Abstract
The present study investigated whether early life exposure to high levels of animal fat increases breast cancer risk in adulthood in rats. Dams consumed a lard-based high-fat (HF) diet (60% fat-derived energy) or an AIN93G control diet (16% fat-derived energy) during gestation or gestation and lactation. Their 7-week-old female offspring were exposed to 7,12-dimethyl-benzo[a]anthracene to induce mammary tumors. Pregnant dams consuming an HF diet had higher circulating leptin levels than pregnant control dams. However, compared to the control offspring, significantly lower susceptibility to mammary cancer development was observed in the offspring of dams fed an HF diet during pregnancy (lower tumor incidence, multiplicity and weight), or pregnancy and lactation (lower tumor multiplicity only). Mammary epithelial elongation, cell proliferation (Ki67) and expression of NFκB p65 were significantly lower and p21 expression and global H3K9me3 levels were higher in the mammary glands of rats exposed to an HF lard diet in utero. They also tended to have lower Rank/Rankl ratios (P=.09) and serum progesterone levels (P=.07) than control offspring. In the mammary glands of offspring of dams consuming an HF diet during both pregnancy and lactation, the number of terminal end buds, epithelial elongation and the BCL-2/BAX ratio were significantly lower and serum leptin levels were higher than in the controls. Our data confirm that the breast cancer risk of offspring can be programmed by maternal dietary intake. However, contrary to our expectation, exposure to high levels of lard during early life decreased later susceptibility to breast cancer.
Keywords: Breast cancer, High-fat diet, Fetal programming, RANK/RANKL pathway, H3K9me3
1. Introduction
Breast cancer is a major global public health problem and the most common type of cancer among women; 16% of all female cancers are breast cancers [1]. Although the origins of breast cancer remain mostly unknown, many risk factors have been identified, including genetic, hormonal and environmental factors, particularly nutrition [2,3]. It has been estimated that 38% of breast cancers are preventable by dietary modifications [4].
Human data initially seem to be highly supportive of high fat intake increasing breast cancer risk [5]. Meta-analysis of different studies in animal models strengthened the idea that dietary fats promote the growth of mammary tumors [6]. However, subsequent studies have generated conflicting data regarding the role of dietary fat in breast cancer, and different types of fats appear to have opposing effects on this disease [7,8]. For example, consumption of animal fat, consisting mainly of saturated fatty acids (SFA), is associated with increased risk of breast cancer in some studies [9,10] but not in others [11–13]. Furthermore, a reduction in dietary fat intake did not prevent the development of breast cancer in high-risk women [14].
As described for many noncommunicable chronic diseases, including cardiovascular diseases, diabetes and obesity [15,16], breast cancers may also have a fetal origin [17]. In 1990, Trichopoulos [18] suggested that some breast cancers are programmed during fetal development by the intrauterine environment, specifically by exposure to high levels of estrogens. It has also been proposed that both maternal undernutrition and maternal overnutrition during pregnancy may epigenetically reprogram gene expression in the mammary gland, resulting in a higher number of terminal end buds (TEBs) with increased susceptibility to malignant transformation [19].
It has been suggested that exposure to a high-fat (HF) diet in utero can modulate later breast cancer risk, perhaps because the mammary gland is particularly sensitive to changes in lipids during early development [20]. Maternal intake of high levels of corn oil, which contains n-6 polyunsaturated fatty acids (PUFA), or Crisco, which contains saturated fat (cottonseed oil) and PUFA (soybean oil), was associated with higher levels of estrogen and leptin during pregnancy, respectively, as well as increased susceptibility to mammary tumorigenesis among the female offspring [21,22]. On the other hand, supplementation with fish or canola oils, important dietary sources of n-3 fatty acids, during lactation or pregnancy and lactation decreased susceptibility to breast cancer in the female offspring [23,24].
In 2003, consumption of vegetable-based oils was 2.3 times higher and animal fat intake was 4.5 times higher in industrialized countries than in developing countries [25]. It is estimated that the ratio of vegetable oil intake in industrialized and developing countries will remain the same for the next 10 years. However, the ratio of animal fat intake will drop to 1.6 because animal fat consumption in the West is decreasing while consumption in developing countries is increasing [25]. It is not known whether increased maternal consumption of animal fat during pregnancy affects breast cancer risk among daughters.
Control of gene expression through epigenetic mechanisms such as DNA methylation and histone modifications during early life can determine cellular fate [26–28]. Maternal fat intake and/or its effects on the fetal hormonal environment are likely to affect offspring's breast cancer risk by inducing epigenetic changes in the genes that control mammary gland development and determine its susceptibility to breast cancer.
The present study aimed to evaluate the effects of maternal consumption of a lard-based HF diet during pregnancy or pregnancy and lactation on mammary tumor development in female rat offspring and the associated changes in the mammary gland morphology, epigenetic markers and gene expression. Our results indicate that lard intake by dams during pregnancy reduced mammary tumorigenesis (incidence and multiplicity, and increased latency) among their female offspring and, during pregnancy and lactation, reduced offspring's mammary tumor multiplicity. Both exposures inhibited mammary epithelial elongation in the offspring. Reduced mammary tumorigenesis in in utero only exposed offspring was associated with reduced cell proliferation (Ki67) and expression of NFκB p65, compared to the control offspring, and higher p21 expression and global H3K9me3 levels. Female rats exposed to high levels of lard both in utero and during nursing exhibited reduced number of TEBs and the BCL-2/BAX ratio. These findings suggest that maternal intake of high levels of lard during pregnancy or pregnancy and lactation reduces mammary tumorigenesis in their offspring, but the mechanisms mediating these protective effects can be different depending if the exposure was only during pregnancy or during pregnancy and lactation.
2. Material and methods
2.1. Experimental protocol
Male and female Sprague–Dawley rats were obtained from the colony of the Faculty of Pharmaceutical Sciences, University of São Paulo, Brazil, and were maintained in an atmosphere of 55%±10% relative humidity, at 22°C±2°C, with a 12-h light/dark cycle (lights on at 07:00 h), receiving water and commercial diet (Nuvital, Brazil) ad libitum. Studies were approved by the Ethics Committee on Animal Experiments of the Faculty of Pharmaceutical Sciences, University of São Paulo (Protocol Number 283). Rats were mated by housing one male together with two females per cage. Gestational day 0 was determined by the presence of sperm in the vaginal smears. Before mating, female rats were divided into three groups (n=20 dams per group) to provide pups: CO [female rats exposed to control diet (AIN-93G) during gestation and lactation; controls], G (female rats exposed to lard-based HF diet during gestation) and GL (female rats exposed to HF diet during gestation and lactation). The HF diet is based on AIN-93G [29] with added lard, representing a Western diet rich in animal fat [30] (Supplementary Table 1). In the CO and HF diets, 10% and 60% of total calories were provided by lipids, respectively. In a pilot study, it was observed that the rats in the HF diet group consumed approximately 30% less diet than the rats in the control group. However, given the higher energy density of the HF diet (5.24 kcal/g) compared to the control diet (3.96 kcal/g), the daily energy intake did not differ between the groups. Thus, to ensure comparable intakes of micro- and macronutrients between the groups, the ingredients (except for starch and lard) were normalized according to the energy density of each diet, as shown in Supplementary Table 1. Additionally, because the HF diet is highly susceptible to oxidation, the content of tert-butylhydroquinone was increased [31]. For adaptation to the experimental diet, feeding with CO or HF diets started 5 days before mating. During lactation, the diet of dams from G group was switched to AIN93-G, while the diets of dams from CO and GL groups remained AIN93-G and HF, respectively. To avoid litter size effect, each dam was kept with six female pups which were weaned on postnatal day 21. Afterwards, all female offspring consumed commercial laboratory chow (Nuvital, Brazil). Rats that did not become pregnant or committed cannibalism were excluded from the study. Body weight was recorded three times per week. Food intake was recorded daily and once a week for mothers during gestation and lactation and for female offspring, respectively.
2.2. Determination of the diets lipid profile
The lipid profile of the CO and HF diets was determined according to the Association of Official Analytical Chemist [32]. Fatty acids were esterified to fatty acid methyl esters according to Hartman and Lago [33], and their composition was analyzed with a gas chromatograph (GC 17 A Shimadzu/Class GC 10, Japan) equipped with a flame ionization detector and a SUPELCOWAX 10 fused silica capillary column (30 mm×0.25 mm i.d.). The temperature was set at 170°C, raised to 225°C at a rate of 1°C/min and held for 25 min. The temperatures of the vaporizer and detector were 250°C and 270°C, respectively. Helium was used as the carrier gas (1 ml/min). Identification of the fatty acids was performed by comparison of the retention times with the standard mixture of fatty acid methyl esters. Each determination was performed in triplicate, using three different samples for each diet.
2.3. Analysis of pregnant dams and female offspring hormone levels
Blood from pregnant dams and female offspring was collected by tail puncture on gestational day 17 and at postnatal weeks 3 and 7 by cardiac puncture during euthanasia, respectively. Serum was obtained and kept at −80°C until use. Levels of circulating estradiol and progesterone were determined using rat radioimmunoassay kits from Siemens Medical Solutions Diagnostics (Munich, Germany), while leptin levels were determined using RADPK-81K Kit from Millipore (Billerica, MA, USA), according to the manufacturer's instructions.
