Abstract
We describe the case of a 67-year-old African American woman with multiple medical problems who presented with septic shock resulting from Sphingobacterium multivorum bacteraemia. S. multivorum, a Gram-negative bacillus, is ubiquitous in nature and is rarely involved in human infections. However, it is intrinsically resistant to many commonly administered antibiotics and can be a life-threatening microorganism.
Keywords: Extended-spectrum β-lactamase, Gram-negative bacilli, metallo-β-lactamase, moist hospital environments, septic shock, sphingophospholipids
Case Report
A 67-year-old African American woman with a medical history of obesity (body mass index 38 kg/m2), chronic active smoker, chronic obstructive pulmonary disease, obstructive sleep apnea receiving continuous positive airway pressure therapy, severe pulmonary hypertension, atrial fibrillation on anticoagulation, well-controlled type 2 diabetes, hypertension and dyslipidemia, who had been discharged to a rehabilitation facility from our institution 4 weeks before after being treated for hypercapnic hypoxic respiratory failure requiring mechanical ventilation, presented with sudden-onset generalized weakness, light-headedness, shortness of breath and dry cough after waking up the morning of admission. One day before admission, the patient was discharged from a rehabilitation center where she received physical therapy/hydrotherapy sessions. The patient was in distress but denied subjective fevers, chills, headache, photophobia, sputum production, nausea, vomiting, diarrhea, dysuria, vaginal discharge, recent travel or exposure to pets. Patient's medications included: simvastatin, metformin, glipizide, valsartan, labetalol, diltiazem, warfarin, fluticasone/salmeterol and albuterol.
Physical examination revealed hypotension (64/36 mm Hg) which required epinephrine drip, tachypnea of 32 breaths/min, O2 saturation of 78% on 2 liters per nasal cannula (91% on biphasic positive airway pressure), pulse 110/min and temperature of 98.9°F (37.2°C).
The patient was alert and oriented to person, place and time, was in distress and showed conjunctival pallor, dry mucous membranes, arrhythmic heart sounds with a 2/6 pansystolic tricuspid murmur, lungs with bilateral crackles at bases and wheezing, nontender abdomen with ascetic wave and 2+ edema in lower extremities with decreased peripheral pulses, but no evidence of skin ulcers. Neurologic examination did not reveal meningeal signs or focal deficits.
Empiric therapy of cefepime and vancomycin was initiated.
Laboratory results showed a leukocyte count of 15.6 cells/mL, neutrophils 85%, no bandemia and platelets 877000/mL. Patient also presented with acute kidney injury with a creatinine of 1.3 mg/dL (baseline 0.6 mg/dL) and hyperglycemia 183 mg/dL. Lactate level was 3 mmol/L. International normalized ratio was 3. HIV test was negative. Urinalysis was negative for blood, nitrates and leukocyte esterase; it had 5 to 10 white blood cells and many bacteria.
Chest and abdominal computed tomography revealed a previously identified right pleural effusion with associated right-sided atelectasis, cardiomegaly, moderate ascites and enlarged caliber hepatic veins.
Transesophageal echocardiogram showed an ejection fraction of 60%, severe pulmonary hypertension, severe tricuspid regurgitation, mild mitral regurgitation and no signs of endocarditis. Cytology of peritoneal and the exudative pleural fluid was negative for malignant cells. Blood, urine, pleural and peritoneal fluid samples were sent for culture.
