Abstract
Background
Corticotropin-releasing factor (CRF) peptides exert profound effects on the secretomotor function of the gastrointestinal tract. Nevertheless, despite the presence of CRF peptides and receptors in colonic tissue, their influence on colonic blood flow (CBF) is unknown.
Aim
To determine the effect and mechanism of members of the CRF peptide family on CBF in isoflurane-anesthetized rats.
Methods
Proximal CBF was measured with laser Doppler flowmetry simultaneously with mean arterial blood pressure (MABP) measurement. Rats were injected with intravenous human/rat CRF (CRF1>CRF2 affinity), mouse urocortin 2 (mUcn2, selective CRF2 agonist) or sauvagine (SVG, CRF2>CRF1 affinity) at 1 – 30 μg/kg. The nitric oxide (NO) synthase inhibitor, L-NAME (3 mg/kg, iv), the cyclooxygenase inhibitor, indomethacin (Indo, 5 mg/kg, ip) or selective CRF2 antagonist, astressin2-B (Ast2B, 50 μg/kg, iv) was given before SVG injection (10 μg/kg, iv).
Results
SVG and mUcn2 dose-dependently increased CBF while decreasing MABP and colonic vascular resistance (CVR). CRF had no effect on CBF, but increased CVR. The hyperemic effect of SVG was inhibited by L-NAME but not by Indo, whereas hypotension was partially reduced by L-NAME. Sensory denervation had no effect on SVG-induced changes. Ast2B inhibited SVG-induced hyperemia and decreased CVR, and partially reduced the hypotension.
Conclusions
Peripheral CRF2 activation induces colonic hyperemia through NO synthesis, without involving prostaglandin synthesis or sensory nerve activation, suggesting a direct action on the endothelium and myenteric neurons. Members of the CRF peptide family may protect the colonic mucosal via the activation of the CRF2 receptor.
Keywords: colonic blood flow, vascular resistance, corticotropin-releasing factor, mouse urocortin 2, sauvagine, astressin2-B, nitric oxide, indomethacin
INTRODUCTION
The gastrointestinal tract receives up to 25% of the total cardiac output of which nearly 80% flows through the mucosal and submucosal layers (1), implying that the high metabolic need of mucosal cells combined with the constant exposure to luminal noxious agents emphasize the importance of the regulation of the enteric microcirculation to physiology. The mucosal microcirculatory defense concept is well established in the upper gastrointestinal tract, where the mucosa is regularly exposed to luminal acid (2-4). Similarly, in the lower intestine, blood flow helps prevent mucosal oxidative stress, increased mucosal permeability, and bacterial translocation (5).
Gastrointestinal blood flow is regulated by sensory afferent nerve-mediated reflexes (6) in series or in parallel with other mediators including nitric oxide (NO), calcitonin gene related peptide (CGRP), and prostaglandins (PGs) (2;7-9). An emerging signaling pathways implicated in the gut response to noxious stimuli includes the action of corticotropin releasing factor- (CRF) related peptides, which mediate and modify stress-induced endocrine, behavioral, autonomic and immune responses (10). The mammalian CRF peptide family consists of 4 distinct paralogs, including CRF and urocortins (Ucn 1, 2 and 3) (10-12). In non-mammalian vertebrates, CRF peptide orthologs include frog sauvagine (13) and fish urotensin-I (14). These peptides exert their effects by activating two class B1 G-protein coupled receptors, the CRF receptor 1 (CRF1) (15) or CRF2 (16-19). CRF is a non-selective peptide with affinity CRF1 > CRF2 whereas urocortin (Ucn1), also non-selective peptide, has equivalent and high affinity to CRF1 and CRF2 receptors. Urocortin 2 (Ucn 2) and 3 (Ucn 3) in contrast are selective agonists to CRF2 receptors (20). Amphibian sauvagine is a non-selective CRF peptide with higher affinity to CRF2 than CRF1 (21).
CRF signaling, in addition to its involvement in stress-related gastrointestinal secretomotor and pain responses (22-24), also affects hemodynamics when injected centrally or peripherally. In sheep with experimental heart failure, intravenous (iv) Ucn 1 improves hemodynamics by increasing cardiac output and decreasing vascular resistance (25). Intracisternal CRF affects hepatic microcirculation via CRF2 receptor activation in rats (26). Topical Ucn 2 increases coronary blood flow via CRF2 receptor activation (27). Ucn 2 injected iv to healthy humans, increases cardiac out put, heart rate and left ventricular ejection while decreasing systemic vascular resistance (28). CRF, sauvagine and urotensin increase mesenteric blood flow when injected centrally (29). The presence of CRF receptors in colonic tissue both in humans (30;31) and rodents (22;32;33) predicts their possible role in the modulation of colonic mucosal blood flow, an enteric defense factor. Yet, the effect of peripheral CRF signaling on colonic mucosal blood flow is thus far unknown.
Here, we investigated the effects of iv injections of members of the CRF peptide family: CRF (CRF1>CRF2 affinity), mouse urocortin 2 (mUcn2, selective CRF2 agonist), and sauvagine (SVG, CRF2>CRF1 affinity) on colonic blood flow, mean arterial pressure and vascular resistance in the anesthetized rat. Colonic CRF2 activation increased colonic blood flow primarily through local NO pathway without involving PGs or extrinsic afferent neurons.
Methods
Animals
Male Sprague-Dawley rats weighing 200-250 g (Harlan, San Diego, CA) were used. All studies were performed with approval of the Veterans Affairs Greater Los Angeles Healthcare System Institutional Animal Care and Use Committee (VA IACUC).
