Abstract
Nitroimidazoles exhibit high microbicidal activity, but mutagenic, genotoxic and cytotoxic properties have been attributed to the presence of the nitro group. However, we synthesised nitroimidazoles with activity against the trypomastigotes of Trypanosoma cruzi, but that were not genotoxic. Herein, nitroimidazoles (11-19) bearing different substituent groups were investigated for their potential induction of genotoxicity (comet assay) and mutagenicity (Salmonella/Microsome assay) and the correlations of these effects with their trypanocidal effect and with megazol were investigated. The compounds were designed to analyse the role played by the position of the nitro group in the imidazole nucleus (C-4 or C-5) and the presence of oxidisable groups at N-1 as an anion receptor group and the role of a methyl group at C-2. Nitroimidazoles bearing NO2 at C-4 and CH3 at C-2 were not genotoxic compared to those bearing NO2 at C-5. However, when there was a CH3 at C-2, the position of the NO2 group had no influence on the genotoxic activity. Fluorinated compounds exhibited higher genotoxicity regardless of the presence of CH3 at C-2 or NO2 at C-4 or C-5. However, in compounds 11 (2-CH3; 4-NO2; N-CH2OHCH2Cl) and 12 (2-CH3; 4-NO2; N-CH2OHCH2F), the fluorine atom had no influence on genotoxicity. This study contributes to the future search for new and safer prototypes and provide.
Keywords: nitroimidazoles, genotoxicity, mutagenicity, trypanocidal activity
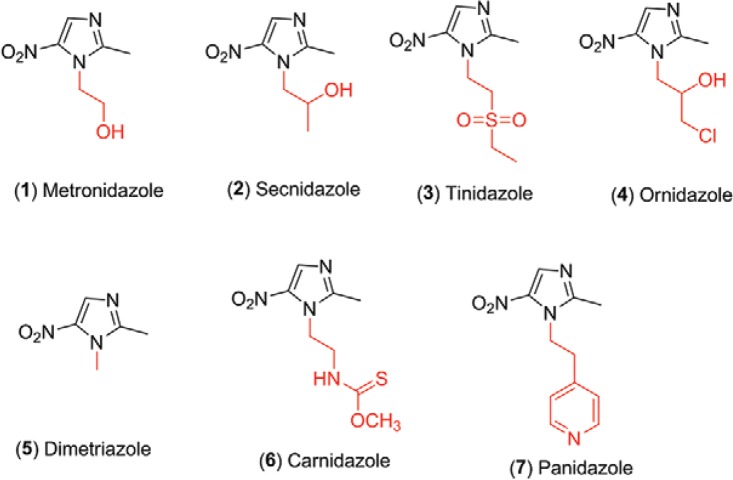
The class of nitroimidazoles includes compounds that are important antiparasitic agents, which have a broad spectrum of action and high biological activity. For instance, metronidazole (1), secnidazole (2), tinidazole (3), ornidazole (4), dimetridazole (5), carnidazole (6) and panidazole (7) (Fig. 1) are some examples of drugs currently used to treat infections of anaerobic Bacteroides sp. and protozoans, such as Trichomonas sp., Entamoeba sp., Giardia sp. and Histomonas sp. (Yakugaku 1971, William et al. 1975, Buschini et al. 2007, Mital 2009, Valdez et al. 2009). In addition, nitroimidazoles have other interesting properties, including antitubercular and antifungal activities, in the control of fertility (Bone et al. 1997, Cooper et al. 1997), as radiosensitisers (Paul & Abdel-Nabi 2007, Khabnadideh et al. 2009, Lee et al. 2011) and against the recombinant reverse transcriptase of human immunodeficiency virus (HIV)-1 (Silvestri et al. 2002, Al-Soud et al. 2007).
Fig. 1. : structures of the nitroimidazole drugs (1-7).
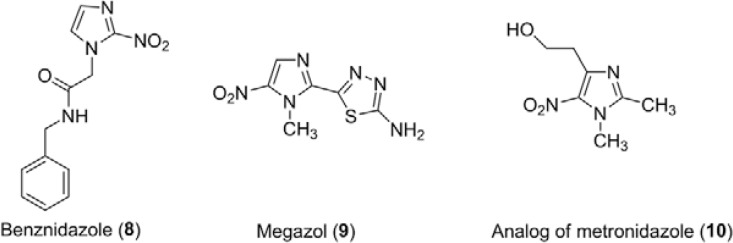
The 2-nitroimidazole benznidazole (BZ) (8) (Fig. 2) and nifurtimox (NFX) are the only available drugs for the treatment of Chagas disease. However, these nitro derivatives exhibit poor activity in the late chronic phase, with severe collateral effects and limited efficacy against different parasitic isolates, justifying the urgent need to identify alternatives to treat chagasic patients (Soeiro & de Castro 2011, Urbina 2014). This disease is caused by Trypanosoma cruzi and affects approximately eight million individuals in Latin America. Furthermore, it is emerging in nonendemic areas, associated with the immigration of infected individuals (Gascon et al. 2010, Schmunis & Yadon 2010, França et al. 2014). Megazol (9) (Fig. 2) is a nitroimidazole-thiadiazole with high in vitro and in vivo activity against Trypanosoma cruzi, including against strains resistant to 8 (Filardi & Brener 1982, d, de Castro & de Meirelles 1986, Lages-Silva et al. 1990, Salomão et al. 2010) and Trypanosoma brucei, the causative agent of human African trypanosomiasis (HAT) (Enanga et al. 1998, 2000, Boda et al. 2004). The mode of action of 9 is associated with the interference with the parasite’s oxygen metabolism, as well as acting as a trypanothione scavenger (Viodé et al. 1999, Maya et al. 2003). Despite its notable trypanocidal activity, 9 was not approved for clinical use due to reports of in vitro mutagenic and genotoxic effects associated with the reduction of the nitro group (Ferreira & Ferreira 1986, Poli et al. 2002, Nesslany et al. 2004), but the nature of the mutagenic metabolite was not yet characterised.
Fig. 2. : chemical structures of the nitroimidazoles: benznidazole (8), megazol (9) and a metronidazole analog (10).
