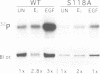
Abstract
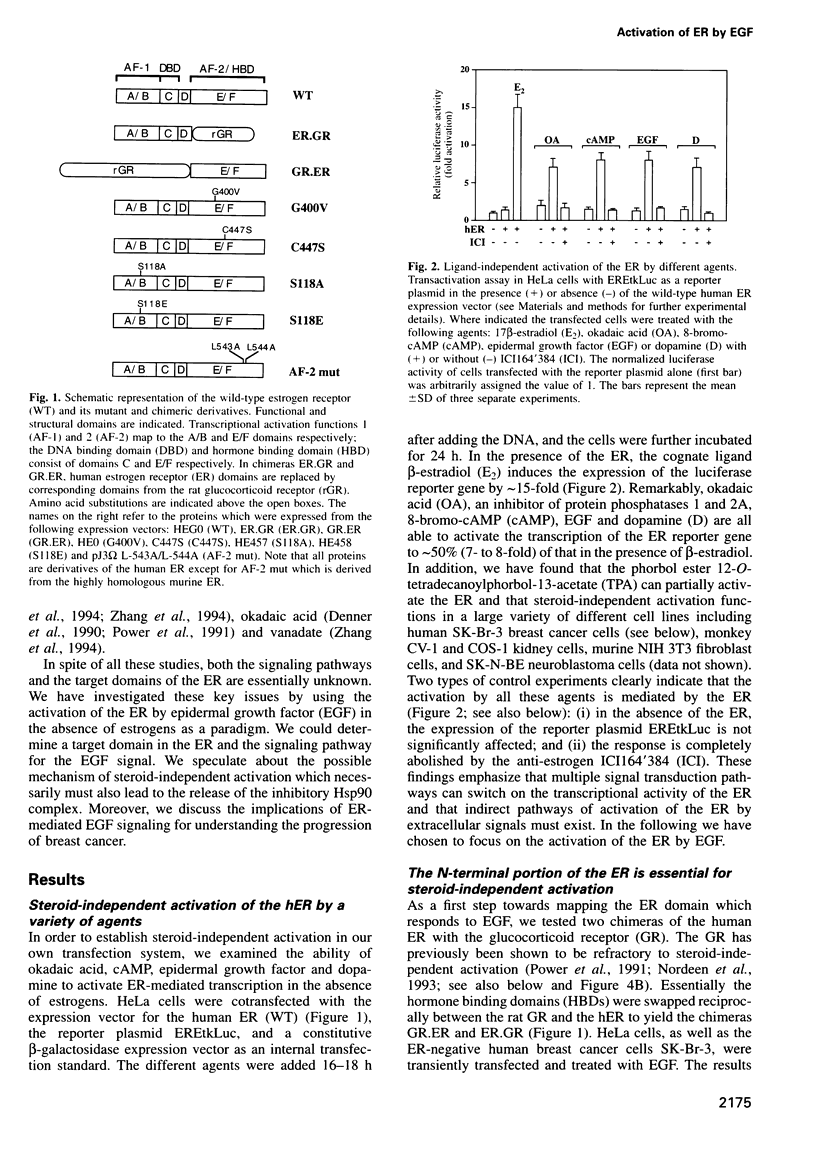
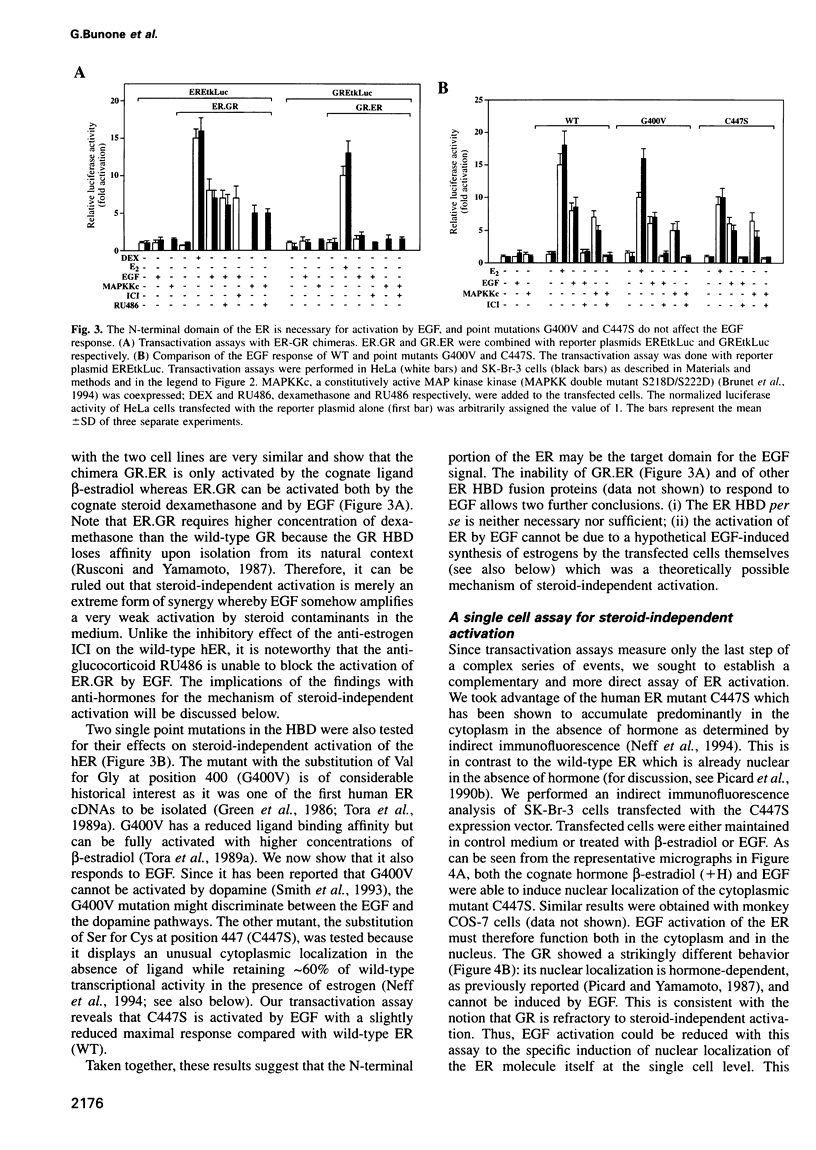
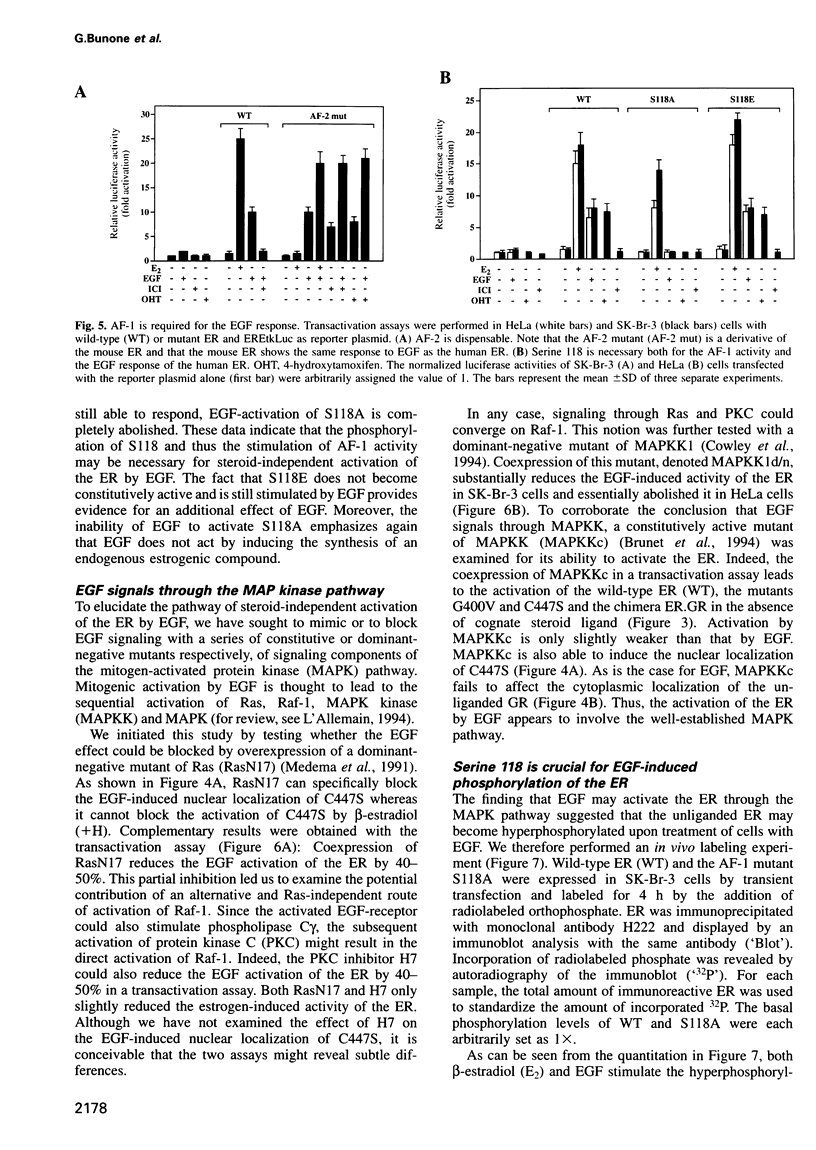
The estrogen receptor (ER) can be activated as a transcription factor either by binding of cognate estrogenic ligand or, indirectly, by a variety of other extracellular signals. As a first step towards elucidating the mechanism of 'steroid-independent activation' of the ER by the epidermal growth factor (EGF), we have mapped the ER target domain and determined the signaling pathway. We show that the N-terminal transcriptional activation function AF-1, but not the C-terminal AF-2, is necessary for the EGF response. Both the EGF-induced hyperphosphorylation and the transcriptional activation of the unliganded ER depend on a phosphorylatable serine residue at position 118. However, its phosphorylation is not sufficient and, hence, there must be other target domains or proteins which fulfill an additional requirement for EGF signaling through the ER. Using dominant-negative Ras and MAP kinase kinase (MAPK kinase) and constitutively active MAPK kinase mutants, we show that EGF activates the ER by signaling through the MAPK pathway suggesting that MAPK directly phosphorylates the critical serine 118. Our results also imply that the steroid-independent activation of a variety of ER mutants, which arise during the malignant progression of breast tumors, may contribute to tamoxifen resistance.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S., Metzger D., Bornert J. M., Chambon P. Modulation of transcriptional activation by ligand-dependent phosphorylation of the human oestrogen receptor A/B region. EMBO J. 1993 Mar;12(3):1153–1160. doi: 10.1002/j.1460-2075.1993.tb05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S. F., Obourn J. D., Jaffe H., Notides A. C. Phosphorylation of the human estrogen receptor on tyrosine 537 in vivo and by src family tyrosine kinases in vitro. Mol Endocrinol. 1995 Jan;9(1):24–33. doi: 10.1210/mend.9.1.7539106. [DOI] [PubMed] [Google Scholar]
- Arnold S. F., Obourn J. D., Jaffe H., Notides A. C. Serine 167 is the major estradiol-induced phosphorylation site on the human estrogen receptor. Mol Endocrinol. 1994 Sep;8(9):1208–1214. doi: 10.1210/mend.8.9.7838153. [DOI] [PubMed] [Google Scholar]
- Arnold S. F., Obourn J. D., Yudt M. R., Carter T. H., Notides A. C. In vivo and in vitro phosphorylation of the human estrogen receptor. J Steroid Biochem Mol Biol. 1995 Feb;52(2):159–171. doi: 10.1016/0960-0760(94)00166-j. [DOI] [PubMed] [Google Scholar]