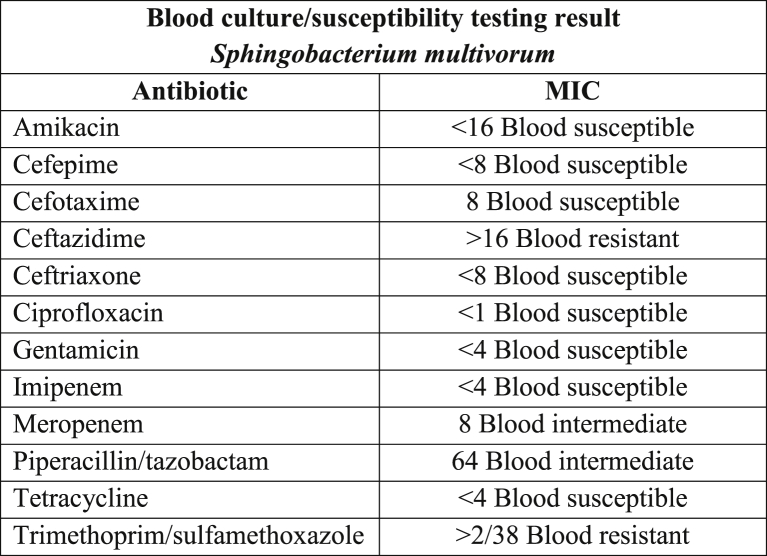
On the second day of hospitalization, the patient no longer required vasopressors. Two sets of blood samples were drawn for culture, and the two sets identified Gram-negative bacilli. Vancomycin was discontinued. On the fourth day of hospitalization, the final identification of blood cultures showed Sphingobacterium multivorum resistant to ceftazidime and trimethoprim/sulfamethoxazole (TMP/SMX) and with intermediate susceptibility to meropenem and piperacillin/tazobactam (Fig. 1). The method used for identification involved using MacConkey agar, a breakpoint panel with biochemical and sensitivity sections for identification and susceptibility tests, respectively, and a MicroScan WalkAway Plus-40 Microbiology System. Cefepime was switched to ciprofloxacin. Gram stain of urine was not reported, but urine culture came negative, as did peritoneal and pleural fluid cultures. The patient was clinically stable to be discharged with a total of 10 days of ciprofloxacin. Repeated blood and urine cultures taken 5 days after admission were negative. Patient was followed up 2 and 4 weeks after discharge and was clinically stable.
Fig. 1.
Sphingobacterium multivorum blood culture susceptibility testing results.
Discussion
Bacteria that belong to the genus Sphingobacterium are Gram-negative, non-lactose-fermentative microorganisms that are positive for catalase and oxidase tests and produce small, circular, convex, smooth and opaque colonies with light yellow pigment when incubated at 35°C in either carbon dioxide or ambient air for a minimum of 24 hours. Growth on MacConkey agar is usually detectable within 24 hours of inoculation [1]. An important feature of bacteria that belong to this genus is the presence of high concentrations of sphingophospholipids in their cell membrane [2].
These microorganisms are ubiquitous in nature but are rarely involved in human infections. Sphingobacterium can be found in soil, on plants, in foodstuffs and in water sources [3]. Sphingobacterium species have the ability to survive in moist hospital environments; these organisms thus also have the potential to contaminate laboratory culture media and blood culture systems. Whenever these species are encountered, their clinical significance and the potential for contamination should be seriously considered [1].
The few reported cases of infections resulting from S. multivorum usually occurred in severely comorbid patients (Table 1), causing, for instance, necrotizing fasciitis and septic shock in a patient with rheumatoid arthritis treated with long-term intermediate-dose corticosteroids (0.25 mg/kg of prednisone per day for more than 30 years) [3], bacteraemia in immunocompromised hosts [4], [5], [6], sepsis in the setting of bacteraemia in a hemodialyzed patient [7], peritonitis [8], chronic respiratory tract infection in patients with cystic fibrosis [9], [10] (where, however, it was not associated with a deterioration of pulmonary function during the follow-up period [10]) and respiratory infection in a patient with chronic obstructive pulmonary disease [11].
Table 1.
