in early sensory processing ability have been increasingly well-documented in schizophrenia, involving both visual and auditory functions known to be related to reading ability (6–8). Moreover, while visual deficits appear to represent a trait component of the illness, auditory deficits develop during the years immediately surrounding illness onset, leading to significant declines in cognitive function (9). Given sensory contributions to reading ability, the present study investigates the degree to which sensory dysfunction produces secondary impairments in reading ability.
Acquisition of reading skills during childhood requires complex integration of visual and auditory functions. Because of this, many individuals have difficulty in initial acquisition of reading skills, a condition termed developmental dyslexia. For most individuals, however, once reading skills are acquired in childhood they remain intact into adulthood. Therefore, formal tests of the ability to fluently read passages of information (“connected text”) are rarely tested in adults. Instead, reading is typically assessed using single-word reading tests such as the Wide Range Achievement Test (WRAT) (10), which assesses an individual’s ability to correctly pronounce irregularly spelled words such as “stalk,” “quarantine,” or “synecdoche.” Because of their irregularity, pronunciation of these words cannot be determined using standard phonological principles. Instead, the ability to pronounce these words correctly indicates prior exposure to them, which in turn, reflects the complexity of reading material to which a person has been exposed. In schizophrenia, performance in single-word reading is typically preserved relative to other cognitive domains (e.g. 11, 12, 13), leading to the tacit assumption that underlying passage reading abilities are relatively retained as well and that literacy is preserved. The present study, however, explicitly tests this assumption using tests drawn from the development reading literature.
In general, reading ability depends upon two sets of interrelated processes. Visual processes permit individuals to accurately scan passages of text, and are linked to function of the visual magnocellular system (14–16). Auditory phonological processes permit individuals to “sound out” words, and are tied to auditory tonal discrimination abilities as reflected in generation of mismatch negativity (MMN) or other early event-related potential components (17–19). Different individuals utilize visual vs. phonological processes to different extents during reading, and, in general, strengths in one set of processes can be used to compensate for weaknesses in the other. Patients with deficits in both sets of processes, however, show a “double deficit” pattern associated with highly impaired reading ability (20, 21).
For the present study, patients with schizophrenia were evaluated using several batteries that assess not only ability to utilize written materials, but also underlying mechanistic processes. The Gray Oral Reading Test (22), for example, assesses both fluency of reading (combined rate and accuracy) and comprehension to obtain an overall reading quotient. The Comprehensive Test of Phonological Processing (23) provides separate indices of phonological awareness vs. rapid naming ability and thus is useful for differentiating phonological vs. visual vs. “double-deficit” subforms of dyslexia. The Woodcock-Johnson III Tests of Achievement (24) assesses a wide range of basic reading skills. The Nelson-Denny Reading Test (25) uniquely provides grade equivalent performance levels through 16th grade. At present, there is no “gold standard” assessment for reading skills. The above tests, therefore, were chosen to provide a comprehensive assessment.
In addition to reading ability, patients were assessed on measures of sensory function, including visual contrast sensitivity (6), auditory tone matching ability (8, 26) and MMN (9), as well as on more global outcome measures including personal/parental socioeconomic status (27) and scores on the Independent Living Scales, which measures capacity for independent living (28). General cognitive ability was assessed using the Processing Speed Index of the Wechsler Adult Intelligence Scale (WAIS-III) (29) and the Working Memory Index of the Wechsler Memory Scale (30), which consistently capture the general cognitive deficit associated with schizophrenia (31, 32).
In addition to a sample of 45 patients with schizophrenia, which represents our primary cohort, we also obtained reading scores from 19 patients who were considered at high clinical risk for schizophrenia vs. age-matched controls. Patterns of deficit were compared both between patients and healthy controls, and between the two patient groups. Given our findings of auditory and visual sensory-level dysfunction, we predicted that schizophrenia patients would show significant deficits in reading ability, and that these deficits were correlate both with impaired visual contrast sensitivity and auditory MMN generation.
Methods
Participants
The primary sample consisted of patients meeting DSM-IV criteria for schizophrenia (n=37) or schizoaffective disorder (n=8) and similar age controls (n=24) recruited at Nathan S. Kline Institute for Psychiatric Research (NKI) and affiliated institutions. All participants performed SCID interviews for diagnostic classification (33) and met the following inclusion criteria: IQ≥85, native English speaking, absence of neurological impairment or current substance abuse or dependence and near vision corrected to 20/32 (Table 1). Patients had been ill on average 16.8±9.4 yrs., and were receiving antipsychotics at a mean daily dose of 944.3±702.7 chlorpromazine equivalents. Twenty-one (51%) patients were inpatients at the time of testing, whereas 20 (49%) were living in supervised residential care settings.
Table 1.
Mean (SD) Demographic and clinical characteristics for Patients and Controls
| Schizophrenia | Clinical high risk | |||||||
|---|---|---|---|---|---|---|---|---|
| Measure | Patients (n=45) | Controls (n=24) | Patients (n=19) | Controls (n=41) | ||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age | 37.6 | 11.6 | 39.6 | 11.3 | 22.8 | 3.8 | 21.0 | 3.5 |
| Education | 12.71 | 2.2 | 14.6 | 1.8 | 15.2 | 2.7 | 14.6 | 3.0 |
| Socioeconomic status-parental | 40.8a | 15.2 | 41.3 | 12.0 | 45.2 | 3.0 | 46.5 | 2.5 |
| Socioeconomic status-individual | 27.8** | 10.9 | 45.3 | 8.6 | --- | --- | ||
| Male | Female | Male | Female | Male | Female | Male | Female | |
| Gender | 40 | 5 | 17 | 7 | 13 | 6 | 24 | 7 |
p<.001 patient vs. control, between-group t-test;
p<.00001
n=26
A second sample consisted of individuals (n=19) ages 12–30 considered to be at high clinical risk for schizophrenia based upon the Structured Interview for Prodromal Syndromes/Scale of Prodromal Symptoms (34) vs. similar age controls (n=41). Recruitment and ascertainment relied on clinician referrals, internet resources and the mailing of brochures. Exclusion criteria included any major medical or neurological disorder, significant risk of harm to self and others, an inability to speak English, and symptoms occurring solely in the context of substance intoxication or withdrawal. Additional exclusion criteria for healthy controls included any current Axis I disorder within the past two years, as assessed by structured diagnostic interview, and any personal or familial (first degree relative) history of psychosis. Because of time constraints, contrast sensitivity scores and MMN were not obtained in this sample.