- Aronica S. M., Katzenellenbogen B. S. Stimulation of estrogen receptor-mediated transcription and alteration in the phosphorylation state of the rat uterine estrogen receptor by estrogen, cyclic adenosine monophosphate, and insulin-like growth factor-I. Mol Endocrinol. 1993 Jun;7(6):743–752. doi: 10.1210/mend.7.6.7689695. [DOI] [PubMed] [Google Scholar]
- Beck C. A., Weigel N. L., Moyer M. L., Nordeen S. K., Edwards D. P. The progesterone antagonist RU486 acquires agonist activity upon stimulation of cAMP signaling pathways. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4441–4445. doi: 10.1073/pnas.90.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M., Metzger D., Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 1990 Sep;9(9):2811–2818. doi: 10.1002/j.1460-2075.1990.tb07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohen S. P., Yamamoto K. R. Isolation of Hsp90 mutants by screening for decreased steroid receptor function. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11424–11428. doi: 10.1073/pnas.90.23.11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick E. H., Dalman F. C., Sanchez E. R., Pratt W. B. Evidence that the 90-kDa heat shock protein is necessary for the steroid binding conformation of the L cell glucocorticoid receptor. J Biol Chem. 1989 Mar 25;264(9):4992–4997. [PubMed] [Google Scholar]
- Brunet A., Pagès G., Pouysségur J. Constitutively active mutants of MAP kinase kinase (MEK1) induce growth factor-relaxation and oncogenicity when expressed in fibroblasts. Oncogene. 1994 Nov;9(11):3379–3387. [PubMed] [Google Scholar]
- Cano E., Mahadevan L. C. Parallel signal processing among mammalian MAPKs. Trends Biochem Sci. 1995 Mar;20(3):117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- Chauchereau A., Cohen-Solal K., Jolivet A., Bailly A., Milgrom E. Phosphorylation sites in ligand-induced and ligand-independent activation of the progesterone receptor. Biochemistry. 1994 Nov 15;33(45):13295–13303. doi: 10.1021/bi00249a016. [DOI] [PubMed] [Google Scholar]
- Cook S. J., McCormick F. Inhibition by cAMP of Ras-dependent activation of Raf. Science. 1993 Nov 12;262(5136):1069–1072. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- Cowley S., Paterson H., Kemp P., Marshall C. J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994 Jun 17;77(6):841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Danielian P. S., White R., Lees J. A., Parker M. G. Identification of a conserved region required for hormone dependent transcriptional activation by steroid hormone receptors. EMBO J. 1992 Mar;11(3):1025–1033. doi: 10.1002/j.1460-2075.1992.tb05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denner L. A., Weigel N. L., Maxwell B. L., Schrader W. T., O'Malley B. W. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science. 1990 Dec 21;250(4988):1740–1743. doi: 10.1126/science.2176746. [DOI] [PubMed] [Google Scholar]
