Abstract
Objective
The aim of this study was to evaluate the prevalence and severity of temporomandibular disorders (TMDs) among male university students in Riyadh, Saudi Arabia. The role of relevant medical and dental histories in the assessment of TMD in this Arab population was also addressed.
Methods
Required information was collected via a questionnaire. The first part of the questionnaire was used to obtain the medical and dental histories of participants. The second part included 10 questions regarding common TMD symptoms. Fonseca’s anamnestic index (FAI) was used to classify TMD severity as “no dysfunction”, “light dysfunction”, “moderate dysfunction”, or “severe dysfunction”.
Results
Of the 600 distributed questionnaires, 400 questionnaires were completed (response rate: 66.6%). Mean age of eligible participants was 21.90 ± 1.79 years. Psychological stress (30.5%) and direct restorations (77%) were the most commonly reported items on the medical and dental histories respectively for the total number of participants. According to the FAI, 53.2% of participants were classified as having no dysfunction, followed by light (36.1%), moderate (9.6%), and severe dysfunction (1.1%).
Conclusions
Based on the FAI, mild to moderate prevalence of TMD appears to exist among male university students in Riyadh. Histories of psychological stress and dental treatment were evident among these students. Information obtained from the FAI may be helpful in assessing the prevalence of TMD and has important implications for the early diagnosis of TMD and the prevention of future TMD-related complications.
Keywords: Temporomandibular disorder, Temporomandibular joint, TMDs, Fonseca’s questionnaire, Fonseca’s anamnestic index, Cross-sectional survey
1. Introduction
Temporomandibular disorders (TMDs) is a collective term that defines a subgroup of painful orofacial disorders, involving complaints of pain in the temporomandibular joint (TMJ) region, fatigue of the cranio cervico facial muscles (especially masticatory muscles), limitation of mandible movement, and the presence of articular clicking. The etiology of TMDs has been linked to multiple factors, including traumatic injury, immune-mediated systemic disease, neoplastic growths, emotional stress, occlusal interferences, mal positioning or loss of teeth, postural changes, dysfunctions of the masticatory musculature and adjacent structures, extrinsic and intrinsic changes of TMJ structure, nonfunctional movements of the mandible (bruxing), tooth clenching habits, or a combination of such factors (deSantis et al., 2014; Manfredini and Lobbezoo, 2010; Bonjardim et al., 2005a,b). Prosthodontic rehabilitation, orthodontic treatment, orthognathic surgery, and mandibular fractures have been associated with TMJ changes and worsening of existing TMD (Goldstein, 1999). Loading, altered jaw position, and mechanical stress in response to the aforementioned treatments induce morphological changes in the TMJ, due to its inherent adaptive capacity (Arnett et al., 1996).
The prevalence of TMDs ranges from 20% to 50%. The variability in prevalence may be attributed to differences in the race of the population, in the sampling design and criteria, and in the methods used for collecting information (Lee et al., 2013; Modi et al., 2012; Ebrahimi et al., 2011; Vojdani et al., 2012; Nomura et al., 2007; de Oliveira et al., 2006; Feteih, 2006; Farsi, 2003). Screening for TMDs in a population is a challenge for researchers and clinicians, and several TMD assessment tools have been proposed in the literature. However, no universal diagnostic criteria have yet been established. In response to this need for a universally accepted TMD assessment tool, Dworkin and Leresche (1992) proposed the RDC/TMDs, which have since been used in several clinical and epidemiological studies. Most recently, Schiffman and colleagues (2014) proposed a new comprehensive version of the RDC/TMDs, known as the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD). They claim that the DC/TMD includes a valid and reliable screening questionnaire, as well as diagnostic algorithms for the most common pain-related TMDs. Despite their advantages, the RDC/TMD and DC/TMD are quite cumbersome assessment tools in that they require the individual to be present in order to render a TMD diagnosis, and they are difficult to use on large samples.
A self-administered questionnaire that includes the Fonseca’s anamnestic index (FAI) has been proposed as a low-cost, easily applied alternative TMD assessment tool for the non-patient population (Da Fonseca et al., 1994). The use of FAI for detecting TMD signs and symptoms offers the advantage of being easily used by either general practitioners or epidemiologists. Thus, the FAI would serve as a preliminary TMD screening tool. After the affected population is identified, a more thorough investigation can be conducted, which would include a complete clinical examination and use of diagnostic instruments to confirm the diagnosis. In a literature review regarding the reliability of using a questionnaire for assessing the severity of TMDs, Campos and colleagues (2009) recommended the use of FAI because of its simplicity, speed, and cost effectiveness. The questionnaire also provides a severity index with less influence from the examiner and less variability in the measures (Nomura et al., 2007; de Oliveira et al., 2006). Fonseca’s questionnaire follows the characteristics of a multidimensional evaluation. It is composed of 10 questions that screen for the presence of pain in the TMJ, head, and back; pain while chewing; parafunctional habits; movement limitations; joint clicking; perception of malocclusion; and sensation of emotional stress (Da Fonseca et al., 1994).