Several nitroimidazoles possess good oral therapeutic activity against protozoal parasites; however, concerns over toxicity, mutagenicity and genotoxicity have made drug development problematic. These adverse properties appear to be related to DNA damage by the products of the bio-reduction of the nitro group. In fact, positive Ames tests were observed for 1 and 8 using Salmonella typhimurium (Rosenkranz Jr et al. 1976). Despite these results, nitroimidazoles are employed for the clinical treatment of bacterial and protozoal infections and 1 was included the World Health Organization list of Essential Medicines, a list of the arsenal of key antimicrobial drugs (WHO 2011).
Studies of the mutagenicity have shown that there are differences in the ability of mammalian cells, bacteria and protozoa to reduce nitroimidazoles (Moreth et al. 2010). However, to date, there are no conclusive results from analogous studies of mutagenicity performed in vitro in animals or in humans (Paula et al. 2009). Voogd et al. (1979) described the influences of different substituent groups in the nitroimidazole ring on the redox system, while Walsh et al. (1987) group demonstrated that the mutagenic action of 1 is affected by different substituents at position 1 of the imidazole nucleus. For example, compound 10 (Fig. 2), which is a position structural analogue of 1, maintained its biological activity without toxic effects. Although some hypotheses exist, active research in this area has yet to produce a comprehensive mechanism to explain the toxic and therapeutic activities of these compounds. Thus, further studies to elucidate the relationship between the biological activity, genotoxicity and mutagenicity of nitroimidazoles are needed.
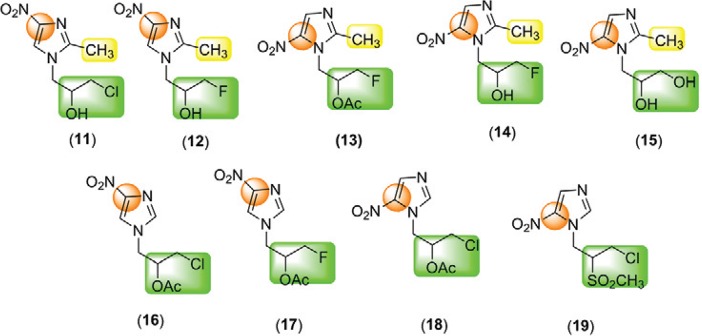
Our group has investigated new nitroimidazoles as trypanocidal agents and their mutagenic/genotoxic activities. We have observed that these activities do not only depend on the nitro group (Boechat et al. 2001, Carvalho et al. 2004, 2006, 2007, 2008, 2014, Mello et al. 2013). Encouraged by this observation, in the present work we evaluated the genotoxic, mutagenic and antitrypanocidal activities of nine nitroimidazoles (11-19) (Fig. 3). The following key structural aspects of the nitroimidazoles were examined: the importance of the position of the nitro group in the imidazole nucleus (C-4 or C-5), the incorporation of an oxidisable group at N-1 of the nitroimidazole ring, as an anion receptor group and the importance of the methyl group at C-2. This study may serve as a guide to the search for new lead compounds for the chemotherapy of human trypanosomiasis and may also identify safer compounds that may serve as the basis for investigation into the mutagenic activity of nitroimidazoles.
Fig. 3. : chemical structures of the studied nitroimidazoles 11-19.
Although the focus of this work is studying the genotoxic and mutagenic effects of different nitroimidazole moieties, the synthesised compounds (11-19) were also assayed against T. cruzi, even though they do not possess the structural requirements required for trypanocidal activity, i.e., two aromatic and/or heteroaromatic rings.
MATERIALS AND METHODS
Chemistry - 4 or 5-nitroimidazoles with different substituents at the 2 position (11-19) were synthesised following the method described by Skupin et al. (1997). Stock solutions of the compounds were prepared in dimethyl sulfoxide (DMSO) and in all subsequent assays control sets with the highest required solvent concentrations (5%) were included.
In vitro trypanocidal assay - Bloodstream trypomastigotes of T. cruzi were obtained from mice intraperitoneally inoculated with the Y strain of T. cruzi and were resuspended in Dulbecco’s modified Eagle’s medium plus 10% foetal calf serum at a concentration of 107 parasites/mL. This suspension (100 μL) was added to an equal volume of 11 to 19 and incubated for 24 h at 37ºC under a 5% CO2 atmosphere. Cell counts were then performed using a Neubauer chamber and the concentration that produced lysis of 50% of the parasites (IC50) was determined. Untreated and BZ-treated parasites were used as controls (Carvalho et al. 2014). The experiments were conducted in accordance with the guidelines established by the Oswaldo Cruz Foundation Committee of Ethics for the Use of Animals (LW 16-13).
In vitro treatment and cytotoxicity assay in whole blood - Heparinised human blood was obtained by venipuncture immediately before the assays. Whole blood was treated for 2 h at 37ºC with different concentrations of 9 (380-4,000 µM) and of 11 to 19 (148-6,400 µM). The cell viability was determined using the fluorescein diacetate (FDA)/ethidium bromide (EtBr)-assay, in which viable cells are labelled in green, while dead ones display orange-stained nuclei (Hartmann & Speit 1997). Whole blood (50 µL) was mixed with an equal volume of a freshly prepared staining solution consisting of 30 µg/mL FDA plus 8 µg/mL EtBr in phosphate-buffered saline (PBS). Samples (50 µL) were spread on a microscope slide, covered with a coverslip and observed using a fluorescence microscope. Two hundred cells were analysed for each treatment. This toxicity test was performed to determine the range of drug concentrations in the subsequent approaches: concentrations that led to more than 30% loss of viability were excluded (Anderson et al. 1998, Henderson et al. 1998).