2.4. Mammary tumor induction
Mammary tumors were induced in 7-week-old offspring female rats (n=24 rats per group) by administration of 7,12-dimethylbenz[a]anthracene (DMBA) (50 mg/kg body weight; Sigma, USA). The carcinogen was dissolved in corn oil and administered by oral gavage. Animals were examined for mammary tumors by palpation three times per week. Latency of tumor appearance, the number of animals with tumors (tumor incidence) and the number of tumors per animal (tumor multiplicity) were evaluated. Those animals in which tumor burden approximated 10% of total body weight were euthanized. All others animals were sacrificed at 19 weeks after carcinogen administration. Tumor histopathological analysis was performed according to Russo and Russo [34].
2.5. Analysis of mammary gland morphology in female offspring
Whole-mount preparations were obtained from 3- and 7-week-old female offspring of fourth abdominal mammary tissue as described by de Assis [35]. TEBs were counted from whole-mount preparations according to the guidelines established by Russo and Russo [36] (n=12/group).
2.6. Analysis of cell proliferation in mammary glands of female offspring
Cell proliferation was assessed in mammary tissues obtained from 7-week-old female rats (n=5 per group) by Ki67 immunohistochemistry. Mammary tissues were fixed in 10% buffered formalin, embedded in paraffin and sectioned. Sections were deparaffinized in xylene and hydrated through graded alcohols, followed by antigen retrieval in citrate buffer at pH 6.0 (0.5% Tween) in a pressure cooker for 20 min. The tissues were incubated with 3% H2O2 for 10 min. Nonspecific protein binding was blocked for 30 min with DAKO protein Block (DAKO, USA). Tissue sections were incubated overnight with the primary monoclonal mouse anti-rat Ki67 (DAKO, USA) at a 1:50 dilution. After several washes, sections were treated with secondary biotinylated anti-mouse IgG antibody (Vector Laboratories, USA) for 30 min at room temperature, followed by treatment with Vectastain ABC reagents (Vector Laboratories, USA) for 30 min at room temperature. Sections were washed and stained with 3,3'-diaminobenzidine in chromogen solution (DAKO, USA) for 4 min and then washed and counterstained for 45 s with Vector's Hematoxylin QS (Vector Laboratories, USA). Proliferation index was determined by calculating the percentage of cells that had positive Ki67 staining among 1000 cells per structure (lobule or duct). Slides were evaluated with Image J software (NIH, USA).
2.7. Analysis of gene expression in mammary glands of female offspring
RNA extraction was performed with the RNeasy Lipid Tissue Mini Kit (QIAGEN, USA), according to the manufacturer's protocol, and its quality was confirmed using an Agilent 2100 Bioanalyzer and RNA 6000 LabChips, from which the RNA integrity numbers were calculated. RNA was treated with DNAse for 20 min, and the reaction was stopped with 0.02 MEDTA at 75°C for 10 min. Treated RNA (1 µg) was used for the synthesis of first-strand cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA) according to the manufacturer's instructions. Expression levels of target genes were measured using a 7900HT Fast Real-Time PCR System (Applied Biosystems). cDNA (2 µl) at a final concentration of 5 ng/µl was mixed with 5 µl EvaGreen qPCR Mastermix (Applied Biosystem Materials, USA) and 0.5 µl of 5 µmol forward and reverse primers in 384-well plates. Amplification reactions were performed with a two-step thermal cycling method, consisting of a 15 min step at 95°C and 40 cycles of 95°C for 15 s, 60°C for 30 s and 72°C for 30 s. Each sample was run in triplicate. Absolute gene expression levels were determined using the Relative Standard Curve Method, and the reaction efficiency was considered adequate when it was between 90% and 110% and the R square value was higher than 0.98. An assay was performed with 18S rRNA, Gapdh, Tpb, β-actin, Hrpt1 and Rpl13a genes to identify the best endogenous control gene using geNorm software. Based on this assay, the expression levels of target genes were normalized to the reference gene Gapdh. Primers were designed using IDT tool primer design, except for 18S rRNA primers, which were from Wang [37]. Primer sequences are listed in Supplementary Table 2.
2.8. Analysis of protein levels in mammary glands of female offspring
Protein levels of BAX, BCL-2 (apoptosis), p21Cip1, p27Kip1 (cell proliferation) estrogen receptor α (ERα), estrogen receptor β (ERβ), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER-2) and nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB p65 unit) were assessed by Western blot in mammary tissues obtained from 7-week-old female rats (n=5 per group). Total protein was extracted from mammary tissues using radioimmunoprecipitation buffer with protease inhibitor (Roche, Switzerland), glycerophosphate (10 mM), sodium orthovanadate (1 mM), pyrophosphate (5 mM) and phenylmethanesulfonyl fluoride (PMSF) (1 mM). The samples were mixed with the buffer for 5 min, then incubated on ice for 30 min and centrifuged for 10 min on high speed. Protein in the supernatant was quantified with BCA Protein Assay Reagent (Pierce, USA). Protein extracts were resolved on a 4%–12% denaturing polyacrylamide gel [sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)]. Proteins were transferred using the iBlot 7-Minute Blotting System (Invitrogen, USA) and blocked with 5% nonfat dry milk for 1 h at room temperature. Membranes were incubated with primary antibodies against BAX, p27, NFκB p65 (Santa Cruz Biotechnology, USA); BCL-2 (Beckman Coulter, USA); ERα, ERβ, HER-2 (Cell Signaling Technology, USA); p21 (Oncogene Research Products, USA) and PR (Abcam antibodies, UK) at dilutions of 1:2000 at 4°C overnight. After several washes, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:5000, Santa Cruz Biotechnology, USA) at room temperature for 1 h. Membranes were developed using the Chemiluminescent HRP antibody detection reagent (Denville Scientific Inc., USA) and exposed to Kodak autoradiography films. Optical density of the bands was quantified using Quantity-one software (BIO-RAD, USA). To control for equal protein loading, expression of the proteins of interest was normalized to the β-actin signal.
2.9. Analysis of global histone acetylation and trimethylation in mammary glands of female offspring
Global acetylation levels of histone H3 lysine 9 (H3K9) and H4 lysine 16 (H4K16) and trimethylation levels of H3K9, H3 lysine 27 (H3K27) and H3 lysine 4 (H3K4) were assessed by Western blot in mammary tissues obtained from 7-week-old female rats (n=6 per group). For histone extraction, 150 mg mammary tissue was homogenized with cold lysis buffer [HEPES (10 mM, pH 7.9, USB, USA), Triton X-100 (1%, USB, USA), MgCl2 (1.5 mM, Sigma Aldrich, USA), KCl (10 mM; Merck, Germany), sodium butyrate (5 mM, Sigma Aldrich, USA), dithiothreitol (0.5 mM; Invitrogen, USA), PMSF (1.5 mM, Sigma Aldrich, USA), protease Inhibitor (1 µg/ml, Sigma Aldrich, USA), phosphatase inhibitor (1 µg/ml, Sigma Aldrich, USA)] for 20–30 min and centrifuged for 5 min at 5000g at 4°C. After centrifugation, the pellet was collected, washed with cold lysis buffer and incubated overnight at 4°C with HCl 0.2 N. Samples were centrifuged for 5 min at 15,000g at 4°C. The pH of the supernatant was adjusted to 7 with 2 N NaOH. The histones were quantified by the Bradford method according to the manufacturer's protocol (Thermo Scientific, USA) and stored at−80°C. Histone extracts were resolved on a 4%–12% denaturing polyacrylamide gel (SDS-PAGE). Histones were transferred to a nitrocellulose membrane (Hybond-ECLTM, Amersham Biosciences, USA) over the course of 35 min and blocked with ECL reagent (Enhanced Chemiluminescence, GE Healthcare, UK) for 1 h at room temperature. Membranes were incubated with antiacetyl H3K9 and H4K16 primary antibodies (Upstate, USA) (1:5000) diluted in ECL reagents overnight at 4°C. The membrane was incubated with HRP-conjugated antirabbit immunoglobulin secondary antibody (GE Healthcare, UK) diluted 1:10,000 in ECL reagent for 1 h. The membrane was then incubated with primary anti-H1 (Upstate, USA) diluted 1:5000 in ECL reagent for 1 h at 37°C under agitation and then incubated with HRP-conjugated anti-rabbit immunoglobulin secondary antibody (GE Healthcare, UK) diluted 1:10,000 in ECL for 1 h at 37°C under agitation. Immunodetection was performed using the ECL chemiluminescence system. The signal was captured using the ImageQuant 400 image acquisition system (GE Healthcare, UK). Membrane stripping was performed in order to evaluate anti-trimethyl H3K9, H3K4 and H3K27 signals. The membrane was incubated with 2% SDS solution containing 100 mM β-mercaptoethanol at 60°C for 30 min, followed by the previously described protocol.