Summary of Sphingobacterium multivorum reports
| Study | Patient characteristics | Comorbidities | Diagnosis | Susceptibility testing results | Treatment and outcome |
|---|---|---|---|---|---|
| Dhawan [8] | 60-year-old man with nausea, vomiting and vague abdominal discomfort for 2 weeks | Alcoholic liver disease, esophageal stricture due to prior suicide attempt by ingesting liquid Drano (a strong alkaline corrosive) | Sepsis due to spontaneous bacterial peritonitis; hospital course complicated with aspiration pneumonia causing acute respiratory failure | Resistant to ampicillin, amikacin, gentamicin, chloramphenicol and cephalosporins; susceptible to tetracycline, carbenicillin, TMP/SMX | Initially treated with ampicillin and gentamicin for 5 days with no improvement, then treated with gentamicin and carbenicillin for 11 days. Full recovery. LOS: 21 days |
| Potvliege [7] | 43-year-old man with chills, fever and myalgias for 1 day | Chronic renal insufficiency on hemodialysis through arteriovenous fistula, bilateral nephrectomy; history of infected arteriovenous fistula | Bacteraemia | Resistant to penicillins, cephalosporins, tobramycin, amikacin, colistin; susceptible to erythromycin, tetracycline, chloramphenicol, vancomycin, gentamicin, sulfonamides, TMP/SMX | Treated with ampicillin (MIC of 8 μg/mL) for 10 days and 1 dose of tobramycin. Full recovery. LOS: no data |
| Freney [4] | 57-year-old man with fever 5 days after starting chemotherapy | Immunoblastic type non-Hodgkin lymphoma treated with chemotherapy complicated by development of bone marrow aplasia | Sepsis in setting of bacteraemia | MICs (mg/L): pefloxacin, 0.5; rifampin, 1; tetracycline, 2; erythromycin, 4; TMP/SMX, 5; chloramphenicol and ceftriaxone, 8; ceftazidime and cefotaxime, 16; carbenicillin and azlocillin, 64; piperacillin and cephalotin, 128; gentamicin, tobramycin, vancomycin, >16; ampicillin, aztreonam and amikacin, >32; fosfomycin, >128 | Treated with a combination of pefloxacin and TMP/SMX. Full recovery. LOS: no data |
| Reina [10] | 20-month-old girl diagnosed with cystic fibrosis at 6 months with fever, cough productive of abundant mucous and respiratory difficulty for 2 days | Cystic fibrosis | Sepsis in setting of acute exacerbation of chronic bronchopathy | Resistant to aztreonam, gentamicin and TMP/SMX; susceptible to carbenicillin, ceftazidime, ceftriaxone, cefuroxime, chloramphenicol, azlocillin, cefotaxime, ticarcillin, ciprofloxacin, imipenem, piperacillin, amikacin | Treated with ceftazidime and amikacin. Full recovery. LOS: no data |
| Areekul [6] | 47-year-old man with fever and chills for 3 months and 10 kg weight loss for 1 month; positive HIV test; cultures from blood and sputum yielded S. multivorum. Patient developed meningitis with negative CSF cultures | Type 2 diabetes; newly diagnosed HIV | Bacteraemia, meningitis, liver failure | Resistant to ampicillin, gentamycin, amikacin, carbenicillin; sensitive to TMP/SMX, chloramphenicol, tetracycline, cefotaxime, ceftazidime, ceftriaxone | Treated initially with gentamycin and ampicillin. Then switched to ceftriaxone and TMP/SMX. Patient died on day 6 of hospitalization |
| Vella [11] | 74-year-old man with 4 days' history of chills, subjective fevers, cough productive of purulent sputum, chest pain; fever, tachypnea, neutrophilia | COPD, history of several hospitalizations due to bronchial infections with Pseudomonas sp. | Sepsis resulting from respiratory infection (authors did specify if pneumonia was present) | Resistant to ceftazidime and aztreonam; intermediate to tobramycin, ticarcillin; susceptible to TMP/SMX, tetracyclines, quinolones, aminoglycosides, β-lactams | Treated initially with ceftazidime and then switched to cefuroxime. Full recovery. LOS: no data |
| Lambiase [9] | Sputum samples obtained from January 2006 to June 2008 from 332 cystic fibrosis patients. S. multivorum was isolated from 3 (2 female, 1 male) patients; 1 was identified as chronically infected by S. multivorum. All patients coinfected by at least 1 other Gram-negative pathogen | Cystic fibrosis, pancreatic insufficiency | Chronic lung infection | Resistant to β-lactams, carbapenems, aminoglycosides; susceptible to TMP/SMX and quinolones | Authors do not specify which therapy was used but mentioned that no subsequent decline in lung function was registered. LOS: no data |
