Abstract
A 73-year-old male metalworker was admitted to our hospital with a 3-year history of progressive dry cough. Chest high-resolution computed tomography revealed emphysematous changes and reticular lesions, which is referred to as combined pulmonary fibrosis and emphysema (CPFE). Surgical lung biopsy specimens revealed unclassified interstitial pneumonia, including a nonspecific interstitial pneumonia pattern and usual interstitial pneumonia pattern. Two years after his first admission he developed rapid progressive renal dysfunction with an elevated level of myeloperoxidase-antineutrophil cytoplasmic antibody (428 EU). A renal biopsy specimen revealed interstitial nephritis and glomerulonephritis. Consequently, microscopic polyangiitis preceded by CPFE was diagnosed. Despite transient exacerbation of renal involvement, his general condition remained mostly stable during a 2-year period of corticosteroid treatment. He ultimately died from severe pneumococcal pneumonia associated with acute lung injury.
Keywords: Microscopic polyangiitis, Interstitial lung disease, Emphysema, Combined pulmonary fibrosis, Emphysema
1. Introduction
Microscopic polyangiitis (MPA) is a systemic necrotizing vasculitis of the small vessels and is associated with various circulating autoantibodies, in particular myeloperoxidase-antineutrophil cytoplasmic antibody (MPO-ANCA). Although diffuse alveolar hemorrhage secondary to pulmonary capillaritis is the most frequent manifestation of pulmonary involvement, an association with pulmonary fibrosis was recently reported. Moreover, oxidation induced by MPO-ANCA may trigger emphysema and fibrosis. In this report we describe the clinicopathologic findings of a case of MPA preceded by combined pulmonary fibrosis and emphysema (CPFE).
2. Case presentation
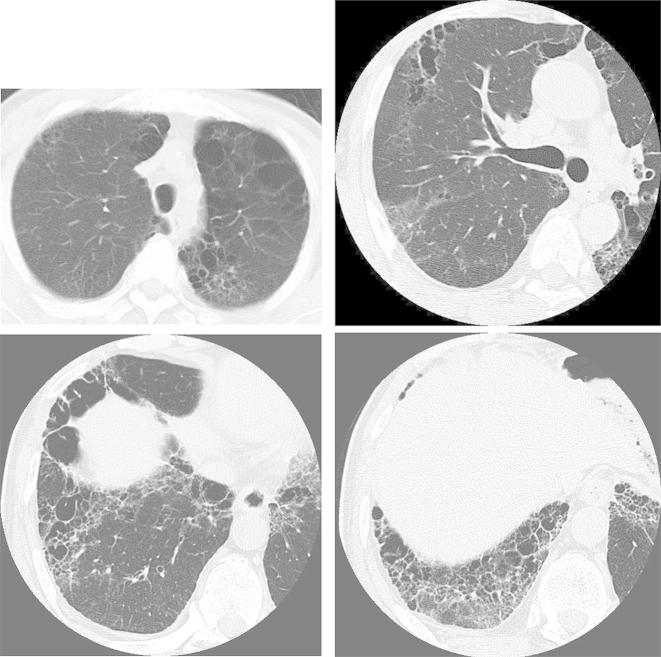
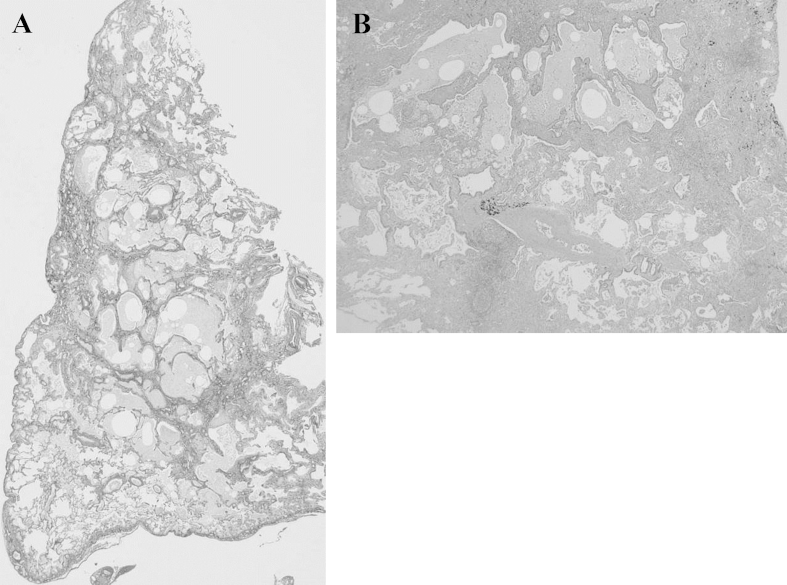
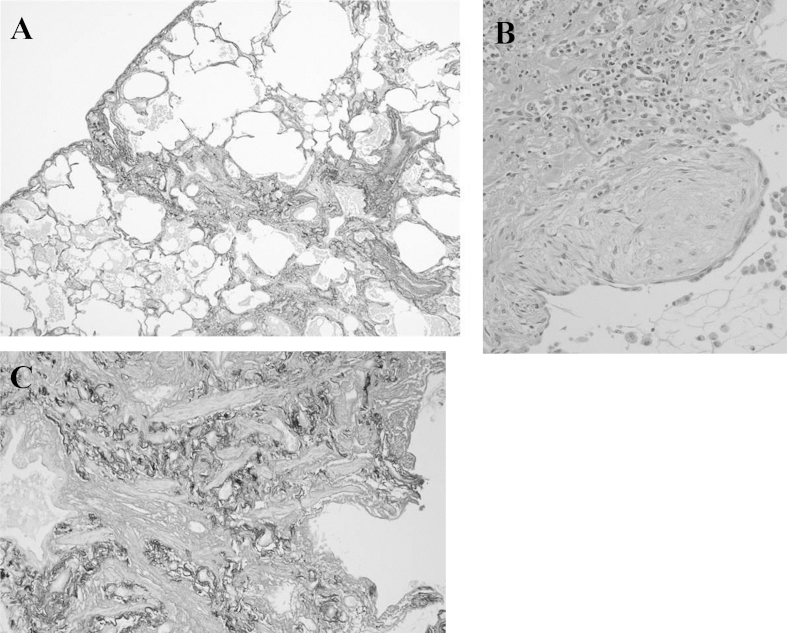
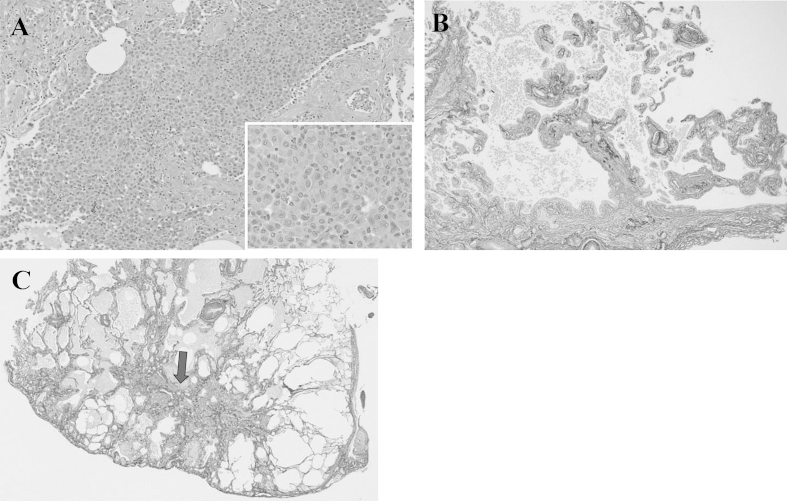
A 73-year-old man with a 3-month history of progressive dyspnea on exertion and dry cough was referred to our hospital for treatment. His medical history was noncontributory, and he had smoked for 25 pack-years. His occupational history included metal work. On admission, his pulse rate was 62/min with regular rhythm, blood pressure was 120/68 mm Hg, body temperature was 36.0 °C, and respiratory rate was 20/min. Chest auscultation revealed fine crackles in bilateral lung bases. Laboratory