In vitro alkaline single cell gel electrophoresis (SCGE) or the comet assay - At the end of a 2 h-treatment, DNA damage in the whole blood was evaluated in duplicate using an alkaline comet assay (Speit & Hartmann 2006). Methyl methane-sulfonate (MMS) (160 µM) (Aldrich, USA) was used as a positive control. Aliquots of 5 µL of whole blood were mixed with 120 µL of 0.5% low melting-point agarose (LMPA) (Sigma-Aldrich, USA) in PBS at 37ºC and were applied to microscope slides previously covered with 1.5% normal melting-point agarose (Sigma-Aldrich). Then, the slides were covered with coverslips and after LMPA solidification (3 min/4-5ºC), the coverslips were removed and the slides were immersed in cold lysing solution (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris, 1% N-lauroylsarcosine sodium salt, 1% Triton X-100, 10% DMSO, pH 10) for at least 1 h at 4-5ºC, protected from light. Afterwards, the slides were placed in a horizontal gel electrophoresis unit (Bio-Rad) filled with a freshly prepared alkaline buffer at a pH > 13 (300 mM NaOH, 1 mM EDTA). The times for the alkali treatment and the electrophoresis of the DNA (0.86 V/cm and 300 mA) were 20 min each in an ice bath. The slides were neutralised by washing three times (5 min) in Tris buffer (0.4 M Tris, pH 7.5), dried, fixed in absolute ethanol (99.8%) for approximately 10 min and left at room temperature overnight. Then, each slide was covered with 30 µL of EtBr (20 µg/mL) and a coverslip and analysed using a fluorescence microscope at 400X magnification.
Fifty random cells per slide (200 cells per treatment) were visually analysed in a blinded manner according to tail size into four classes of damage: 0 (undamaged, i.e., no visible tail), 1 (slightly damaged), 2 (moderately damaged) and 3 (maximally damaged, i.e., the head of comet was very small and most of the DNA was in the tail) (Kobayashi et al. 1995). DNA damage was expressed as arbitrary units (AU) as percentage of cells into each of the four classes according to the formula AU = (0 x M0) + (1 x M1) + (2 x M2) + (3 x M3).
The codes M0, M1, M2 and M3 correspond to the number of cells within damage classes 0, 1, 2 and 3, respectively. Thus, the total DNA damage score [total arbitrary units (TAU)] for 200 comets range from zero (undamaged) to 600 (maximally damaged). The experiments were performed in triplicate and the statistical significance (p < 0.05) between the treated and control groups was evaluated using Student’s one-tailed t test (Kobayashi et al. 1995, OECD iLibrary 1997)
Salmonella/Microsome mutagenicity test - Four samples with nitro group at positions 4 (16 and 17) and 5 (13 and 18) and different substituent groups at position N-1 were selected for this analysis (Fig. 3). The Salmonella/Microsome mutagenicity test evaluates mutations in the DNA of genetically modified Salmonella enterica serovar Typhimurium strains that lack the ability to synthesise histidine (His -). The strains TA97, TA98, TA100, TA102 and TA1535 were utilised following the guidelines for the testing of chemicals (Maron & Ames 1983). At least five strains of bacteria were used, including four strains of S. enterica serovar typhimurium (TA1535; TA1537 or TA97a or TA97; TA98 and TA100).
A volume of 100 µL of the nitroimidazole (25-2,500 µM), 500 µL of PBS and 100 µL of the bacterial suspension (2 x 109 cells/mL) were combined in a test tube. Next, 2 mL of top agar (7 g/L agar; 5 g/L NaCl; 0.0105 g/L L-histidine; 0.0122 g/L biotin; pH 7.4, 45ºC) was added and the mixture was poured into a Petri dish containing minimal agar {15 g/L agar, Vogel-Bonner E medium 10X [10 g/L MgSO4.7H2O; 100 g/L C6H8O7.H2O; 500 g/L K2HPO4; 175 g/L Na(NH4)HPO4.4H2O]} with 20 g/L glucose. The plates were incubated at 37ºC for 72 h and the His + revertant colonies were counted. The positive controls included 1.0 µg/plate 4-nitroquinoline-N-oxide (4-NQO) for TA97 and TA98, 0.5 µg/plate sodium azide for TA100 and TA1535 and 0.5 µg/plate mitomycin C for TA102. All of the chemicals were obtained from Sigma Co (USA). DMSO was used as the negative control. The sample was considered positive for mutagenicity when the following conditions were observed: the number of revertant colonies in the assay was at least twice the number of spontaneous revertants [mutagenicity index (MI) ≥ 2], a significant response to the analysis of variance (p ≤ 0.05) was observed and a reproducible, positive dose-response curve (p ≤ 0.01) was obtained. The MI was calculated by dividing the number of His+ colonies induced in the sample by the number of His+ colonies in the negative control. All the experiments were performed in triplicate and were repeated twice. Statistical significance was evaluated using analysis of variance, including a one-way ANOVA and Tukey’s honestly significant difference HSD post hoc analysis (p ≤ 0.01). The mutagenic potency slope was obtained using the Bernstein model (Bernstein et al. 1982).
Survival experiments - Quantitative evaluations were performed to determine the cytotoxic effects of the nitroimidazoles by the Ames test. Ten microlitres of each treated bacterial suspension (108 cells/mL) was diluted in a saline solution (NaCl, 9 g/L-0.9%). Then, 100 µL of the solution was placed on a Petri dish containing nutrient agar [0.8% bacto nutrient broth (Difco), 0.5% NaCl and 1.5% agar] and incubated at 37ºC for 24 h. The colonies were counted and the survival percentage was determined with respect to the negative control. All the experiments were performed in triplicate and were repeated twice (Aiub et al. 2004).
RESULTS
The present study focused on the use of SCGE to evaluate DNA damage induced by the nitroimidazoles 11-19 with the objective of mapping genotoxic activity, as displayed in Table I. The treatment of blood cells with 9 over the range of 380-4,000 µM for 2 h at 37°C did not reduce the cell viability. However, a highly significant (p < 0.01) genotoxic effect was observed at concentrations higher than of 1,562 µM. The positive control, MMS, was highly active at 160 µM (p < 0.0001), causing a clear genotoxic effect with a TAU value of 454.3 ± 9.2. In the range of 148-6,400 µM, the nitroimidazoles 11, 12, 16 and 18 did not alter cellular viability and caused no DNA damage compared to the control group (p > 0.05). On the other hand, at the same concentrations, 13, 15 and 19 caused moderate alterations to the DNA, whereas 14, 17 and 9 caused strong alterations of the DNA structure.