- Faure M., Bourne H. R. Differential effects on cAMP on the MAP kinase cascade: evidence for a cAMP-insensitive step that can bypass Raf-1. Mol Biol Cell. 1995 Aug;6(8):1025–1035. doi: 10.1091/mbc.6.8.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure M., Voyno-Yasenetskaya T. A., Bourne H. R. cAMP and beta gamma subunits of heterotrimeric G proteins stimulate the mitogen-activated protein kinase pathway in COS-7 cells. J Biol Chem. 1994 Mar 18;269(11):7851–7854. [PubMed] [Google Scholar]
- Frödin M., Peraldi P., Van Obberghen E. Cyclic AMP activates the mitogen-activated protein kinase cascade in PC12 cells. J Biol Chem. 1994 Feb 25;269(8):6207–6214. [PubMed] [Google Scholar]
- Fujimoto N., Katzenellenbogen B. S. Alteration in the agonist/antagonist balance of antiestrogens by activation of protein kinase A signaling pathways in breast cancer cells: antiestrogen selectivity and promoter dependence. Mol Endocrinol. 1994 Mar;8(3):296–304. doi: 10.1210/mend.8.3.7517003. [DOI] [PubMed] [Google Scholar]
- Fuqua S. A., Chamness G. C., McGuire W. L. Estrogen receptor mutations in breast cancer. J Cell Biochem. 1993 Feb;51(2):135–139. doi: 10.1002/jcb.240510204. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Raden D. L., Davis R. J. Identification of substrate recognition determinants for human ERK1 and ERK2 protein kinases. J Biol Chem. 1991 Nov 25;266(33):22159–22163. [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H. Transcription activation by estrogen and progesterone receptors. Annu Rev Genet. 1991;25:89–123. doi: 10.1146/annurev.ge.25.120191.000513. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H. Transcription activation by nuclear receptors. J Recept Res. 1993;13(1-4):667–691. doi: 10.3109/10799899309073686. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B. How do breast cancers become hormone resistant? J Steroid Biochem Mol Biol. 1994 Jun;49(4-6):295–302. doi: 10.1016/0960-0760(94)90271-2. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B. When tamoxifen turns bad. Endocrinology. 1995 Mar;136(3):821–823. doi: 10.1210/endo.136.3.7867589. [DOI] [PubMed] [Google Scholar]
- Häfner S., Adler H. S., Mischak H., Janosch P., Heidecker G., Wolfman A., Pippig S., Lohse M., Ueffing M., Kolch W. Mechanism of inhibition of Raf-1 by protein kinase A. Mol Cell Biol. 1994 Oct;14(10):6696–6703. doi: 10.1128/mcb.14.10.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignar-Trowbridge D. M., Teng C. T., Ross K. A., Parker M. G., Korach K. S., McLachlan J. A. Peptide growth factors elicit estrogen receptor-dependent transcriptional activation of an estrogen-responsive element. Mol Endocrinol. 1993 Aug;7(8):992–998. doi: 10.1210/mend.7.8.8232319. [DOI] [PubMed] [Google Scholar]