2.10. Analysis of global DNA methylation in mammary glands of female offspring
Global DNA methylation was assessed by high-performance liquid chromatography with diode-array detection (HPLC-DAD) in mammary tissues obtained from 7-week-old female rats (n=6 per group). For DNA extraction, 100 mg of mammary tissue was homogenized with lysis buffer (Gentra Puregene Kit, QIAGEN Mayland Sciences, USA) containing 0.5 mM deferoxamine (Sigma Aldrich, USA). The homogenized samples were incubated with proteinase K (20 mg/ml, Sigma Aldrich, USA) overnight at room temperature. Samples were treated with RNase A (15 mg/ml, Sigma Aldrich, USA) for 2 h, and protein was precipitated with protein precipitating solution (Gentra Puregene Kit, QIAGEN, Maryland, USA). The homogenate was centrifuged at 3000 rpm for 15 min at 4°C, and DNA was precipitated with cold isopropanol. After centrifugation, Tris buffer (10 mM Tris with 1 mM deferoxamine, pH=7.0) and chloroform (96%) and isoamyl alcohol (4%) were added to the pellet. After centrifugation, 5 M NaCl and 70% ethanol were added to the supernatant and incubated overnight. Samples were centrifuged for 10 min at 3000 rpm, and the DNA was resuspended in water with 0.1mMdeferoxamine. The samples were quantified by spectrophotometry (Nanodrop1000, ThermoScientific). DNA (5 µg) was hydrolyzed with Tris buffer including 200 mM HCl/MgCl2 (pH 7.4) and DNAse 1 (Sigma Aldrich, EUA) for 1 h at 37°C. DNA was incubated with phosphodiesterase (Sigma Aldrich, USA) and alkaline phosphatase for 1 h at 37°C. Aliquots of hydrolyzed DNA were injected into the HPLC-DAD analytical system (Shimadzu Corporation, Japan) using Luna column C18(2), 250 mm×4.6 mm ID, 5 µm (Phenomenex, Torrance, CA), with a C18 precolumn of 4.0×3.0 mm (Phenomenex, Torrance, CA) eluted with a gradient of formic acid (0.1% in water) and CH3OH (0–25 min, 0%–18% CH3OH; 25–27 min, 18%–0% CH3OH; 27–37 min, 0% CH3OH) with a flow rate of 1 ml/min at 30°C. The DAD detector was set at 260 nm for quantification of dC and at 286 nm for quantification of the 5-methyl-dC. Calibration curves were performed in the ranges of 0.05 to 6 nmol for dC and 0.005 to 0.4 nmol for 5-methyl-dC.
2.11. Statistical analysis
Results are expressed as the mean±standard error of mean (S.E.M.), and all analyses were conducted with STATISTICA 8.0 (Statsoft, USA). Multiple-group comparisons were performed using one-way analysis of variance (ANOVA) followed by Duncan's post hoc tests, and two-group comparisons were performed using Student's t test. Repeated-measures ANOVA test was applied for caloric intake data evaluation, and Kaplan–Meier curve and log-rank test were applied for determining differences in tumor incidence. For all data analysis, P≤.05 was applied as the threshold for statistical significance.
3. Results
3.1. Effects of HF diet on pregnancy and lactation
Caloric intake was not different (P>.05) among the dams in any of the diet groups during gestation and lactation (Supplementary Figure 1). Due to tight homeostatic control of food intake and energy expenditure, it is well described that pregnant rats consuming an energy-rich HF diet reduce their food intake in order to adjust their calorie intake [38]. This indicates that the reported effects of HF diet during gestation or gestation and lactation seem to be related to increased fat consumption rather than caloric intake. Body weight gain during pregnancy, number of pups/litter, female/male ratio and male and female offspring birth weight were not different (P>.05) among the groups (Table 1). Maternal exposure to the HF diet marginally increased the dam's leptin level (P=.07) on gestation day 17 but did not affect estradiol levels (P>.05) (Table 1).
Table 1.
Effects of HF diet on pregnancy and pups variables
| Variables | Group | ||
|---|---|---|---|
| CO | G | GL | |
| Body weight gain during pregnancy (g) | 152.2±9.3 | 157.6±5.4 | 146.8±7.1 |
| Gestational hormonal levels of Estradiol (pg/ml) | 36.4±4.3 | 34.4±2.5 | 40.4±2.2 |
| Leptin (ng/ml) | 6.8±1.3 | 13.8±2.1 * | 13.5 ±3.1 * |
| Number of pups/litter | 12.5±1.0 | 12.8±0.5 | 12.3±0.94 |
| Female/male ratio | 1.32±0.2 | 1.1±0.1 | 1.5±0.4 |
| Offspring birth weight | |||
| Male | 7.2±0.1 | 7.1±0.2 | 6.9±0.1 |
| Female | 7.1±0.2 | 6.8±0.1 | 6.8±0.2 |
The data presented were obtained from 11, 13 and 12 dams of CO, G and GL groups, respectively. For hormonal levels, n=6 per group. The data are expressed as mean±S.E.M.
Marginal difference (P=.07) compared to CO group according to ANOVA followed by Duncan's test.
3.2. Fatty acid composition of CO and HF diets
Fatty acid composition was determined in the lipid fraction of the diets. The control diet contained 17%, 27% and 55% (% of total fat) of SFA, monounsaturated (MUFA) and PUFA, respectively, while the HF diet contained 37%, 38% and 24% of these fatty acids, respectively (Supplementary Table 3). Total daily fat intake was 1.42 g during pregnancy in the control rats, 2.70 g in the rats fed HF diet during gestation only and 2.63 g in the rats fed HF diet both during pregnancy and lactation (Table 2). During lactation, total fat intakes were 2.49 g in the controls, 2.36 g in the rats fed HF diet only during pregnancy, and 4.84 g in the rats fed HF diet both during pregnancy and lactation (Table 2). Rats exposed to the HF diet during gestation consumed increased levels of SFA (4.2-fold) and MUFA (2.7-fold) (P≤.05) and decreased levels of PUFA (−1.2-fold) (P>.05) compared to the CO group (Table 2). Similarly, dams exposed to the HF diet during lactation consumed at that time period increased levels of SFA (4.3-fold) and MUFA (2.8-fold) (P≤.05) and decreased levels of PUFA (−1.2-fold) (P>.05) compared to the CO group (Table 2).
Table 2.
Daily intake of fatty acids (g/animal) by dams during gestation and lactation
| Fatty acids |
Gestation | Lactation | ||||
|---|---|---|---|---|---|---|
| CO | G | GL | CO | G | GL | |
| SFA | 0.24±0.01 | 1.01±0.01a | 0.99±0.02a | 0.42±0.01 | 0.40±0.01 | 1.81±0.05 a |
| MUFA | 0.39±0.01 | 1.04±0.01a | 1.01±0.02a | 0.67±0.01 | 0.64±0.02 | 1.86±0.05 a |
| PUFA | 0.79±0.02 | 0.65±0.01a | 0.63±0.01a | 1.40±0.03 | 1.32±0.04 | 1.17±0.03 a |
The data presented were obtained from 11, 13 and 12 dams of CO, G and GL, respectively. The data are expressed as mean±S.E.M.
Statistically significant difference compared to CO group according to Kruskal–Wallis test.
3.3. Effects of maternal HF diet consumption on hormonal levels of female offspring
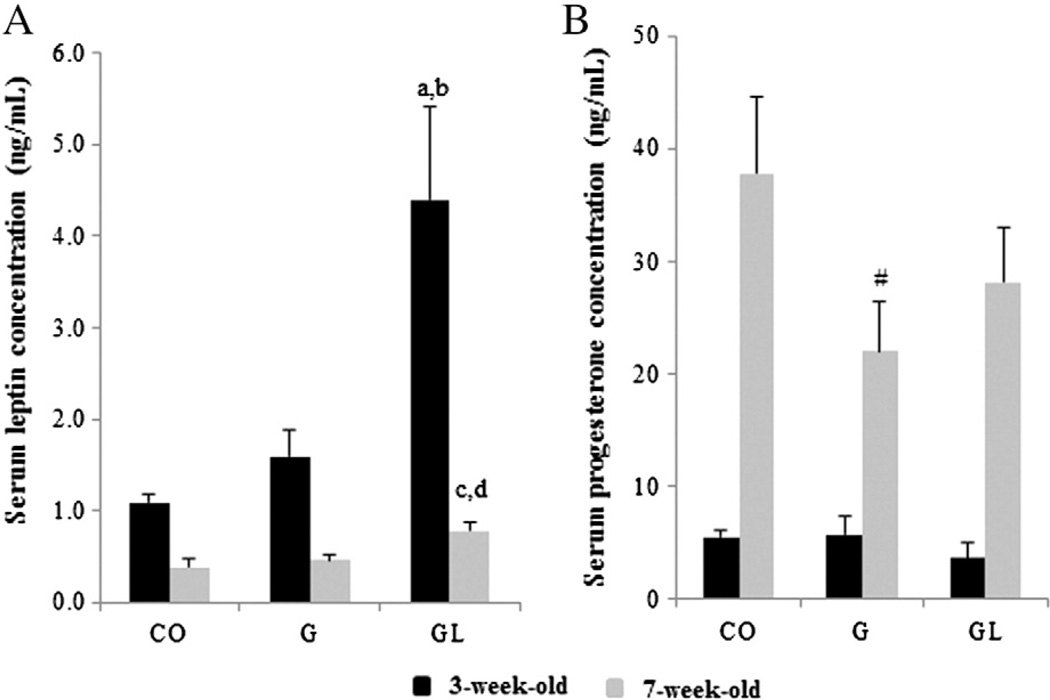
Serum estradiol levels did not differ among the groups (P>.05, data not shown). Compared to the offspring of the CO and G groups, 3- and 7-week-old GL female offspring showed increased serum leptin levels (Fig. 1A). Compared to the CO group offspring, only 7-week-old G female offspring showed a marginally decreased (P=.07) progesterone level (Fig. 1B).