TABLE I. Anti-Trypanosoma cruzi and genotoxic in vitro activities of nitroimidazoles 11-19.
| Compound | IC50/24 h (µM) | Genotoxic |
|---|---|---|
| 9 | 9.9 ± 0.8 | 3 |
| 11 | 1,690.9 ± 371.8 | 0 |
| 12 | 1,147.3 ± 111.6 | 0 |
| 13 | 1,749.7 ± 184.6 | 1 |
| 14 | 1,170.7 ± 100.5 | 3 |
| 15 | 1,585.8 ± 218.3 | 1 |
| 16 | 1,132.4 ± 86.3 | 0 |
| 17 | 1,049.4 ± 63.7 | 3 |
| 18 | > 2,000 | 0 |
| 19 | 902.6 ± 83.4 | 1 |
0: undamaged; 1: slightly damaged; 2: moderately damaged; 3: maximally damaged (see Materials and Methods for more details).
The nitroimidazoles were also assayed against T. cruzi. Compounds 11-17 and 19 exhibited an IC50 in the range of 902-1,749 µM, whereas for 18, this parameter was > 2000 µM. Under the same experimental conditions, the values for 8 and 9 were 9.7 ± 2.4 and 9.9 ± 0.8 µM, respectively (Table I). As expected, the compounds were not active trypanocides because they do not possess the structural requirements required for trypanocidal activity, i.e., two aromatic and/or heteroaromatic rings.
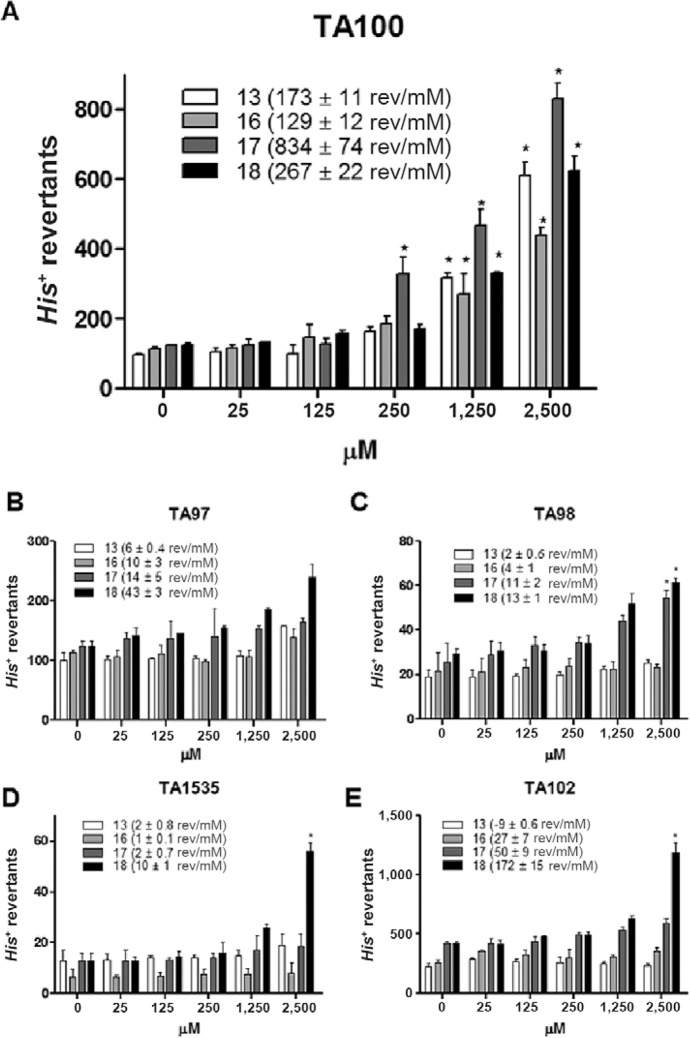
Fig. 4 illustrates the mutagenicity and cytotoxicity induced by nitroimidazoles 13, 16, 17 and 18 using S. typhimurium strains TA97, TA98, TA100, TA102 and TA1535. At concentrations between 25-2,500 µM, these compounds did not induce frameshift mutations in TA97. However, compounds 17 and 18 were capable of inducing frameshift mutations in TA98, whereas 18 also induced transversions and transitions in TA102 and base pair substitutions in TA1535. Furthermore, all of the nitroimidazoles were capable of inducing dose-dependent base pair substitutions for at least two tested doses in TA100, the most mutagen-responsive strain (Fig. 5). In addition, cytotoxic activity was present only in the higher concentration (2,500 µM) for at least one sample and strain. Using the Bernstein correlation, the number of revertant colonies per µg (rev/µg) was deduced. In addition, higher mutagenic potency correlated with an increased risk of being a mutagen.
Fig. 4. : Salmonella/Microsome assay of the nitroimidazoles 13, 16, 17 and 18. rev/mM: revertant colonies per nM.
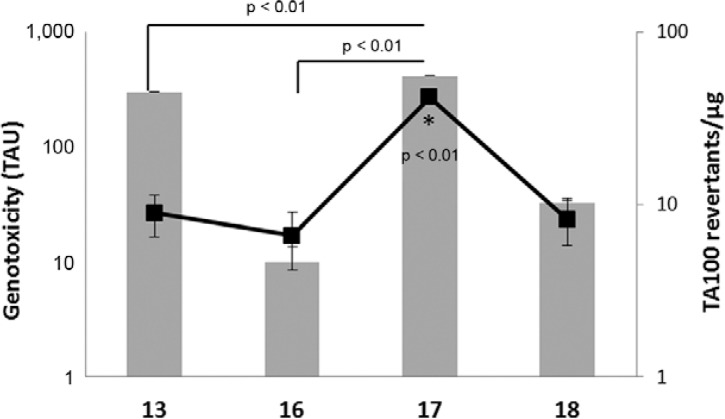
Fig. 5. : genotoxic (comet assay) and mutagenic (Salmonella/Microsome assay) response of the nitroimidazoles 13, 16, 17 and 18.