Chemicals
CRF, mUcn2, SVG and astressin2-B (Ast2B) were synthesized (34) and provided by Dr. Rivier (Salk Institute, Peptide Laboratories). NG-nitro-L-arginine methyl ester (L-NAME), indomethacin (Indo), capsaicin, HEPES, and other chemicals were obtained from Sigma Chemical (St. Louis, MO). Krebs solution contained (in mM) 136 NaCl, 2.6 KCl, 1.8 CaCl2, and 10 HEPES at pH 7.4. Each solution was prewarmed to 37 °C using a water bath, with temperature maintained during the experiment with a heating pad. For stock solutions, capsaicin was dissolved in 10% Tween©-80, 10% ethanol, and 80% saline, and the solvent was used as a vehicle. For iv injection, agonists or antagonists were freshly dissolved in saline containing 0.1 % bovine serum albumin (BSA). Saline containing BSA was used as a vehicle control.
Measurement of colonic blood flow
Under isoflurane anesthesia (1.5-2.0 %) using a rodent anesthesia inhalation system (Summit Medical Systems, Bend, OR), rats were placed supine on a heating block system with warmed recirculating water (Summit Medical) to maintain body temperature at 36-37 °C, as monitored by a rectal thermistor. Prewarmed saline was infused via the right femoral vein at 1.08 ml/hr using a Harvard infusion pump (Harvard Apparatus, Holliston MA); blood pressure was monitored via a catheter placed in the left femoral artery using a pressure transducer (Kent Scientific, Torrington, CT). Colonic blood flow was measured with laser Doppler flowmetry by modified method as previously described (3).
The cecum and proximal colon were exposed via a midline incision. The cecum and mid-colon, 3-cm caudal from the cecum, were loosely ligated with nylon suture, and the proximal colon segment was filled with 1 ml saline prewarmed at 37 °C. A ~ 2-cm incision was made in the anterior wall of the segment using a thermal cautery (Geiger Medical Technologies, Inc., Monarch Beach, CA) to expose the colonic mucosa. Luminal contents and feces were gently removed, followed by rinsing the lumen with warmed saline. A concave stainless steel disk (16-mm diameter and 1-2 mm deep) with a 3-mm central aperture was fixed watertight on the mucosal surface with a silicone plastic adherent (Silly Putty, Binney & Smith, Easton, PA). The serosal surface of the colon was supported with a laser-Doppler flow probe (described below). A thin plastic cover slip was fixed to the disk with the silicone adherent to permit closed superfusion with solutions at a rate of 0.25 ml/min by means of a Harvard infusion pump.
For the measurement of colonic blood flow, a right-angle probe (R-type, Transonic, Ithaca, NY) was surrounded with a silicone plastic adherent and attached on the colonic serosa just below the chambered mucosa. Blood flow was measured as the voltage output of the laser-Doppler instrument (model BLF21, Transonic) and was expressed relative to the stable level (the basal level) ~ 60 min after the superfusion started. Blood flow and blood pressure were continuously recorded with a strip chart recorder.
Animals had free access to water and food before the experiments, since our preliminary studies showed that, regardless of the surgical stress, colonic blood flow was unstable over time and the effects of CRF peptides on blood flow irreproducible following an overnight fast, presumably due to excessive stress.
Experimental protocol
Blood flow was stabilized during a continuous perfusion of pH 7.4 Krebs buffer, after which the time was set as t = 0 min. Because of the laparotomy and surgical stress, the stabilization of colonic blood flow usually took > 1 hr. The colonic mucosa was continuously superfused at 0.25 ml/min with pH 7.4 Krebs buffer throughout the experiments as in our previous duodenal superfusion (35;36),. Colonic blood flow (CBF) and mean arterial blood pressure (MABP) were simultaneously measured every 5 min and expressed as % basal and mmHg, respectively. Colonic vascular resistance (CVR) was calculated as an equation; CVR (% basal) = MABP (% basal at t = 0)/ CBF (% basal) × 100 as in a previous study (37).
To examine the dose-dependent effect of the CRF peptide family on the proximal colonic blood flow, after 10-min baseline measurement (t = 0 to 10 min), 1 – 30 μg/kg of CRF, mUcn2 or SVG in 0.1 ml BSA-saline solution was iv injected every 10 min followed by gentle flushing iv line with 0.1 ml BSA-saline.
To investigate the involvement of NO or PGs in the effects of SVG, the animals were given the NO synthase (NOS) inhibitor L-NAME (3 mg/kg, iv at t = 10 min) or pretreated, 1 hr before the experiments, with indomethacin (Indo (5 mg/kg, ip)) an inhibitor of cyclo-oxygenase and production of prostaglandins (PGs) that are known to affect intestinal blood flow (2;7-9), followed by SVG (10 μg/kg) iv injection at t = 20 min. Some animals were pretreated with the high dose of capsaicin (Cap-t, 125 mg/kg, sc) or vehicle (veh-t) in order to denervate sensory nerves 10-14 days prior to the experiments as previously described (3).
To confirm the receptor specificity of SVG, a selective CRF2 receptor antagonist Ast2B (50 μg/kg, iv) (34) was injected 10 min before SVG injection. The doses of CRF ligands and Ast2B were adopted from our prior studies (38;39). SVG was used in these studies as a pharmacological tool based on its higher potency, than Ucn2, in inducing CRF2 mediated vagal efferent activity (40) and in attenuating visceral pain responses in rats (unpublished data).
Statistics
All data from six rats in each group are expressed as means ± SEM. Comparisons between groups were made by one-way ANOVA followed by Fischer's least significant difference test. P values of 0.05 were taken as significant.