Psychological factors are known to play a role in the etiology and persistence of TMDs. In particular, a high incidence of exposure to stressful life events and elevated levels of anxiety and stress-related symptoms have been reported in TMD patients (Pesqueira et al., 2010; Pallegama et al., 2005). Anxiety and depression are the most frequent clinical disorders in the general population and are highly present among university students. The repercussions of academic stress on the health of university students have been reported in the literature (Bonjardim et al., 2009). The university setting provides an ideal context for studying the mental health of young adults. University students are often undergoing role transitions, such as moving away from the family home for the first time, residing with other students, and experiencing reduced adult supervision. These changes may increase the risk of depression (Bonjardim et al., 2009; Pesqueira et al., 2010).
The aim of this study was to use a cross-sectional epidemiological survey to investigate the prevalence and severity of TMDs in male Saudi university students using Fonseca’s questionnaire. The role of relevant medical and dental histories in the assessment of TMD was also investigated. We hypothesized that using the FAI to characterize TMJ dysfunction would be helpful in furthering the understanding of TMD prevalence among Saudi university students and would provide information important for the early diagnosis and management of TMDs.
2. Methods
2.1. Study design
This descriptive cross-sectional research project was approved by the Ethics Committee of the College of Dentistry Research Center, King Saud University, Riyadh. The study was carried out between September 2013 and March 2014. Required information was collected via an anonymous questionnaire that was adopted from previous studies (Modi et al., 2012; Ebrahimi et al., 2011; Vojdani et al., 2012; Nomura et al., 2007; de Oliveira et al., 2006; Feteih, 2006; Farsi, 2003) and modified to suit the requirements of our study. An Arabic translation of the questionnaire was provided to students who could not read English. Questionnaires and a cover letter stating the instructions, rationale, and purpose of the survey were distributed to a conveniently selected sample of 600 male university students from the Colleges of Medicine, Dentistry, Pharmacy, Applied Medical Sciences and Engineering at King Saud University, Riyadh, Saudi Arabia. Students who were willing to participate in the study filled the questionnaire by hand and returned it in person. Participants were not given a time limit for completing the questionnaire.
2.2. Questionnaire
The questionnaire comprised two main parts. The first part collected demographic information and past medical, dental, TMJ, and facial trauma histories. The second part asked Fonseca’s 10 questions (Da Fonseca et al., 1994). Participants were requested to select one answer: yes, no, or sometimes. Each “yes” answer was assigned a value of 10, each “sometimes” answer a value of 5, and each “no” answer a value of 0. The sum of the values for all 10 answers was used to classify each subject according to the criteria shown in Table 1. Participants having a history of TMJ trauma, receiving orthodontic treatment or treatment for TMD, and/or suffering from any immunocompromised disease were excluded from the study (Hiz et al., 2012).
Table 1.
TMDs severity classification based on Fonseca’s anamnestic index.
| Without dysfunction | Score b/w 0–15 |
| With light dysfunction | Score b/w 20–40 |
| With moderate dysfunction | Score b/w 45–65 |
| With severe dysfunction | Score b/w 70–100 |
2.3. Statistical analysis
Descriptive statistics and frequency analyses of the collected data were performed in the Statistical Package for Social Sciences (SPSS) software (version 17 SPSS, Chicago, Illinois, USA). Age of the participants was expressed as the mean ± standard deviation (SD), and 95% confidence intervals were reported. Chi-square tests for statistical significance were applied to the frequencies within each Fonseca’s severity category and to the frequencies of responses to each Fonseca’s question, with p < 0.05 regarded as statistically significant.
3. Results
Of 600 questionnaires distributed, 400 completed questionnaires were received (response rate: 66.6%).Of these 400 respondents, 120 (20%) were excluded from the study based on the aforementioned criteria, leaving a total of 280 (46.66%) participants for the analysis. Eligible respondents included 141 (50.5%) from the College of Dentistry, 56 (20%) from the College of Medicine, 31 (11.25%) from the College of Pharmacy, 30 (9.25%) from the College of Engineering, and 25 (9%) from the College of Applied Medical Sciences. The mean age of participants was 21.90 ± 1.79 years.