The study was approved by the Institutional Review Board associated with the New York State Office of Mental Health. Adult participants provided written consent after being informed of all study procedures, while minors provided assent, with written informed consent provided by a parent.
Instruments
Reading Measures
The full battery consisted of complete versions of the 4 test batteries described in the Introduction (see Table 2). The primary measure used to assess overall current (passage) reading level across analyses was the Oral Reading Quotient of the Gray Oral Reading Test Visual reading ability was assessed using an average of the rapid naming and alternative rapid naming measures in the Comprehensive Test of Phonological Processing, while phonological ability was assessed using an average of the phonological awareness and alternate phonological awareness scores.
Table 2.
Mean and standard deviation (SD) for neurocognitive and reading test measures for schizophrenia patients and controls
| Patients | Controls | |||||||
|---|---|---|---|---|---|---|---|---|
| Test | Mean | SD | Mean | SD | F | df | p | Effect size (d) |
| General neurocognitive measures | ||||||||
|
Wechsler Adult Intelligence Scale - Processing speed index |
81.9 | 1.3 | 105.0 | 18.9 | 25.9 | 1,54 | <.001 | −1.4 |
|
Wechsler Memory Scale - Working memory Index |
90.5 | 18.6 | 105.8 | 12.2 | 8.73 | 1,53 | .005 | −1.0 |
| WRAT (Scaled score) | 95.6 | 12.6 | 105.9 | 5.9 | 14.5 | 1,67 | <.001 | −1.1 |
| Gray Oral Reading Test (4th-edition) – scaled scores (F=30.3, df=2,66, p<.001) | ||||||||
| Rate1 | 6.6 | 3.1 | 11.2 | 1.6 | 46.0 | 1,67 | <.001 | −2.0 |
| Accuracy1 | 7.0 | 4.3 | 13.3 | 2.3 | 45.7 | 1,67 | <.001 | −1.9 |
| Fluency1 | 5.7 | 5.2 | 13.8 | 2.4 | 53.1 | 1,67 | <.001 | −2.1 |
| Comprehension1 | 5.9 | 2.6 | 9.0 | 1.8 | 29.5 | 1,67 | <.001 | −1.5 |
| Oral Reading Quotient2 | 74.8 | 20.3 | 108.1 | 9.2 | 61.8 | 1,67 | <.001 | −2.3 |
| Comprehensive Test of Phonological Processing – scaled scores (F=9.91,df=3,43, p<.001) | ||||||||
| Phonological awareness | 79.1 | 16.3 | 88.4 | 18.5 | 3.05 | 1,45 | .087 | −.5 |
| Alternate phonological awareness2 | 71.7 | 13.4 | 88.9 | 14.3 | 24.6 | 1,67 | <.001 | −1.2 |
| Phonological memory | 93.4 | 13.3 | 100.4 | 14.4 | 2.7 | 1,45 | .11 | −.5 |
| Rapid Naming | 87.3 | 17.0 | 106.8 | 17.5 | 13.1 | 1,45 | .001 | −1.1 |
| Alternative rapid naming | 72.3 | 14.7 | 99.6 | 15.4 | 34.0 | 1,45 | <.001 | −1.8 |
| Woodcock Johnson III Tests of Achievement (Scaled scores) (F=20.3, df=5,37, p<.001) | ||||||||
| Fluency | 85.8 | 6.2 | 108.7 | 8.4 | 103.8 | 1,41 | <.001 | −3.1 |
| Spelling | 99.4 | 10.2 | 109.1 | 10.8 | 8.40 | 1,41 | .006 | −1.0 |
| Listening comprehension | 93.4 | 7.8 | 104.1 | 9.0 | 16.5 | 1,41 | <.001 | −1.3 |
| Basic Reading Skills | 98.2 | 6.6 | 106.3 | 8.7 | 12.0 | 1,41 | .001 | −1.1 |
| Reading comprehension | 93.9 | 7.2 | 104.9 | 10.6 | 16.4 | 1,44 | <.001 | −1.3 |
| Phoneme/Grapheme Knowledge | 95.5 | 6.7 | 105.1 | 8.1 | 17.2 | 1,41 | <.001 | −1.3 |
| Broad Reading | 90.8 | 5.6 | 109.9 | 9.1 | 71.9 | 1,44 | <.001 | −2.8 |
| Nelson-Denny Reading Test – grade equivalent scores (F=13.51, df=3,39, p<.001) | ||||||||
| Total | 8.6 | 3.4 | 15.1 | 2.9 | 39.3 | 1,41 | <.001 | −2.0 |
| Vocabulary | 10.2 | 3.2 | 16.1 | 2.2 | 40.8 | 1,41 | <.001 | −2.1 |
| Comprehension | 7.6 | 3.8 | 13.9 | 3.6 | 27.5 | 1,41 | <.001 | −1.7 |
normed based upon mean =10, sd=3
included in brief battery
Based upon initial experience with these tests we also developed a briefer battery consisting only of a subset of tests that required only approx. 45 minutes (Table 2). Most, but not all, reading tests are normed with a mean of 100 and standard deviation of 15, permitting direct comparison to standard IQ scores. The Nelson Denny Reading Test provides grade equivalent scores, and has been validated through grade 16.
Clinical, Measures
Neurocognitive
Neurocognitive ability was assessed using the Processing Speed Index of the Wechsler Adult Intelligence Scale-III (29) and the Working Memory Index of the Wechsler Memory Scale-III (30) (Table 2).
Symptoms
Ratings were obtained for patients using the Positive and Negative Symptoms Scale (PANSS) (35). Mean scores were 72.0±13.6, 17.4±5.5, 18.7±4.5, and 36.6±8.8 for total, positive, negative and general factors, respectively.
Functional
Both individual and parental socioeconomic status were assessed using the Hollingshead Index, which considers both education and occupational function (27) (Table 1). Functional cognition for patients was assessed using the Independent Living Scales, Problem-solving Factor subscale (36, 37). Mean score for patients was 41.7±11.7, reflecting, in general, need for supervised living.
