Abstract
Sensory processing deficits, first investigated by Kraeplin and Bleuler as possible pathophysiological mechanisms in schizophrenia, are now being re-characterized in the context of modern understanding of the involved molecular and neurobiological brain mechanisms. The National Institute of Mental Health Research Domain Criteria position these deficits as intermediaries between molecular and cellular mechanisms and clinical symptoms of schizophrenia such as hallucinations. The pre-pulse inhibition of startle responses by a weaker preceding tone, the inhibitory gating of response to paired sensory stimuli characterized using the auditory P50 evoked response, and the detection of slightly different stimuli that elicits the cortical Mismatch Negativity potential demonstrate deficits in early sensory processing mechanisms, whose molecular and neurobiological bases are increasingly well understood. Deficits in sensory processing underlie more complex cognitive dysfunction and, vice versa, are affected by higher-level cognitive difficulties. These deficits are now being used to identify genes involved in familial transmission of the illness and to monitor potentially therapeutic drug effects for both treatment and prevention. This research also provides a clinical reminder that patients’ sensory perception of the surrounding world, even during treatment sessions, may differ considerable from others’ perceptions. A person’s ability to understand and interact effectively with surrounding world ultimately depends upon an underlying sensory experience of it.
Keywords: Schizophrenia, Sensory Gating, Startle, Evoked Response Auditory, Mismatch Negativity, Evoked Response Visual
When patients tell us that they are hearing voices or seeing visions, we often assume that these experiences are layered onto a perception of the world that is basically similar to our own. Yet patients report instead more globally distorted perceptions of their world that underlie their psychotic symptoms. Elyn Saks thus describes the first day that she became psychotic:
“I suddenly decided that I needed to get up, leave school, and walk home….As I walked along, I began to notice that the colors and shapes of everything around me were becoming more intense. And, at some point, I began to realize that the houses I was passing were sending special messages to me: Look closely. You are special. You are especially bad. Look closely and ye shall find. There are many things you must see. See. See.” (1)
Her suddenly heightened perception of her familiar neighborhood quickly became delusional. We may easily overlook these basic perceptual disturbances in diagnostic interviews, yet they are a basis for the more bizarre distortions of reality that lead to clinical diagnosis of schizophrenia and other psychoses. Deficits in auditory and visual sensory processing may not fully explain patients’ psychotic experience, but they are a paradigmatic example of research that has been guided by patients’ own descriptions of their experience of the world. Clinicians attuned to similar descriptions from their own patients of their sensory overload may find that their understanding of their patients’ experience of the world around them is sharpened (2).
Although both Kraeplin and Bleuler included descriptions of perceptual abnormalities in schizophrenia in their textbooks (3), the detailed methodology involved in their characterization often makes the research advances in their characterization obscure. The strategy of using simple stimuli, tones or light patterns, helps isolate specific mechanisms but it can also leave the impression that only initial sensation is being tested. The processing of such simple stimuli is actually complex and involves both cortical and subcortical circuitry. Deficits in processing the simplest stimuli found in schizophrenia may underlie more complex hallucinations and delusions, as for Professor Saks, in two ways: [1] neuropsychologically, failure to register basic sensory information correctly makes poor decisions about it inevitable, and [2] neurobiologically, the same neuronal mechanisms that register the information are utilized throughout the brain, so that deficits in neuronal mechanisms detected in sensory areas are likely to be present also in regions that have more complex executive functions. Accordingly, many of the neurotransmitter mechanisms most associated with schizophrenia, such as glutamate, GABA, dopamine and acetylcholine, as well as their receptors, are widely distributed throughout brain as are many of the associated risk genes, suggesting that their effects on brain function should be equally detectable across brain regions.
David Hubel and Torsten Wiesel similarly described their Nobel Prize research in vision:
“To examine the workings of this visual pathway our strategy since the late 1950’s has been (in principle) simple. Beginning, say, with the fibers of the optic nerve, we record with microelectrodes from a single nerve fiber and try to find out how we can most effectively influence the firing by stimulating the retina with light (p. 152)….There was a time, not so long ago, when one looked at the millions of neurons in the various layers of the cortex and wondered if anyone would ever have any idea of their function….For the visual cortex the answer seems now to be known in broad outline: Particular stimuli turn neurons on or off; groups of neurons do indeed perform particular transformations. It seems reasonable to think that if the secrets of a few regions such as this one can be unlocked, other regions will also in time give up their secrets (4).”
Elementary sensation, such as auditory detection thresholds or visual acuity, is generally considered to be normal in patients, reflecting integrity of the peripheral sensory apparatus. However, research like Hubel and Wiesel’s points to the many stages of processing that occur as sight and sound pass from the eyes and ears through successive brain regions before becoming recognizable as images and words. Much of this processing occurs outside the realm of conscious awareness, so unless it is specifically tested patients will be unaware of their deficits. Moreover, because basic sensory systems have been extensively characterized not only in humans but also in animal models, patterns of deficit can be directly linked to underlying biology.
Over recent decades, both the nature of the deficits and their consequences for function have become increasingly clear. This review highlights the important role played by sensory deficits in both the subjective and objective functioning of individuals with schizophrenia, and how an increasing focus on sensory dysfunction may improve functional outcome for patients with schizophrenia and other severe neuropsychiatric disorders.
Deficits in sensory processing are most effectively demonstrated using neurophysiological measures such as event-related potentials, which uniquely trace the flow of information from sense organs, through brainstem and subcortical regions, and into sensory and then higher cortical brain regions. Results of these event-related potential studies, however, are strongly corroborated by both behavioral and neuroimaging based measures that provide additional information regarding location and nature of underlying cortical impairments (Table 1).
Table 1.
Sensory processing dysfunctions in schizophrenia
| Modality | Patient experience | Neurocognitive disturbance |
Neurophysiological paradigms |
Neuroimaging paradigms |
Neurotransmitter mechanisms |
Candidate Genes |
|---|---|---|---|---|---|---|
| Auditory | Noises appear louder; misperception of sounds; hallucinations (6); reduced response to environmental change as a chronic adaptation (110) |
Decreased detection of target signals in digit-vigilance and coding tasks; poor pitch perception ; phonological reading deficits (74); amusia (121) |
Diminished PPI (7, 11), and P50/N100 sensory gating (10); reduced N100, MMN, and P300 amplitude (12, 71) |
Increased frontotemporal /thalamic activity during gating (14); Reduced auditory activation to deviant auditory stimuli (68) |
Diminished nicotinic cholinergic and NMDAR glutamate activation of inhibitory interneurons; increased catecholaminergic sensitization of neuron responses (36, 37) |
CHRNA7, NRG1, COMT, DISC1 (122) |
| Visual | Objects appear fragmented and distorted (102); decreased sensitivity to dim, rapidly presented, or moving objects (48) |
Reduced “closure” ability (98); impaired face emotion recognition (99); visual reading deficits (104, 105) |
Diminished visual P1 to low spatial frequency stimuli (89, 123, 124); diminished event- related desynchronization of ongoing rhythms (121) |
Reduced activation of low spatial frequency regions of visual cortex (91, 92) |
Non-linear amplification failure in subcortical visual pathways ; decreased glutamate NMDAR activation (90) |
DTNBP1 (125); NOS1 (126) |
Auditory dysfunction in schizophrenia
Brain functions that differentiate humans from other primates include our superior reasoning and problem solving ability. These functions related to a dramatic expansion of human frontal brain regions as compared to other primates (Figure 1A). It is less appreciated that an equally marked evolutionary expansion has occurred within the human auditory system (5). This expansion underlies not only our unique linguistic abilities, but also our greatly expanded ability to appreciate pitch, rhythm and other musical features. Like other recently evolving regions, auditory cortex completes its maturation late in human development (5). Thus, like prefrontal regions, these systems may remain vulnerable to disruption even during late adolescence and early adulthood when schizophrenia typically develops. In general, sensory systems play a dual role – first to orient attention to critical regions and features of the environment and, second, to decode the information emanating from those regions to enable subsequent voluntary processing. In schizophrenia, both the attentional and informational roles of the sensory systems are impaired, contributing to the overall pattern of symptoms and neurocognitive deficits we frequently associate with schizophrenia
Figure 1.
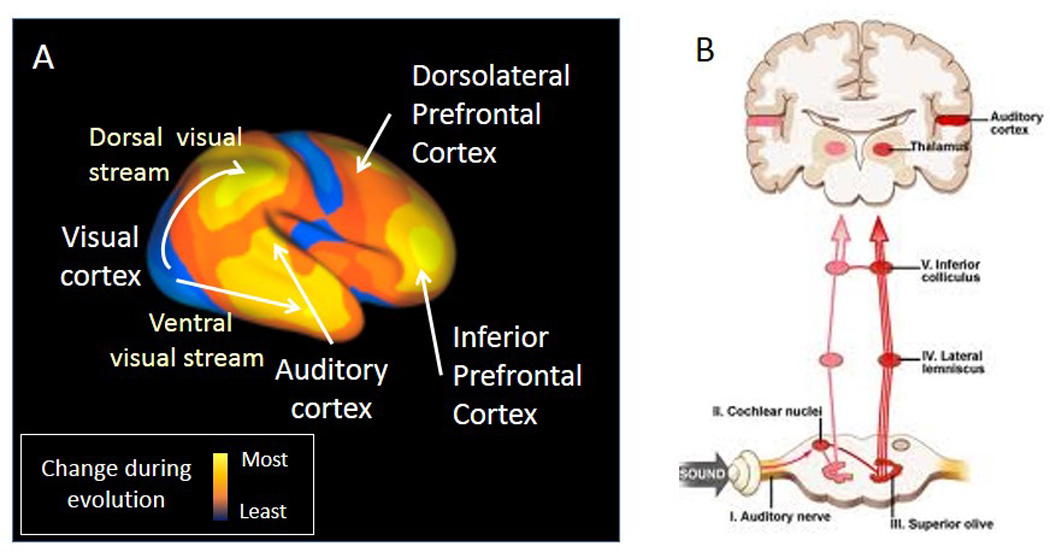
Anatomy of brain sensory systems, A. Cortical areas responsible for perception and executive function have both experienced especially enhanced expansion in the evolution of human brain (from 5). B. Right side of the brain shows auditory responses from the inner ear, conveyed through brainstem relay nuclei, arriving at the auditory cortex through the medical geniculate nucleus. Left side shows how a branch from the brainstem relay nuclei contact the reticular brainstem formation, which in turns activtes the medial septal nucleus. The medial septal nucleus cholinergic input acts in the hippocampus to help filter all the sensory information from the cortex, including auditory cortex, that is funneled to it through the Superior Temporal Gyrus.