- Ince B. A., Montano M. M., Katzenellenbogen B. S. Activation of transcriptionally inactive human estrogen receptors by cyclic adenosine 3',5'-monophosphate and ligands including antiestrogens. Mol Endocrinol. 1994 Oct;8(10):1397–1406. doi: 10.1210/mend.8.10.7531820. [DOI] [PubMed] [Google Scholar]
- Joel P. B., Traish A. M., Lannigan D. A. Estradiol and phorbol ester cause phosphorylation of serine 118 in the human estrogen receptor. Mol Endocrinol. 1995 Aug;9(8):1041–1052. doi: 10.1210/mend.9.8.7476978. [DOI] [PubMed] [Google Scholar]
- Kang K. I., Devin J., Cadepond F., Jibard N., Guiochon-Mantel A., Baulieu E. E., Catelli M. G. In vivo functional protein-protein interaction: nuclear targeted hsp90 shifts cytoplasmic steroid receptor mutants into the nucleus. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):340–344. doi: 10.1073/pnas.91.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S., Endoh H., Masuhiro Y., Kitamoto T., Uchiyama S., Sasaki H., Masushige S., Gotoh Y., Nishida E., Kawashima H. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995 Dec 1;270(5241):1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- Kushner P. J., Baxter J. D., Duncan K. G., Lopez G. N., Schaufele F., Uht R. M., Webb P., West B. L. Eukaryotic regulatory elements lurking in plasmid DNA: the activator protein-1 site in pUC. Mol Endocrinol. 1994 Apr;8(4):405–407. doi: 10.1210/mend.8.4.8052261. [DOI] [PubMed] [Google Scholar]
- L'Allemain G. Deciphering the MAP kinase pathway. Prog Growth Factor Res. 1994;5(3):291–334. doi: 10.1016/0955-2235(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Le Goff P., Montano M. M., Schodin D. J., Katzenellenbogen B. S. Phosphorylation of the human estrogen receptor. Identification of hormone-regulated sites and examination of their influence on transcriptional activity. J Biol Chem. 1994 Feb 11;269(6):4458–4466. [PubMed] [Google Scholar]
- Ma Z. Q., Santagati S., Patrone C., Pollio G., Vegeto E., Maggi A. Insulin-like growth factors activate estrogen receptor to control the growth and differentiation of the human neuroblastoma cell line SK-ER3. Mol Endocrinol. 1994 Jul;8(7):910–918. doi: 10.1210/mend.8.7.7984152. [DOI] [PubMed] [Google Scholar]