Sensory measures
Auditory
Auditory function was assessed using simple tone-matching and mismatch negativity (MMN) paradigms, as previously described (8, 9). MMN recordings were obtained with a Biosemi system (Amsterdam, the Netherlands) using a standard 10–10 channel layout. Separate measures were obtained for pitch, duration and intensity MMN at the FCz electrode using analysis approach and latency intervals as described previously (9).
Contrast Sensitivity
Early visual processing was evaluated using contrast sensitivity, as previously described (6). Stimuli (0.5, 7 or 21 cycles/degree) were presented for 32 ms in a 3-down/1-up adaptive staircase method to determine detection threshold. Contrast sensitivity was calculated as 1/(detection threshold).
Statistical Analyses
Demographic characteristics between groups were analyzed with t-tests and Mann-Whitney U tests as appropriate. Group differences on main dependent measures were analyzed with univariate or repeated measures MANOVA, with post-hoc analyses by least significant difference (LSD). The relationship between measures was assessed using stepwise multiple regression, which yields measures for R2 change for successive steps of the model, as well as partial correlations (rp) for individual variables. Categorical analyses were performed using likelihood ratio (LR) chi-square (χ2). All statistical tests were performed using SPSS v20 (SPSS Inc., Chicago, IL). Data in text represent mean ± sd unless otherwise specified. All statistics are two-tailed, with preset α level for significance of p<.05.
Results
Patients showed highly significant impairments in reading relative to controls across all test batteries (F=6.70, df=18, 24, p<.001) with an overall large effect size across batteries (mean d=1.6) (Table 2). Effect-sizes for summary measures such as the Oral Reading Quotient of the Gray Oral Reading Test (d=2.3) or the Broad Reading measure of the Woodcock-Johnson (d=2.8) were substantially larger than deficits in either general cognitive measures (Table 2) or sensory measures alone (Table 3).
Table 3.
Mean and standard deviation (SD) for sensory measures for schizophrenia patients and controls
| Measure | Patient | Control | F | df | p | d | ||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| Tone matching (% correct) | 74.4 | 12.4 | 87.8 | 9.5 | 14.6 | 1,54 | <.001 | −1.2 |
| Visual Contrast Sensitivity (CS, 1/threshold) | ||||||||
| 0.5 cycles/degree (c/d) | 64.3 | 31.6 | 105.0 | 31.8 | 20.7 | 1,54 | <.001 | −1.3 |
| 7 cycles/degree (c/d) | 77.2 | 27.3 | 82.6 | 23.9 | .53 | 1,54 | .5 | −.2 |
| 21 cycles/degree (c/d) | 2.8 | 1.7 | 3.4 | 1.7 | 1.71 | 1,54 | .2 | −.4 |
| Mismatch negativity (MMN) amplitude (μV) | ||||||||
| Frequency deviant | −2.4 | 1.8 | −3.5 | 1.7 | 9.71 | 1,49 | .037 | −.9 |
| Duration deviant | −2.4 | 2.0 | −4.1 | 1.9 | 4.62 | 1,49 | .003 | −.6 |
| Intensity deviant | −2.9 | 1.9 | −4.5 | 2.1 | 7.71 | 1,49 | .008 | −.8 |
On subscales, patients showed significantly greater impairment in reading fluency than reading comprehension in both the Gray Oral Reading Test (group X subtest: F=21.6, df=1,67, p<.0001) and Woodcock-Johnson (group X test: F=21.6, df=1,41, p<.0001) which provide parallel measures (Table 2). On the Comprehensive Test of Phonological Processing (Table 2), phonological processing scores in schizophrenia patients were strongly different from controls (scaled score=74.5 ± 13.7 vs. 89.1± 14.2, t=4.17, df=45, p<.0001, d=1.0), as were visual reading scores (scaled score=79.8 ± 14.1 vs. 103.2 ± 14.9, t=5.21, df=45, p<.0001, d=1.6). As a result, there was a strongly significant main effect of group (F=15.9, df=1,45, p<.001) whereas the group X test interaction was not significant (F=2.18, df=1,45, p=.15). Present vs. premorbid reading level: In order to assess the degree to which reading impairments reflected an acquired dyslexia (i.e. regression in performance from a prior non-impaired state) versus neurodevelopmental dyslexia in schizophrenia, we performed two sets of analyses.
First, we compared performance on passage reading ability (i.e. ability to read paragraphs of information) to performance predicted based upon single-word reading (WRAT) (Figure 1A). For controls, these two scores were highly similar (paired t=1.46, df=23, p=.16). In contrast, for patients, passage reading ability was significantly reduced both versus controls (p<.0001) and versus within-subject levels predicted by WRAT reading (paired t=8.97, df=44, p<.0001). The group X test interaction was therefore highly significant (F=46.6, df=1,67, p<.0001), confirming differential impairment in passage vs. single-word reading ability in schizophrenia.
Figure 1.
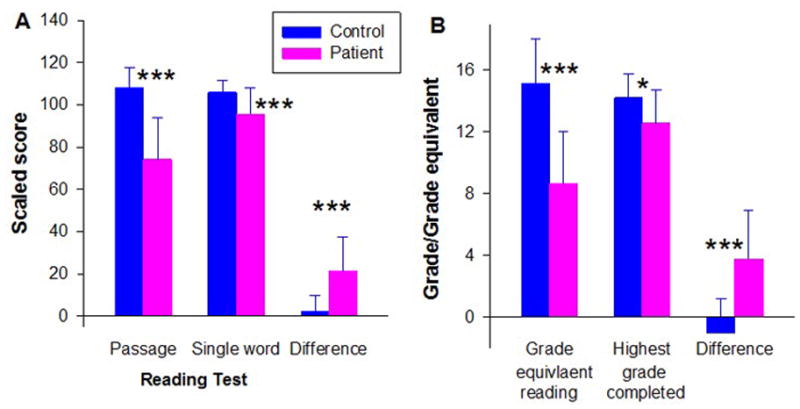
Difference between present (passage) and estimated premorbid reading ability in schizophrenia patients and healthy controls. A. Passage reading ability (± std dev) as determined using the Gray Oral Reading Test oral reading quotient vs. single-word reading assessed using the WRAT. B. Present grade-equivalent reading level (± std dev) assessed using the Nelson-Denny test vs. years of education completed.