Normal auditory physiology (Figure 1B)
Most clinical tests of auditory function, such as audiological assessments or far field brainstem potentials, are tests of the most peripheral portions of the system in the inner ear and the auditory nerve leading to the brainstem, which do not appear to be affected in schizophrenia (6). The auditory information from the inner ear is initially processed in brainstem relay neurons, primarily to separate tones and regulate sensitivity to high and low volumes, and to orient the person to the location of sound. Auditory information then ascends through the medial geniculate nucleus to the auditory cerebral cortex, which plays a critical role in decoding of simple auditory features such as pitch, intensity or location, which then get elaborated in subsequent auditory regions into more complex auditory percepts such as language or tonality. Dysfunction within the cortical regions may thus contribute to the complex patterns of social and communicative disturbance observed in schizophrenia.
Sensory and sensorimotor gating dysfunction
Auditory gating relies on the interplay of auditory input and brainstem reticular formation function. Reticular neurons have rapidly habituating responses to repeated stimulation and thus form the brain’s initial sensory gating mechanism, which prevents other brain regions from being flooded with repeated and presumed less important sensory information, e.g., in a noisy environment. The habituation to repeated stimulation leaves the brain ready to respond to more novel and thus potentially more meaningful sensory stimuli.
The effect of the brainstem’s habituation is observed experimentally in two ways (Table 1). First, a descending spinal pathway from the larger reticular neurons initiates the startle response. A weak tone initiates habituation of the reticular neurons’ response to a successive louder tone, leading to the phenomenon of prepulse inhibition (PPI) of the acoustic startle response (7, 8).
In parallel, projections from other reticular neurons ascend to the midbrain where they contact cholinergic neurons in the medial septal nucleus, which in turn project to the hippocampus, thalamus, and neocortex. These cholinergic projections activate inhibitory interneurons in the hippocampus and other areas that modulate local excitatory glutamatergic neurotransmission (9). The hippocampus then inhibits response to less important information coming from the auditory cortex and other sensory regions, funneled to it through the Superior Temoral Gyrus. In humans, the largest initial cerebral response to an auditory stimulus is a positive wave that occurs at 50 ms and is thus termed the P50 potential. The P50 response to the second of paired stimuli in is decreased relative to the first in normal persons, an example of inhibitory sensory gating (10).
Deficits in the integrity of both PPI and P50 gating in schizophrenia have been extensively replicated since their first demonstrations, pointing to dysfunction even within these low level circuits. The deficit in PPI is most prominent in schizophrenia when the weaker tone precedes the startling sound by 60 msec (7). The deficit is more pronounced in women, who normally show less inhibition than men. Patients with schizophrenia, either unmedicated or treated with most antipsychotic drugs, also have reduced inhibition of the P50 auditory evoked potential (Figure 2A). The inhibition of the second stimulus is maximal at 500 msec, later than the maximal PPI interval of 60 msec. In many patients the two deficits do not occur together, and thus they represent distinct aspects of pathophysiology (11). The N100 also shows impaired gating in schizophrenia (12).
Figure 2.
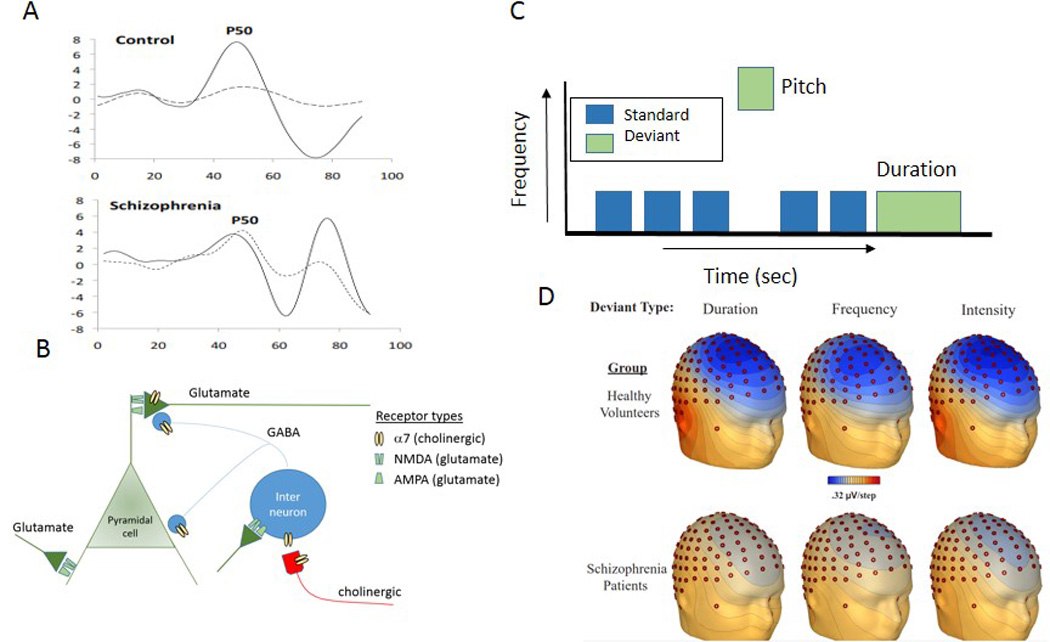
Dysfunctions in auditory sensory processing in schizophrenia.
A. Representative tracing of the P50 sensory gating deficit in schizophrenia. Voltage in microvolts is shown on the vertical axis, time in milliseconds after each stimulus is shown on the horizontal. The response to the first stimulus is solid land the response to the second is dashed. The second response is less inhibited in the schizophrenia patient.
B. Brain circuits responsible for sensory processing in the neocortex or hippocampus. AMPA-type glutamate receptors are responsible for the excitatoy transmission of sensory input to the pyramidal cells, where integration into images and memory occurs. Inhibitory feedback to help the pyramidal cells’ selective focusing of their response cccurs through GABA transmission from inhibitory interneurons. Interneuron function is modulated by α7-nicotinic cholinergic receptors that are activated by subcortical midbrain cholinergic pathways, which convey sensory gating responses from subcortical mechanims, such as those shown in Figure 1, and NMDA-type glutamate receptors that convey information about unusual or mismatched stimulus characteristics.
C. Paradigm for mismatch negativity (MMN) generation; the detection of the deviant tone generates the MMN. Diminished MMN is seen in schizophrenia regardless of whether the tone is deviant in duration, pitch or frequency, or intensity from a train of otherwise identical stimuli.
D. Topographical distribution of MMN deficits in schizophrenia (65).
For both PPI and P50 gating, despite the origin of the effect in reticular brain stem neurons, the response engages an entire network of brain circuits, the dopaminergic circuits of the basal ganglia for PPI and the basal cholinergic nuclei and cortex for P50 gating (8, 9). Rodent models of P50 gating emphasize the response in the hippocampus, but sources for human P50 have been found in the superior temporal gyrus and cortical rhythms are altered during the sensory gating paradigm, with a decrease in beta electroencephalographic waves (13). Deficits in the response to repeated sounds have also been localized to the hippocampus and dorsal lateral prefrontal cortex by both dipole analysis and functional magnetic resonance imaging (fMRI) (14, 15).
P50 inhibitory gating deficits in schizophrenia have been associated with the frequency and intensity of auditory hallucinations and with neurocognitive measures of decreased vigilance and attention, consistent with the role of inhibitory sensory gating in protecting the brain from being responsive to extraneous stimuli while it is trying to focus attention on a task (16, 17). The association between patients’ self-report of sensory phenomena and physiological measures is confounded by their psychopathology (18). Thus, several investigations in patients themselves have not shown association between P50 inhibition and self-reports of sensory gating difficulties (19), but in schizotypal adults deficiencies in P50 inhibition are associated with difficulties in “hearing everything at once” and with a sense of unreality, including perceptual anomalies and magical beliefs (20, 21). PPI deficits have been correlated with deficient social perception (22).
Genetic endophenotypes
Inhibitory gating measures have also contributed significantly to the search for genes involved in schizophrenia. Deficits in both PPI and P50 gating are found in schizotypal individuals who have a family history of schizophrenia and in adolescents at high risk for schizophrenia (23–25). As a complex genetic illness, schizophrenia has not been linked to a single major gene. However, these specific endophenotype measures, each thought to underlie one aspect of the genetic basis of schizophrenia, were hypothesized to have closer relationship to particular elements of genetic risk (20).