Results
Dose-dependent effect of CRF peptides on CBF, MABP and CVR
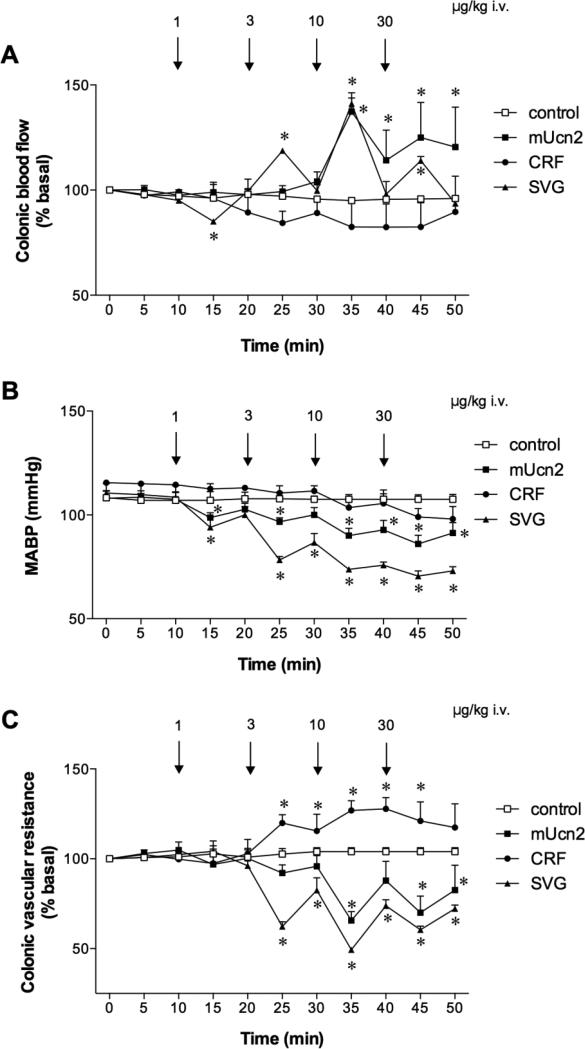
CBF and MABP were stable during perfusion with pH 7.4 Krebs solution in control group for ~ 1 hr (Fig. 1A-C). CRF iv injection somewhat decreased CBF with stable MABP, significantly increasing CVR at the 3 and 10 μg/kg doses. In contrast, mUcn2 or SVG dose-dependently increased CBF (Fig. 1A) with a decrease in MABP (Fig. 1B), decreasing CVR (Fig. 1C). The effects of SVG on MABP and CVR were more potent than those of mUcn2 at 3 and 10 μg/kg (p < 0.05). Fig. 1D and 1E depict dose-response curve of the peak response in CBF and CVR to CRF peptides. The maximum response to mUcn2 and SVG was achieved at the 10 μg/kg dose.
Fig. 1. Effects of mouse urocortin 2 (mUcn2), corticotropin-releasing factor (CRF) and sauvagine (SVG) on blood flow and vascular resistance in rat proximal colon.
Colonic blood flow was measured with laser-Doppler flowmetry with colonic vascular resistance calculated from blood flow and mean arterial blood pressure (MABP) monitored simultaneously under isoflurane anesthesia. SVG and mUcn2 (1-30 μg/kg, iv) dose-dependently increased colonic blood flow (A) and decreased MABP (B), decreasing vascular resistance (C), whereas CRF increased vascular resistance. Each datum is expressed as mean ± SEM (n = 6). *p < 0.05 vs. control group. Dose-response effects of mUcn2, CRF and SVG on blood flow (D) and vascular resistance (E) are shown, where the peak values after the drug iv injection were plotted. Each datum is expressed as mean ± SEM (n = 6).
Effects of L-NAME and Indo on SVG-induced colonic hyperemia
Based on the dose-dependent effect and potency on CBF as above, we chose SVG iv injection at the 10 μg/kg dose for the following experiments to examine the mechanism involved in the blood flow response in rat colon.
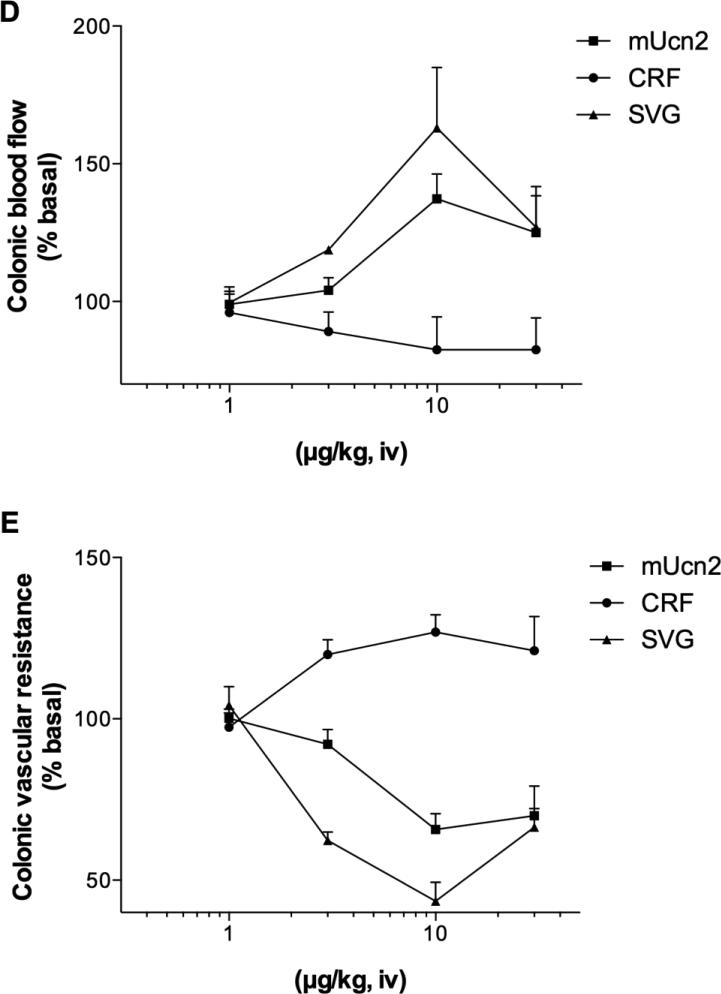
SVG iv injection (10 μg/kg) increased CBF (Fig. 2A) with a decrease in MABP (Fig. 2B), markedly decreasing CVR (Fig. 2C). L-NAME iv injection (3 mg/kg) rapidly increased MABP with no effect on CBF (t = 10 to 20 min). L-NAME pretreatment abolished SVG-induced hyperemia and reduced SVG-induced hypotension, preventing the SVG-induced CVR decrease. Alternatively, SVG injection after L-NAME treatment decreased CBF and reversed L-NAME-induced hypertension to hypotension. In contrast, Indo pretreatment (5 mg/kg, ip) had no effect on SVG-induced hypotension, but partially enhanced hyperemia, resulting in more decrease in CVR.