*p<.05 patients vs. controls, between group t-test; ***p<.001
Second, we compared grade equivalent reading as determined using the Nelson-Denny Reading Test to years of education completed. In this analysis, controls showed grade-equivalent scores closely matching their years of education completed (Figure 1B). In contrast, patients showed grade-equivalent scores that were substantially reduced relative to years of education completed (paired t=6.35, df=27, p<.0001), leading again to a highly significant group X type of achievement (reading level/grade level) interaction (F=16.37, df=1, 41, p<.001).
Moreover, although patients had completed fewer years of education, between-group differences in Nelson-Denny scores remained strongly significant (F=27.4, df-1,40, p<.0001) even after co-variation for this difference. Patients were far more likely than controls to have attended special school (12/41 vs. 1/23, LR χ2=7.2, p=.007). However, there was no significant relationship between school type and reading scores (F=.7, df=1,39, p=.4), and between-group deficits remained highly significant even when differences in school type were considered (F=26.1, df-1,39, p<.0001). All subjects denied a history of developmental dyslexia.
Finally, passage reading scores were similar among patients drawn from inpatient (72.3 ± 19.5) vs. outpatient (77.5 ± 21.4) settings (t=.8, df=39, p=.4), were unrelated to length of illness (r=−.04, n=41, p=.8) and were significantly reduced vs. controls even in the small subgroup (n=6) who were within their first year of illness (reading score= 66.7 ± 12.9, t=9.07, df=28, p<.0001 vs. controls).
Reading vs. sensory measures
Integrity of early auditory processing was evaluated using MMN (Figure 2A) and tone matching (Table 3), while integrity of visual processing was assessed using contrast sensitivity across spatial frequencies (Table 3). As reported previously (6, 9) patients showed significant deficits compared with controls across all measures.
Figure 2. Relationship between sensory deficits and reading impairments in schizophrenia.

A. Mismatch negativity (MMN) scalp distributions in controls, non-reading impaired patients, and reading-impaired patients, along with difference map between controls and reading impaired patients.
B. Correlation between reduced MMN amplitude and impaired reading ability assessed using the Gray Oral Reading Test oral reading quotient. Correlations were significant across groups even when controlled for group status (rp =.53, n=50, p=.006), and in patients alone (r=.62, n=31, p<.0001).
C. Correlation between impaired sensory function, using a combined tone matching (TM) X contrast sensitivity (CS) sensory index, and scores on the Oral Reading Quotient of Gray Oral Reading Test. Correlations were significant across groups even when controlled for group status (rp=.43, n=47, p=.003), and were independently significant in patients (r=.42, n=33, p=.016) and control (r=.73, n=15, p=.002) groups independently.
Relationship to overall reading ability was assessed using stepwise regression controlled, in step 1, for group status. A significant multivariate correlation was observed for MMN across deviant types (multivariate R2=.13, p=.001). This correlation was driven primarily by response to duration deviants (rp =.50, df=46, p<.001) (Figure 2B) and remained significant even within patients alone (rp=.62, n=30, p<.001).
Significant independent correlations were also observed for tone matching (rp =.44, df=45, p=.002) and contrast sensitivity (rp =.37, df=45, p=.01). A composite measure combining these two factors correlated highly both across groups controlling for group status (rp =.43, df=45, p=.003), and within patients (r=.42, n=33, p=.016) and controls (r=.73, df=15, p=.002) independently (Figure 2C). The relationship between reading ability and sensory measures also remained significant even when Processing Speed was used to control for between-group differences in overall cognition (rp =.48, df=47, p=.001).
Prevalence of Impairment
Thirty of 41 (73.%) schizophrenia patients met criteria for current dyslexia based upon a score >1.5 sd below adult norms for passage reading vs. 0 of 24 controls (χ2=42.0, p<.0001). By contrast, only 1 of 41 (2.4%) of patients showed single-word reading (WRAT) scores >1.5 sd below adult norms, suggesting relatively intact premorbid function. Thus, the large majority of the patients with present reading impairment (29 of 30, 97.0%) showed discrepancy between premorbid and present reading abilities. When reading was considered at a grade-equivalent level based upon the Nelson Denny, 14 of 28 (50%) of patients receiving the full battery were found to be reading at below 8th grade reading level vs. only one control (χ2=9.45, df=1, p=.002). Eight of the 28 (29%) were reading at 5th grade level, which represents the lower cutoff of the test.
When schizophrenia patients who had received the full Comprehensive Test of Phonological Processing (n=32) were subdivided into subtypes based upon impaired visual (rapid naming/alternative rapid naming) vs. phonological (phonological awareness/alternate phonological awareness) scores <1.5 sd below norms, 11 (34.4%) were categorized as normal readers, 4 (12.5%) as visual subtype, 10 (31.3%) as phonological subtype and 7 (21.9%) as double deficit dyslexia. Overall reading scaled scores varied significantly across subtypes (F=3.96, df=3,28, p=.02) and were worse in patients with phonological (66.20±11.5, post-hoc p=.008) and double-deficit (65.3±8.6, post-hoc p=.012) dyslexia than in those with neither visual nor phonological impairments (86.6±19.6). MMN to duration stimuli (Figure 2A) also differentiated strongly between patients with and without reading impairment (t=3.56, df=29, p=.001).
Relationship to socioeconomic status, functional outcome and symptoms
As expected, schizophrenia patients in this study differed significantly in socioeconomic status not only from controls (Figure 3A), but also from their parents (paired t=3.94, df=25, p=.001). In contrast, healthy controls showed similar scores to their parents (paired t=1.62, df=20, p=.12), leading to a highly significant group X generation interaction (F=15.7, df=1,45, p<.0001). The group X generation interaction remained significant even following covariation for reduced educational achievement (F=7.86, df=1,44, p=.008).
Figure 3. Relationship of reading deficits to reduced socioeconomic status in schizophrenia.