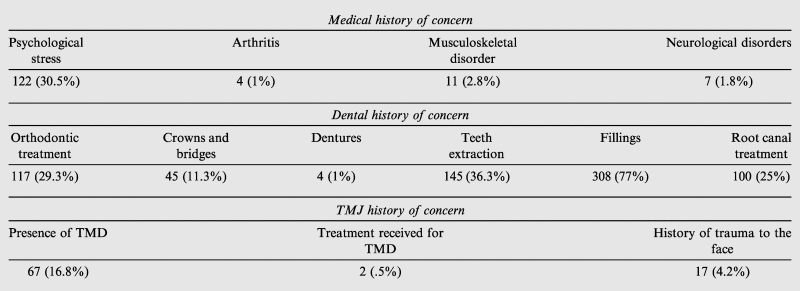
The medical, dental, and TMJ histories of all the 400 participants are presented in Table 2. With respect to past medical history, 30.5% of participants reported a history of psychological stress. Over three-fourths (77%) of participants reported a history of dental fillings. Whereas 67 participants (16.8%) reported a history of TMD, only 2 of them (0.5%) had received treatment for it.
Table 2.
Medical, dental and TMJ history of all the participants (N = 400).
 |
The number and percentage of participants with different levels of TMJ dysfunction based on the FAI are presented in Table 3 and Fig. 1. Almost half of participants (53.2%) were classified as having no dysfunction, whereas only 1.1% was classified as having severe dysfunction. There was no significant difference in the mean age of participants among the Fonseca’s severity categories.
Table 3.
Classification of severity of temporomandibular disorders based on Fonseca’s anamnestic index (N = 280).
| Fonseca’s classification | N (%) | Mean age ± SD | 95% Confidence interval for mean |
Minimum | Maximum | |
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| Without dysfunction | 149 (53.2) | 21.84 ± 1.78 | 21.53 | 22.16 | 19.00 | 27.00 |
| With light dysfunction | 101 (36.1) | 21.76 ± 1.85 | 21.34 | 22.17 | 19.00 | 26.00 |
| With moderate dysfunction | 27 (9.6) | 22.55 ± 1.57 | 21.81 | 23.28 | 20.00 | 25.00 |
| With severe dysfunction | 3 (1.1) | 23.66±.57 | 22.23 | 25.10 | 23.00 | 24.00 |
| Total | 280 | 21.90 ± 1.79 | 21.66 | 22.17 | 19.00 | 27.00 |
Figure 1.

Classification on the basis of severity of TMDs (n = 400).
The frequencies of the participants in Fonseca’s severity categories with respect to past medical and dental histories are listed in Table 4. Most participants who reported a history of psychological stress (42.2%), teeth extraction (45.67%), and dentures (66.66%) were classified by the FAI as having light dysfunction. Participants with a history of crowns and bridges were classified equally as having no dysfunction (29.72%) or light dysfunction (43.24%) for each category. The majority of participants in the moderate dysfunction category had a history of dental fillings (10.90%), teeth extraction (13.58%), and root canal treatment (8.41%). Most participants in the severe dysfunction category had a history of dental fillings (1.42%) or teeth extraction (2.46%).
Table 4.
Level of TMJ dysfunction with medical and dental history of concern of the participants included in the analysis (N = 280).
| Medical or dental complaint | Fonseca’s classification |
Chi square p value | |||
|---|---|---|---|---|---|
| Without dysfunction N (%) | Light dysfunction N (%) | Moderate dysfunction N (%) | Severe dysfunction N (%) | ||
| Psychological stress N = 83 (29.64%) | 35 (42.2) | 35 (42.2) | 12 (14.4) | 1 (1.2) | .079 |
| Root canal treatment N = 202 (72.14%) | 29 (14.35) | 31 (15.34) | 17 (8.41) | 1 (0.49) | .000 |
| Fillings N = 211 (75.35%) | 103 (48.81) | 82 (38.86) | 23 (10.90) | 3 (1.42) | .062 |
| Teeth extraction N = 81 (28.92%) | 31 (38.27) | 37 (45.67) | 11 (13.58) | 2 (2.46) | .009 |
| Dentures N = 3 (1.07%) | 0 (0) | 2 (66.66) | 1 (33.33) | 0 (0) | .241 |
| Crowns and bridges N = 37 (13.21%) | 11 (29.72) | 16 (43.24) | 10 (27.02) | 0 (0) | .000 |
Participants’ responses to Fonseca’s 10 questions are shown in Table 5. Chi-square analysis of the frequencies of responses to every question revealed a statistically significant difference (p < 0.05). Among the “yes” responses, the most frequently reported TMJ-related problem was poor articulation of the teeth (23.2%). Difficulty in opening the mouth (1.8%) was the least frequently reported problem. Among the “sometimes” responses, nervousness (26.1%) was the most frequently reported problem. Earaches or pain in the cranio mandibular joints (5.7%) was the least frequently reported problem.