Fig. 2. Effects of L-NAME and indomethacin (Indo) pretreatment on sauvagine (SVG)-induced hyperemia in rat proximal colon.
SVG (10 μg/kg, iv) increased colonic blood flow (A) accompanied by a decrease in MABP (B), decreasing vascular resistance (C). L-NAME (3 mg/kg, iv) increased vascular resistance and inhibited SVG-induced hyperemia and vascular dilatation. Indo pretreatment (5 mg/kg, sc) sustained SVG-induced hyperemia and enhanced vascular resistance decrease. Each datum is expressed as mean ± SEM (n = 6). *p < 0.05 vs. control, †p < 0.05 vs. SVG iv alone.
Capsaicin-sensitive afferent nerves in SVG-induced hyperemia
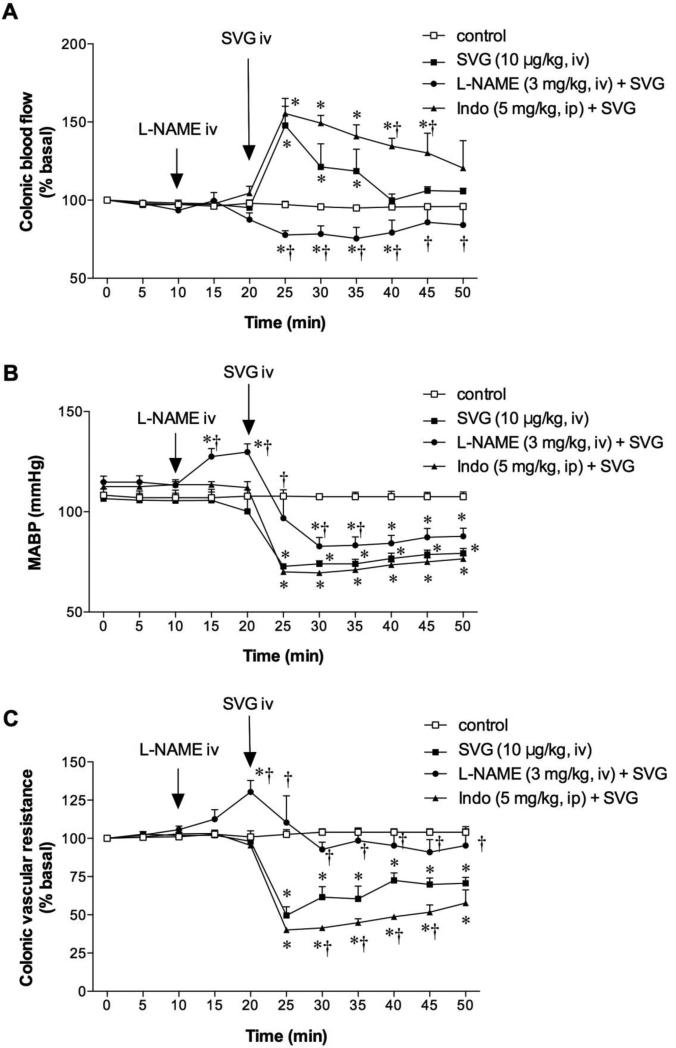
In vehicle-treated rats, SVG (10 μg/kg, iv) increased CBF (Fig. 3A) accompanied by hypotension (Fig. 3B) and decreased CVR (Fig. 3C). Capsaicin pretreatment (Cap-t, 125 mg/kg, sc) had no effect on CBF, MABP or CVR during the basal period (t = 0 to 10 min). Cap-t had no effect on SVG-induced changes in CBF (Fig. 3A), MABP (Fig. 3B) or CVR (Fig. 3C). This result suggests that the activation of sensory nerves is not involved in SVG-induced colonic vasodilation.
Fig. 3. Effect of afferent denervation on sauvagine (SVG)-induced hyperemia in rat proximal colon.
Capsaicin treatment (125 mg/kg, sc; Cap-t) had no effect on SVG-induced hyperemia (A), hypotension (B) or vascular dilatation (C). Each datum is expressed as mean ± SEM (n = 6). *p < 0.05 vs. control.