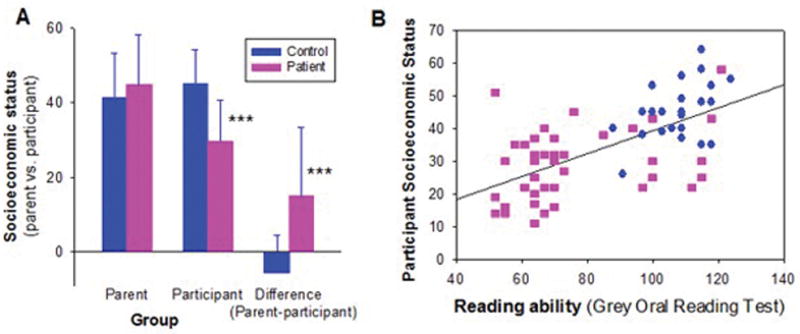
A. Mean (sd) parental vs. participant socioeconomic status in schizophrenia patients vs. healthy controls
B. Correlation of impaired reading ability to participant socioeconomic status across patients and controls (whole group: r=.66, n=70, p<.0001; patients alone: r=.36, n=45, p=.016).
***p<.001, between-group t-test
Given the potential importance of reading to occupational function, a multivariate regression analysis was performed assessing effects of reading and control variables on socioeconomic status. Impaired reading ability correlated significantly with reduced socioeconomic achievement over and above contribution of diagnosis (rp=.37, n=68, p=.002) (Figure 3B). By contrast, no significant correlation was observed for either processing speed (rp=.13, n=54, p=.4) or working memory (rp=.11, n=55, p=4).
Furthermore, when control (education, processing speed, working memory) and reading scores were entered simultaneously only reading remained a significant predictor of socioeconomic status (rp=.37, n=50, p=.01). Finally, as observed in our pilot study, reading ability in patients correlated strongly with score on the Independent Living Scales (r=.42, n=41, p=.007). No significant correlations of reading ability with PANSS scores, or with medication dose were observed.
Clinical high-risk patients
Reading measures were also obtained in a group of 19 high-risk individuals vs. 41 comparison subjects of similar age and gender. On global indices, high-risk patients showed single word (WRAT) and passage reading scores that were not significantly different from controls (Figure 4A). By contrast, reading rate was significantly reduced (t=2.24, df=58, p=.03) (Figure 4B), although comprehension and accuracy scores were not significantly different from either controls or published norms.
Figure 4. Reading scores (± std dev) of clinical high risk individuals vs. controls.

A. Scores of premorbid (single-word) reading ability assessed using the WRAT vs. present (passage) reading ability assessed using the Gray Oral Reading Test Oral Reading Quotient (GORT-ORQ).
B. Score on subscales of the Gray Oral Reading Test
C. Scores on the Comprehensive Test of Phonological Processing showing discrepancy between visual reading reflected by mean rapid naming (RN)/alternative rapid naming (ARN) scores vs. auditory (phonological) reading reflected by mean phonological awareness (PA)/alternate phonological awareness (APA) vs. phonological memory components in high-risk individuals.
*p<.05 clinical high-risk vs. control, between-group t-test; ***p<.001
On the Comprehensive Test of Phonological Processing (Figure 4C), phonological processing scores in clinical high-risk patients were not significantly different from controls (t=1.47, df=56, p=.15, d=.4). By contrast, rapid naming scores were significantly reduced (t=4.30, df=56, p<.0001, d=1.1), resulting in both a significant main effect of group (F=13.2, df=1,56, p=.001) and a significant group X test interaction (F=7.15, df=1,56, p=.01). Furthermore, visual reading scores were statistically similar to those in patients with established schizophrenia (t=.8, df=45, p=.4), whereas phonological measures were significantly superior (t=4.97, df=58, p<.0001, d=1.1), again leading to a significant group X test interaction (F=7.12, df=1,45, p=.011). Tone matching scores (90.6± 7.5% correct) were also not significantly different between high-risk individuals and healthy volunteers.
Finally, despite similar age (21.0±2.8 yr), schizophrenia patients in their first year of illness nevertheless had both passage reading scores (66.7±12.9 vs. 100.8±23.1, t=3.42, df=23, p=.002) and phonological processing scores (71.3±10.7 vs. 94.7±15.3, t=3.46, df=23, p=.002) that were significantly reduced versus controls, whereas visual reading scores were not significantly different (85.7±12.0 vs. 82.8±19.7, t=.34, df=23, p=.7).
Discussion
The ability to read is one of the most basic necessities for personal and occupational success in developed countries. For some individuals, learning to read may be difficult. However, for the vast majority of individuals, once these skills are developed they are retained for life. The present study demonstrates that such a situation may not be true in schizophrenia. In the present study, even relatively young schizophrenia patients showed deficits that were not only extreme in magnitude (effect size d >2.0), but were also much larger than the approximately 1 sd general cognitive impairment and sensory dysfunction associated with the disorder.
In a previous pilot study that focused only on individuals with schizophrenia who nevertheless had preserved overall cognitive abilities, we observed that these patients were reading on average at the 10th grade level despite having completed >12 years of education. In this more representative sample of persistently ill schizophrenia patients drawn primarily from long-term inpatient and residential care facilities, over 70% of patients met criteria for dyslexia, with 50% of patients reading below 8th grade level, and 29% reading at or below 5th, grade levels corresponding to percentile scores <10%. These levels constituted reductions of about 20 scaled score points relative to premorbid reading ability (Figure 1A), corresponding to approximately 4 grade levels vs. objective years of schooling completed or estimated premorbid reading levels (Figure 1B).
Furthermore, deficits in reading significantly predicted poor “real world” function as reflected in socioeconomic status (Figure 3B). In contrast, parental socioeconomic status did not affect either educational achievement or reading ability, suggesting that the “downward drift” associated with schizophrenia is a consequence of impaired functional ability rather than familial circumstances (38, 39). Prior studies have demonstrated significant contributions of cognitive disability in schizophrenia to a range of real-world outcome measures, including interpersonal skills, community activity, work skills (40) and quality of life measures (41, 42). How reading disability interrelates with other cognition and outcome impairments in schizophrenia beyond socioeconomic status will need to be pursued in future investigations.