In families affected with multiple cases of schizophrenia, the deficit in P50 gating segregates as an autosomal dominant and is linked to the CHRNA7 gene complex, which forms the α7-nicotinic acetylcholine receptor (26). Polymorphisms of CHRNA7 have been associated with PPI as well (27). Another related gene is NRG1 which forms neuregulin (28, 29). Neuregulin helps assemble both α-nicotinic and N-methyl-D-aspartate-type glutamate receptors (NMDARs) (30), and thus its association with both sensory processing deficits is not surprising. The α7-nicotinic receptor is found presynaptically on glutamate nerve terminals and postsynaptically on inhibitory interneurons (Figure 2B), particularly in the hippocampus and nucleus reticularis thalamis (9). Thus, there is convergence between the genetic findings and the neurobiological model, corroborated by postmortem findings of decreased α7-nicotinic receptors in schizophrenia (31). As the scope of genomic analysis has increased, both deficits have been associated with entire networks of genetic variants in schizophrenia in the NIMH Consortium of Genetics Studies of Schizophrenia study, which examines the relationships of these phenotypes to the cognitive and genetic aspects of schizophrenia (28).
Drug Development
Although schizophrenia cannot be re-produced in animals, mouse and rat analogues of PPI and P50 deficits can be produced. Increased dopaminergic neurotransmission from stimulants and psychotomimetic antagonists of NMDARs such as phencyclidine (PCP) induce the deficit pharmacologically (8). Deficits in CHRNA7 null mutant mice for P50 gating (32) and in NRG-1 null mutants for PPI have also been reported (8). Intriguingly, the NRG1-induced deficits do not appear until adolescence (33), modeling the time course of onset of schizophrenia. (34)
Animal models were first used to examine effects of nicotine and clozapine, with good corroboration in clinical observations (10). Cigarette smoking increases P50 inhibition and PPI in schizophrenia (35). Further animal and clinical investigation confirmed nicotine itself, acting through α7-nicotinic receptors, as the mechanism of effects on P50 inhibition (36, 37). Clozapine, unlike other antipsychotics, significantly normalizes P50 inhibition, through increased release of acetylcholine in the hippocampus and the resultant indirect activation of α7-nicotinic receptors on inhibitory interneurons (38).
Investigational therapeutics followed to develop specific α7-nicotinic receptor agonists to enhance cognition. The initial proof of principle for several of the agents included the demonstration of normalization of P50 inhibition in schizophrenia (39). Human studies are mixed with several but not all showing enhancement of cognition and relief of negative symptoms (39–41). Nevertheless, at least one compound is presently in late stage clinical testing, with definitive results expected within the next several years (42).
Development
P50 deficits are already present in many newborn infants of parents with schizophrenia Table 2; (43). In the perinatal period, α7-nicotinic receptors are critical to the development of cerebral inhibition. However, cholinergic inputs do not reach the hippocampus until shortly before birth. Therefore, they are not stimulated by synaptically released acetylcholine. Choline, in the millimolar concentrations found in amniotic fluid, activates α7-nicotinic receptors. Dietary supplementation of choline stimulates the development of cerebral inhibition as measured by P50 inhibition after birth (44). Deficits in development of P50 inhibition in newborns are correlated with the first appearance of attention deficit symptoms in later childhood (45).
Table 2.
Development of sensory processing dysfunctions
| Period | Fetal newborn offspring of parent with schizophrenia |
Adolescents at risk for schizophrenia |
Schizophrenia childhood and adult |
Unaffected adult relatives |
Adult schizotypal persons |
|---|---|---|---|---|---|
|
Deficit in PPI |
Increased incidence of poor startle habituation |
Increased prevalence |
Increased prevalence, decreased with second generation drugs, smoking |
Increased prevalence |
Increased prevalence |
|
Deficit in P50 gating |
Increased incidence |
Increased prevalence |
Increased prevalence, decreased with clozapine and nicotinic agonists |
Increased prevalence, segregates as autosomal dominant |
Increased Prevalence Particularly with positive family history |
|
Deficits in MMN |
Increased prevalence |
Increased prevalence, unaffected by antipsychotics or clozapine |
Variable results (127, 128) |
Increased Prevalence (128, 129) |
|
|
Deficits in visual P1 |
Unknown | Increased prevalence, unaffected by antipsychotics or clozapine |
Increased prevalence |
Deficits in neocortical discrimination of auditory stimuli
The brainstem-based gating mechanisms operate at a basic filtering level to enable people to operate effectively in noisy, stimulus filled environments. Once information reaches the cerebral cortex, then fine discrimination of differences is needed for complex feature extraction needed for uses like language and more emotive features of sound, including voice tone and music. The primary auditory cortex contains different regions, which support the extraction of these different features, including the localization of the source vs. the content of sounds (46).
Sound localization in schizophrenia
One of the strongest clues to auditory dysfunction in schizophrenia is the presence of auditory hallucinations. Although neural substrates of hallucinations remain incompletely understood, it has been known since Penfield (47) that stimulation of auditory regions induces auditory hallucinations similar to those of schizophrenia. Consistent with this literature, auditory hallucinations in schizophrenia are increasingly tied to auditory cortex function. For example, hallucinations are accompanied by objective hyperactivity of left sided auditory regions (48). Similarly, increased ability of the left hemisphere to localize sound in the right side of space correlate with increased severity of hallucinations (49). Increased development of the superior longitudinal fasiculi white matter fiber tracts connecting the auditory areas in the temporal lobe with the prefrontal cortex also correlate with increased severity of auditory hallucinations and provide a possible anatomical correlate (50).
Neither sound localization nor the cortical anatomical substrate are enhanced in schizophrenia, compared to controls, but their preservation in patients with more severe hallucinations compared to those without this symptom intensity may reflect the output of a relatively intact cerebral cortex that is attempting to analyze an unregulated stream of sensory information resulting from earlier gating dysfunctions and from specific synaptic dysfunction within the cortex itself. Consistent with this hypothesis, deficits in P50 gating and PPI are both associated with increased severity of auditory hallucinations (51, 52).
Tone matching ability
One of the most widely studied measures of cortical auditory dysfunction is the mismatch negativity (MMN), which is elicited whenever a stimulus differs from what is expected based upon recent auditory experience. MMN is elicited most frequently in an auditory “oddball” task, in which a series of repetitive stimuli are interrupted infrequently by a physically deviant “oddball” stimulus. Deviants can differ on any of several physical dimensions, including pitch, duration, intensity, or location (53) (Figure 2C).
MMN has been tied to impaired neurotransmission at NMDARs based upon both intracranial (54) and surface (55) recordings in non-human primates and ketamine challenge studies in healthy human volunteers (rev. in 3). Unlike PPI, MMN generation is little affected by either dopaminergic or serotonergic manipulations (56). However, as with P50 gating, ketamine-induced deficits in MMN generation are reversed by nicotinic agonists (57), possibly by presynaptic stimulation of nicotinic receptors on the glutamate nerve terminals (40, 58) (Figure 2B), with similar effects observed in schizophrenia (39). Other compounds that may reverse MMN deficits in schizophrenia include N-acetylcysteine, a precursor of the brain antioxidant glutathione (59) and D-serine, an endogenous NMDAR modulator (60).
Deficits in MMN generation were first reported in the early 1990’s, and this finding has since been a widely replicated neurophysiological finding in schizophrenia (rev. in 3, 61, 62, 63) (Figure 2D). Deficits in MMN generation correlate highly with overall level of function (64) and age at which symptoms of the illness first appear (65). Like P50 and PPI deficits, MMN deficits are heritable across the general population (66). In persons at high clinical risk for schizophrenia, MMN has emerged as a predictor of who will progress to schizophrenia (rev. in 67), and therefore may require most intensive remediation. Although the majority of studies of auditory dysfunction in schizophrenia have used neurophysiological measures such as MMN, similar deficits may be detected using fMRI to evaluate response to stimulus change within auditory cortical regions (68).
In keeping with the continued maturation of auditory cortex in adolescence (Figure 1A), impairments in MMN continue to develop over initial stages of the illness (69) in parallel with progressive volume reduction within auditory brain regions (70). In addition to MMN deficits, patients with schizophrenia also show deficits in generation of other cortical auditory responses, such as the auditory steady-state response to rapidly presented stimuli (rev. in 71). Patients fail to entrain their neuronal response to the rhythm of repeatedly presented stimuli, and so make less efficient use of sensory processing resources (72).
On a behavioral level, deficits in MMN generation are associated with impairments of basic auditory discrimination, such as tone matching (3) and auditory spatial discrimination (49), both underappreciated aspects of the clinical features of schizophrenia. Important social information, including emotion and attitude, is conveyed by variation in vocal intonation (“prosody”). Consequently, impaired ability to detect vocal intonation may contribute significantly to impaired social function (73).
Deficits in tone matching contribute significantly to ability to detect emotional prosody (74, 75), as well as to communications such as sarcasm that require patients to appreciate what another person may be thinking (76, 77), a trait called “theory of mind”, a form of empathy. When tested on a standardized tests of musical ability, such as the Montreal Battery for Evaluation of Amusia (78), nearly 50% of schizophrenia patients test positive vs. only 10% of the general population (79)
Deficits in MMN generation, particularly to duration deviants, are also prominent in developmental dyslexia, reflecting impaired ability to perform phonological operations (i.e. “sounding out” words) required for successful reading (80). Recently, similar reductions in phonological reading ability have been observed in patients with established, but not prodromal, schizophrenia, suggesting that progressive reductions in auditory function during the peri-onset period may result in a significant regression in reading ability in schizophrenia from premorbid levels (81). Such findings may identify pathways through which deficits in sensory function lead to impaired functional outcome when schizophrenia itself develops later in life.
Visual sensory systems
Although sensory studies in schizophrenia to date have focused mostly on auditory dysfunction, visual function is increasingly investigated as well. Like the auditory system, visual function in the brain is subserved by two separate pathways: a dorsal stream “where” system that is also termed “perception for action”, and a ventral stream “what” system that is also termed “perception for identification” (Figure 1A).