Effect of CRF2 receptor antagonist on SVG-induced changes
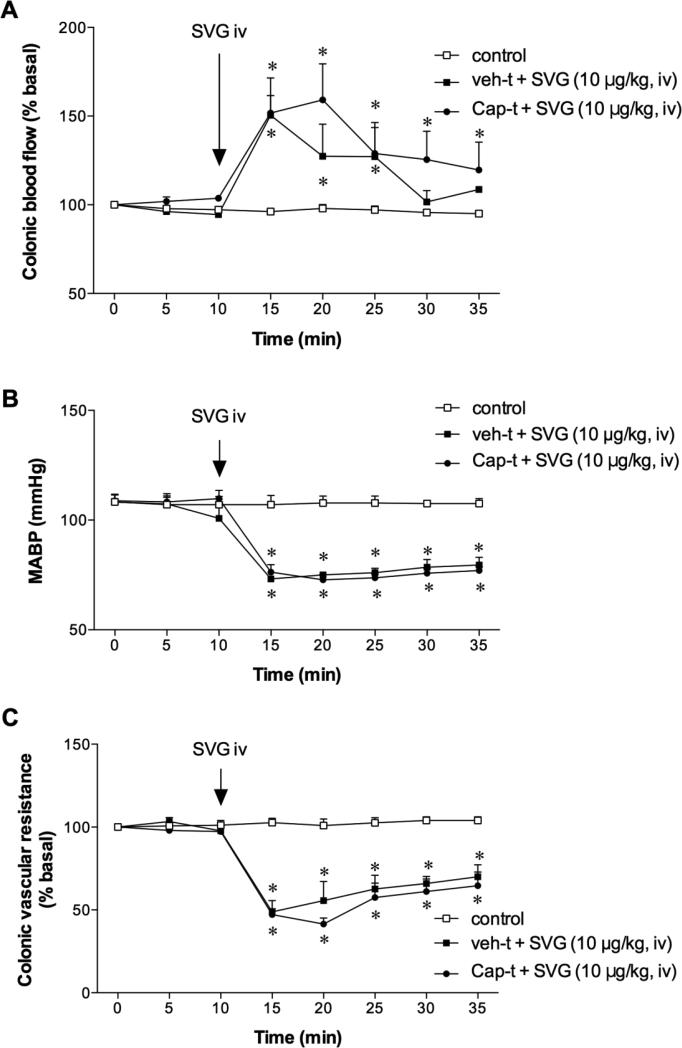
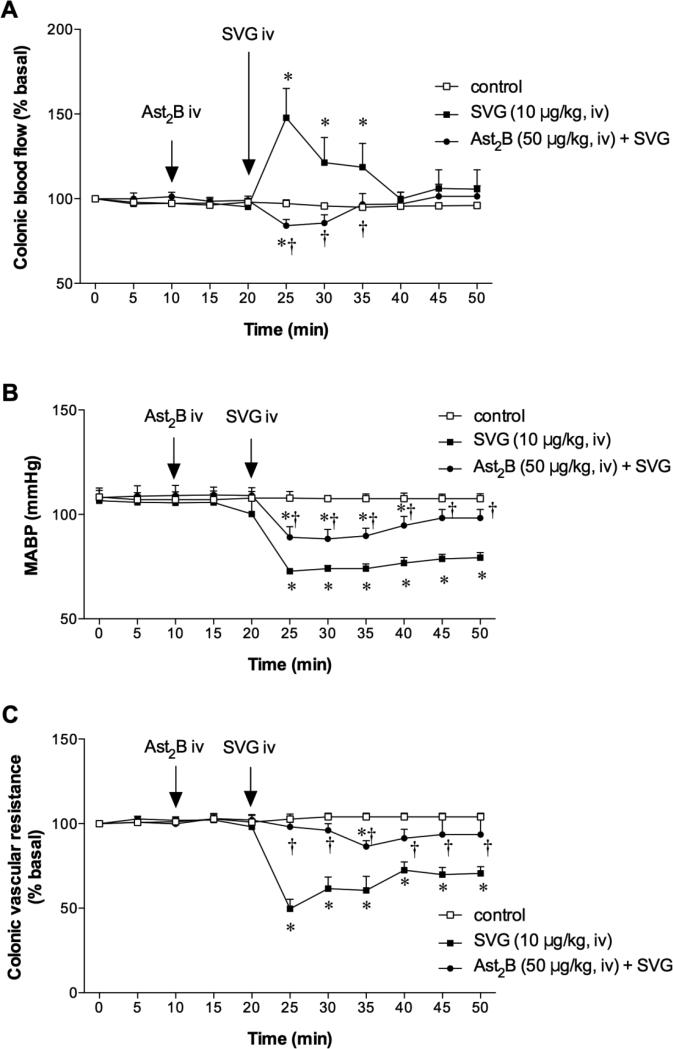
Since SVG is a preferable CRF2 receptor agonist, we confirmed whether SVG-induced changes were mediated via CRF2 receptor activation. The selective CRF2 receptor antagonist Ast2B (50 μg/kg, iv) was injected prior to SVG injection. Ast2B injection had no effect on CBF, MABP or CVR (t = 10 to 20 min). Ast2B injection abolished SVG-induced hyperemia (Fig. 4A) and inhibited the associated hypotension (Fig. 4B), strongly inhibiting the CVR decrease (Fig. 4C). This result suggests that SVG-induced colonic hyperemia is selectively mediated via CRF2 receptor activation.
Fig. 4. Effect of CRF2 receptor antagonist on sauvagine (SVG)-induced hyperemia in rat proximal colon.
Astressin2-B (Ast2B, 50 μg/kg, iv) abolished SVG-induced hyperemia (A) and vascular dilatation (C), and inhibited hypotension (B). Each datum is expressed as mean ± SEM (n = 6). *p < 0.05 vs. control, †p < 0.05 vs. SVG iv alone.
Discussion
We demonstrate that, in isoflurane-anesthetized rats, peripheral CRF2 receptor activation increases colonic blood flow through a NO-dependent mechanism but not involving PG synthesis or sensory nerve activation. Similarly, iv injection of CRF2 agonists potently induced hypotension in part through NO. L-NAME abolished the iv SVG-induced decrease in CVR while only modestly reducing SVG-induced hypotension. The data suggest that the CRF2-mediated hypotension is in part due to the peripheral vasodilatation via NO, but may also involve cardiac effects on the systemic hemodynamics as previously reported (27).
The regulation of intestinal blood flow involves capsaicin-sensitive afferent C-fiber-mediated reflex and several locally produced enteric neuromodulators and transmitters including PGs, CGRP and NO_(2;6-9). The present study provides the first evidence that CRF peptides induce colonic hyperemia in anesthetized rats through CRF2 receptor activation, demonstrated by the robust blood flow response following iv administration of the selective CRF2 agonist mUcn2, (20) and SVG, a peptide with affinity CRF2 > CRF1 (21) in contrast to the effect of native CRF with higher affinity to CRF1 (21) which exerted little to no effect. This is further confirmed by the blockade of the effects of SVG by the selective CRF2 receptor antagonist, Ast2B. Given that the peripherally administered CRF peptides do not cross the blood brain barrier (29;41;42), the likely site of action of the observed colonic blood flow is decidedly peripheral. The effect of mUcn2 and SVG was dose-dependent up to the 10 μg/kg dose, but became less robust at 30 μg/kg, possibly due to action on CRF1 or due to a CRF2-CRF1 receptor cross talk where lower doses of centrally injected mUcn2 showed no effect on colonic motor response but when given at a 5-fold higher doses than CRF, it stimulated colonic motor response through a CRF1 activation (38;43).