Table 5.
Response of the participants to Fonseca’s 10 questions (N = 280).
| S. No. | Questions | Yes N (%) | Sometimes N (%) | No N (%) | Chi square p value |
|---|---|---|---|---|---|
| 1. | Is it hard for you to open your mouth? | 5 (1.8) | 37 (13.2) | 238 (85) | .000 |
| 2. | Is it hard for you to move your mandible from side to side? | 8 (2.9) | 25 (8.9) | 244 (87.1) | .000 |
| 3. | Do you get tired/muscular pain while chewing? | 35 (12.5) | 62 (22.1) | 180 (64.3) | .000 |
| 4. | Do you have frequent headaches? | 42 (15) | 52 (18.6) | 186 (66.4) | .000 |
| 5. | Do you have pain on the nape or stiff neck? | 22 (7.9) | 58 (20.7) | 199 (71.1) | .000 |
| 6. | Do you have earaches or pain in cranio mandibular joints? | 8 (2.9) | 16 (5.7) | 255 (91.1) | .000 |
| 7. | Have you noticed any TMJ clicking while chewing or when you open your mouth? | 34 (12.1) | 62 (22.1) | 182 (65) | .000 |
| 8. | Do you clench or grind your teeth? | 48 (17.1) | 31 (11.1) | 199 (71.1) | .000 |
| 9. | Do you feel your teeth do not articulate well? | 65 (23.2) | 30 (10.7) | 184 (65.7) | .000 |
| 10. | Do you consider yourself a tense (nervous) person? | 54 (19.3) | 73 (26.1) | 152 (54.3) | .000 |
4. Discussion
The present study provides information about the prevalence and severity of TMDs, based on the FAI, in male university students of Riyadh, Saudi Arabia. The response rate of the questionnaire (∼ 67%) compared to other studies (de Oliveira et al., 2006; Modi et al., 2012; Nomura et al., 2007) was regarded as satisfactory. The prevalence of TMDs based on the FAI varies among published studies. In our study 46.8% of participants were classified by the FAI as having light, moderate, or severe TMD. This prevalence is within the range of FAI-based TMD prevalence rates (42–68%) reported by other investigators (Modi et al., 2012; Conti et al., 1996; Nomura et al., 2007; Shiau and Chang, 1992; de Oliveira et al., 2006). In addition to factors such as ethnic background and sample size, the variation in TMD prevalence among these studies may be attributed to gender distribution. According to some studies (de Oliveira et al., 2006; Nomura et al., 2007), females have a greater risk of TMD than males. In our study, only male students were included; therefore, our TMD prevalence estimate might be underestimated.
Our study also examined the impact of relevant medical and dental histories on the prevalence of TMDs in Arabic university students. Nearly one-third of participants had a history of psychological stress, and out of these 57.8% was classified as having some degree of TMD dysfunction ranging from light to severe. These outcomes are in agreement with those of Pesqueira and colleagues (2010) and Bonjardim and colleagues (2009), who asserted that stress and anxiety play important roles in TMDs by acting as predisposing or aggravating factors. However, it is difficult to measure a variable such as stress or anxiety. Moreover, although efforts have been made to find the prevalence of stress among TMD patients, there is a need for long-term studies of this issue in the local population.
Historically, dental professionals have thought that occlusion is strongly linked to the occurrence of TMDs. This notion is supported by little scientific data, and literature reviews by Higdon (2009) and Badel et al. (2012), suggest that occlusion is not the dominant cause of TMDs. Nevertheless, occlusion is a basic issue associated with dental restorative procedures that change or supplement the compromised or lost occlusal relations. Any error in occlusion caused by dental treatment results in increased muscle tension and pain, which cause a mild unloading of the TMJ. This malocclusion sometimes leads to a TMD, depending on the degree of occlusal error.