Basic visual function
The visual system begins in the retina and projects via the visual lateral geniculate nucleus to cortex. Subcortical visual systems are divided into two main pathways (82). The magnocellular pathways consists of large neurons that rapidly conduct low resolution visual information to the dorsal visual stream, and thus is most analogous to the brainstem pathways involved in rapid response to auditory stimuli, subserving “where”. By contrast, the parvocellular system consists of smaller, more numerous neurons that conduct slower but higher resolution information primarily to ventral visual stream, subserving “what.”
Consistent with their different functions, magnocellular and parvocellular neurons have divergent physiological properties. For example, magnocellular neurons are uniquely sensitive to dim and low contrast stimuli, giving rise to our familiar ability to detect extremely dim objects in the near dark even if we cannot identify what those objects are. Furthermore, magnocellular neurons are highly sensitive to moving or flickering objects, accounting for the strong influence of such objects on our attentional systems. These properties depend heavily upon function of NMDARs within both subcortical and cortical visual regions (83).
By contrast, parvocellular neurons are smaller and more numerous, and so far more able than magnocellular neurons to transmit fine details of the visual scene. Furthermore, the parvocellular system responds in a more graded fashion to shades of grey than the magnocellular system, and is sensitive to color, permitting fine object recognition (3). Parvocellular neurons show more linear responses than magnocellular neurons, suggesting relatively lesser engagement of NMDARs. Relative dysfunction of the systems, therefore, will depend upon both the pathways involved and stimulus paradigms used.
As with the auditory system, routine clinical visual testing, such as use of an eye chart, does not adequately assess potential disturbances in schizophrenia. Magnocellular function can be assessed only through use of specially designed stimuli or tasks, such as measures of contrast sensitivity to low spatial frequency stimuli or related neurophysiological measures. Furthermore, although we are consciously aware of events occurring in the ventral visual system, we are typically unaware of processing taking place within our dorsal stream. For example, we may turn our heads in response to a flickering object even before we are consciously aware of it. A consequence of such unconscious processing is that patients experiencing dorsal stream dysfunction may be unaware of what they are missing and may not know that their subjective experience differs from that of other people.
Visual deficits in schizophrenia
Using the newly invented photographic shutter, Kraeplin and colleagues observed that dementia praecox patients showed severe deficits in detecting stimuli presented for brief (20 ms) durations, confirming even earlier findings with a rotating striped drum apparatus (84). Nevertheless, modern studies of visual processing in schizophrenia were not conducted until the 1960’s when Holzman observed that both patients and their unaffected family members showed more variability of eye movement during eye tracking than did healthy volunteers (85, 86). Subsequent studies of visual backward masking(87) demonstrated that patients not only required longer time to detect a target, a parameter termed “critical stimulus duration,” (as in the earlier Kraeplin studies) but also showed more sensitivity to masking of the stimulus by a later, more intense stimulus, suggesting impaired interplay between different visual pathways (88).
Since then, more specific paradigms for demonstrating visual dysfunction have been developed based upon improved understanding of magnocellular and parvocellular system function (63). Neurophysiological studies have demonstrated impaired generation of the visual P1 potential to stimuli biased toward the magnocellular visual system (65, 89–91), Other potentially useful paradigms include steady-state visual evoked potentials, contour integration and coherent motion tasks (63). fMRI literature regarding early visual deficits is more limited. Nevertheless, impaired activation have been observed to low spatial frequency stimulation (91, 92), along with visual behavioral deficits. Visual regions are often not assessed in studies of higher cognitive dysfunction; nevertheless impaired activation of visual regions has been observed in meta-analyses of visual working memory tasks (93), supporting recent suggestions that low level visual deficits cascade up the system to undermine higher order cognitive impairments, as in the auditory system (3).
Overall, deficits in sensory response are observed even in the absence of the attentional impairments (94), suggesting that visual deficits are more likely a cause than consequence of attentional dysfunction. As in the early Holzman studies, deficits in visual processing are consistently observed not only in schizophrenia patients but also in unaffected family members, suggesting that they represent a possible heritable vulnerability factor for the disorder (95).
Functional implications
As with auditory deficits, visual dysfunction in schizophrenia may go undetected unless specifically evaluated. Nevertheless, as in the auditory system low level deficits may cascade up the system to produce higher order cognitive impairments.
One critical function tied to the early visual system is the ability to “close” objects based upon fragmented information. This process, often referred to as the “cat behind the Venetian blind effect,” depends upon rapid transmission of low resolution information through the magnocellular/dorsal stream visual system to prefrontal cortex even in advance of more detailed information reaching ventral stream visual cortex via the parvocellular visual system (96, 97). In schizophrenia, this framing function is lost due to impaired transmission within the magnocellular visual system (98) , leading not only to impaired ability to recognize fragmented objects but also to impaired ability to discriminate facial emotion (99) or to engage in tasks that require sustained attention to visual information (91, 100). Patients during the early stages of schizophrenia often experience visual illusions and feelings that images are fragmentary (101, 102). Loss of this framing function may serve as a physiological substrate for this common perceptual experience.
Similarly, the magnocellular visual system plays a critical role in organizing visual space during reading of connected passages of text, such as paragraphs (103, 104). Although single-word reading has been extensively studied in schizophrenia, studies of paragraph reading have only recently been initiated based upon emerging “whole brain” theories of schizophrenia. As predicted, schizophrenia patients show severe deficits in passage, but not single word, reading (105)that correlate with both behavioral (106, 107) and fMRI-based (108) assessment of visual magnocellular function. Consistent with other visual findings, visual reading deficits extend to individuals at clinical high risk for schizophrenia (81) as well as to unaffected family members (106).
Auditory-visual interaction
Given the parallel deficits in auditory and visual function, processes that rely on the synergistic interplay between these systems should be most affected in schizophrenia. To date, these interactions have been mostly studied in regard to social cognition and, more recently reading, where expected synergistic consequences have been observed.
In social cognition, subjects infer emotion both from auditory cues and facial expression. Individuals with congenital amusia, like schizophrenia patients, show impaired auditory emotion recognition (79), but nevertheless may compensate using visual cues and higher order cognitive reasoning. In schizophrenia, the auditory and visual emotion processing deficits are correlated but independent (74), suggesting that the confluence of deficits contributes in parallel to the social disconnection of schizophrenia patients.
Similarly, with regard to reading, individuals with isolated visual vs. phonological deficts may compensate by becoming “auditory” vs. “visual” readers. In schizophrenia, the parallel deficits in auditory and visual processing combine to produce a “double deficit” pattern of dyslexia that leads to significant impairments in socioeconomic outcome (81). Such relationships are observed even in individuals who do not suffer from schizophrenia. However, whereas developmental dyslexia occurs during school years, permitting early detection and remediation, schizophrenia-related dyslexia represents a regression from a higher level of function and thus may go undetected unless specifically assessed.
Deficits in non-schizophrenia disorders
Sensory dysfunction has been studied most extensively in the context of schizophrenia, but additional studies suggest that sensory deficits may also be present dimensionally across other neuropsychiatric disorders. For example, deficits in PPI appears in conditions other than schizophrenia, including bipolar disorder (109) and Huntington’s disease (110), consistent with the hyperarousal often seen in these conditions. Similarly, P50 deficits may be associated with psychotic mania and childhood autism spectrum disorder (ASD), consistent with impairments in excitatory/inhibitory balance (111, 112). By contrast, MMN appears to be differentially impaired in schizophrenia relative to either bipolar disorder or depression (113). In higher functioning ASD alterations are found in consistency, although not in overall amplitude, of unimodal sensory response (114). Similarly, patients may have superior, rather than reduced, performance in basic unimodal functions such as pitch processing (115) or visual search capabilities (116) that are impaired in schizophrenia. ASD patients show particular deficits in multisensory facilitation perhaps leading to a fragmented experience of the environment, (117, 118). The NIMH has recently proposed the Research Domain Criterion initiative (119), which views neurocognitive deficits as being present dimensionally across neuropsychiatric disorders. Sensory processing measures are included within this initiative, and may make appropriate targets for intervention across, as well as within, specific nosological entities.
Discussion
The cognitive dysfunction of schizophrenia encompasses a profound disturbance in sensory processing. As Hubel and Wiesel found in their investigations, the understanding of how sensory information is processed has proven to be a valuable strategy for identifying specific neuronal dysfunctions. Assessments of sensory dysfunction, including PPI, P50 inhibitory gating, auditory MMN, and visual P1, steady state, and magnocellular responses reveal heritable neurobiological abnormalities in schizophrenia that include deficits in nicotinic cholinergic, NMDA glutamatergic, dopaminergic, and GABAergic synaptic mechanisms. Many of these abnormalities are manifest before the onset of clinical signs of psychosis. The synaptic mechanisms and associated genes are now targets for new drug development to improve cognitive function in schizophrenia, one of its most limiting clinical features.
While research tools for assessing sensory dysfunction are not used in clinical practice, clinical observations of patients’ sensory function also have a role in treatment. The first clinical report of chlorpromazine’s effects in schizophrenia included its effects on the patients’ sensory perception of their environment, described by Delay and Deniker as “apparent indifference or the slowing of responses to external stimuli,” in contrast to their baseline state of hyper-excitability (120). Clinicians treating acutely ill patients look for the subsidence of the hyper-vigilant state as an early therapeutic milestone. Both isolation in a quiet environment and antipsychotic drugs are used to achieve this initial therapeutic effect. Dr. Saks’s description of her hyper-awareness of her neighborhood is an example of a personal experience of this phase of psychosis.
Patients are often disturbed by their sensory distortions, but may not volunteer this information to clinicians, particularly if they are already developing parnaoia. If the clinician can communicate that many patients find colors may seem more intense, visual perception may be more fragmented, and sounds may seem too loud or too intrusive, patients are often relieved that their situation is understood. These topics may be simpler to explore with patients than their more cognitively elaborated hallucinations and paranoid delusions. Clinicians must be aware as well that patients may be unable to detect the variations in tonality that are used to communicate information, such as emotion or even attitude (e.g. humor and sarcasm) or to process the visual information needed to correctly interpret facial expression, contributing to their difficulty in interpersonal and social interactions.