Interestingly, the effect of SVG was similar to those of mUcn2, with regard to blood flow, although SVG more strongly reduced MABP. Although SVG binds to CRF1, albeit at a lesser affinity than to CRF2, its more pronounced hypotensive effect cannot be due to its potential effect on CRF1, because CRF, with its higher affinity to CRF1 does not affect any of the hemodynamic endpoints measured. In dogs injected intracerebroventriculalry (icv) with either SVG or CRF, only SVG increased mesenteric blood flow (29). SVG injected subcutaneously (sc) is also more potent than CRF (sc) in inhibiting food intake in rats (44). Nonetheless, the maximal effects of SVG (10 μg/kg), on colonic blood flow, systemic blood pressure, and vascular resistance were all prevented by pretreatment with the selective CRF2 antagonist Ast2B suggesting that the effects of mUcn2 or SVG on colonic blood flow and vascular resistance are mediated through CRF2.
Our data further show that CRF2-mediated colonic hyperemia involves NO but not PG or afferent nerve fibers as shown by the blockade of the response by pretreatment with L-NAME but not by Indo or capsaicin. Although NO and PG affect acid-induced gastric mucosal hyperemia (45;46) and the gastric microcirculation (47), PG has no effect on the CRF2-mediated colonic hyperemia. Similarly, CRF2-mediated colonic hyperemia does not involve capsaicin-sensitive afferent fibers. Taken together, these data suggest that CRF2 receptor ligands induce colonic hyperemia most likely through a direct release of NO, similar to our prior report that CRF2 receptors are present in the colonic vascular endothelium (33) and co-localize with neuronal NOS in enteric neurons (22). Endothelial and neuronal NO induces capsaicin-induced gastric mucosal hyperemia (48;49) reinforcing the notion that CRF2-mediated endothelial and neuronal NO release in the colon be directly vasodilatory.
An intriguing finding in the present study is the anomalous increase of CRF2-mediated colonic mucosal blood flow and the decrease of MABP and vascular resistance following pretreatment with Indo, which cannot be explained from the data we present in this study, but may be inferred from the variable vascular responses reported to EP receptor activation. For instance, in mouse kidney, low-dose PGE2 vasoconstricts through EP1 and EP3 receptors but at a higher dose vasodilates through activation of EP2 and EP4 (50). Similarly, PGs administered centrally and systemically exert differing effects dependent on the receptor types involved (51). Alternatively Indo may exert differential effects depending on the physiological state of the tissue. For instance, in hypertensive but not in normotensive dogs, Indo increases renal blood flow (52). Whether the presence of CRF2 activation in rats constitutes a different state in which Indo enhances the CRF2-mediated blood flow response needs further studies. Taken together it is likely that Indo, by preventing or reducing PG synthesis, reduces EP1 or EP3-mediated vasoconstriction, thereby enhancing the CRF2-mediates NO-dependent vasodilatation.
In conclusion, peripheral CRF2 receptor activation induces colonic hyperemia through NO but not through the PG pathway or through capsaicin-sensitive afferent fibers. The data suggest that members of the CRF peptide family may protect the mucosa via the activation of the CRF2 receptors in the rat lower intestine. Overall, the protective effects of CRF2 against ischemia/reperfusion-induced cardiac myocyte injury [see (53)], its anti-angiogenic effect in the inflamed colon (54) and our finding of colonic mucosal hyperemia strongly suggest that peripheral CRF2 signaling is a key component of the hemodynamic regulatory system in normal and in disease states. Of note is that the effect of CRF peptides on mesenteric blood flow could be different in awake vs anesthetized state, as intestinal motility per-se can influences blood flow (55). However, in awake rats CRF2 activation by Ucn 2 in awake rodents does not have effect on the basal colonic transit. Thus, the increased mucosal blood flow due to Ucn2 or SVG is unlikely to be due to increased intestinal motility. As such, while the anesthetized rat model is a limitation in the current study and extrapolation to awake state mucosal blood flow needs caution, the model has the advantage to study the direct effect of CRF peptides on mucosal blood flow without the confounding factor of the awake-state intestinal motility.
Acknowledgements
We would like to thank Dr. Yvette Taché (UCLA) and Dr. Jean Rivier (Salk Institute, Peptide Laboratories) for their suggestions and peptide supply.
Study support: NIHDDK-41303 Animal Model Core (MM, JDK), NIHDDK DK 57238-01A1S1 & DK078676 (MM), Department of Veterans Affairs Merit Review Award, and NIH-NIDDK RO1 DK54221 (JDK).