DISCUSSION
Although nitroimidazoles have been clinically used for chemotherapy against several parasites, the mechanisms underlying their genotoxic and biological activities are not fully understood. On the basis of the high activity of 9 against trypanosomatids (Filardi & Brener 1982, Enanga et al. 1998, Darsaud et al. 2004) together with the success of NFX-eflornithine for the treatment of HAT (Yun et al. 2010), the Drugs for Neglected Diseases initiative triggered a systematic review of more than 700 nitroheterocycles (mostly nitroimidazoles) (Torreele et al. 2010). In this context, fexinidazole (5-nitroimidazole), first described in 1978 by Winkelmann and Raether (1978), emerged as a potential candidate. The development of this compound was previously abandoned largely due to the prejudice against nitroaromatic compounds (Patterson & Wyllie 2014). More recently, analysis of pharmacological and toxicological profiles suggested that fexinidazole is a promising candidate for both HAT (Torreele et al. 2010) and Chagas disease (Bahia et al. 2012). These facts and our previous results prompted us to re-evaluate the role of the nitro group in the toxic effects of nitroimidazoles. The presence of a nitro group in a compound can result in several toxicity issues, including genotoxicity and mutagenicity (Walsh & Miwa 2011). However, at the same time, this functional group is necessary for the desired biological activity. Consequently, nitrocompounds are not included in screening libraries due to this unwanted functionality (Brenk et al. 2008) and are not synthesised in medicinal chemistry programs [reviewed in Patterson and Wyllie (2014)].
This work investigated the importance of different functional groups of C-4 or C-5 nitroimidazoles having oxidisable groups bonded at N-1 on the biological and mutagenic activities of the compounds.
The results of the genotoxicity assays are described in Table I. Nitroimidazoles 11 and 12, which contain a NO2 group at C-4 and a CH3 group at C-2, were not genotoxic compared to 13, 14 and 15, which possess a NO2 group at C-5 and exhibited moderate to high genotoxicity. Nitroimidazole 13 had moderate mutagenic effects. We also observed a comparable result between 11 (2-CH3 and 4-NO2), which was not genotoxic and its analogue 4 (2-CH3 and 5-NO2), which is known to be a mutagenic agent (Ferreiro et al. 2002). However, when the nitroimidazole had no CH3 group at C-2, the position of the NO2 group had no influence on genotoxic activity. This is the case for 16 (4-NO2) and 18 (5-NO2), which exhibited behaviours similar to that of 11 and 12 (no genotoxicity). When comparing pairs of similar compounds, for instance, 16 (4-NO2 and N-CH2OAcCH2Cl) with 17 (4-NO2 and N-CH2OAcCH2F) and 14 (2-CH3; 5-NO2; N-CH2OHCH2F) with 15 (2-CH3; 5-NO2; N-CH2OHCH2OH), we observed that the presence of fluorine induced genotoxicity. The fluorinated compounds 14 and 17 showed higher genotoxicity regardless of the presence of CH3 at C-2 or NO2 at C-4 or C-5. However, when comparing compounds 11 (2-CH3; 4-NO2; N-CH2OHCH2Cl) and 12 (2-CH3; 4-NO2; N-CH2OHCH2F), the fluorine atom had no influence on genotoxicity.
Four compounds were selected for the Ames assays [nitro group at positions 4 (16 and 17) and 5 (13 and 18) and different substituent groups at position N-1] with the aim of clarifying whether the increase in mutagenicity of nitroimidazoles was dependent only on the position of the nitro group or was related to the presence of more or less reactive halogens.
The results of the cytotoxicity and mutagenicity analyses are described in Fig. 4 and Table II. The four S. typhimurium strains have GC base pairs at the primary reversion site and as a result, it is known that these strains may not detect certain oxidising mutagens, cross-linking agents and hydrazines. Such substances may be detected by S. typhimurium TA102, which has an AT base pair at the primary reversion site (OECD iLibrary 1997). The data showed that 17 is a highly potent mutagen (41.7 rev/µg to TA100), whereas 13, 16 and 18 are low-potency mutagens, causing approximately 8-9 rev/µg. The data in Fig. 5 further corroborate this finding, on the basis that the number of TAU in the comet assay is high for 17, similar to the number of rev/µg.
TABLE II. Genotoxicity, mutagenicity and mutagenic potency slope of nitroimidazoles 13, 16, 17 and 18.
| Compound | Genotoxicity | Ames test (MI > 2) - Mutagenicity | Mutagenic potency slope (revertants/µg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1,562 µM | 250 µM | 1,250 µM | 2,500 µM | TA97 | TA98 | TA100 | TA102 | TA1535 | |
| 13 | 1 (300 TAU) | - | TA100 | TA100 | 0.30 ± 0.22 | NA | 8.98 ± 0.71 | NA | NA |
| 16 | 0 (10 TAU) | - | TA100 | TA100 | NA | NA | 6.60 ± 0.63 | NA | NA |
| 17 | 3 (411 TAU) | TA100 | TA100 | TA98, TA100 | NA | 0.55 ± 0.08 | 41.70 ± 3.71 | 2.51 ± 0.44 | NA |
| 18 | 0 (32 TAU) | - | TA100 | TA98, TA100, TA102, TA1535 | 2.13 ± 0.14 | 0.69 ± 0.05 | 8.18 ± 0.15 | NA | 0.51 ± 0.07 |
MI: mutagenicity inde; NA: not applicable; TAU: total arbitrary units for the positive control, methyl methane-sulfonate value was 454.3 ± 9.2.
In conclusion, we observed that the type and position of different substituents bonded to the imidazole ring have a significant influence on the toxicological activity. Whereas nitroimidazoles 11 and 12 were not genotoxic, nitroimidazoles 13, 15 and 19 were moderately genotoxic and mutagenic. Nitroimidazole 16 was neither genotoxic nor mutagenic and 18 was moderately mutagenic but not genotoxic. These results demonstrate that the nitro group is not solely responsible for the mutagenic or genotoxic activity.
Furthermore, the data suggest that the nitroimidazole may be the moiety most likely to be responsible for the genotoxic and mutagenic effects, but in the analogues tested, this moiety was unable to provide anti-T. cruzi activity.