- Mahfoudi A., Roulet E., Dauvois S., Parker M. G., Wahli W. Specific mutations in the estrogen receptor change the properties of antiestrogens to full agonists. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4206–4210. doi: 10.1073/pnas.92.10.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C. J. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev. 1994 Feb;4(1):82–89. doi: 10.1016/0959-437x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- McGuire W. L., Chamness G. C., Fuqua S. A. Estrogen receptor variants in clinical breast cancer. Mol Endocrinol. 1991 Nov;5(11):1571–1577. doi: 10.1210/mend-5-11-1571. [DOI] [PubMed] [Google Scholar]
- Medema R. H., Wubbolts R., Bos J. L. Two dominant inhibitory mutants of p21ras interfere with insulin-induced gene expression. Mol Cell Biol. 1991 Dec;11(12):5963–5967. doi: 10.1128/mcb.11.12.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M. E., Pornon A., Ji J. W., Bocquel M. T., Chambon P., Gronemeyer H. Agonistic and antagonistic activities of RU486 on the functions of the human progesterone receptor. EMBO J. 1990 Dec;9(12):3923–3932. doi: 10.1002/j.1460-2075.1990.tb07613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksicek R. J. Steroid receptor variants and their potential role in cancer. Semin Cancer Biol. 1994 Oct;5(5):369–379. [PubMed] [Google Scholar]
- Miner J. N., Yamamoto K. R. Regulatory crosstalk at composite response elements. Trends Biochem Sci. 1991 Nov;16(11):423–426. doi: 10.1016/0968-0004(91)90168-u. [DOI] [PubMed] [Google Scholar]
- Nathan D. F., Lindquist S. Mutational analysis of Hsp90 function: interactions with a steroid receptor and a protein kinase. Mol Cell Biol. 1995 Jul;15(7):3917–3925. doi: 10.1128/mcb.15.7.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff S., Sadowski C., Miksicek R. J. Mutational analysis of cysteine residues within the hormone-binding domain of the human estrogen receptor identifies mutants that are defective in both DNA-binding and subcellular distribution. Mol Endocrinol. 1994 Sep;8(9):1215–1223. doi: 10.1210/mend.8.9.7838154. [DOI] [PubMed] [Google Scholar]
- Newton C. J., Buric R., Trapp T., Brockmeier S., Pagotto U., Stalla G. K. The unliganded estrogen receptor (ER) transduces growth factor signals. J Steroid Biochem Mol Biol. 1994 Apr;48(5-6):481–486. doi: 10.1016/0960-0760(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Nordeen S. K., Bona B. J., Moyer M. L. Latent agonist activity of the steroid antagonist, RU486, is unmasked in cells treated with activators of protein kinase A. Mol Endocrinol. 1993 Jun;7(6):731–742. doi: 10.1210/mend.7.6.8395651. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Schrader W. T., Mani S., Smith C., Weigel N. L., Conneely O. M., Clark J. H. An alternative ligand-independent pathway for activation of steroid receptors. Recent Prog Horm Res. 1995;50:333–347. doi: 10.1016/b978-0-12-571150-0.50020-2. [DOI] [PubMed] [Google Scholar]