Interestingly, a history of dental treatment was a very common finding among the participants in our study. Almost one-fourth of participants who were classified as having moderate to severe dysfunction had a history of past dental treatment. However, such an association should be interpreted carefully in light of the limitations of this study, such as the use of a brief questionnaire, a conveniently selected sample, a sample population comprised of only male students, and distribution of the questionnaire among all levels of undergraduate and graduate students. Although our study provided some information regarding the prevalence and severity of TMDs in young male Saudis, long-term clinical studies should be conducted in this region. Early diagnosis and prevention of future complications associated with TMDs are key for successful TMD treatment.
5. Conclusions
Based on the FAI, mild to moderate prevalence of TMD appears to be evident among male Saudi university students. The FAI is a useful TMD screening tool that has important implications for the early diagnosis of TMD and the prevention of future complications from it. Histories of psychological stress and dental treatment were found in students with mild to severe TMJ dysfunction. Therefore, the collection of relevant medical and dental histories might be useful in the TMD screening process. Longitudinal studies in this population are warranted to follow the prevalence of TMD and the healthcare needs of TMD patients.
Conflict of interest
Authors declare no conflict of interest associated with this publication.
Acknowledgements
The authors are thankful to all the participating students for their participation and cooperation in the study. Also the authors would like to thank Mr. Nassr Maflehi for his help in the statistical analysis. The research project was approved by the College of Dentistry Research Center (Registration number IR0058).
Footnotes
Peer review under responsibility of King Saud University.
References
- Arnett G.W., Milam S.B., Gottesman L. Progressive mandibular retrusion- idiopathic condylar resorption, 1. Am. J. Orthod. Dentofac. Orthop. 1996;110:8–15. doi: 10.1016/s0889-5406(96)70081-1. [DOI] [PubMed] [Google Scholar]
- Badel T., Marotti M., Pavicin I.S., Kes V.B. Temporomandibular disorders and occlusion. Acta Clin. Croat. 2012;51:419–424. [PubMed] [Google Scholar]
- Bonjardim L.R., Gaviao M.B., Pereira L.J., Castelo P.M. Anxiety and depression in adolescents and their relationship with signs and symptoms of temporomandibular disorders. Int. J. Prosthodont. 2005;18:347–352. [PubMed] [Google Scholar]
- Bonjardim L.R., Gavião M.B.D., Pereira L.J., Castelo P.M., Garcia R.C.M.R. Signs and symptoms of temporomandibular disorders in adolescents. Braz. Oral Res. 2005;19(2):93–98. doi: 10.1590/s1806-83242005000200004. [DOI] [PubMed] [Google Scholar]
- Bonjardim L.R., Lopes-Filho R.J., Amado G., Albuquerque R.L., Jr., Goncalves S.R. Association between symptoms of temporomandibular disorders and gender, morphological occlusion, and psychological factors in a group of university students. Indian J. Dent. Res. 2009;20:190–194. doi: 10.4103/0970-9290.52901. [DOI] [PubMed] [Google Scholar]
- Campos J.A.D.B., Goncalves D.A.G., Camparis C.M., Speciali J.G. Reliability of a questionnaire for diagnosing the severity of temporomandibular disorder. Rev. Bras. Fisioter. 2009;13(1):38–43. [Google Scholar]
- Conti P.C.R., Ferreira P.M., Pegoraro L.F., Conti J.V., Salvador M.C.G. A cross sectional study of prevalence and etiology of signs and symptoms of temporomandibular disorders in high school and university students. J. Orofac. Pain. 1996;10(3):254–262. [PubMed] [Google Scholar]
- Da Fonseca D.M., Bonfante G., Valle A.L., de Freitas S.F.T. Diagnósticopelaanamnese da disfunçãocraniomandibular. Rev. Gauch de Odontol. 1994;4(1):23–32. [Google Scholar]
- de Oliveira A.S., Dias E.M., Contato R.G., Berzin F. Prevalence study of signs and symptoms of temporomandibular disorder in Brazilian college student. Braz. Oral Res. 2006;20(1):3–7. doi: 10.1590/s1806-83242006000100002. [DOI] [PubMed] [Google Scholar]