Footnotes
Financial support: FAPERJ, CNPq, FIOCRUZ, Organisch-Chemisches Institut, Westfälischen Wilhelms-Universität Münster
REFERENCES
- Aiub CAF, Stankevicins L, Costa V, Ferreira F, Mazzei JL, Ribeiro AS, Moura RS, Felzenszwalb I. Genotoxic evaluation of a vinifera skin extract that present pharmacological activities. Food Chem Toxicol. 2004;42:969–973. doi: 10.1016/j.fct.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Al-Soud YA, Al-Masoudi NA, Hassan HG, Clercq E, Pannecouque C. Nitroimidazoles. V. Synthesis and anti-HIV evaluation of new 5-substituted piperazinyl-4-nitroimidazole derivatives. Acta Pharm. 2007;57:379–393. doi: 10.2478/v10007-007-0031-7. [DOI] [PubMed] [Google Scholar]
- Anderson D, Yu TW, Mcgregor DB. Comet assay responses as indicators of carcinogen exposure. Mutagenesis. 1998;13:539–555. doi: 10.1093/mutage/13.6.539. [DOI] [PubMed] [Google Scholar]
- Bahia MT, Andrade IM, Martins TA, Nascimento AF, Diniz LF, Caldas IS, Talvani A, Trunz BB, Torreele E, Ribeiro I. Fexinidazole: a potential new drug candidate for Chagas disease. PLoS Negl Trop Dis. 2012;6: doi: 10.1371/journal.pntd.0001870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein L, Kaldor MJ, Pike MC. An empirical approach to the statistical analysis of mutagenesis data from Salmonella test. Mutat Res. 1982;97:267–281. doi: 10.1016/0165-1161(82)90026-7. [DOI] [PubMed] [Google Scholar]
- Boda C, Enanga B, Dumet H, Chauviere G, Labrousse F, Couquet C, Saivin S, Houin G, Perie J, Dumas M, Bouteille B. Plasma kinetics and efficacy of oral megazol treatment in Trypanosoma brucei brucei-infected sheep. Vet Parasitol. 2004;121:213–223. doi: 10.1016/j.vetpar.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Boechat N, Carvalho AS, Fernandez-Ferreira E, Soares ROA, Souza AS, Gibaldi D, Bozza M, Pinto AC. Novel nitroimidazoles with trypanocidal and cell growth inhibition activities. Cytobios. 2001;105:83–90. [PubMed] [Google Scholar]
- Bone W, Yeung CH, Skupin R, Haufe G, Cooper TG. Toxicity of ornidazole and its analogues on rat spermatozoa as reflected in motility parameters. Int J Androl. 1997;20:347–355. doi: 10.1046/j.1365-2605.1998.00077.x. [DOI] [PubMed] [Google Scholar]
- Brenk R, Schipani A, James D, Krasowski A, Gilbert IH, Frearson J, Wyatt PG. Lessons learnt from assembling screening libraries for drug discovery for neglected diseases. Chem Med Chem. 2008;3:435–444. doi: 10.1002/cmdc.200700139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschini A, Giordani F, Albuquerque CN, Pellacani C, Pelosi G, Rossi C, Zucchi TMAD, Poli P. Trypanocidal nitroimidazole derivatives: relationships among chemical structure and genotoxic activity. Biochem Pharmacol. 2007;73:1537–1547. doi: 10.1016/j.bcp.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Carvalho AS, Gibaldi D, Bozza M, Pinto AC, Boechat N. Synthesis and trypanocidal evaluation of news 5-[N-(3-(5-substituted)-1,3,4-thiadiazolyl)]amino-1-methyl-4-nitroimidazoles. Lett Drug Des Discov. 2006;3:98–101. [Google Scholar]
- Carvalho AS, Menna-Barreto RFS, Romeiro NC, Castro S, Boechat N. Design, synthesis and activity against Trypanosoma cruzi of azaheterocyclic analogs of megazol. Med Chem Res. 2007;3:460–465. doi: 10.2174/157340607781745519. [DOI] [PubMed] [Google Scholar]
- Carvalho AS, Salomão K, Castro SL, Conde TR, Zamith HPS, Caffarena ER, Hall BS, Wilkinson SR, Boechat N. Megazol and its bioisostere 4H-1,2,4-triazole: comparing the trypanocidal, cytotoxic and genotoxic activities and their in vitro and in silico interactions with the Trypanosoma brucei nitroreductase enzyme. Mem Inst Oswaldo Cruz. 2014;109:315–323. doi: 10.1590/0074-0276140497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho SA, Silva EF, Santa-Rita RM, Castro SL, Fraga CAM. Synthesis and antitrypanosomal profile of new functionalyzed 1,3,4-thiadiazole-2-arylhydrazone derivatives, designed as non-mutagenic megazol analogues. Bioorg Med Chem Lett. 2004;14:5967–5970. doi: 10.1016/j.bmcl.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Carvalho SA, Lopes FAS, Salomão K, Romeiro NC, Wardell SMVS, Castro SL, Silva EF, Fraga CAM. Studies toward the structural optimization of new brazilizone-related trypanocidal 1,3,4-thiadiazole-2-arylhydrazone derivatives. Bioorg Med Chem. 2008;16:413–421. doi: 10.1016/j.bmc.2007.09.027. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Yeung CH, Skupin R, Haufe G. The antifertility potential of ornidazole analogues in rats. J Androl. 1997;18:431–438. [PubMed] [Google Scholar]
- Darsaud A, Chevrier C, Bourdon L, Dumas M, Buguet A, Bouteille B. Megazol combined with suramin improves a new diagnosis index of the early meningo-encephalitic phase of experimental African trypanosomiasis. Trop Med Health. 2004;9:83–91. doi: 10.1046/j.1365-3156.2003.01154.x. [DOI] [PubMed] [Google Scholar]