Fig. 1.
(A) Serum leptin levels in 3- and 7-week-old female offspring from the CO, G and GL groups. There are statistically significant differences (P≤.05) compared to COa and Gb groups (3 weeks old) and COc and Gd groups (7 weeks old) according to ANOVA followed by Duncan's test. (B) Serum progesterone levels in 3-and 7-week-old female offspring from the CO, G and GL groups. #Marginal difference (P=.07) compared to the CO group (7 weeks old) according to ANOVA followed by Duncan’s test. The data are expressed as the mean±S.E.M (n=6 per group for leptin and progesterone analysis).
3.4. Effects of maternal HF diet consumption on female offspring mammary carcinogenesis
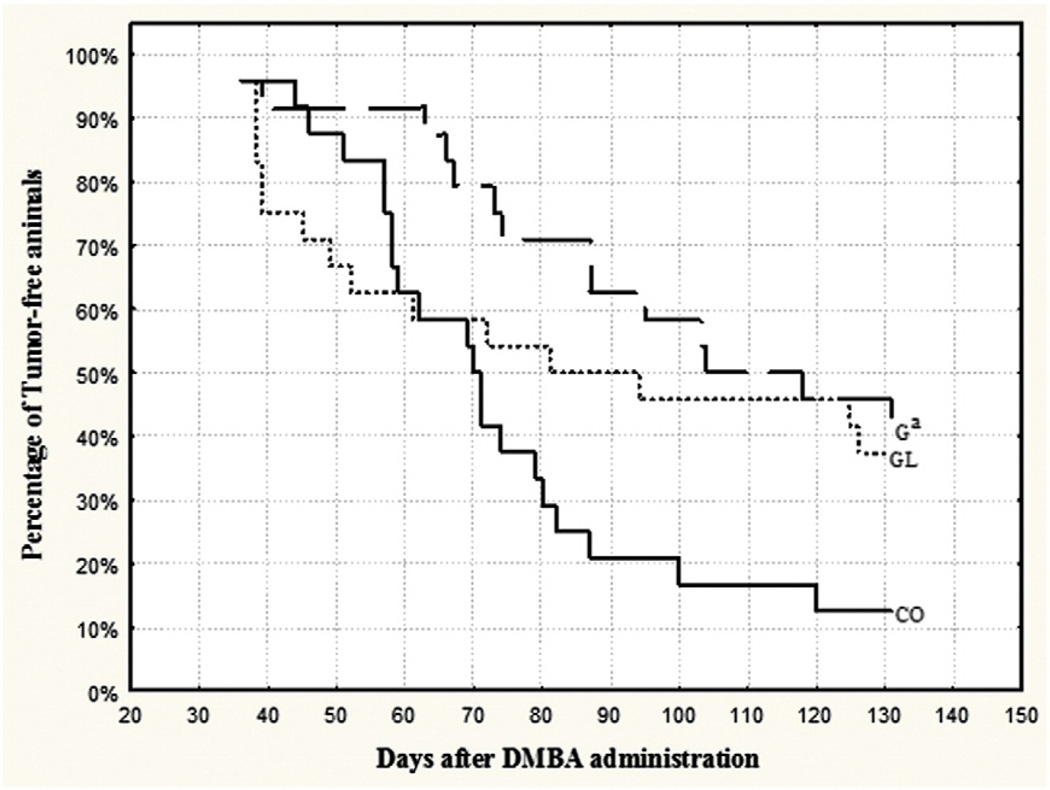
Female offspring exposed to a high-animal-fat diet during gestation only (G group) had lower (P≤.05) mammary tumor incidence and multiplicity (Fig. 2, Table 3) compared to the CO group and longer (P≤.05) first tumor latency compared to offspring exposed to the HF diet during both gestation and nursing (GL group). Weights of the mammary tumors were lower (P≤.05) in the G group than in the CO group. Compared to the CO group, female offspring of the GL group had lower (P≤.05) mammary tumor multiplicity (Table 3). In all groups, mammary tumors were predominantly malignant and classified as tubular or papillary adenocarcinomas. Compared to the G group, female offspring in the GL group had a higher (P≤.05) proportion of grade 2 mammary adenocarcinomas.
Fig. 2.
Mammary tumor incidence in female offspring from the CO, G and GL groups (n=24 per group). aStatistically significant difference (P≤.05) compared to CO group according to Kaplan–Meier analysis and log-rank test.
Table 3.
Effects of maternal HF diet consumption on female offspring mammary carcinogenesis
| Parameters | Group | ||
|---|---|---|---|
| CO | G | GL | |
| Effective number of rats | 24 | 24 | 24 |
| Period of first tumor appearance (days) | 68±3.9 | 82±5.6b | 62±6.3 |
| Multiplicity1 (no./rat) | 2.3±0.3 | 1.2±0.3a | 1.4±0.3a |
| Tumors weight | |||
| ≤0.50 g | 43.6% | 65.6% | 46.9% |
| 0.51–2.0 g | 38.2% | 21.9%c | 25.0% |
| ≥2.1 g | 18.2% | 12.5% | 28.1% |
| Total number of tumors | 56 | 29 | 35 |
| Benign | 3.4% | 12.5% | 12.9% |
| Malignant | 96.6% | 87.5% | 87.1% |
| Histological types2 | |||
| Tubular | 50.9% | 39.2% | 59.3% |
| Papillary | 35.1% | 53.6% | 29.6% |
| Cribiform | 7.0% | 0% | 7.4% |
| Mixed | 7.0% | 7.2% | 3.7% |
| Grade2 | |||
| 1 | 73.7% | 78.6% | 48.2% |
| 2 | 21.0% | 10.7% | 37.0%d |
| 3 | 5.3% | 10.7% | 14.8% |
Multiplicity: number of tumors/total number of rats per group. Statistically significant difference (P≤.05) compared to aCO and bGL group according to ANOVA followed by Duncan's test and compared to cCO and dG group according Fisher’s Exact Test.
Of malignant tumors.
3.5. Effects of maternal HF diet consumption on female offspring mammary gland morphology
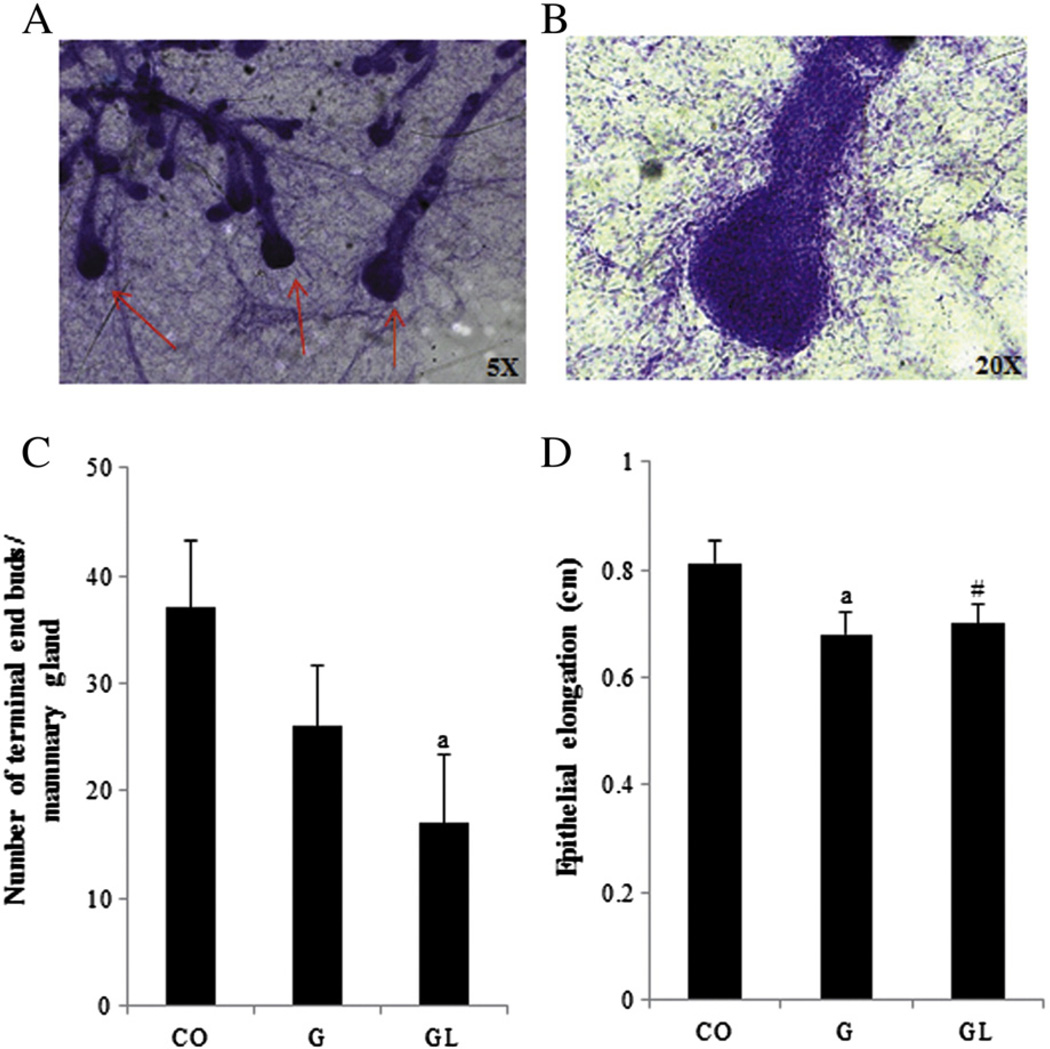
At 3 weeks of age, both the G and GL offspring had fewer numbers of TEBs than the CO offspring, although statistical significance (P≤.05) was only reached in the GL group (Fig. 3A). Compared to the CO group, epithelial elongation was lower in the G (P≤.05) and GL (P=.06) groups at this age (Fig. 3B). No differences (P>.05) were observed in any of the mammary morphological parameters among the 7-week-old CO, G and GL groups (data not shown).