- deSantis T.O., Motta L.J., Gonzalez D.A.B., Ferrari R.A.M., Fernandes K.P.S., de Godoy C.H.L., Alfaya T.A., Bussadori S.K. Accuracy study of the main screening tools for temporomandibular disorder in children and adolescents. J. Bodyw. Mov. Ther. 2014;18:87–91. doi: 10.1016/j.jbmt.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Dworkin S.F., LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J. Carniomandib. Disord. 1992;6(4):301–355. [PubMed] [Google Scholar]
- Ebrahimi M., Dashti H., Mehrabkhani M., Arghavani M., Daneshvar-Mozafari A. Temporomandibular disorders and related factors in a group of Iranian adolescents: a cross sectional survey. J. Dent. Res. Dent. Clin. Dent. Prospects. 2011;5(4):123–127. doi: 10.5681/joddd.2011.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsi N.M.A. Symptoms and signs of temporomandibular disorders and oral parafunctions among Saudi children. J. Oral Rehabil. 2003;30:1200–1208. doi: 10.1111/j.1365-2842.2003.01187.x. [DOI] [PubMed] [Google Scholar]
- Feteih R.M. Signs and symptoms of temporomandibular disorders and oral parafunctions in urban Saudi Arabian adolescents: a research report. Head Face Med. 2006;2:25. doi: 10.1186/1746-160X-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B.H. Temporomandibular disorders: a review of current understanding. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999;88:379–385. doi: 10.1016/s1079-2104(99)70048-x. [DOI] [PubMed] [Google Scholar]
- Higdon, S.J., 2009. Occlusion and temporomandibular disorders: is there a causal relationship? A critique of the existing scientific literature. TMJ Oregon [Internet] <http://tmjoregon.com/wp/wp-content/themes/azurio/images/pdfs/providers_pdfs/Critique%20of%20Existing%20Science%20 Related%20to%20Occlusion%20and%20TMD.pdf>.
- Hiz Ozcan, Ediz L., Ozkan Y., Bora A. Clinical and magnetic resonance imaging findings of the temporomandibular joint in patients with rheumatoid arthritis. J. Clin. Med. Res. 2012;4(5):323–331. doi: 10.4021/jocmr1084w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.Y., Kim Y.K., Kim S.G., Yun P.Y. Evaluation of Korean teenagers with temporomandibular joint disorders. J. Korean Assoc. Oral Maxillofac. Surg. 2013;39:231–237. doi: 10.5125/jkaoms.2013.39.5.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredini D., Lobbezoo F. Relationship between bruxism and temporomandibular disorders: a systematic review of literature from 1998 to 2008. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010;109:e26–e50. doi: 10.1016/j.tripleo.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Modi P., Shaikh S.S., Munde A. A cross sectional study of prevalence of temporomandibular disorders in university students. Int. J. Sci. Res. Publ. 2012;2(9):1–3. [Google Scholar]
- Nomura K., Vitti M., Oliveira A.S., Chaves T.C., Semprini M., Siéssere S., Hallak J.E., Regalo S.C. Use of the Fonseca’s questionnaire to assess the prevalence and severity of temporomandibular disorders in Brazilian dental undergraduate. Braz. Dent. J. 2007;18(2):163–167. doi: 10.1590/s0103-64402007000200015. [DOI] [PubMed] [Google Scholar]
- Pallegama R.W., Ranasinghe A.W., Weerasinghe V.S., Sitheeque M.A.M. Anxiety and personality traits in patients with muscle related temporomandibular disorders. J. Oral Rehabil. 2005;32:701–707. doi: 10.1111/j.1365-2842.2005.01503.x. [DOI] [PubMed] [Google Scholar]
- Pesqueira A.A., Zuim P.R., Monteiro D.R., RibeiroPdo P., Garcia A.R. Relationship between psychological factors and symptoms of TMD in university undergraduate students. Acta Odontol. Latinoam. 2010;23(3):182–187. [PubMed] [Google Scholar]
- Schiffman E., Ohrbach R., Truelove E., Look J., Anderson G., Goulet J.P. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for clinical and research applications: recommendations of the international RDC/TMD Consortium Network∗ and Orofacial Pain Special Interest Group†. J. Oral Facial Pain Headache. 2014;28(1):6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau Y., Chang C. An epidemiological study of temporomandibular disorders in university students of Taiwan. Commun. Dent. Oral Epidemiol. 1992;20:43–47. doi: 10.1111/j.1600-0528.1992.tb00672.x. [DOI] [PubMed] [Google Scholar]
- Vojdani M., Bahrani F., Ghadiri P. The study of relationship between reported temporomandibular symptoms and clinical dysfunction index among university students in Shiraz. Dent. Res. J. 2012;9(2):221–225. doi: 10.4103/1735-3327.95240. [DOI] [PMC free article] [PubMed] [Google Scholar]