Because current first and second generation anti-dopaminergic antipsychotic drugs do not affect all the mechanisms that are associated with sensory disturbance in schizophrenia, many aspects of patients’ sensory dysfunction will persist chronically. Persistent abnormalities in P50 gating are associated with impaired ability to focus attention on specific tasks, a cognitive deficits associated with poor psychosocial rehabilitation (16). Deficits in MMN are tied to impairments in both orientation to critical environmental events and detection of the sensory modulations that are used to communicate social information such as emotion or attitude by tone of voice (3). Deficits in visual function are tied to impaired facial discrimination and reading. The result is a loss of connection to people and the environment experienced by many patients especially during the chronic phases. Clinicians and families, as well as patients themselves, who are aware of these impairments may be able to compensate for them by more explicit explanations of communications, with the realization that patients may easily misperceive even the most helpful intent.
Figure 3.
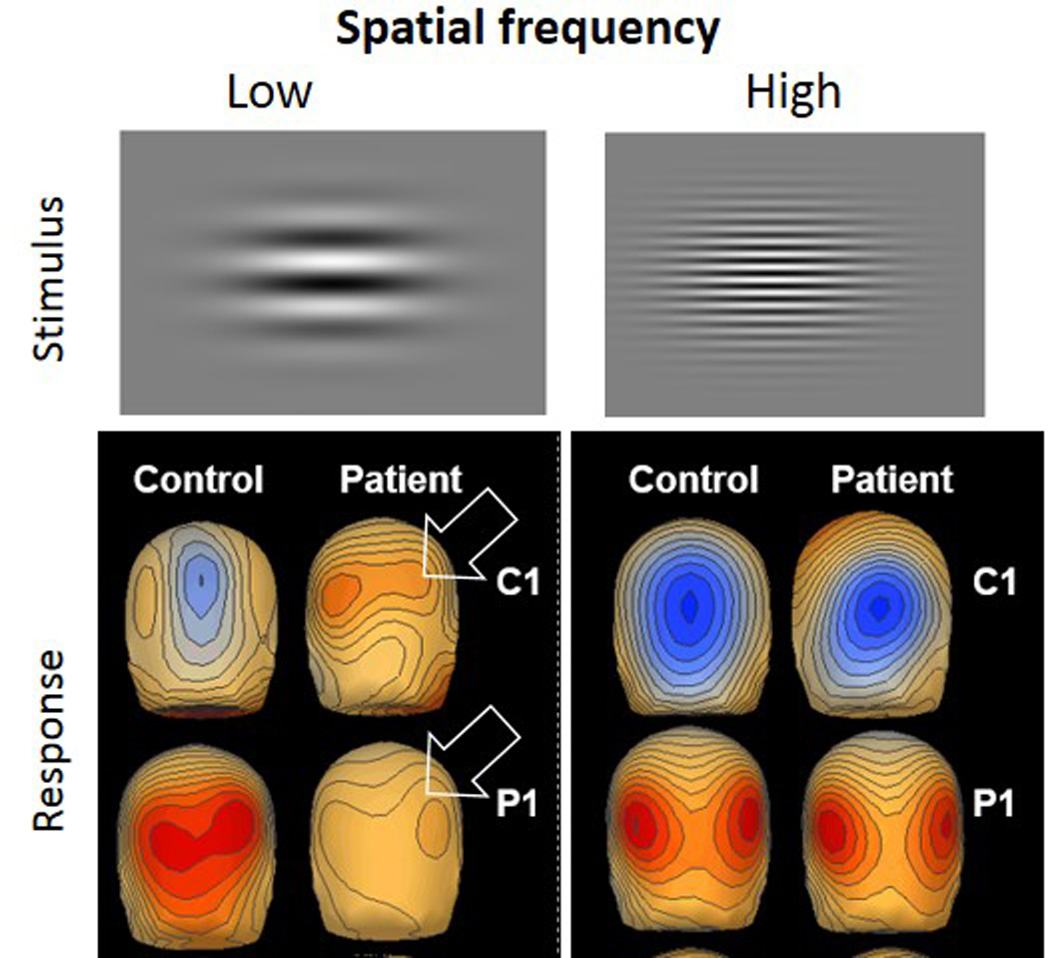
Visual event-related potential deficits in schizophrenia in response to low versus high spatial frequency stimuli in schizophrenia. Top panel shows example of these visual stimuli, which appear as waves. The C1 component of the response at 94 msec is followed by the P1 at 138 msec. The arrows show lower amplitudes of both early components in the patients at low frequency stimulation. The lower frequency stimuli preferentially activate the neuronal mechamisms that help draw patients’ attention to more important features of their surroundings (from 90)
Acknowledgments
Disclosures
Within the past 36 months, Dr. Javitt reports receiving honoraria from Sunovion, BMS, Eli Lilly, Takeda, Omeros, Otsuka, Consensus Medical Communications, Guidepoint Global, American Capital, Clearpoint Communications, Vindico Medical Communication, Clearview Healthcare, Rockpointe Communications and Health and Wellness Partners; research support from Pfizer and Roche; equity in Glytech, Inc. and AASI; intellectual property rights for use of glycine, D-serine and glycine transport inhibitors in schizophrenia, and serving on scientific advisory board of Promentis.
Dr. Freedman has a patent through the Department of Veterans Affairs on CHRNA7 sequences. He derives no income from it.
Funding: Supported by NIH grants R01 MH49334 and P50 MH086385 to DCJ and a VA Medical Research Service award and grant P50 MH-086383 to RF
References
- 1.Saks ER. The Center Cannot Hold. New York: Hyperion; 2007. [Google Scholar]
- 2.Gottschalk LA, Haer JL, Bates DE. Effect of sensory overload on psychological state. Changes in social alienation-personal disorganization and cognitive-intellectual impairment. Arch Gen Psychiatry. 1972;27(4):451–457. doi: 10.1001/archpsyc.1972.01750280019004. [DOI] [PubMed] [Google Scholar]
- 3.Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annual review of clinical psychology. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hubel DH, Wiesel TN. Brain mechanisms of vision. Sci Am. 1979;241(3):150–162. doi: 10.1038/scientificamerican0979-150. [DOI] [PubMed] [Google Scholar]
- 5.Hill J, Inder T, Neil J, Dierker D, Harwell J, Van Essen D. Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci U S A. 2010;107(29):13135–13140. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKay CM, Headlam DM, Copolov DL. Central auditory processing in patients with auditory hallucinations. Am J Psychiatry. 2000;157(5):759–766. doi: 10.1176/appi.ajp.157.5.759. [DOI] [PubMed] [Google Scholar]
- 7.Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatry. 2006;63(12):1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- 8.Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156(2–3):117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- 9.Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998;18(4):1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman R, Adler LE, Waldo MC, Pachtman E, Franks RD. Neurophysiological evidence for a defect in inhibitory pathways in schizophrenia: comparison of medicated and drug-free patients. Biol Psychiatry. 1983;18(5):537–551. [PubMed] [Google Scholar]
- 11.Braff DL, Light GA, Swerdlow NR. Prepulse inhibition and P50 suppression are both deficient but not correlated in schizophrenia patients. Biol Psychiatry. 2007;61(10):1204–1207. doi: 10.1016/j.biopsych.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Turetsky BI, Greenwood TA, Olincy A, Radant AD, Braff DL, Cadenhead KS, Dobie DJ, Freedman R, Green MF, Gur RE, Gur RC, Light GA, Mintz J, Nuechterlein KH, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Calkins ME. Abnormal auditory N100 amplitude: a heritable endophenotype in first-degree relatives of schizophrenia probands. Biol Psychiatry. 2008;64(12):1051–1059. doi: 10.1016/j.biopsych.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smucny J, Wylie K, Rojas D, Stevens K, Olincy A, Kronberg E, Zheng L, Tregellas J. Evidence for gamma and beta sensory gating deficits as translational endophenotypes for schizophrenia. Psychiatry Res. 2013;214(2):169–174. doi: 10.1016/j.pscychresns.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tregellas JR, Davalos DB, Rojas DC, Waldo MC, Gibson L, Wylie K, Du YP, Freedman R. Increased hemodynamic response in the hippocampus, thalamus and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophr Res. 2007;92(1–3):262–272. doi: 10.1016/j.schres.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer AR, Ruhl D, Merideth F, Ling J, Hanlon FM, Bustillo J, Canive J. Functional imaging of the hemodynamic sensory gating response in schizophrenia. Hum Brain Mapp. 2013;34(9):2302–2312. doi: 10.1002/hbm.22065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cullum CM, Harris JG, Waldo MC, Smernoff E, Madison A, Nagamoto HT, Griffith J, Adler LE, Freedman R. Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophr Res. 1993;10(2):131–141. doi: 10.1016/0920-9964(93)90048-n. [DOI] [PubMed] [Google Scholar]
- 17.Smith AK, Edgar JC, Huang M, Lu BY, Thoma RJ, Hanlon FM, McHaffie G, Jones AP, Paz RD, Miller GA, Canive JM. Cognitive abilities and 50- and 100-msec paired-click processes in schizophrenia. Am J Psychiatry. 2010;167(10):1264–1275. doi: 10.1176/appi.ajp.2010.09071059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Light GA, Braff DL. Do self-reports of perceptual anomalies reflect gating deficits in schizophrenia patients? Biol Psychiatry. 2000;47(5):463–467. doi: 10.1016/s0006-3223(99)00280-2. [DOI] [PubMed] [Google Scholar]
- 19.Jin Y, Potkin SG, Patterson JV, Sandman CA, Hetrick WP, Bunney WE., Jr Effects of P50 temporal variability on sensory gating in schizophrenia. Psychiatry Res. 1997;70(2):71–81. doi: 10.1016/s0165-1781(97)03091-6. [DOI] [PubMed] [Google Scholar]
- 20.Greenwood TA, Light GA, Swerdlow NR, Radant AD, Braff DL. Association analysis of 94 candidate genes and schizophrenia-related endophenotypes. PLoS ONE. 2012;7(1):e29630. doi: 10.1371/journal.pone.0029630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Croft RJ, Lee A, Bertolot J, Gruzelier JH. Associations of P50 suppression and desensitization with perceptual and cognitive features of “unreality” in schizotypy. Biol Psychiatry. 2001;50(6):441–446. doi: 10.1016/s0006-3223(01)01082-4. [DOI] [PubMed] [Google Scholar]
- 22.Wynn JK, Sergi MJ, Dawson ME, Schell AM, Green MF. Sensorimotor gating, orienting and social perception in schizophrenia. Schizophr Res. 2005;73(2–3):319–325. doi: 10.1016/j.schres.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Cadenhead KS, Light GA, Geyer MA, Braff DL. Sensory gating deficits assessed by the P50 event-related potential in subjects with schizotypal personality disorder. Am J Psychiatry. 2000;157(1):55–59. doi: 10.1176/ajp.157.1.55. [DOI] [PubMed] [Google Scholar]