| Grimaldi [3] | 64-year-old woman with leg pain and fever 24 hours after her dog scratched her right leg; patient confused, skin mottled from legs to abdominal wall, right leg was erythematous, edematous and tender to palpation | Morbid obesity (BMI 33.5 kg/m2), coronary artery disease, type 2 diabetes, rheumatoid arthritis treated with long-term intermediate-dose corticosteroids | Septic shock, encephalopathy, acute kidney injury requiring hemodialysis, respiratory failure in setting of necrotizing fasciitis | Resistance to penicillins, cephalosporins, carbapenems, aminoglycosides; susceptible to amoxicillin-clavulanate, ticarcillin-clavulanate, quinolones, TMP/SMX | Treated with amoxicillin-clavulanate for 10 days; patient discharged to rehabilitation unit after 7 weeks; no physical sequelae |
| Nielsen [12] | Report of 3 cases of infections after TRUS-Bx. All received oral prophylaxis with pivmecillinam and amoxicillin/clavulanic acid the night before and 2 hours before TRUS-Bx. Case 1: 79-year-old man hospitalized with fever, chills, malaise 3 days after TRUS-Bx; case 2: 59-year-old man with dysuria and frequent voiding 2 weeks after TRUS-Bx; case 3: 69-year-old-man followed in active surveillance program for low-risk prostate cancer consulted the emergency room 3 days after TRUS-Bx with dysuria and hematuria | Case 1: end-stage renal disease resulting from glomerulonephritis on hemodialysis, benign prostatic hyperplasia, prostate cancer; case 2: benign prostatic hyperplasia; case 3: benign prostatic hyperplasia | Case 1: cystitis and bacteraemia; case 2: cystitis; case 3: cystitis | Cases 1 and 2 had the same antibiogram: resistant to ampicillin, cefuroxime, piperacillin/tazobactam, mecillinam, gentamicin; susceptible to ciprofloxacin and TMP/SMX. Antibiogram not performed for case 3 | Case 1: initially started on piperacillin/tazobactam, ciprofloxacin and discharged after 6 days with oral ciprofloxacin; case 2: did not receive antibiotic treatment; case 3: empiric treatment with trimethoprim for 10 days. All patients recovered |
| This study | 67-year-old African American woman discharged to rehabilitation facility from our institution 4 weeks before, after being treated for hypercapnic hypoxic respiratory failure requiring mechanical ventilation, presented with sudden onset of generalized weakness, light-headedness, shortness of breath and dry cough the morning of admission | Obesity (BMI 38 kg/m2), chronic active smoker, COPD, obstructive sleep apnea, severe pulmonary hypertension, atrial fibrillation, type 2 diabetes, hypertension, dyslipidemia | Septic shock, acute kidney injury in setting of bacteraemia | Resistant to ceftazidime and TMP/SMX; intermediate to meropenem and piperacillin/tazobactam; susceptible to amikacin, cefepime, cefotaxime, ceftriaxone, ciprofloxacin, gentamicin, imipenem, tetracycline | Initially treated with cefepime (4 days) and vancomycin (2 days), then switched to ciprofloxacin for 10 days. Full recovery. LOS: 10 days, 4 in ICU |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; LOS, length of stay; TMP/SMX, trimethoprim-sulfamethoxazole; TRUS-Bx, transrectal ultrasound-guided prostate biopsy.
In addition, the medical literature mentions a report of three cases of cystitis, one of which was complicated with bacteraemia, within a period of 10 days after transrectal ultrasound-guided prostate biopsy where an infection hygiene evaluation identified and changed a step in the biopsy process in order to reduce the risk of inocula [12].
Although we could not establish the source of the pathogen, we speculate that it was present in the water or soil of the rehabilitation facility.
Sphingobacterium species are intrinsically resistant to many commonly used antibiotics and can grow in antiseptics and disinfectants [2], [13]. S. multivorum can produce an extended-spectrum β-lactamase and a metallo-β-lactamase, conferring resistance to third-generation cephalosporins and carbapenems, respectively [14]. In our review of the literature, the three most common susceptible antibiotic therapies were TMP/SMX, quinolones and tetracycline; however, in our case, there was resistance to TMP/SMX.
Including our case, we found a total of 14 cases of S. multivorum infection in nine male and five female subjects. Of the 11 cases where age was reported, ten occurred in adults; mean age was 56 years. One of the 14 patients died.