Compared to patients with established schizophrenia, high-risk patients showed passage reading abilities that did not differ significantly from controls in this limited sample, although further studies are required to determine whether some degree of deficit may nevertheless be present. Despite the lack of a significant between-group difference in overall passage reading ability in this sample, their level of visual processing was nevertheless significantly reduced relative to both test norms and matched controls (Figure 4), and was similar to levels observed in patients with established schizophrenia, suggesting that visual deficits may pre-exist illness onset, and may predispose to development of later functional reading impairments. Whether or not phonological processing deficits also exist prior to illness onset remains to be determined.
In addition to confirming prior findings of impaired reading in established schizophrenia, the present findings are consistent with an emergent literature suggesting that visual processing deficits represent a stable, trait/risk factor for schizophrenia, whereas loss of auditory function occurs in the years immediately surrounding illness onset and thus may represent the proximate cause of the decline in absolute reading ability associated with schizophrenia onset (9). Thus, deficits in visual neurophysiological measures are present even in unaffected family members of schizophrenia patients (43), and, in schizophrenia, are unrelated either to premorbid function or length of illness (9). In contrast, deficits in MMN generation in response to duration deviants first develop during the years preceding illness onset and are among the strongest cognitive predictors of conversion to psychosis (44, 45), while deficits in MMN to frequency deviants may progress even following illness onset (46, 47), and thus may represent extension of dysfunction to primary sensory regions. In follow-back studies, MMN deficits, especially to duration deviants, are strongly related to premorbid educational attainment, consistent with the notion that regression of these pathways during the peri-onset period limits achievement (9).
Database studies
The present findings are also consistent with follow-back studies of reading ability performed using retrospective databases. For example, in studies using Iowa Scholastic Test scores of individuals who ultimately developed schizophrenia, reading scores were observed to decline from 45th to 37th percentile from 8th to 11th grade even prior to illness onset (31, 48). Similarly, small (d=.2–.4), but significant (p<.05) deficits in reading and reading comprehension were found in follow-back using the Israeli army conscription database (49, 50). Those follow-back scores correspond well to the premorbid, 41st percentile reading levels estimated based on WRAT reading scores in the present study.
Our present study demonstrates that further reduction to 17.5th percentile scores on passage reading (equivalent to scaled score of 74.2) occurs early in the course of the illness, suggesting functional decline in the years either directly preceding or immediately following illness onset. The decline in performance is not only larger than that for overall cognitive function (Table 2) or simple sensory measures (Table 3) considered independently, but may be more disruptive in terms of occupational function, given the extremely strong correlation between reading ability and socioeconomic status (Figure 3)
Clinical implications
On a clinical level, individuals with schizophrenia typically present in the 2nd to 3nd decades of life - i.e. during late high school or early college years. Individuals developing schizophrenia typically experience academic difficulty even prior to symptom onset and rarely return to their prior level of academic performance (51, 52). Although academic difficulties are often attributed to symptoms, general cognitive dysfunction or outside factors (e.g., substance abuse), the present study suggests that regression in reading ability, best conceptualized as a secondary or acquired dyslexia, must also be considered as a potential cause. A major unexplained finding in schizophrenia in general is the superior outcome in developing vs. developed countries, especially with outcomes such as employment (53, 54). Given the present findings, the differences in base literacy rates in such countries might represent at least a partial explanation for the differential outcome.
In summary, the present study demonstrates deficits in reading ability that were more severe than would be expected based upon general cognitive impairment alone in this sample, with 73% of schizophrenia patients meeting criteria for dyslexia despite intact prior reading ability, including 29% of patients reading at the 5th grade level or below, which is a widely used cutoff for functional illiteracy (2). Significant, albeit less pronounced, deficits in visual reading scores were present in individuals at high clinical risk for schizophrenia and may be deserving of future investigation. Although deficits in phonological processing were not observed in the limited sample of high risk patients who were tested, these may emerge when studies are extended to larger populations. The decline in reading ability from premorbid levels, which appears to occur during early stage of the illness correlates highly with the failure to meet socioeconomic expectations, and thus may represent a remediable cause of persistent occupational disability in schizophrenia.
Patients were selected for this study only if they showed IQ levels ≥85, and thus were “underimpaired” on measures such as processing speed and working memory compared to typical schizophrenia outpatients (e.g. 55). It might be that in a more globally impaired sample, the reading scores would be even more impaired and contribute even more strongly to functional outcome, but this issue can only be addressed through future, large scale investigation.
Supplementary Material
Acknowledgments
Funding: Supported by NIH grants R01 MH49334; P50 MH086385 and P50 MH086385-S1 to DCJ
Footnotes
Disclosures: Within the past 36 mo., Dr. Javitt reports receiving honoraria from Sunovion, BMS, Eli Lilly, Takeda, Omeros, Otsuka, Consensus Medical Communications, Guidepoint global, American Capital, Clearpoint communications, Vindico Medical Communication, and Clearview Healthcare; research support from Pfizer and Roche; equity in Glytech, Inc. and AASI; intellectual property rights for use of glycine, D-serine and glycine transport inhibitors in schizophrenia, and serving on scientific advisory board of Promentis. Other authors report no financial relationships with commercial interests.