Fig. 3.
Mammary gland morphology in 3-week-old female offspring from the CO, G and GL groups. Photomicrography of TEBs (A, 5×; B, 20×). (C) Number of TEBs per mammary gland. (D) Epithelial elongation (cm) of mammary gland. aStatistically significant difference (P≤.05) and #marginal difference (P=.06) compared to the CO group according to ANOVA followed by Duncan's test. The data are expressed as the mean±S.E.M (n=8–11).
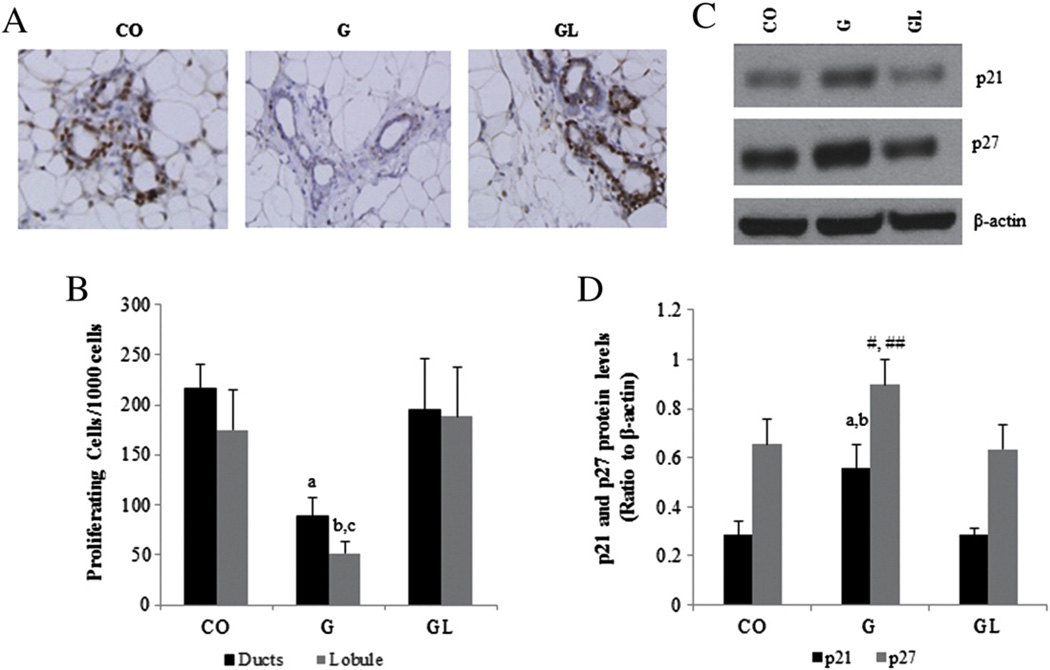
3.6. Effects of maternal HF diet consumption on mammary gland cell proliferation, apoptosis, and p21 and p27 protein levels in 7-weekold offspring
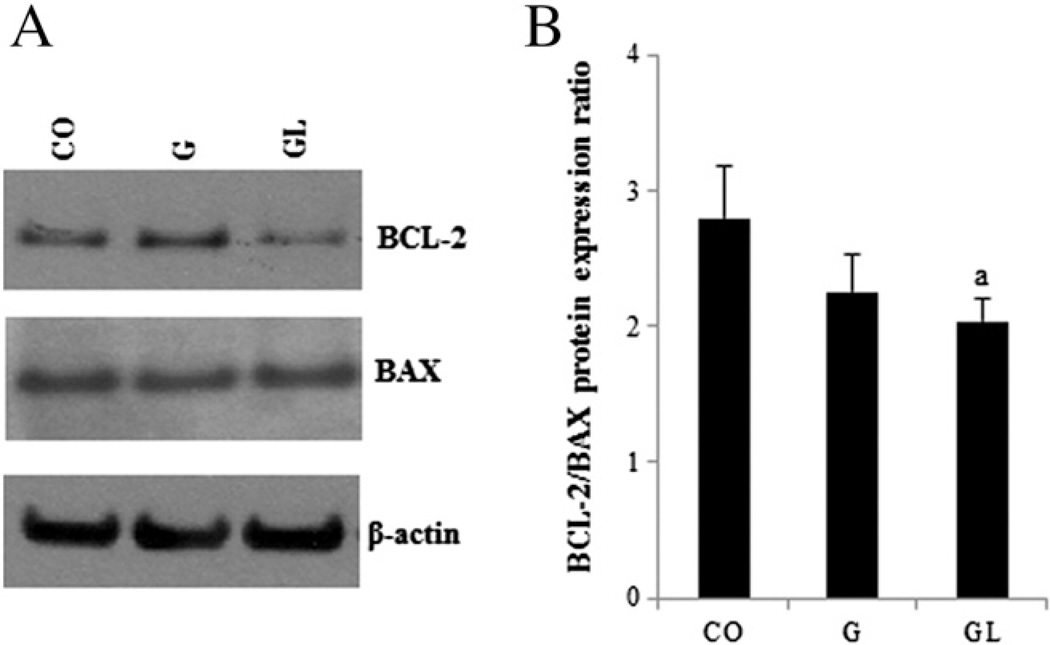
Cell proliferation was significantly lower (P≤.05) in both ducts and lobules in the offspring of dams consuming the HF diet during pregnancy compared to the CO group (Fig. 4A and B). Protein levels of p21 (P≤.05) and p27 (P=.07) were increased in the G offspring (Fig. 4C and D). Mammary glands of GL female offspring showed a lower (P≤.05) ratio between the antiapoptotic protein BCL-2 and the proapoptotic protein BAX compared to the CO group (Fig. 5). The G group female offspring followed the same trend, although the differences were not statistically significant (P>.05).
Fig. 4.
Photomicrography (40×) of Ki67 staining (A) and quantification of cell proliferation (B) in mammary glands of 7 week-old female offspring from the CO, G and GL groups. Statistically significant difference compared to aCO group (ducts) and to bCO and cGL groups (lobule) according to ANOVA followed by Duncan's test. Western blot analysis (C) and quantification (D) of p21 and p27 protein expression in mammary glands of 7-week-old female offspring from the CO, G and GL groups. Statistically significant difference compared to aCO and bGL groups and marginal difference compared to #CO (P=.08) and ##GL (P=.07) groups according to ANOVA followed by Duncan's test. The data are expressed as the mean±S.E.M (n=6 per group for Ki67 analysis; n=5 per group for Western blot analysis).
Fig. 5.
Western blot analysis of BCL-2 and BAX protein expression (A) and the BCL-2/BAX protein expression ratio (B) in mammary glands of 7-week-old female offspring from the CO, G, and GL groups. The data are expressed as the mean±S.E.M (n=5 per group). aSignificant difference compared to CO according to ANOVA followed by Duncan's test (P≤.05).