- Okret S., Wikström A. C., Wrange O., Andersson B., Gustafsson J. A. Monoclonal antibodies against the rat liver glucocorticoid receptor. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1609–1613. doi: 10.1073/pnas.81.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne C. K., Fuqua S. A. Mechanisms of tamoxifen resistance. Breast Cancer Res Treat. 1994;32(1):49–55. doi: 10.1007/BF00666205. [DOI] [PubMed] [Google Scholar]
- Pellegrini R., Mariotti A., Tagliabue E., Bressan R., Bunone G., Coradini D., Della Valle G., Formelli F., Cleris L., Radice P. Modulation of markers associated with tumor aggressiveness in human breast cancer cell lines by N-(4-hydroxyphenyl) retinamide. Cell Growth Differ. 1995 Jul;6(7):863–869. [PubMed] [Google Scholar]
- Picard D., Khursheed B., Garabedian M. J., Fortin M. G., Lindquist S., Yamamoto K. R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990 Nov 8;348(6297):166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Picard D., Kumar V., Chambon P., Yamamoto K. R. Signal transduction by steroid hormones: nuclear localization is differentially regulated in estrogen and glucocorticoid receptors. Cell Regul. 1990 Feb;1(3):291–299. doi: 10.1091/mbc.1.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D., Salser S. J., Yamamoto K. R. A movable and regulable inactivation function within the steroid binding domain of the glucocorticoid receptor. Cell. 1988 Sep 23;54(7):1073–1080. doi: 10.1016/0092-8674(88)90122-5. [DOI] [PubMed] [Google Scholar]
- Picard D. Steroid-binding domains for regulating the functions of heterologous proteins in cis. Trends Cell Biol. 1993 Aug;3(8):278–280. doi: 10.1016/0962-8924(93)90057-8. [DOI] [PubMed] [Google Scholar]
- Picard D., Yamamoto K. R. Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J. 1987 Nov;6(11):3333–3340. doi: 10.1002/j.1460-2075.1987.tb02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras R. J., Arboleda J., Reese D. M., Wongvipat N., Pegram M. D., Ramos L., Gorman C. M., Parker M. G., Sliwkowski M. X., Slamon D. J. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene. 1995 Jun 15;10(12):2435–2446. [PubMed] [Google Scholar]
- Power R. F., Conneely O. M., O'Malley B. W. New insights into activation of the steroid hormone receptor superfamily. Trends Pharmacol Sci. 1992 Aug;13(8):318–323. doi: 10.1016/0165-6147(92)90099-r. [DOI] [PubMed] [Google Scholar]