References
- 1.Mead S. Reading for life. The American Prospect. 2010;21:A2–A5. [Google Scholar]
- 2.Kirsch IS, Jungeblat AJL, Kolstad A. Adult literacy in America: A first look at findings of the National Adult Literacy Survey. 3. Washington, DC: U.S. Department of Education, Office of Educational Research and Improvement; 2002. [Google Scholar]
- 3.Talcott JB, Witton C, Hebb GS, Stoodley CJ, Westwood EA, France SJ, Hansen PC, Stein JF. On the relationship between dynamic visual and auditory processing and literacy skills; results from a large primary-school study. Dyslexia (Chichester, England) 2002;8:204–25. doi: 10.1002/dys.224. [DOI] [PubMed] [Google Scholar]
- 4.Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62:816–47. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor JS, Rastle K, Davis MH. Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychological bulletin. 2013;139:766–91. doi: 10.1037/a0030266. [DOI] [PubMed] [Google Scholar]
- 6.Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, Lim KO, Revheim N, Silipo G, Javitt DC. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annual review of clinical psychology. 2009;5:249–75. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gold R, Butler P, Revheim N, Leitman DI, Hansen JA, Gur RC, Kantrowitz JT, Laukka P, Juslin PN, Silipo GS, Javitt DC. Auditory emotion recognition impairments in schizophrenia: relationship to acoustic features and cognition. Am J Psychiatry. 2012;169:424–32. doi: 10.1176/appi.ajp.2011.11081230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman T, Sehatpour P, Dias E, Perrin M, Javitt DC. Differential relationships of mismatch negativity and visual p1 deficits to premorbid characteristics and functional outcome in schizophrenia. Biol Psychiatry. 2012;71:521–9. doi: 10.1016/j.biopsych.2011.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkinson GS. The Wide Range Achievement Test 3 Administrative Manual. Wide Range, Inc; 1993. [Google Scholar]
- 11.Dalby JT, Williams R. Preserved reading and spelling ability in psychotic disorders. Psychol Med. 1986;16:171–5. doi: 10.1017/s0033291700002609. [DOI] [PubMed] [Google Scholar]
- 12.Harvey PD, Moriarty PJ, Friedman JI, White L, Parrella M, Mohs RC, Davis KL. Differential preservation of cognitive functions in geriatric patients with lifelong chronic schizophrenia: less impairment in reading compared with other skill areas. Biol Psychiatry. 2000;47:962–8. doi: 10.1016/s0006-3223(00)00245-6. [DOI] [PubMed] [Google Scholar]
- 13.Kravariti E, Morgan K, Fearon P, Zanelli JW, Lappin JM, Dazzan P, Morgan C, Doody GA, Harrison G, Jones PB, Murray RM, Reichenberg A. Neuropsychological functioning in first-episode schizophrenia. Br J Psychiatry. 2009;195:336–45. doi: 10.1192/bjp.bp.108.055590. [DOI] [PubMed] [Google Scholar]
- 14.Vidyasagar TR. Neural underpinnings of dyslexia as a disorder of visuo-spatial attention. Clin Exp Optom. 2004;87:4–10. doi: 10.1111/j.1444-0938.2004.tb03138.x. [DOI] [PubMed] [Google Scholar]
- 15.Stein J. The magnocellular theory of developmental dyslexia. Dyslexia (Chichester, England) 2001;7:12–36. doi: 10.1002/dys.186. [DOI] [PubMed] [Google Scholar]
- 16.Iles J, Walsh V, Richardson A. Visual search performance in dyslexia. Dyslexia (Chichester, England) 2000;6:163–77. doi: 10.1002/1099-0909(200007/09)6:3<163::AID-DYS150>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 17.Bishop DV. Using mismatch negativity to study central auditory processing in developmental language and literacy impairments: where are we, and where should we be going? Psychological bulletin. 2007;133:651–72. doi: 10.1037/0033-2909.133.4.651. [DOI] [PubMed] [Google Scholar]
- 18.Kujala T, Naatanen R. The mismatch negativity in evaluating central auditory dysfunction in dyslexia. Neuroscience and biobehavioral reviews. 2001;25:535–43. doi: 10.1016/s0149-7634(01)00032-x. [DOI] [PubMed] [Google Scholar]
- 19.Baldeweg T, Richardson A, Watkins S, Foale C, Gruzelier J. Impaired auditory frequency discrimination in dyslexia detected with mismatch evoked potentials. Annals of neurology. 1999;45:495–503. doi: 10.1002/1531-8249(199904)45:4<495::aid-ana11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 20.Wolf M, Bowers PG. Naming-speed processes and developmental reading disabilities: an introduction to the special issue on the double-deficit hypothesis. Journal of learning disabilities. 2000;33:322–4. doi: 10.1177/002221940003300404. [DOI] [PubMed] [Google Scholar]
- 21.Miller CJ, Miller SR, Bloom JS, Jones L, Lindstrom W, Craggs J, Garcia-Barrera M, Semrud-Clikeman M, Gilger JW, Hynd GW. Testing the double-deficit hypothesis in an adult sample. Ann Dyslexia. 2006;56:83–102. doi: 10.1007/s11881-006-0004-4. [DOI] [PubMed] [Google Scholar]
- 22.Wiederholt JL, Bryant BR. Gray Oral Reading Tests (GORT-4): Examiner’s Manual. 4. Austin, TX: Pro-Ed; 2001. [Google Scholar]
- 23.Wagner RK, Torgesen JK, Rashotte CA. CTOPP: Comprehensive Test of Phonological Processing (Examiner’s Manual.) Austin, TX: Pro-Ed; 1999. [Google Scholar]
- 24.Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- 25.Brown JI, Fishco VV, Hanna G. Nelson-Denny Reading Test: Manual for Scoring and Interpretation. Itasca, IL: The Riverside Publishing Company; 1993. [Google Scholar]
- 26.Strous RD, Cowan N, Ritter W, Javitt DC. Auditory sensory (“echoic”) memory dysfunction in schizophrenia. Am J Psychiatry. 1995;152:1517–9. doi: 10.1176/ajp.152.10.1517. [DOI] [PubMed] [Google Scholar]
- 27.Hollingshead AB. Two factor index of social position. 1957 [Google Scholar]
- 28.Revheim N, Schechter I, Kim D, Silipo G, Allingham B, Butler P, Javitt DC. Neurocognitive and symptom correlates of daily problem-solving skills in schizophrenia. Schizophr Res. 2006;83:237–45. doi: 10.1016/j.schres.2005.12.849. [DOI] [PubMed] [Google Scholar]
- 29.Wechsler D. The Wechsler Adult Intelligence Scale. 3. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 30.Wechsler D. The Wechsler Memory Scale-III. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 31.Fuller R, Nopoulos P, Arndt S, O’Leary D, Ho BC, Andreasen NC. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. Am J Psychiatry. 2002;159:1183–9. doi: 10.1176/appi.ajp.159.7.1183. [DOI] [PubMed] [Google Scholar]