- 24.Myles-Worsley M, Ord L, Blailes F, Ngiralmau H, Freedman R. P50 sensory gating in adolescents from a pacific island isolate with elevated risk for schizophrenia. Biol Psychiatry. 2004;55(7):663–667. doi: 10.1016/j.biopsych.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Brockhaus-Dumke A, Schultze-Lutter F, Mueller R, Tendolkar I, Bechdolf A, Pukrop R, Klosterkoetter J, Ruhrmann S. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biol Psychiatry. 2008;64(5):376–384. doi: 10.1016/j.biopsych.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A. 1997;94(2):587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Hong X, Chan RC, Kong F, Peng Z, Wan X, Wang C, Cheng L. Association study of polymorphisms in the alpha 7 nicotinic acetylcholine receptor subunit and catechol-o-methyl transferase genes with sensory gating in first-episode schizophrenia. Psychiatry Res. 2013;209(3):431–438. doi: 10.1016/j.psychres.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 28.Greenwood TA, Lazzeroni LC, Murray SS, Cadenhead KS, Calkins ME, Dobie DJ, Green MF, Gur RE, Gur RC, Hardiman G, Kelsoe JR, Leonard S, Light GA, Nuechterlein KH, Olincy A, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Freedman R, Braff DL. Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am J Psychiatry. 2011;168(9):930–946. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keri S, Beniczky S, Kelemen O. Suppression of the P50 evoked response and neuregulin 1-induced AKT phosphorylation in first-episode schizophrenia. Am J Psychiatry. 2010;167(4):444–450. doi: 10.1176/appi.ajp.2009.09050723. [DOI] [PubMed] [Google Scholar]
- 30.Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54(4):583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995;38(1):22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- 32.Adams CE, Yonchek JC, Schulz KM, Graw SL, Stitzel J, Teschke PU, Stevens KE. Reduced Chrna7 expression in mice is associated with decreases in hippocampal markers of inhibitory function: implications for neuropsychiatric diseases. Neuroscience. 2012;207:274–282. doi: 10.1016/j.neuroscience.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Law AJ, Wang Y, Sei Y, O’Donnell P, Piantadosi P, Papaleo F, Straub RE, Huang W, Thomas CJ, Vakkalanka R, Besterman AD, Lipska BK, Hyde TM, Harrison PJ, Kleinman JE, Weinberger DR. Neuregulin 1-ErbB4-PI3K signaling in schizophrenia and phosphoinositide 3-kinase-p110delta inhibition as a potential therapeutic strategy. Proc Natl Acad Sci U S A. 2012;109(30):12165–12170. doi: 10.1073/pnas.1206118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deakin IH, Law AJ, Oliver PL, Schwab MH, Nave KA, Harrison PJ, Bannerman DM. Behavioural characterization of neuregulin 1 type I overexpressing transgenic mice. Neuroreport. 2009;20(17):1523–1528. doi: 10.1097/WNR.0b013e328330f6e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150(12):1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- 36.Olincy A, Freedman R. Nicotinic mechanisms in the treatment of psychotic disorders: a focus on the alpha7 nicotinic receptor. Handb Exp Pharmacol. 2012;(213):211–232. doi: 10.1007/978-3-642-25758-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miwa JM, Freedman R, Lester HA. Neural systems governed by nicotinic acetylcholine receptors: emerging hypotheses. Neuron. 2011;70(1):20–33. doi: 10.1016/j.neuron.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagamoto HT, Adler LE, McRae KA, Huettl P, Cawthra E, Gerhardt G, Hea R, Griffith J. Auditory P50 in schizophrenics on clozapine: improved gating parallels clinical improvement and changes in plasma 3-methoxy-4-hydroxyphenylglycol. Neuropsychobiology. 1999;39(1):10–17. doi: 10.1159/000026553. [DOI] [PubMed] [Google Scholar]
- 39.Preskorn SH, Gawryl M, Dgetluck N, Palfreyman M, Bauer LO, Hilt DC. Normalizing effects of EVP-6124, an alpha-7 nicotinic partial agonist, on event-related potentials and cognition: a proof of concept, randomized trial in patients with schizophrenia. J Psychiatr Pract. 2014;20(1):12–24. doi: 10.1097/01.pra.0000442935.15833.c5. [DOI] [PubMed] [Google Scholar]
- 40.Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, Allensworth D, Guzman-Bonilla A, Clement B, Ball MP, Kutnick J, Pender V, Martin LF, Stevens KE, Wagner BD, Zerbe GO, Soti F, Kem WR. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165(8):1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lieberman JA, Javitch JA, Moore H. Cholinergic agonists as novel treatments for schizophrenia: the promise of rational drug development for psychiatry. Am J Psychiatry. 2008;165(8):931–936. doi: 10.1176/appi.ajp.2008.08050769. [DOI] [PubMed] [Google Scholar]
- 42.Preskorn SH. The role of proof of concept (POC) studies in drug development using the EVP-6124 POC study as an example. J Psychiatr Pract. 2014;20(1):59–60. doi: 10.1097/01.pra.0000442938.61575.c2. [DOI] [PubMed] [Google Scholar]
- 43.Hunter SK, Kisley MA, McCarthy L, Freedman R, Ross RG. Diminished cerebral inhibition in neonates associated with risk factors for schizophrenia: parental psychosis, maternal depression, and nicotine use. Schizophr Bull. 2011;37(6):1200–1208. doi: 10.1093/schbul/sbq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross RG, Hunter SK, McCarthy L, Beuler J, Hutchison AK, Wagner BD, Leonard S, Stevens KE, Freedman R. Perinatal choline effects on neonatal pathophysiology related to later schizophrenia risk. Am J Psychiatry. 2013;170(3):290–298. doi: 10.1176/appi.ajp.2012.12070940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hutchison AK, Hunter SK, Wagner BD, Calvin EA, Zerbe GO, Ross RG. Diminished Infant P50 Sensory Gating Predicts Increased 40-Month-Old Attention, Anxiety/Depression, and Externalizing Symptoms. J Atten Disord. 2013 doi: 10.1177/1087054713488824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaas JH, Hackett TA. ‘What’ and ‘where’ processing in auditory cortex. Nat Neurosci. 1999;2(12):1045–1047. doi: 10.1038/15967. [DOI] [PubMed] [Google Scholar]
- 47.Penfield W, Perot P. The Brain’s Record of Auditory amd Visual Experience. A Final Summary and Discussion. Brain. 1963;86:595–696. doi: 10.1093/brain/86.4.595. [DOI] [PubMed] [Google Scholar]
- 48.Kompus K, Westerhausen R, Hugdahl K. The “paradoxical” engagement of the primary auditory cortex in patients with auditory verbal hallucinations: a meta-analysis of functional neuroimaging studies. Neuropsychologia. 2011;49(12):3361–3369. doi: 10.1016/j.neuropsychologia.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 49.Perrin MA, Butler PD, DiCostanzo J, Forchelli G, Silipo G, Javitt DC. Spatial localization deficits and auditory cortical dysfunction in schizophrenia. Schizophr Res. 2010;124(1–3):161–168. doi: 10.1016/j.schres.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shergill SS, Kanaan RA, Chitnis XA, O’Daly O, Jones DK, Frangou S, Williams SC, Howard RJ, Barker GJ, Murray RM, McGuire P. A diffusion tensor imaging study of fasciculi in schizophrenia. Am J Psychiatry. 2007;164(3):467–473. doi: 10.1176/ajp.2007.164.3.467. [DOI] [PubMed] [Google Scholar]
- 51.Smith DM, Grant B, Fisher DJ, Borracci G, Labelle A, Knott VJ. Auditory verbal hallucinations in schizophrenia correlate with P50 gating. Clin Neurophysiol. 2013;124(7):1329–1335. doi: 10.1016/j.clinph.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Kumari V, Peters ER, Fannon D, Premkumar P, Aasen I, Cooke MA, Anilkumar AP, Kuipers E. Uncontrollable voices and their relationship to gating deficits in schizophrenia. Schizophr Res. 2008;101(1–3):185–194. doi: 10.1016/j.schres.2007.12.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naatanen R, Escera C. Mismatch negativity: clinical and other applications. Audiol Neurootol. 2000;5(3–4):105–110. doi: 10.1159/000013874. [DOI] [PubMed] [Google Scholar]
- 54.Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci U S A. 1996;93(21):11962–11967. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gil-da-Costa R, Stoner GR, Fung R, Albright TD. Nonhuman primate model of schizophrenia using a noninvasive EEG method. Proc Natl Acad Sci U S A. 2013;110(38):15425–15430. doi: 10.1073/pnas.1312264110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leung S, Croft RJ, Guille V, Scholes K, O’Neill BV, Phan KL, Nathan PJ. Acute dopamine and/or serotonin depletion does not modulate mismatch negativity (MMN) in healthy human participants. Psychopharmacology (Berl) 2010;208(2):233–244. doi: 10.1007/s00213-009-1723-0. [DOI] [PubMed] [Google Scholar]
- 57.Knott V, Shah D, Millar A, McIntosh J, Fisher D, Blais C, Ilivitsky V. Nicotine, Auditory Sensory Memory, and sustained Attention in a Human Ketamine Model of Schizophrenia: Moderating Influence of a Hallucinatory Trait. Frontiers in pharmacology. 2012;3:172. doi: 10.3389/fphar.2012.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin H, Hsu FC, Baumann BH, Coulter DA, Lynch DR. Cortical synaptic NMDA receptor deficits in alpha7 nicotinic acetylcholine receptor gene deletion models: Implications for neuropsychiatric diseases. Neurobiol Dis. 2014;63:129–140. doi: 10.1016/j.nbd.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, Boulat O, Bovet P, Bush AI, Conus P, Copolov D, Fornari E, Meuli R, Solida A, Vianin P, Cuenod M, Buclin T, Do KQ. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33(9):2187–2199. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- 60.Kantrowitz JT, Javitt DC. Amelioration of neurophysiological deficits in schizophrenia by high-dose D-serine: Relevance for clinical treatment development. Biol Psychiatry. 2014;75:11S. [Google Scholar]