- Power R. F., Mani S. K., Codina J., Conneely O. M., O'Malley B. W. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science. 1991 Dec 13;254(5038):1636–1639. doi: 10.1126/science.1749936. [DOI] [PubMed] [Google Scholar]
- Pratt W. B. Interaction of hsp90 with steroid receptors: organizing some diverse observations and presenting the newest concepts. Mol Cell Endocrinol. 1990 Nov 12;74(1):C69–C76. doi: 10.1016/0303-7207(90)90198-h. [DOI] [PubMed] [Google Scholar]
- Pratt W. B. The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor. J Biol Chem. 1993 Oct 15;268(29):21455–21458. [PubMed] [Google Scholar]
- Rafestin-Oblin M. E., Couette B., Radanyi C., Lombes M., Baulieu E. E. Mineralocorticosteroid receptor of the chick intestine. Oligomeric structure and transformation. J Biol Chem. 1989 Jun 5;264(16):9304–9309. [PubMed] [Google Scholar]
- Rusconi S., Yamamoto K. R. Functional dissection of the hormone and DNA binding activities of the glucocorticoid receptor. EMBO J. 1987 May;6(5):1309–1315. doi: 10.1002/j.1460-2075.1987.tb02369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatcioglu F., Claret F. X., Karin M. Negative transcriptional regulation by nuclear receptors. Semin Cancer Biol. 1994 Oct;5(5):347–359. [PubMed] [Google Scholar]
- Sakai D. D., Helms S., Carlstedt-Duke J., Gustafsson J. A., Rottman F. M., Yamamoto K. R. Hormone-mediated repression: a negative glucocorticoid response element from the bovine prolactin gene. Genes Dev. 1988 Sep;2(9):1144–1154. doi: 10.1101/gad.2.9.1144. [DOI] [PubMed] [Google Scholar]
- Sartorius C. A., Groshong S. D., Miller L. A., Powell R. L., Tung L., Takimoto G. S., Horwitz K. B. New T47D breast cancer cell lines for the independent study of progesterone B- and A-receptors: only antiprogestin-occupied B-receptors are switched to transcriptional agonists by cAMP. Cancer Res. 1994 Jul 15;54(14):3868–3877. [PubMed] [Google Scholar]
- Sartorius C. A., Tung L., Takimoto G. S., Horwitz K. B. Antagonist-occupied human progesterone receptors bound to DNA are functionally switched to transcriptional agonists by cAMP. J Biol Chem. 1993 May 5;268(13):9262–9266. [PubMed] [Google Scholar]
- Schramm K., Niehof M., Radziwill G., Rommel C., Moelling K. Phosphorylation of c-Raf-1 by protein kinase A interferes with activation. Biochem Biophys Res Commun. 1994 Jun 15;201(2):740–747. doi: 10.1006/bbrc.1994.1763. [DOI] [PubMed] [Google Scholar]