3.7. Effects of maternal HF diet consumption on mammary gland ERα, ERβ and HER-2 expression and PR–receptor activator of nuclear factor κB (RANK)–receptor activator of nuclear factor κB ligand (RANKL) pathway in 7-week-old offspring
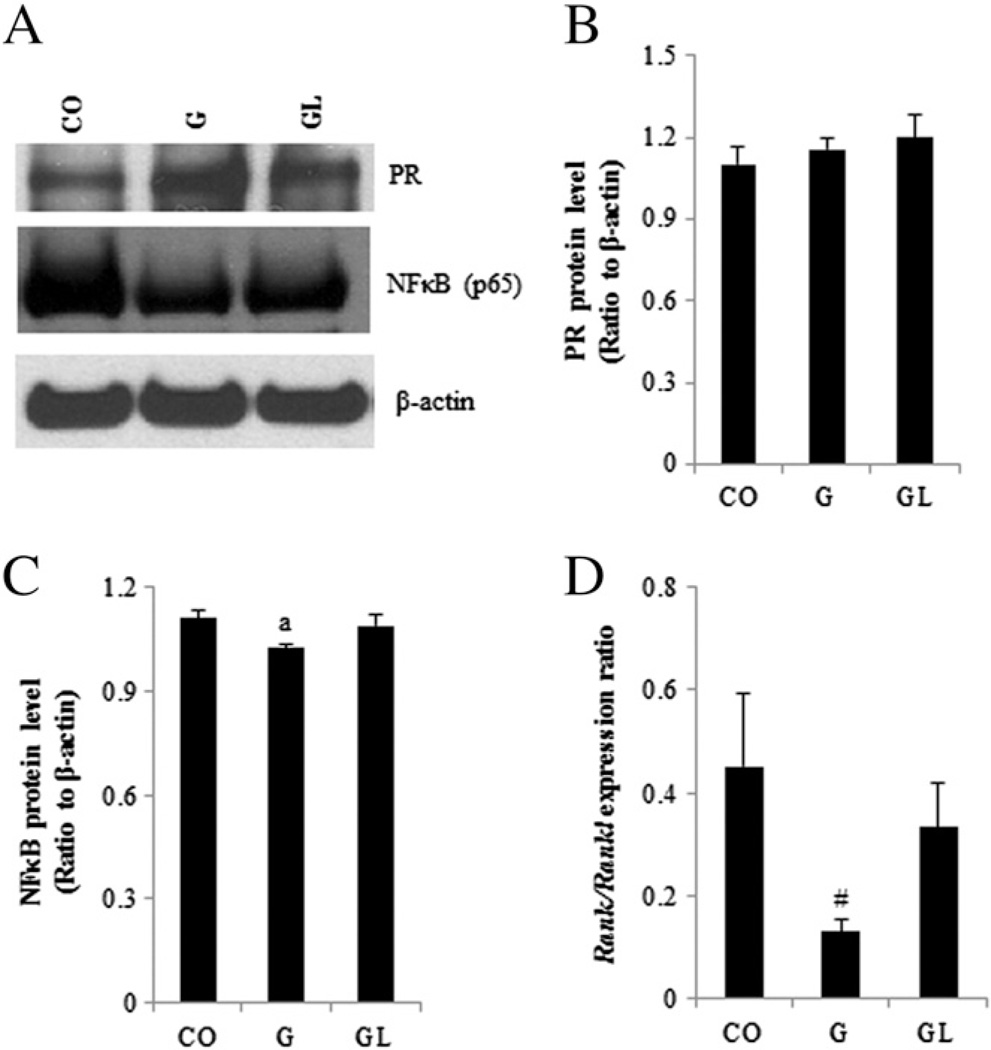
We used Western blotting for ER, HER-2 and PR and the qPCR Rank/Rankl expression ratio to evaluate the long-term effects of early life HF on mammary tissues. There were no differences (P>.05) among groups regarding ERα, ERβ and HER-2 protein expression (Supplementary Figure 2). Compared to the CO group, the G group expressed decreased levels of the NFκB p65 subunit (P≤.05) and a lower Rank/Rankl (P=.09) gene expression ratio, but no differences (P>.05) were observed regarding PR expression (Fig. 6). These changes were not seen in the GL offspring exposed to animal fat during both fetal development and nursing.
Fig. 6.
Western blot analysis of PR and NFκB p65 subunit expression (A) and quantification of PR (B), NFκB p65 subunit protein (C) and Rank/Rankl gene expression (D) in mammary glands of 7-week-old female offspring from CO, G and GL groups. The data are expressed as the mean±S.E.M (n=5 per group). aSignificant (P≤.05) and #marginal (P=.09) differences compared to the CO group according to Student's t test.
3.8. Effects of maternal HF diet consumption on global DNA methylation and histone modifications in the mammary glands of female offspring
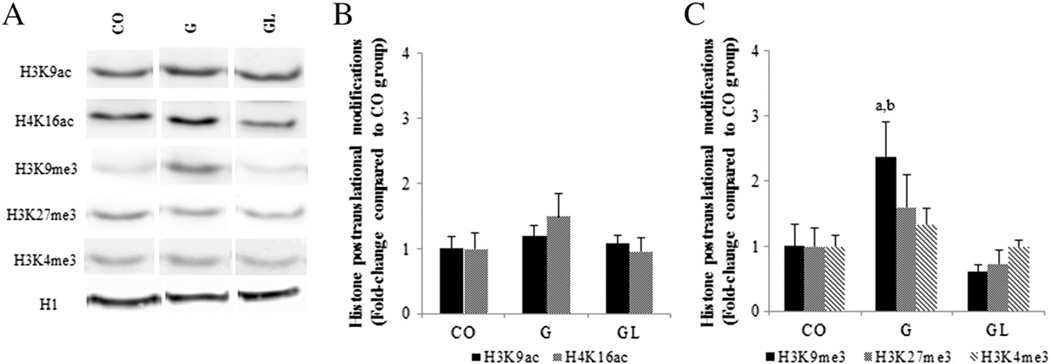
Maternal exposure to an HF diet did not change the global DNA methylation pattern in mammary glands of female offspring (data not shown). Compared to the CO group, female offspring in the G group had increased (P≤.05) levels of H3K9me3 in mammary gland, while no differences were observed regarding H3K9ac, H4K16ac, H3K27me3 and H3K4me3 (Fig. 7). No differences in histone modifications were seen between the CO group and the GL offspring exposed to animal fat during both fetal development and nursing.
Fig. 7.
Western blot analysis of H3K9ac, H4K16ac, H3K9me3, H3K27me3 and H3K4me3 in mammary glands of 7-week-old female offspring from the CO, G and GL groups (A). Quantification of H3K9 and H4K16 acetylation (B) and H3K9, H3K27 and H3K4 trimethylation (C) in mammary glands of 7-week-old female offspring from the CO, G and GL groups. The data are expressed as the mean±S.E.M (n=5 per group). There are statistically significant differences (P≤.05) compared to aCO and bGL groups according to ANOVA followed by the Tukey test.
4. Discussion
We found that female offspring of rat dams consuming high levels of lard during pregnancy or during pregnancy and lactation were at a reduced risk of developing mammary cancer. In the group exposed to an HF lard diet only in utero, all aspects of mammary tumorigenesis were reduced; i.e., latency to tumor appearance was lengthened, and tumor incidence, multiplicity and weight were lower compared to the control group. Offspring of dams consuming the HF lard diet both during pregnancy and lactation exhibited only reduced mammary tumor multiplicity. Reduced risk in the offspring exposed to the HF lard diet during gestation was associated with reduced mammary epithelial elongation and cell proliferation, and p21 and H3K9me3 upregulation. Expression of NFkB p65 was also down-regulated in the mammary glands or rats exposed to HF lard diet in utero. Reduced risk in the offspring exposed to the HF lard diet during both gestation and nursing was associated also with reduced mammary epithelial elongation. In addition, their mammary glands contained less TEBs and exhibited lower BCL-2/BAX ratio than the control rats. Our data thus confirm previous observations that the risk of developing breast cancer can be programmed during fetal and early postnatal development bymaternal dietary intake [18,19,21,39]. However, contrary to our expectation, offspring born to mothers that consumed an HF diet containing lard (saturated and monounsaturated fats) rather than PUFA exhibited reduced susceptibility to developing breast cancer in adulthood.
Fatty acid profile analysis of the HF diet used in our study indicated that saturated [mainly palmitic (16:0) and stearic acids (C18:0)] and monounsaturated [mainly oleic acid (18:1 n-9)] fatty acids comprised 37% and 38% of total fatty acids, respectively. No clear association of fat consumption in adult life, especially of animal origin, with breast cancer risk has been established [11,13]. Although epidemiologic studies have reported an association between consumption of two saturated fatty acids, palmitic and stearic fatty acids, and increased breast cancer risk [40,41], opposite data have been obtained in both in vitro and in vivo studies. Specifically, treatment of breast cancer cells with palmitate promoted cell death and inhibited cell growth [42,43]. Stearate inhibited breast cancer cell cycle progression by inducing p21CIP1/WAF1 and p27KIP1 expression in vitro, and N-nitroso-N-methylurea induced breast carcinogenesis in vivo in rats [44,45].
We are not aware of any previous studies that have investigated the impact of early life consumption of an HF lard diet on breast cancer risk in adulthood. Medvedovic [46] observed that isocaloric intake of butter-based and olive-oil-based HF diets, rich in palmitic and n-9 MUFA fatty acids, respectively, by dams during gestation and lactation and by offspring during the first 7 weeks of life increased cell proliferation in the offspring's mammary gland. On the other hand, consumption of an olive-oil-based diet by the dams from 5 weeks prior to gestation to the end of lactation decreased chemically induced mammary tumorigenesis in the offspring [47]. We suggest that higher intake of both saturated and monounsaturated fatty acids and lower intake of polyunsaturated fat by dams consuming the HF lard diet are associated with the protective effects against breast cancer among the offspring.
The timing of dietary fat exposure represents a major factor influencing its impact on breast cancer risk [19]. Exposure to a cornoil-based HF diet rich in n-6 PUFA during gestation [21] or during gestation and lactation [47] but not during prepuberty [23] increased the susceptibility to mammary carcinogenesis in rats. It is likely that the timing of exposure to the HF lard diet is also important in determining its effect on breast cancer risk. Its protective effect was partly lost in the offspring exposed to the HF lard diet during both the fetal period and nursing.
Higher numbers of TEBs in the mammary gland are often associated with increased breast cancer risk, possibly because this structure is a site of tumor initiation [36,48]. Epithelial elongation reflects rapid growth of the mammary gland, directed by TEBs [49]. Maternal intake of an HF diet (corn oil and Crisco) increased the number of TEBs in the offspring's mammary glands and was associated with increased mammary tumorigenesis [21,22]. In the present study, the number of TEBs was reduced and epithelial elongation was delayed in the mammary gland of 3-week-old female offspring of dams consuming the HF lard diet, consistent with their lower mammary cancer risk.