- 61.Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76(1):1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Light GA, Swerdlow NR, Rissling AJ, Radant A, Sugar CA, Sprock J, Pela M, Geyer MA, Braff DL. Characterization of neurophysiologic and neurocognitive biomarkers for use in genomic and clinical outcome studies of schizophrenia. PLoS ONE. 2012;7(7):e39434. doi: 10.1371/journal.pone.0039434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Green MF, Butler PD, Chen Y, Geyer MA, Silverstein S, Wynn JK, Yoon JH, Zemon V. Perception measurement in clinical trials of schizophrenia: promising paradigms from CNTRICS. Schizophr Bull. 2009;35(1):163–181. doi: 10.1093/schbul/sbn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry. 2005;62(2):127–136. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- 65.Friedman T, Sehatpour P, Dias E, Perrin M, Javitt DC. Differential relationships of mismatch negativity and visual p1 deficits to premorbid characteristics and functional outcome in schizophrenia. Biol Psychiatry. 2012;71(6):521–529. doi: 10.1016/j.biopsych.2011.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hall MH, Schulze K, Rijsdijk F, Kalidindi S, McDonald C, Bramon E, Murray RM, Sham P. Are auditory P300 and duration MMN heritable and putative endophenotypes of psychotic bipolar disorder? A Maudsley Bipolar Twin and Family Study. Psychol Med. 2009;39(8):1277–1287. doi: 10.1017/S0033291709005261. [DOI] [PubMed] [Google Scholar]
- 67.Perez VB, Woods SW, Roach BJ, Ford JM, McGlashan TH, Srihari VH, Mathalon DH. Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biol Psychiatry. 2014;75(6):459–469. doi: 10.1016/j.biopsych.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wible CG, Kubicki M, Yoo SS, Kacher DF, Salisbury DF, Anderson MC, Shenton ME, Hirayasu Y, Kikinis R, Jolesz FA, McCarley RW. A functional magnetic resonance imaging study of auditory mismatch in schizophrenia. Am J Psychiatry. 2001;158(6):938–943. doi: 10.1176/appi.ajp.158.6.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCarley RW. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch Gen Psychiatry. 2002;59(8):686–694. doi: 10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- 70.Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64(5):521–529. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nature reviews. 2008;7(1):68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lakatos P, Schroeder CE, Leitman DI, Javitt DC. Predictive suppression of cortical excitability and its deficit in schizophrenia. J Neurosci. 2013;33(28):11692–11702. doi: 10.1523/JNEUROSCI.0010-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brekke J, Kay D, Lee K, Green M. Biosocial pathways to functional outcome in schizophrenia. Schizophrenia Research. 2005;80(2–3):213–225. doi: 10.1016/j.schres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 74.Gold R, Butler P, Revheim N, Leitman DI, Hansen JA, Gur RC, Kantrowitz JT, Laukka P, Juslin PN, Silipo GS, Javitt DC. Auditory emotion recognition impairments in schizophrenia: relationship to acoustic features and cognition. Am J Psychiatry. 2012;169(4):424–432. doi: 10.1176/appi.ajp.2011.11081230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leitman DI, Foxe JJ, Butler PD, Saperstein A, Revheim N, Javitt DC. Sensory contributions to impaired prosodic processing in schizophrenia. Biol Psychiatry. 2005;58(1):56–61. doi: 10.1016/j.biopsych.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 76.Leitman DI, Ziwich R, Pasternak R, Javitt DC. Theory of Mind (ToM) and counterfactuality deficits in schizophrenia: misperception or misinterpretation? Psychol Med. 2006;36(8):1075–1083. doi: 10.1017/S0033291706007653. [DOI] [PubMed] [Google Scholar]
- 77.Kantrowitz JT, Hoptman MJ, Leitman DI, Silipo G, Javitt DC. The 5% difference: early sensory processing predicts sarcasm perception in schizophrenia and schizo-affective disorder. Psychol Med. 2014;44(1):25–36. doi: 10.1017/S0033291713000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sloboda JA, Wise KJ, Peretz I. Quantifying tone deafness in the general population. Ann N Y Acad Sci. 2005;1060:255–261. doi: 10.1196/annals.1360.018. [DOI] [PubMed] [Google Scholar]
- 79.Kantrowitz JT, Scaramello N, Jakubovitz A, Lehrfeld JM, Laukka P, Elfenbein HA, Silipo G, Javitt DC. Amusia and protolanguage impairments in schizophrenia. Psychol Med. doi: 10.1017/S0033291714000373. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kujala T, Naatanen R. The mismatch negativity in evaluating central auditory dysfunction in dyslexia. Neuroscience and biobehavioral reviews. 2001;25(6):535–543. doi: 10.1016/s0149-7634(01)00032-x. [DOI] [PubMed] [Google Scholar]
- 81.Revheim N, Corcoran CM, Dias E, Hellmann E, Martinez APDB, Lehrfeld JM, DiCostanzoa J, Albert J, Javitt DC. Reading deficits in schizophrenia and individuals at high clinical risk: relation to sensory function, course of illness, and psychosocial outcome. Am J Psychiatry. 2014 doi: 10.1176/appi.ajp.2014.13091196. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Milner AD, Goodale MA. Two visual systems re-viewed. Neuropsychologia. 2008;46(3):774–785. doi: 10.1016/j.neuropsychologia.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 83.Nelson SB, Sur M. NMDA receptors in sensory information processing. Curr Opin Neurobiol. 1992;2(4):484–488. doi: 10.1016/0959-4388(92)90184-m. [DOI] [PubMed] [Google Scholar]
- 84.Busch A. The power of comprehension and observation in Dementia Praecox. Psychol Arbeiten. 1904 [Google Scholar]
- 85.Holzman P. Assessment of perceptual functioning in schizophrenia. Psychopharmacol. 1972;24:29–41. doi: 10.1007/BF00402904. [DOI] [PubMed] [Google Scholar]
- 86.Levy DL, Lajonchere CM, Dorogusker B, Min D, Lee S, Tartaglini A, Lieberman JA, Mendell NR. Quantitative characterization of eye tracking dysfunction in schizophrenia. Schizophr Res. 2000;42(3):171–185. doi: 10.1016/s0920-9964(99)00122-x. [DOI] [PubMed] [Google Scholar]
- 87.Saccuzzo DP, Braff DL. Early information processing deficit in schizophrenia. New findings using schizophrenic subgroups and manic control subjects. Arch Gen Psychiatry. 1981;38(2):175–179. doi: 10.1001/archpsyc.1981.01780270061008. [DOI] [PubMed] [Google Scholar]
- 88.Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. I. Specifying a mechanism. Arch Gen Psychiatry. 1994;51(12):939–944. doi: 10.1001/archpsyc.1994.03950120011003. [DOI] [PubMed] [Google Scholar]
- 89.Schechter I, Butler PD, Zemon VM, Revheim N, Saperstein AM, Jalbrzikowski M, Pasternak R, Silipo G, Javitt DC. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clin Neurophysiol. 2005;116(9):2204–2215. doi: 10.1016/j.clinph.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Butler PD, Martinez A, Foxe JJ, Kim D, Zemon V, Silipo G, Mahoney J, Shpaner M, Jalbrzikowski M, Javitt DC. Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain. 2007;130(Pt 2):417–430. doi: 10.1093/brain/awl233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martinez A, Hillyard SA, Bickel S, Dias EC, Butler PD, Javitt DC. Consequences of magnocellular dysfunction on processing attended information in schizophrenia. Cereb Cortex. 2012;22(6):1282–1293. doi: 10.1093/cercor/bhr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martinez A, Hillyard SA, Dias EC, Hagler DJ, Jr, Butler PD, Guilfoyle DN, Jalbrzikowski M, Silipo G, Javitt DC. Magnocellular pathway impairment in schizophrenia: evidence from functional magnetic resonance imaging. J Neurosci. 2008;28(30):7492–7500. doi: 10.1523/JNEUROSCI.1852-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luck S, Fuller R, Braun E, Robinson B, Summerfelt A, Gold J. The speed of visual attention in schizophrenia: Electrophysiological and behavioral evidence. Schizophrenia Research. 2006;85(1–3):174–195. doi: 10.1016/j.schres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 95.Holzman PS. Eye movements and the search for the essence of schizophrenia. Brain Res Brain Res Rev. 2000;31(2–3):350–356. doi: 10.1016/s0165-0173(99)00051-x. [DOI] [PubMed] [Google Scholar]