- Segnitz B., Gehring U. Subunit structure of the nonactivated human estrogen receptor. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2179–2183. doi: 10.1073/pnas.92.6.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Conneely O. M., O'Malley B. W. Modulation of the ligand-independent activation of the human estrogen receptor by hormone and antihormone. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6120–6124. doi: 10.1073/pnas.90.13.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. F. Dynamics of heat shock protein 90-progesterone receptor binding and the disactivation loop model for steroid receptor complexes. Mol Endocrinol. 1993 Nov;7(11):1418–1429. doi: 10.1210/mend.7.11.7906860. [DOI] [PubMed] [Google Scholar]
- Smith D. F., Toft D. O. Steroid receptors and their associated proteins. Mol Endocrinol. 1993 Jan;7(1):4–11. doi: 10.1210/mend.7.1.8446107. [DOI] [PubMed] [Google Scholar]
- Tora L., Mullick A., Metzger D., Ponglikitmongkol M., Park I., Chambon P. The cloned human oestrogen receptor contains a mutation which alters its hormone binding properties. EMBO J. 1989 Jul;8(7):1981–1986. doi: 10.1002/j.1460-2075.1989.tb03604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M. J., O'Malley B. W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Turgeon J. L., Waring D. W. Activation of the progesterone receptor by the gonadotropin-releasing hormone self-priming signaling pathway. Mol Endocrinol. 1994 Jul;8(7):860–869. doi: 10.1210/mend.8.7.7984148. [DOI] [PubMed] [Google Scholar]
- Tzukerman M. T., Esty A., Santiso-Mere D., Danielian P., Parker M. G., Stein R. B., Pike J. W., McDonnell D. P. Human estrogen receptor transactivational capacity is determined by both cellular and promoter context and mediated by two functionally distinct intramolecular regions. Mol Endocrinol. 1994 Jan;8(1):21–30. doi: 10.1210/mend.8.1.8152428. [DOI] [PubMed] [Google Scholar]
- Whitehurst C. E., Owaki H., Bruder J. T., Rapp U. R., Geppert T. D. The MEK kinase activity of the catalytic domain of RAF-1 is regulated independently of Ras binding in T cells. J Biol Chem. 1995 Mar 10;270(10):5594–5599. doi: 10.1074/jbc.270.10.5594. [DOI] [PubMed] [Google Scholar]
- Wu J., Dent P., Jelinek T., Wolfman A., Weber M. J., Sturgill T. W. Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3',5'-monophosphate. Science. 1993 Nov 12;262(5136):1065–1069. doi: 10.1126/science.7694366. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Godowski P. J., Picard D. Ligand-regulated nonspecific inactivation of receptor function: a versatile mechanism for signal transduction. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):803–811. doi: 10.1101/sqb.1988.053.01.091. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Bai W., Allgood V. E., Weigel N. L. Multiple signaling pathways activate the chicken progesterone receptor. Mol Endocrinol. 1994 May;8(5):577–584. doi: 10.1210/mend.8.5.8058067. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]