Altered expression of genes involved in promoting cell cycle progression and inhibiting apoptosis in the offspring's mammary gland during different developmental phases by maternal dietary fat exposures can program increased susceptibility to diseases such as cancer in adulthood [48]. In the present study, we observed a decrease in cell proliferation and an increase in the expression of the cell cycle regulators p21 and p27 in the mammary gland of 7-week-old female offspring of dams that consumed the HF lard diet during gestation. p21 and p27 are cyclin-dependent kinase inhibitors and key G1-checkpoint proteins inhibiting the progression fromG1to S phase [50]. Reduction in their expression is associated with more aggressive breast tumor phenotypes and metastasis [51]. Continuous exposure to the HF lard diet during gestation and nursing decreased the ratio of antiapoptotic BCL-2/proapoptotic BAX proteins in the mammary gland of 7-week-old female offspring. Our data suggest that maternal exposure to HF diet during pregnancy reduced offspring's breast cancer risk by inhibiting cell proliferation and during pregnancy and lactation perhaps by inducing apoptosis.
Higher leptin levels in dams induced by consumption of an HF diet based on Crisco during gestation have been associated with increased breast cancer risk in the offspring [19,22]. In our study, leptin levels were slightly but nonsignificantly higher in pregnant rats exposed to the HF lard diet. These results are unexpected because reduction in breast cancer risk was observed in the female offspring of dams consuming the HF diet during either pregnancy or pregnancy and lactation. The role of higher leptin levels in utero on later breast cancer risk should be investigated deeper.
Progesterone increases cell proliferation and morphogenesis of the mammary luminal epithelium and promotes mammary tumorigenesis [52]. In the present study, female offspring of dams that consumed the HF lard diet during gestation showed nonsignificantly lower levels of progesterone than the control offspring. It has been suggested that progesterone-induced tumorigenesis involves expansion of the adult mammary stem cell population [53] through stimulation of RANKL expression in luminal cells [52]. RANKL is released to the extracellular space and binds to the RANK receptors in the stem cells, leading to activation of the downstreamIKK/IB/NFκB/cyclin D1 signaling pathway to stimulate proliferation [54]. Interestingly, in our study, slightly decreased progesterone levels were accompanied by decreases in the Rank/Rankl ratio and NFκB p65 subunit expression, suggesting that this pathway is suppressed and may be associated with reduced mammary tumorigenesis in the offspring of dams fed the HF lard diet during gestation.
Global patterns of histone modifications change during early stages of development and are associated with cellular processes [28] that mediate development and differentiation of the mammary gland [55]. In the present study, maternal consumption of the HF lard diet only during gestation increased H3K9me3 levels in offspring's mammary tissue, but the functional significance of this histone modification in modulating breast cancer risk remains to be elucidated. Increased levels of this histone marker cause transcriptional repression [56] and are associated with inhibition of the tumorigenic potential of MCF-7 breast cancer cells [57]. However, higher levels of H3K9me3 are found in breast cancer patients' serum [58], and increased expression of H3K9me3 promotes migration and invasion of breast cancer cells [59]. It remains to be studied whether some oncogenes are silenced by histone modifications in the mammary glands of rats exposed to HF lard diet in utero and during nursing. Although we did not observe differences in global DNA methylation, it is important in future studies to investigate possible changes in DNA methylation levels of oncogenes and tumor suppressor genes' promoter regions.
In conclusion, our data reinforce earlier findings that maternal diet during pregnancy can determine the susceptibility of offspring to the development of breast cancer in adult life [48]. We found that female offspring of rat dams consuming high levels of lard during pregnancy were at a reduced risk of developing DMBA-induced mammary tumors. It is important to confirm in future studies if the protective effects apply to other breast cancer models as well as genetically modified mouse models. Because other studies in rodents have reported that maternal consumption of diets high in saturated fat led to increased incidence of cardiovascular and metabolic dysfunctions in the offspring [60,38,61], it is not advisable for a pregnant mother to consume high levels of lard to protect her daughter from developing breast cancer. Further studies should focus on identifying the optimal dietary fat combination and quantity that can be recommended for pregnant mothers to reduce disease risk in their offspring; such a diet likely involves high levels of MUFA, n-3 PUFA and some saturated fats, and less n-6 PUFA.
Supplementary Material
Acknowledgments
We would like to thank Mrs. Sandra de Jesus Santana and Rosangela Pavan Torres for technical support. This work was supported by grant 2011/23259-4 and scholarships 2010/11742-0 and 2012/03330-9, São Paulo State Research Foundation (FAPESP).
Footnotes
Financial support: This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; 2010/11742-0; 2011/23259-4; 2012/03330-9).
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jnutbio.2014.02.002.
References
- 1.World Health Organization (WHO) The global burden of disease: 2004 update 2008
- 2.Teegarden D, Romieu I, Lelièvre SA. Redefining the impact of nutrition on breast cancer incidence: is epigenetics involved? Nutr Res Rev. 2012;25(1):68–95. doi: 10.1017/S0954422411000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peto J. Cancer epidemiology in the last century and the next decade. Nature. 2001;411(6835):390–395. doi: 10.1038/35077256. [DOI] [PubMed] [Google Scholar]
- 4.American Institute for Cancer Research (AICR) The continuous update project, 2010 [Google Scholar]
- 5.Lubin F, Wax Y, Modan B. Role of fat, animal protein, and dietary fiber in breast cancer etiology: a case–control study. J Natl Cancer Inst. 1986;77(3):605–612. doi: 10.1093/jnci/77.3.605. [DOI] [PubMed] [Google Scholar]
- 6.Freedman LS, Clifford C, Messina M. Analysis of dietary fat, calories, body weight, and the development of mammary tumors in rats and mice: a review. Cancer Res. 1990;50(18):5710–5719. [PubMed] [Google Scholar]
- 7.Hulka BS. Dietary fat and breast cancer: case–control and cohort studies. Prev Med. 1989;18(2):180–193. doi: 10.1016/0091-7435(89)90065-0. [DOI] [PubMed] [Google Scholar]
- 8.Willett WC. Diet and cancer. Oncologist. 2000;5(5):393–404. doi: 10.1634/theoncologist.5-5-393. [DOI] [PubMed] [Google Scholar]
- 9.Cho E, Spiegelman D, Hunter DJ, Chen WY, Stampfer MJ, Colditz GA, Willett WC. Premenopausal fat intake and risk of breast cancer. J Natl Cancer Inst. 2003;95(14):1079–1085. doi: 10.1093/jnci/95.14.1079. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez CA, Riboli E. Diet and cancer prevention: contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Cancer. 2010;46(14):2555–2562. doi: 10.1016/j.ejca.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 11.Löf M, Sandin S, Lagiou P, Hilakivi-Clarke L, Trichopoulos D, Adami HO, Weiderpass E. Dietary fat and breast cancer risk in the Swedish women's lifestyle and health cohort. Br J Cancer. 2007;97(11):1570–1576. doi: 10.1038/sj.bjc.6604033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Key TJ, Appleby PN, Cairns BJ, Luben R, Dahm CC, Akbaraly T, et al. Dietary fat and breast cancer: comparison of results from food diaries and food-frequency questionnaires in the UK Dietary Cohort Consortium. Am J Clin Nutr. 2011;94(4):1043–1052. doi: 10.3945/ajcn.111.015735. [DOI] [PubMed] [Google Scholar]
- 13.Park SY, Kolonel LN, Henderson BE, Wilkens LR. Dietary fat and breast cancer in postmenopausal women according to ethnicity and hormone receptor status: the Multiethnic Cohort Study. Cancer Prev Res (Phila) 2012;5(2):216–228. doi: 10.1158/1940-6207.CAPR-11-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin LJ, Li Q, Melnichouk O, Greenberg C, Minkin S, Hislop G, Boyd NF. A randomized trial of dietary intervention for breast cancer prevention. Cancer Res. 2011;71(1):123–133. doi: 10.1158/0008-5472.CAN-10-1436. [DOI] [PubMed] [Google Scholar]
- 15.Langley-Evans SC, McMullen S. Developmental origins of adult disease. Med Princ Pract. 2010;19(2):87–98. doi: 10.1159/000273066. [DOI] [PubMed] [Google Scholar]
- 16.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 17.Hilakivi-Clarke L, de Assis S. Fetal origins of breast cancer. Trends Endocrinol Metab. 2006;17(9):340–348. doi: 10.1016/j.tem.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Trichopoulos D. Hypothesis: does breast cancer originate in utero? Lancet. 1990;335(8695):939–940. doi: 10.1016/0140-6736(90)91000-z. [DOI] [PubMed] [Google Scholar]
- 19.De Assis S, Hilakivi-Clarke L. Timing of dietary estrogenic exposures and breast cancer risk. Ann N Y Acad Sci. 2006;1089:14–35. doi: 10.1196/annals.1386.039. [DOI] [PubMed] [Google Scholar]