- Castro SL, Meirelles MNL. Effect of drugs on Trypanosoma cruzi and on its interaction with heart muscle cell in vitro. Mem Inst Oswaldo Cruz. 1986;82:209–218. doi: 10.1590/s0074-02761987000200009. [DOI] [PubMed] [Google Scholar]
- Enanga B, Keita M, Chauvière G, Dumas M, Bouteille B. Megazol combined with suramin: a chemotherapy regimen which reversed the CNS pathology in a model of human African trypanosomiasis in mice. Trop Med Int Health. 1998;3:736–741. doi: 10.1046/j.1365-3156.1998.00291.x. [DOI] [PubMed] [Google Scholar]
- Enanga B, Ndong JMM, Boudra H, Debrauwer L, Dubreuil G, Bouteille B, Chauviere G, Labat C, Dumas M, Perie J, Houin G. Pharmacokinetics, metabolism and excretion of megazol in a Trypanosoma brucei gambiense primate model of human African trypanosomiasis: preliminary study. Arzneimittelforschung. 2000;50:158–162. doi: 10.1055/s-0031-1300182. [DOI] [PubMed] [Google Scholar]
- Ferreira RC, Ferreira LC. Mutagenicity of CL 64855, a potent anti-Trypanosoma cruzi drug. Mutat Res. 1986;171:11–15. doi: 10.1016/0165-1218(86)90003-0. [DOI] [PubMed] [Google Scholar]
- Ferreiro GR, Badías LC, Lopez-Nigro M, Palermo A, Mudry M, Elio PG, Carballo MA. DNA single strand breaks in peripheral blood lymphocytes induced by three nitroimidazole derivatives. Toxicol Lett. 2002;132:109–115. doi: 10.1016/s0378-4274(02)00039-5. [DOI] [PubMed] [Google Scholar]
- Filardi LS, Brener ZA. Nitroimidazole-thiadiazole derivative with curative action in experimental Trypanosoma cruzi infections. Ann Trop Med Parasitol. 1982;76:293–297. doi: 10.1080/00034983.1982.11687544. [DOI] [PubMed] [Google Scholar]
- França RRF, Carvalho AS, Branco FSC, Pinto AC, Boechat N. Inibidores potentes da enzima esterol 14α-desmetilase contra Trypanosoma cruzi. Rev Virtual Quim. 2014;6:1483–1516. [Google Scholar]
- Gascon J, Bern C, Pinazo MJ. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 2010;115:22–27. doi: 10.1016/j.actatropica.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Hartmann A, Speit G. The contribution of cytotoxicity to DNA - effects in the single cell gel test (comet assay) Toxicol Lett. 1997;90:183–188. doi: 10.1016/s0378-4274(96)03847-7. [DOI] [PubMed] [Google Scholar]
- Henderson L, Wolfreys A, Fedyk J, Bourner C, Windebank S. The ability of the comet assay to discriminate between genotoxins and cytotoxins. Mutagenesis. 1998;13:89–94. doi: 10.1093/mutage/13.1.89. [DOI] [PubMed] [Google Scholar]
- Khabnadideh S, Rezaei Z, Khalafi-Nezhad A, Pakshir K, Heiran MJ, Shobeiri H. Design and synthesis of 2-methyl and 2-methyl-4-nitro imidazole derivatives as antifungal agents. Iranian J Pharm Sci. 2009;5:31–36. [Google Scholar]
- Kobayashi H, Sugiyama C, Morikawa Y, Hayashi M, Sofuni T. A comparison between manual microscopic analysis and computerized image analysis in the single cell gel electrophoresis assay. MMS Commun. 1995;3:103–115. [Google Scholar]
- Lages-Silva E, Filardi LS, Brener Z. Effect of the host specific treatment in the phagocytosis of Trypanosoma cruzi blood forms by mouse peritoneal macrophages. Mem Inst Oswaldo Cruz. 1990;85:401–405. doi: 10.1590/s0074-02761990000400003. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim S, Yun M, Lee YS, Cho S, Oh T, Kim P. Synthesis and antitubercular activity of monocyclic nitroimidazoles: insights from econazole. Bioorg Med Chem Lett. 2011;21:1515–1518. doi: 10.1016/j.bmcl.2010.12.128. [DOI] [PubMed] [Google Scholar]
- Maron DM, Ames BN. Revised methods for the Salmonella mutagenity test. Mutat Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- Maya JD, Bollo S, Nuñez-Vergara LJ, Squella JA, Repetto Y, Morello A, Périé J, Chauvière G. Trypanosoma cruzi: effect and mode of action of nitroimidazole and nitrofuran derivatives. Biochem Pharmacol. 2003;65:999–1006. doi: 10.1016/s0006-2952(02)01663-5. [DOI] [PubMed] [Google Scholar]
- Mello FVC, Carvalho AS, Bastos MM, Boechat N, Aiub CAF, Felzenszwalb I. Evaluation of genotoxic effects of new molecules with possible trypanocidal activity for Chagas disease treatment. Scientific World Journal. 2013;2013:287–319. doi: 10.1155/2013/287319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mital A. Synthetic nitroimidazoles: biological activities and mutagenicity relationships. Sci Pharm. 2009;77:497–520. [Google Scholar]
- Moreth M, Ornelas D, Gomes CRB, Souza MVN. Nitroimidazóis - uma promissora classe de substâncias para o tratamento da tuberculose. Rev Virtual Quim. 2010;2:105–117. [Google Scholar]
- Nesslany F, Brugier S, Mouries M, Le Curieux F, Marzin D. In vitro and in vivo chromosomal aberrations induced by megazol. Mutat Res. 2004;560:147–158. doi: 10.1016/j.mrgentox.2004.02.013. [DOI] [PubMed] [Google Scholar]
- OECD iLibrary - Organisation for Economic Cooperation and Development OECD Guidelines for the Testing of Chemicals, Section 4. Test No. 471: Bacterial Reverse Mutation Test. 1997 oecd-ilibrary.org/environment/test-no-471-bacterial-reverse-mutation-test_9789264071247-en.