- 32.Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–42. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- 33.Press AP. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington DC: American Psychiatric Association; 1994. (DSM-IV) [Google Scholar]
- 34.Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–15. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- 35.Kay SR. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin. 1987;13:261. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 36.Revheim N, Medalia A. The independent living scales as a measure of functional outcome for schizophrenia. Psychiatr Serv. 2004;55:1052–4. doi: 10.1176/appi.ps.55.9.1052. [DOI] [PubMed] [Google Scholar]
- 37.Green MF, Schooler NR, Kern RS, Frese FJ, Granberry W, Harvey PD, Karson CN, Peters N, Stewart M, Seidman LJ, Sonnenberg J, Stone WS, Walling D, Stover E, Marder SR. Evaluation of functionally meaningful measures for clinical trials of cognition enhancement in schizophrenia. Am J Psychiatry. 2011;168:400–7. doi: 10.1176/appi.ajp.2010.10030414. [DOI] [PubMed] [Google Scholar]
- 38.Dohrenwend BP, Levav I, Shrout PE, Schwartz S, Naveh G, Link BG, Skodol AE, Stueve A. Socioeconomic status and psychiatric disorders: the causation-selection issue. Science. 1992;255:946–52. doi: 10.1126/science.1546291. [DOI] [PubMed] [Google Scholar]
- 39.Goldberg S, Fruchter E, Davidson M, Reichenberg A, Yoffe R, Weiser M. The relationship between risk of hospitalization for schizophrenia, SES, and cognitive functioning. Schizophr Bull. 2011;37:664–70. doi: 10.1093/schbul/sbr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowie CR, Reichenberg A, Patterson TL, Heaton RK, Harvey PD. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry. 2006;163:418–25. doi: 10.1176/appi.ajp.163.3.418. [DOI] [PubMed] [Google Scholar]
- 41.Mohamed S, Rosenheck R, Swartz M, Stroup S, Lieberman JA, Keefe RS. Relationship of cognition and psychopathology to functional impairment in schizophrenia. Am J Psychiatry. 2008;165:978–87. doi: 10.1176/appi.ajp.2008.07111713. [DOI] [PubMed] [Google Scholar]
- 42.Kahn R, Keefe R. JAMA Psychiatry. 2013. Schizophrenia is a cognitive illness: time for a change in focus. epub. [DOI] [PubMed] [Google Scholar]
- 43.Yeap S, Kelly SP, Sehatpour P, Magno E, Javitt DC, Garavan H, Thakore JH, Foxe JJ. Early visual sensory deficits as endophenotypes for schizophrenia: high-density electrical mapping in clinically unaffected first-degree relatives. Arch Gen Psychiatry. 2006;63:1180–8. doi: 10.1001/archpsyc.63.11.1180. [DOI] [PubMed] [Google Scholar]
- 44.Atkinson RJ, Michie PT, Schall U. Duration mismatch negativity and P3a in first-episode psychosis and individuals at ultra-high risk of psychosis. Biol Psychiatry. 2012;71:98–104. doi: 10.1016/j.biopsych.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 45.Bodatsch M, Ruhrmann S, Wagner M, Muller R, Schultze-Lutter F, Frommann I, Brinkmeyer J, Gaebel W, Maier W, Klosterkotter J, Brockhaus-Dumke A. Prediction of psychosis by mismatch negativity. Biol Psychiatry. 2011;69:959–66. doi: 10.1016/j.biopsych.2010.09.057. [DOI] [PubMed] [Google Scholar]
- 46.Javitt DC, Shelley AM, Silipo G, Lieberman JA. Deficits in auditory and visual context-dependent processing in schizophrenia: defining the pattern. Arch Gen Psychiatry. 2000;57:1131–7. doi: 10.1001/archpsyc.57.12.1131. [DOI] [PubMed] [Google Scholar]
- 47.Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–9. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ho B-C, Mrcpsych, Andreasen N, Nopoulos P, Fuller R, Arndt S, Cadoret R. Secondary Prevention of Schizophrenia: Utility of Standardized Scholastic Tests in Early Identification. Annals of Clinical Psychiatry. 2005;17:11–8. doi: 10.1080/10401230590905272. [DOI] [PubMed] [Google Scholar]
- 49.Reichenberg A, Weiser M, Rabinowitz J, Caspi A, Schmeidler J, Mark M, Kaplan Z, Davidson M. A population-based cohort study of premorbid intellectual, language, and behavioral functioning in patients with schizophrenia, schizoaffective disorder, and nonpsychotic bipolar disorder. Am J Psychiatry. 2002;159:2027–35. doi: 10.1176/appi.ajp.159.12.2027. [DOI] [PubMed] [Google Scholar]
- 50.Weiser M, Reichenberg A, Rabinowitz J, Nahon D, Kravitz E, Lubin G, Knobler HY, Davidson M, Noy S. Impaired reading comprehension and mathematical abilities in male adolescents with average or above general intellectual abilities are associated with comorbid and future psychopathology. J Nerv Ment Dis. 2007;195:883–90. doi: 10.1097/NMD.0b013e31815928b0. [DOI] [PubMed] [Google Scholar]
- 51.Monte RC, Goulding SM, Compton MT. Premorbid functioning of patients with first-episode nonaffective psychosis: a comparison of deterioration in academic and social performance, and clinical correlates of Premorbid Adjustment Scale scores. Schizophr Res. 2008;104:206–13. doi: 10.1016/j.schres.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allen DN, Frantom LV, Strauss GP, van Kammen DP. Differential patterns of premorbid academic and social deterioration in patients with schizophrenia. Schizophr Res. 2005;75:389–97. doi: 10.1016/j.schres.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 53.Jablensky A, Sartorius N, Ernberg G, Anker M, Korten A, Cooper JE, Day R, Bertelsen A. Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten-country study Psychological medicine. Monograph supplement. 1992;20:1–97. doi: 10.1017/s0264180100000904. [DOI] [PubMed] [Google Scholar]
- 54.Menezes NM, Arenovich T, Zipursky RB. A systematic review of longitudinal outcome studies of first-episode psychosis. Psychol Med. 2006;36:1349–62. doi: 10.1017/S0033291706007951. [DOI] [PubMed] [Google Scholar]
- 55.Kern RS, Gold JM, Dickinson D, Green MF, Nuechterlein KH, Baade LE, Keefe RS, Mesholam-Gately RI, Seidman LJ, Lee C, Sugar CA, Marder SR. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr Res. 2011;126:124–31. doi: 10.1016/j.schres.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