- 96.Sehatpour P, Molholm S, Javitt DC, Foxe JJ. Spatiotemporal dynamics of human object recognition processing: an integrated high-density electrical mapping and functional imaging study of “closure” processes. Neuroimage. 2006;29(2):605–618. doi: 10.1016/j.neuroimage.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 97.Sehatpour P, Molholm S, Schwartz TH, Mahoney JR, Mehta AD, Javitt DC, Stanton PK, Foxe JJ. A human intracranial study of long-range oscillatory coherence across a frontal-occipital-hippocampal brain network during visual object processing. Proc Natl Acad Sci U S A. 2008;105(11):4399–4404. doi: 10.1073/pnas.0708418105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sehatpour P, Dias EC, Butler PD, Revheim N, Guilfoyle DN, Foxe JJ, Javitt DC. Impaired visual object processing across an occipital-frontal-hippocampal brain network in schizophrenia: an integrated neuroimaging study. Arch Gen Psychiatry. 2010;67(8):772–782. doi: 10.1001/archgenpsychiatry.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schneider F, Gur RC, Koch K, Backes V, Amunts K, Shah NJ, Bilker W, Gur RE, Habel U. Impairment in the specificity of emotion processing in schizophrenia. Am J Psychiatry. 2006;163(3):442–447. doi: 10.1176/appi.ajp.163.3.442. [DOI] [PubMed] [Google Scholar]
- 100.Dias EC, Butler PD, Hoptman MJ, Javitt DC. Early sensory contributions to contextual encoding deficits in schizophrenia. Arch Gen Psychiatry. 2011;68(7):654–664. doi: 10.1001/archgenpsychiatry.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Brit J Med Psychol. 1961;34:103–116. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 102.Klosterkotter J, Schultze-Lutter F, Ruhrmann S. Kraepelin and psychotic prodromal conditions. Eur Arch Psychiatry Clin Neurosci. 2008;258(Suppl 2):74–84. doi: 10.1007/s00406-008-2010-5. [DOI] [PubMed] [Google Scholar]
- 103.Stein J. The magnocellular theory of developmental dyslexia. Dyslexia (Chichester, England) 2001;7(1):12–36. doi: 10.1002/dys.186. [DOI] [PubMed] [Google Scholar]
- 104.Laycock R, Crewther SG. Towards an understanding of the role of the ‘magnocellular advantage’ in fluent reading. Neuroscience and biobehavioral reviews. 2008;32(8):1494–1506. doi: 10.1016/j.neubiorev.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 105.Revheim N, Butler PD, Schechter I, Jalbrzikowski M, Silipo G, Javitt DC. Reading impairment and visual processing deficits in schizophrenia. Schizophr Res. 2006;87(1–3):238–245. doi: 10.1016/j.schres.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roberts EO, Proudlock FA, Martin K, Reveley MA, Al-Uzri M, Gottlob I. Reading in Schizophrenic Subjects and Their Nonsymptomatic First-Degree Relatives. Schizophr Bull. 2012 doi: 10.1093/schbul/sbr191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Whitford V, O’Driscoll GA, Pack CC, Joober R, Malla A, Titone D. Reading impairments in schizophrenia relate to individual differences in phonological processing and oculomotor control: evidence from a gaze-contingent moving window paradigm. J Exp Psychol Gen. 2013;142(1):57–75. doi: 10.1037/a0028062. [DOI] [PubMed] [Google Scholar]
- 108.Martinez A, Revheim N, Butler PD, Guilfoyle DN, Dias EC, Javitt DC. Impaired magnocellular/dorsal stream activation predicts impaired reading ability in schizophrenia. NeuroImage Clinical. 2012;2:8–16. doi: 10.1016/j.nicl.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Giakoumaki SG, Roussos P, Rogdaki M, Karli C, Bitsios P, Frangou S. Evidence of disrupted prepulse inhibition in unaffected siblings of bipolar disorder patients. Biol Psychiatry. 2007;62(12):1418–1422. doi: 10.1016/j.biopsych.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 110.Swerdlow NR, Paulsen J, Braff DL, Butters N, Geyer MA, Swenson MR. Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington’s disease. J Neurol Neurosurg Psychiatry. 1995;58(2):192–200. doi: 10.1136/jnnp.58.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Franks RD, Adler LE, Waldo MC, Alpert J, Freedman R. Neurophysiological studies of sensory gating in mania: comparison with schizophrenia. Biological Psychiatry. 1983;18(9):989–1005. [PubMed] [Google Scholar]
- 112.Orekhova EV, Stroganova TA, Prokofyev AO, Nygren G, Gillberg C, Elam M. Sensory gating in young children with autism: relation to age, IQ, and EEG gamma oscillations. Neurosci Lett. 2008;434(2):218–223. doi: 10.1016/j.neulet.2008.01.066. [DOI] [PubMed] [Google Scholar]
- 113.Umbricht D, Koller R, Schmid L, Skrabo A, Grubel C, Huber T, Stassen H. How specific are deficits in mismatch negativity generation to schizophrenia? Biol Psychiatry. 2003;53(12):1120–1131. doi: 10.1016/s0006-3223(02)01642-6. [DOI] [PubMed] [Google Scholar]
- 114.Dinstein I, Heeger DJ, Lorenzi L, Minshew NJ, Malach R, Behrmann M. Unreliable evoked responses in autism. Neuron. 2012;75(6):981–991. doi: 10.1016/j.neuron.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ouimet T, Foster NE, Tryfon A, Hyde KL. Auditory-musical processing in autism spectrum disorders: a review of behavioral and brain imaging studies. Ann N Y Acad Sci. 2012;1252:325–331. doi: 10.1111/j.1749-6632.2012.06453.x. [DOI] [PubMed] [Google Scholar]
- 116.Joseph RM, Keehn B, Connolly C, Wolfe JM, Horowitz TS. Why is visual search superior in autism spectrum disorder? Developmental science. 2009;12(6):1083–1096. doi: 10.1111/j.1467-7687.2009.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Collignon O, Charbonneau G, Peters F, Nassim M, Lassonde M, Lepore F, Mottron L, Bertone A. Reduced multisensory facilitation in persons with autism. Cortex. 2013;49(6):1704–1710. doi: 10.1016/j.cortex.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 118.Brandwein AB, Foxe JJ, Butler JS, Russo NN, Altschuler TS, Gomes H, Molholm S. The development of multisensory integration in high-functioning autism: high-density electrical mapping and psychophysical measures reveal impairments in the processing of audiovisual inputs. Cereb Cortex. 2013;23(6):1329–1341. doi: 10.1093/cercor/bhs109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 120.Delay J, Deniker P. Neuroleptic effects of chlorpromazine in therapeutics of neuropsychiatry. J Clin Exp Psychopathol. 1955;16(2):104–112. [PubMed] [Google Scholar]
- 121.Dias EC, Bickel S, Epstein ML, Sehatpour P, Javitt DC. Abnormal task modulation of oscillatory neural activity in schizophrenia. Front Psychol. 2013;4:540. doi: 10.3389/fpsyg.2013.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Greenwood TA, Swerdlow NR, Gur RE, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Green MF, Gur RC, Lazzeroni LC, Nuechterlein KH, Olincy A, Radant AD, Ray A, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Sugar CA, Tsuang DW, Tsuang MT, Turetsky BI, Light GA, Braff DL. Genome-wide linkage analyses of 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am J Psychiatry. 2013;170(5):521–532. doi: 10.1176/appi.ajp.2012.12020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59(11):1011–1020. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- 124.Luck SJ, Fuller RL, Braun EL, Robinson B, Summerfelt A, Gold JM. The speed of visual attention in schizophrenia: electrophysiological and behavioral evidence. Schizophr Res. 2006;85(1–3):174–195. doi: 10.1016/j.schres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 125.Donohoe G, Morris DW, De Sanctis P, Magno E, Montesi JL, Garavan HP, Robertson IH, Javitt DC, Gill M, Corvin AP, Foxe JJ. Early visual processing deficits in dysbindin-associated schizophrenia. Biol Psychiatry. 2008;63(5):484–489. doi: 10.1016/j.biopsych.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 126.O’Donoghue T, Morris DW, Fahey C, Da Costa A, Foxe JJ, Hoerold D, Tropea D, Gill M, Corvin A, Donohoe G. A NOS1 variant implicated in cognitive performance influences evoked neural responses during a high density EEG study of early visual perception. Hum Brain Mapp. 2012;33(5):1202–1211. doi: 10.1002/hbm.21281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Michie PT, Innes-Brown H, Todd J, Jablensky AV. Duration mismatch negativity in biological relatives of patients with schizophrenia spectrum disorders. Biol Psychiatry. 2002;52(7):749–758. doi: 10.1016/s0006-3223(02)01379-3. [DOI] [PubMed] [Google Scholar]
- 128.Elliot Hong L, Moran LV, Du X, O’Donnell P, Summerfelt A. Mismatch negativity and low frequency oscillations in schizophrenia families. Clin Neurophysiol. 2012;123(10):1980–1988. doi: 10.1016/j.clinph.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Niznikiewicz MA, Spencer KM, Dickey C, Voglmaier M, Seidman LJ, Shenton ME, McCarley RW. Abnormal pitch mismatch negativity in individuals with schizotypal personality disorder. Schizophr Res. 2009;110(1–3):188–193. doi: 10.1016/j.schres.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