Footnotes
Conflict of Interest: None
Reference List
- 1.Hasibeder W. Gastrointestinal microcirculation: still a mystery? Br J Anaesth. 2010;105:393–396. doi: 10.1093/bja/aeq236. [DOI] [PubMed] [Google Scholar]
- 2.Holzer P. Efferent-like roles of afferent neurons in the gut: Blood flow regulation and tissue protection. Auton Neurosci. 2006;125:70–75. doi: 10.1016/j.autneu.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akiba Y, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Acid-sensing pathways of rat duodenum. Am J Physiol. 1999;277:G268–G274. doi: 10.1152/ajpgi.1999.277.2.G268. [DOI] [PubMed] [Google Scholar]
- 4.Akiba Y, Kaunitz JD. Lafutidine, a protective H. (2) receptor antagonist, enhances mucosal defense in rat esophagus. Dig Dis Sci. 2010;55:3063–3069. doi: 10.1007/s10620-010-1379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kvietys PR. The Gastrointestinal Circulation. Morgan & Claypool Life Sciences . 2010 Ref Type: Abstract. [PubMed] [Google Scholar]
- 6.Holzer P, Livingston EH, Guth PH. Sensory neurons signal for the increase in rat gastric mucosal blood flow in the face of pending acid injury. Gastroenterology. 1991;101:416–423. doi: 10.1016/0016-5085(91)90020-l. [DOI] [PubMed] [Google Scholar]
- 7.Akiba Y, Nakamura M, Nagata H, Kaunitz JD, Ishii H. Acid-sensing pathways in rat gastrointestinal mucosa. J Gastroenterol. 2002;37(Suppl 14):133–8. 133–138. doi: 10.1007/BF03326432. [DOI] [PubMed] [Google Scholar]
- 8.Wallace JL, Miller MJ. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000;119:512–520. doi: 10.1053/gast.2000.9304. [DOI] [PubMed] [Google Scholar]
- 9.Holzer P, Lippe IT, Jocic M, Wachter C, Erb R, Heinemann A. Nitric oxide-dependent and -independent hyperaemia due to calcitonin gene-related peptide in the rat stomach. Br J Pharmacol. 1994;110:404–410. doi: 10.1111/j.1476-5381.1993.tb13824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vale W, Vaughan J, Perrin M. Corticotropin-releasing factor (CRF) family of ligands and their receptors. The Endocrinologist. 1997;7:S3–S9. [Google Scholar]
- 11.Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- 12.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pallai PV, Mabilia M, Goodman M, Vale W, Rivier J. Structural homology of corticotropin-releasing factor, sauvagine, and urotensin I: circular dichroism and prediction studies. Proc Natl Acad Sci U S A. 1983;80:6770–6774. doi: 10.1073/pnas.80.22.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lederis K, Letter A, McMaster D, Moore G, Schlesinger D. Complete amino acid sequence of urotensin I, a hypotensive and corticotropin-releasing neuropeptide from Catostomus. Science. 1982;218:162–165. doi: 10.1126/science.6981844. [DOI] [PubMed] [Google Scholar]
- 15.Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci U S A. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishimoto T, Pearse RV, Lin CR, Rosenfeld MG. A sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle. Proc Natl Acad Sci U S A. 1995;92:1108–1112. doi: 10.1073/pnas.92.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci USA. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perrin M, Donaldson C, Chen R, Blount A, Berggren T, Bilezikjian L, et al. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc Natl Acad Sci U S A. 1995;92:2969–2973. doi: 10.1073/pnas.92.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenzel P, Kesterson R, Yeung W, Cone RD, Rittenberg MB, Stenzel-Poore MP. Identification of a novel murine receptor for corticotropin-releasing hormone expressed in the heart. Mol Endocrinol. 1995;9:637–645. doi: 10.1210/mend.9.5.7565810. [DOI] [PubMed] [Google Scholar]
- 20.Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI. Current Status of the Nomenclature for Receptors for Corticotropin-Releasing Factor and Their Ligands. Pharmacol Rev. 2003;55:21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- 21.Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann N Y Acad Sci. 1999;885:312–328. doi: 10.1111/j.1749-6632.1999.tb08687.x. [DOI] [PubMed] [Google Scholar]
- 22.Gourcerol G, Wu SV, Yuan PQ, Pham H, Miampamba M, Larauche M, et al. Activation of corticotropin-releasing factor receptor 2 mediates the colonic motor coping response to acute stress in rodents. Gastroenterology. 2011;140:1586–1596. doi: 10.1053/j.gastro.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tache Y, Perdue MH. Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Motil. 2004;16(Suppl 1):137–142. doi: 10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 24.Tache Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33–40. doi: 10.1172/JCI30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rademaker MT, Charles CJ, Espiner EA, Fisher S, Frampton CM, Kirkpatrick CM, et al. Beneficial hemodynamic, endocrine, and renal effects of urocortin in experimental heart failure: comparison with normal sheep. J Am Coll Cardiol. 2002;40:1495–1505. doi: 10.1016/s0735-1097(02)02170-8. [DOI] [PubMed] [Google Scholar]
- 26.Yoneda M, Nakamura K, Nakade Y, Tamano M, Kono T, Watanobe H, et al. Effect of central corticotropin releasing factor on hepatic circulation in rats: the role of the CRF2 receptor in the brain. Gut. 2005;54:282–288. doi: 10.1136/gut.2003.036426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grossini E, Molinari C, Mary DA, Marino P, Vacca G. The effect of urocortin II administration on the coronary circulation and cardiac function in the anaesthetized pig is nitric-oxide-dependent. Eur J Pharmacol. 2008;578:242–248. doi: 10.1016/j.ejphar.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Davis ME, Pemberton CJ, Yandle TG, Fisher SF, Lainchbury JG, Frampton CM, et al. Urocortin 2 infusion in healthy humans: hemodynamic, neurohormonal, and renal responses. J Am Coll Cardiol. 2007;49:461–471. doi: 10.1016/j.jacc.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 29.Lenz HJ, Fisher LA, Vale WW, Brown MR. Corticotropin-releasing factor, sauvagine, and urotensin I: effects on blood flow. Am J Physiol. 1985;249:R85–R90. doi: 10.1152/ajpregu.1985.249.1.R85. [DOI] [PubMed] [Google Scholar]