- Patterson S, Wyllie S. Nitro drugs for the treatment of try- panosomatid diseases: past, present and future prospects. Trends Parasitol. 2014;30:289–298. doi: 10.1016/j.pt.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul AK, Abdel-Nabi H. Cancer imaging agents for positron emission tomography: beyond FDG. Curr Med Imaging Rev. 2007;3:178–185. [Google Scholar]
- Paula FR, Serrano SHP, Tavares LC. Aspectos mecanísticos da bioatividade e toxicidade de nitrocompostos. Química Nova. 2009;32:1013–1020. [Google Scholar]
- Poli P, Mello MA, Buschini A, Mortara RA, Albuquerque CN, Silva S, Rossi C, Zucchi TMAD. Cytotoxic and genotoxic effects of megazol, an anti-Chagas disease drug, assessed by different short-term tests. Biochem Pharmacol. 2002;64:1617–1627. doi: 10.1016/s0006-2952(02)01390-4. [DOI] [PubMed] [Google Scholar]
- Rosenkranz HS, Jr, Speck WT, Stambaugh JE. Mutagenicity of metronidazole: structure-activity relationships. Mutat Res. 1976;38:203–206. doi: 10.1016/0165-1161(76)90191-6. [DOI] [PubMed] [Google Scholar]
- Salomão K, Souza EM, Carvalho AS, Silva EF, Fraga CAM, Barbosa HS, Castro SL. In vitro and in vivo activity of 1,3,4-thiadiazole-2-arylhydrazone derivatives of megazol on Trypanosoma cruzi. Antimicrob Agents Chemother. 2010;54:2023–2031. doi: 10.1128/AAC.01241-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmunis GA, Yadon ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 2010;115:14–21. doi: 10.1016/j.actatropica.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Silvestri R, Artico M, Martino G, Ragno R, Massa S, Loddo R, Murgioni C, Loi AG, La Colla P, Pani A. Synthesis, biological evaluation and binding mode of novel 1-[2-(diarylmethoxy)ethyl]-2-methyl-5-nitroimidazoles targeted at the HIV-1 reversed transcriptase. J Med Chem. 2002;45:1567–1576. doi: 10.1021/jm010904a. [DOI] [PubMed] [Google Scholar]
- Skupin R, Cooper TG, Frohlich R, Prigge J, Haufe G. Lipase-catalyzes resolution of both enantiomers of ornidazole and some analogues. Tetrahedron Asymmetry. 1997;8:2453–2464. [Google Scholar]
- Soeiro MNC, Castro SL. Screening of potential anti-Trypanosoma cruzi candidates: In vitro and in vivo studies. Open Med Chem J. 2011;5:21–30. doi: 10.2174/1874104501105010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speit G, Hartmann A. The comet assay: a sensitive genotoxicity test for the detection of DNA damage and repair. Methods Mol Biol. 2006;314:275–286. doi: 10.1385/1-59259-973-7:275. [DOI] [PubMed] [Google Scholar]
- Torreele E, Trunz BB, Tweats D, Kaiser M, Brun R, Mazué G, Bray MA, Pécoul B. Fexinidazole - a new oral nitroimidazole drug candidate entering clinical development for the treatment of sleeping sickness. PLoS Negl Trop Dis. 2010;4: doi: 10.1371/journal.pntd.0000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbina JA. Recent clinical trials for the etiological treatment of chronic Chagas disease: advances, challenges and perspectives. J Eukaryot Microbiol. 2014;62:149–156. doi: 10.1111/jeu.12184. [DOI] [PubMed] [Google Scholar]
- Valdez C, Tripp JC, Miyamoto Y, Kalisiak J, Hruz P, Andersen YS, Brown SE, Kangas K, Arzu LV, Davids BJ, Gillin FD, Upcroft JA, Upcroft P, Fokin VV, Smith DK, Sharpless KB, Eckmann L. Synthesis and electrochemistry of 2-ethenyl and 2-ethanyl derivatives of 5-nitroimidazole and antimicrobial activity against Giardia lamblia. J Med Chem. 2009;52:4038–4053. doi: 10.1021/jm900356n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viodé C, Bettache N, Cenas N, Krauth-Siegel RL, Chauvière G, Bakalara N, Périé J. Enzymatic reduction studies of nitroheterocycles. Biochem Pharmacol. 1999;57:549–557. doi: 10.1016/s0006-2952(98)00324-4. [DOI] [PubMed] [Google Scholar]
- Voogd CE, van der Stel JJ, Jacobs JJ. The mutagenic action of nitroimidazoles. IV. A comparison of the mutagenic action of several nitroimidazoles and some imidazoles. Mutat Res. 1979;66:207–221. doi: 10.1016/0165-1218(79)90082-x. [DOI] [PubMed] [Google Scholar]
- Walsh JS, Miwa GT. Bioactivation of drugs: risk and drug design. Annu Rev Pharmacol Toxicol. 2011;51:145–167. doi: 10.1146/annurev-pharmtox-010510-100514. [DOI] [PubMed] [Google Scholar]
- Walsh JS, Wang R, Bagan E, Wang CC, Wislock P, Miwa GT. Structural alterations that differentially affect the mutagenic and antitrichomonal activities of 5-nitroimidazoles. J Med Chem. 1987;30:150–156. doi: 10.1021/jm00384a025. [DOI] [PubMed] [Google Scholar]
- WHO 17th list of essential medicines. 2011 whqlibdoc.who.int/hq/2011/a95053_eng.pdf.
- William J, Ross WJ, Jamieson WB. Antiparasitic nitroimidazoles. 8. Derivatives of 2-(4-formylstyryl)-5-nitro-1-vinylimidazole. J Med Chem. 1975;18:2158–2161. doi: 10.1021/jm00236a009. [DOI] [PubMed] [Google Scholar]
- Winkelmann E, Raether W. Chemotherapeutically active nitro-compounds. 4. 5-nitroimidazoles (Part III) Arzneimttelforschung. 1978;28:739–749. [PubMed] [Google Scholar]
- Yakugaku Z. Studies on chemotherapeutical drugs. VII. Synthesis of nitroimidazole derivatives. 2. Preparation of 4(or 5)-nitro-5(or 4)-styrylimidazole derivatives and 4 (or 5)-(beta-phenyl-beta-hydroxy)ethyl-5 (or 4)-nitro-imidazole derivatives and their antimicrobial activity. J Pharm Soc Japan. 1971;91:231–239. doi: 10.1248/yakushi1947.91.2_231. [DOI] [PubMed] [Google Scholar]
- Yun O, Priotto G, Tong J, Flevaud L, Chappuis F. NECT is next: implementing the new drug combination therapy for Trypanosoma brucei gambiense sleeping sickness. PLoS Negl Trop Dis. 2010;4: doi: 10.1371/journal.pntd.0000720. [DOI] [PMC free article] [PubMed] [Google Scholar]