- 20.MacLennan M, Ma DW. Role of dietary fatty acids in mammary gland development and breast cancer. Breast Cancer Res. 2010;12(5):211. doi: 10.1186/bcr2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilakivi-Clarke L, Clarke R, Onojafe I, Raygada M, Cho E, Lippman M. A maternal diet high in n-6 polyunsaturated fats alters mammary gland development, puberty onset, and breast cancer risk among female rat offspring. Proc Natl Acad Sci U S A. 1997;94(17):9372–9377. doi: 10.1073/pnas.94.17.9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Assis S, Khan G, Hilakivi-Clarke L. High birth weight increases mammary tumorigenesis in rats. Int J Cancer. 2006;119(7):1537–1546. doi: 10.1002/ijc.21936. [DOI] [PubMed] [Google Scholar]
- 23.Olivo SE, Hilakivi-Clarke L. Opposing effects of prepubertal low- and high-fat n-3 polyunsaturated fatty acid diets on rat mammary tumorigenesis. Carcinogenesis. 2005;26(9):1563–1572. doi: 10.1093/carcin/bgi118. [DOI] [PubMed] [Google Scholar]
- 24.Ion G, Akinsete JA, Hardman WE. Maternal consumption of canola oil suppressed mammary gland tumorigenesis in C3(1) TAg mice offspring. BMC Cancer. 2010;10:81. doi: 10.1186/1471-2407-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kearney J. Food consumption trends and drivers. Philos Trans R Soc Lond B Biol Sci. 2010;365(1554):2793–2807. doi: 10.1098/rstb.2010.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gluckman PD, Hanson MA, Beedle AS. Non-genomic transgenerational inheritance of disease risk. Bioessays. 2007;29(2):145–154. doi: 10.1002/bies.20522. [DOI] [PubMed] [Google Scholar]
- 27.Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27(5):358–368. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vastenhouw NL, Schier AF. Bivalent histone modifications in early embryogenesis. Curr Opin Cell Biol. 2012;24(3):374–386. doi: 10.1016/j.ceb.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123(11):1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, et al. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci. 2010;30(10):3826–3830. doi: 10.1523/JNEUROSCI.5560-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pang J, Choi Y, Park T. Ilex paraguariensis extract ameliorates obesity induced by high-fat diet: potential role of AMPK in the visceral adipose tissue. Arch Biochem Biophys. 2008;476(2):178–185. doi: 10.1016/j.abb.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 32.Association of Official Analytical Chemist (AOAC) Official methods of analysis, Arlington, official method. 2002;41:20A–24A. n996.06. [Google Scholar]
- 33.Hartman L, Lago RC. Rapid preparation of fatty acid methyl esters from lipids. Lab Pract. 1973;22(6):475–476. [PubMed] [Google Scholar]
- 34.Russo J, Russo IH. Atlas and histologic classification of tumors of the rat mammary gland. J Mammary Gland Biol Neoplasia. 2000;5(2):187–200. doi: 10.1023/a:1026443305758. [DOI] [PubMed] [Google Scholar]
- 35.De Assis S, Warri A, Cruz MI, Hilakivi-Clarke L. Changes in mammary gland morphology and breast cancer risk in rats. J Vis Exp. 2010;(44) doi: 10.3791/2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russo J, Russo IH. Biological and molecular bases of mammary carcinogenesis. Lab Invest. 1987;57(2):112–137. [PubMed] [Google Scholar]
- 37.Wang XY, Yin Y, Yuan H, Sakamaki T, Okano H, Glazer RI. Musashi1 modulates mammary progenitor cell expansion through proliferin-mediated activation of the Wnt and Notch pathways. Mol Cell Biol. 2008;28(11):3589–3599. doi: 10.1128/MCB.00040-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armitage JA, Taylor PD, Poston L. Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. J Physiol. 2005;565(Pt 1):3–8. doi: 10.1113/jphysiol.2004.079756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Assis S, Warri A, Cruz MI, Laja O, Tian Y, Zhang B, Wang Y, Huang TH, Hilakivi-Clarke L. High-fat or ethinyl-oestradiol intake during pregnancy increases mammary cancer risk in several generations of offspring. Nat Commun. 2012;3:1053. doi: 10.1038/ncomms2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saadatian-Elahi M, Norat T, Goudable J, Riboli E. Biomarkers of dietary fatty acid intake and the risk of breast cancer: a meta-analysis. Int J Cancer. 2004;111(4):584–591. doi: 10.1002/ijc.20284. [DOI] [PubMed] [Google Scholar]
- 41.Sczaniecka AK, Brasky TM, Lampe JW, Patterson RE, White E. Dietary intake of specific fatty acids and breast cancer risk among postmenopausal women in the VITAL cohort. Nutr Cancer. 2012;64(8):1131–1142. doi: 10.1080/01635581.2012.718033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hardy S, Langelier Y, Prentki M. Oleate activates phosphatidylinositol 3-kinase and promotes proliferation and reduces apoptosis of MDA-MB-231 breast cancer cells, whereas palmitate has opposite effects. Cancer Res. 2000;60(22):6353–6358. [PubMed] [Google Scholar]
- 43.Kourtidis A, Srinivasaiah R, Carkner RD, Brosnan MJ, Conklin DS. Peroxisome proliferator-activated receptor-gamma protects ERBB2-positive breast cancer cells from palmitate toxicity. Breast Cancer Res. 2009;11(2):R16. doi: 10.1186/bcr2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wickramasinghe NS, Jo H, McDonald JM, Hardy RW. Stearate inhibition of breast cancer cell proliferation. A mechanism involving epidermal growth factor receptor and G-proteins. Am J Pathol. 1996 Mar;148(3):987–995. [PMC free article] [PubMed] [Google Scholar]
- 45.Li C, Zhao X, Toline EC, Siegal GP, Evans LM, Ibrahim-Hashim A, Desmond RA, Hardy RW. Prevention of carcinogenesis and inhibition of breast cancer tumor burden by dietary stearate. Carcinogenesis. 2011;32(8):1251–1258. doi: 10.1093/carcin/bgr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medvedovic M, Gear R, Freudenberg JM, Schneider J, Bornschein R, Yan M, et al. Influence of fatty acid diets on gene expression in rat mammary epithelial cells. Physiol Genomics. 2009;38(1):80–88. doi: 10.1152/physiolgenomics.00007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stark AH, Kossoy G, Zusman I, Yarden G, Madar Z. Olive oil consumption during pregnancy and lactation in rats influences mammary cancer development in female offspring. Nutr Cancer. 2003;46(1):59–65. doi: 10.1207/S15327914NC4601_08. [DOI] [PubMed] [Google Scholar]
- 48.Hilakivi-Clarke L. Nutritional modulation of terminal end buds: its relevance to breast cancer prevention. Curr Cancer Drug Targets. 2007;7(5):465–474. doi: 10.2174/156800907781386641. [DOI] [PubMed] [Google Scholar]
- 49.Williams JM, Daniel CW. Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev Biol. 1983;97(2):274–290. doi: 10.1016/0012-1606(83)90086-6. [DOI] [PubMed] [Google Scholar]
- 50.Abukhdeir AM, Park BH. P21 and p27: roles in carcinogenesis and drug resistance. Expert Rev Mol Med. 2008;10:e19. doi: 10.1017/S1462399408000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dressler AC, Hudelist G, Fink-Retter A, Gschwantler-Kaulich D, Pfeiler G, Rosner M, et al. Tuberin and p27 expression in breast cancer patients with or without BRCA germline mutations. J Cancer Res Clin Oncol. 2013;139(8):1349–1355. doi: 10.1007/s00432-013-1443-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obr AE, Edwards DP. The biology of progesterone receptor in the normal mammary gland and in breast cancer. Mol Cell Endocrinol. 2012;357(1–2):4–17. doi: 10.1016/j.mce.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465(7299):803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- 54.Schramek D, Leibbrandt A, Sigl V, Kenner L, Pospisilik JA, Lee HJ, et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature. 2010;468(7320):98–102. doi: 10.1038/nature09387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rijnkels M, Kabotyanski E, Montazer-Torbati MB, Hue Beauvais C, Vassetzky Y, Rosen JM, Devinoy E. The epigenetic landscape of mammary gland development and functional differentiation. J Mammary Gland Biol Neoplasia. 2010;15(1):85–100. doi: 10.1007/s10911-010-9170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Füllgrabe J, Kavanagh E, Joseph B. Histone onco-modifications. Oncogene. 2011;30(31):3391–3403. doi: 10.1038/onc.2011.121. [DOI] [PubMed] [Google Scholar]
- 57.Khanal P, Kim G, Lim SC, Yun HJ, Lee KY, Choi HK, Choi HS. Prolyl isomerase Pin1 negatively regulates the stability of SUV39H1 to promote tumorigenesis in breast cancer. FASEB J. 2013;27(11):4606–4618. doi: 10.1096/fj.13-236851. [DOI] [PubMed] [Google Scholar]
- 58.Leszinski G, Gezer U, Siegele B, Stoetzer O, Holdenrieder S. Relevance of histone marks H3K9me3 and H4K20me3 in cancer. Anticancer Res. 2012;32(5):2199–2205. [PubMed] [Google Scholar]
- 59.Yokoyama Y, Hieda M, Nishioka Y, Matsumoto A, Higashi S, Kimura H, et al. Cancer-associated upregulation of histone H3lysine 9 trimethylation promotes cell motility in vitro and drives tumor formation in vivo. Cancer Sci. 2013;104(7):889–895. doi: 10.1111/cas.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taylor PD, McConnell J, Khan IY, Holemans K, Lawrence KM, Asare-Anane H, et al. Impaired glucose homeostasis and mitochondrial abnormalities in offspring of rats fed a fat-rich diet in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R134–R139. doi: 10.1152/ajpregu.00355.2004. [DOI] [PubMed] [Google Scholar]
- 61.Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, et al. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension. 2003;41(1):168–175. doi: 10.1161/01.hyp.0000047511.97879.fc. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.