- 30.Hill LT, Kidson SH, Michell WL. Corticotropin-releasing factor is present in intestinal tissue of patients with gastrointestinal dysfunction following shock and abdominal surgery. Nutrition. 2013;29:650–654. doi: 10.1016/j.nut.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Chatzaki E, Anton PA, Million M, Lambropoulou M, Constantinidis T, Kolios G, et al. Corticotropin-releasing factor receptor subtype 2 in human colonic mucosa: down-regulation in ulcerative colitis. World J Gastroenterol. 2013;19:1416–1423. doi: 10.3748/wjg.v19.i9.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porcher C, Juhem A, Peinnequin A, Sinniger V, Bonaz B. Expression and effects of metabotropic CRF1 and CRF2 receptors in rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1091–G1103. doi: 10.1152/ajpgi.00302.2004. [DOI] [PubMed] [Google Scholar]
- 33.Chatzaki E, Crowe PD, Wang L, Million M, Taché Y, Grigoriadis D. CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J Neurochem. 2004;90:309–316. doi: 10.1111/j.1471-4159.2004.02490.x. [DOI] [PubMed] [Google Scholar]
- 34.Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, Kunitake K, et al. Potent and long-acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J Med Chem. 2002;45:4737–4747. doi: 10.1021/jm0202122. [DOI] [PubMed] [Google Scholar]
- 35.Akiba Y, Kaunitz JD. Regulation of intracellular pH and blood flow in rat duodenal epithelium in vivo. Am J Physiol. 1999;276:G293–G302. doi: 10.1152/ajpgi.1999.276.1.G293. [DOI] [PubMed] [Google Scholar]
- 36.Akiba Y, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Dynamic regulation of mucus gel thickness in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2000 Aug;279(2):G437–47. doi: 10.1152/ajpgi.2000.279.2.G437. 2000; 279:G437-G447. [DOI] [PubMed] [Google Scholar]
- 37.Kawakubo K, Akiba Y, Adelson D, Guth PH, Engel E, Taché Y, Kaunitz JD. Role of gastric mast cells in the regulation of central TRH analog-induced hyperemia in rats. Peptides. 2005;26:1580–1589. doi: 10.1016/j.peptides.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 38.Martinez V, Wang L, Rivier J, Grigoriadis D, Tache Y, Central CRF. urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol. 2004;556.1:221–234. doi: 10.1113/jphysiol.2003.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Million M, Maillot C, Saunders P, Rivier J, Vale W, Tache Y. Human urocortin II, a new CRF-related peptide, displays selective CRF(2)-mediated action on gastric transit in rats. Am J Physiol Gastrointest Liver Physiol. 2002;282:G34–G40. doi: 10.1152/ajpgi.00283.2001. [DOI] [PubMed] [Google Scholar]
- 40.Kosoyan HP, Wei JY, Tache Y. Intracisternal sauvagine is more potent than corticotropin-releasing factor to decrease gastric vagal efferent activity in rats. Peptides. 1999;20:851–858. doi: 10.1016/s0196-9781(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 41.Martins JM, Kastin AJ, Banks WA. Unidirectional specific and modulated brain to blood transport of corticotropin-releasing hormone. Neuroendocrinology. 1996;63:338–348. doi: 10.1159/000126974. [DOI] [PubMed] [Google Scholar]
- 42.Kastin AJ, Akerstrom V. Differential interactions of urocortin/corticotropin-releasing hormone peptides with the blood-brain barrier. Neuroendocrinology. 2002;75:367–374. doi: 10.1159/000059433. [DOI] [PubMed] [Google Scholar]
- 43.Martinez V, Wang L, Million M, Rivier J, Tache Y. Urocortins and the regulation of gastrointestinal motor function and visceral pain. Peptides. 2004;25:1733–1744. doi: 10.1016/j.peptides.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 44.Negri L, Noviello L, Noviello V. Effects of sauvagine, urotensin I and CRF on food intake in rats. Peptides. 1985;6(Suppl 3):53–57. doi: 10.1016/0196-9781(85)90350-x. [DOI] [PubMed] [Google Scholar]
- 45.Kato S, Hirata T, Takeuchi K. Nitric oxide, prostaglandin, and sensory neurons in gastric mucosal blood flow response during acid secretion in rats. Gen Pharmacol. 1997;28:513–519. doi: 10.1016/s0306-3623(96)00350-3. [DOI] [PubMed] [Google Scholar]
- 46.Sugamoto S, Kawauch S, Furukawa O, Mimaki TH, Takeuchi K. Role of endogenous nitric oxide and prostaglandin in duodenal bicarbonate response induced by mucosal acidification in rats. Dig Dis Sci. 2001;46:1208–1216. doi: 10.1023/a:1010603026913. [DOI] [PubMed] [Google Scholar]
- 47.Wallace JL. Cooperative modulation of gastrointestinal mucosal defence by prostaglandins and nitric oxide. Clin Invest Med. 1996;19:346–351. [PubMed] [Google Scholar]
- 48.Raimura M, Tashima K, Matsumoto K, Tobe S, Chino A, Namiki T, et al. Neuronal nitric oxide synthase-derived nitric oxide is involved in gastric mucosal hyperemic response to capsaicin in rats. Pharmacology. 2013;92:60–70. doi: 10.1159/000351853. [DOI] [PubMed] [Google Scholar]
- 49.Chen RY, Guth PH. Interaction of endogenous nitric oxide and CGRP in sensory neuron-induced gastric vasodilation. Am J Physiol. 1995;268:G791–G796. doi: 10.1152/ajpgi.1995.268.5.G791. [DOI] [PubMed] [Google Scholar]
- 50.Schweda F, Klar J, Narumiya S, Nusing RM, Kurtz A. Stimulation of renin release by prostaglandin E2 is mediated by EP2 and EP4 receptors in mouse kidneys. Am J Physiol Renal Physiol. 2004;287:F427–F433. doi: 10.1152/ajprenal.00072.2004. [DOI] [PubMed] [Google Scholar]
- 51.Yang T, Du Y. Distinct roles of central and peripheral prostaglandin E2 and EP subtypes in blood pressure regulation. Am J Hypertens. 2012;25:1042–1049. doi: 10.1038/ajh.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kedar A, Wajsman Z, Williams P, Moore R, Murphy GP. Paradoxical increase of renal blood flow in anesthetized hypertensive dog treated with indomethacin. Urology. 1979;14:256–259. doi: 10.1016/0090-4295(79)90495-3. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi K, Totsune K, Murakami O, Shibahara S. Urocortins as cardiovascular peptides. Peptides. 2004;25:1723–1731. doi: 10.1016/j.peptides.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 54.Im E, Rhee SH, Park YS, Fiocchi C, Tache Y, Pothoulakis C. Corticotropin-releasing hormone family of peptides regulates intestinal angiogenesis. Gastroenterology. 2010;138:2457–67, 2467. doi: 10.1053/j.gastro.2010.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Konturek SJ, Bilski J, Pawlik W, Thor P, Czarnobilski K, Szoke B, et al. Gastrointestinal secretory, motor and circulatory effects of corticotropin releasing factor (CRF). Life Sci. 1985;37:1231–1240. doi: 10.1016/0024-3205(85)90135-3. [DOI] [PubMed] [Google Scholar]