Here we present a case of septic shock resulting from Sphingobacterium multivorum bacteraemia, likely nosocomial in origin, which, although rare, can cause life-threatening infection.
Conflict of Interest
None declared.
References
- 1.Forbes B., Sahm D., Weissfeld A. 12th ed. Elsevier; Philadelphia: 2007. Diagnostic microbiology; pp. 358–362. [Google Scholar]
- 2.Mandell G., Bennett J., Dolin R. 7th ed. Elsevier; Philadelphia: 2010. Principles and practice of infectious diseases. 3016–3017, 3027. [Google Scholar]
- 3.Grimaldi D., Doloy A., Fichet J., Bourgeois E., Zuber B., Wajsfisz A., et al. Necrotizing fasciitis and septic shock related to the uncommon Gram-negative pathogen Sphingobacterium multivorum. J Clin Microbiol. 2012;50:202–203. doi: 10.1128/JCM.05151-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freney J., Hansen W., Ploton C., Meugnier H., Madier S., Bornstein N., et al. Septicemia caused by Sphingobacterium multivorum. J Clin Microbiol. 1987;25:1126–1128. doi: 10.1128/jcm.25.6.1126-1128.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manfredi R., Nanetti A., Ferri M., Mastroianni A., Coronado O.V., Chiodo F. Flavobacterium spp. organisms as opportunistic bacterial pathogens during advanced HIV disease. J Infect. 1999;39:146–152. doi: 10.1016/s0163-4453(99)90007-5. [DOI] [PubMed] [Google Scholar]
- 6.Areekul S., Vongsthongsri U., Mookto T., Chettanadee S., Wilairatana P. Sphingobacterium multivorum septicemia: a case report. J Med Assoc Thai. 1996;79:395–398. [PubMed] [Google Scholar]
- 7.Potvliege C., Dejaegher-Bauduin C., Hansen W., Dratwa M., Collart F., Tielemans C., et al. Flavobacterium multivorum septicemia in a hemodialyzed patient. J Clin Microbiol. 1984;19:568–569. doi: 10.1128/jcm.19.4.568-569.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhawan V.K., Rajashekaraiah K.R., Metzger W.I., Rice T.W., Kallick C.A. Spontaneous bacterial peritonitis due to a group IIk-2 strain. J Clin Microbiol. 1980;11:492–495. doi: 10.1128/jcm.11.5.492-495.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambiase A., Rossano F., Del Pezzo M., Raia V., Sepe A., de Gregorio F., et al. Sphingobacterium respiratory tract infection in patients with cystic fibrosis. BMC Res Notes. 2009;2:262. doi: 10.1186/1756-0500-2-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reina J., Borrell N., Figuerola J. Sphingobacterium multivorum isolated from a patient with cystic fibrosis. Eur J Clin Microbiol Infect Dis. 1992;11:81–82. doi: 10.1007/BF01971283. [DOI] [PubMed] [Google Scholar]
- 11.Vella J., Rodríguez A. [Respiratory infection caused by Sphingobacterium multivorum] An Med Interna. 2001;18:655–656. [PubMed] [Google Scholar]
- 12.Nielsen T.K., Pinholt M., Norgaard N., Mikines K.J. Inoculation of Sphingobacterium multivorum in the prostate by prostate biopsy. Scand J Urol. 2014;48:116–118. doi: 10.3109/21681805.2013.807871. [DOI] [PubMed] [Google Scholar]
- 13.Fass R., Barnishan J. In vitro susceptibilities of nonfermentative Gram-negative bacilli other than Pseudomonas aeruginosa to 32 antimicrobial agents. Rev Infect Dis. 1980;2:841–853. doi: 10.1093/clinids/2.6.841. [DOI] [PubMed] [Google Scholar]
- 14.Blahová J., Králiková K., Krcméry V., Sr., Kubonová K. Hydrolysis of imipenem, meropenem, ceftazidime, and cefepime by multiresistant nosocomial strains of Sphingobacterium multivorum. Eur J Clin Microbiol Infect Dis. 1997;16:178–180. doi: 10.1007/BF01709484. [DOI] [PubMed] [Google Scholar]