analysis revealed high levels of lactate dehydrogenase (632 IU/L), Krebs von den Lungen-6 (701 U/mL), surfactant protein-D (195 ng/mL), and creatinine (1.03 mg/dL); MPO and PR3-ANCA were negative. Urinalysis revealed microscopic hematuria with red blood cells and proteinuria. Arterial blood gas analysis showed a pH of 7.38, PaCO2 of 38.6 Torr, and PaO2 of 77.8 Torr in room air. Findings from pulmonary function testing were as follows: vital capacity, 3.3 L (105.8% of predicted); FEV1, 2.45 L (116.1% of predicted); and diffusing capacity for carbon monoxide, 8.51 mL/min/mmHg (59.9% of predicted). A chest radiograph revealed diffuse reticular shadows in the bilateral lower lung fields. A chest computed tomography (CT) scan showed paraseptal emphysema in the bilateral upper lobes and reticular lesions with enlarged cysts in the bilateral lower lobes (Fig. 1). Lung biopsy specimens from the right S6 were obtained by video-assisted thoracoscopic surgery and revealed prominent, uniform thickening of alveolar septa by fibrosis (fibrotic nonspecific interstitial pneumonia [f-NSIP] pattern) (Fig. 2A). The alveolar septal thickening mainly consisted of collagen deposition with mild inflammatory cell infiltration and lymphoid hyperplasia (Fig. 2B). Lung biopsy specimens from the right S8 revealed subpleural and perilobular fibrosis adjacent to relatively normal alveoli (usual interstitial pneumonia [UIP] pattern) (Fig. 3A). There were fibroblastic foci with dense collagen fibrosis (Fig. 3B) and smooth muscle cell proliferation in areas of dense collagen fibrosis (Fig. 3C). In addition, numerous macrophages with a few eosinophils were present in the alveolar lumen (DIP-like lesion) (Fig. 4A), and alveolar wall destruction with enlargement was seen in lung parenchyma (Fig. 4B). Patchy fibrotic lesions were also scattered around the terminal and respiratory bronchioles (Fig. 4C). There was no evidence of pulmonary vasculitis. These findings were consistent with a diagnosis of CPFE composed of unclassified interstitial pneumonia and paraseptal emphysema.
Fig. 1.
Chest computed tomography (CT) scans show paraseptal emphysema in the bilateral upper lobes and reticular lesions with enlarged cysts in the bilateral lower lobes.
Fig. 2.
A: Lung biopsy specimens from the right S6 obtained by video-assisted thoracoscopic surgery show prominent uniform thickening of alveolar septa by fibrosis (fibrotic nonspecific interstitial pneumonia [f-NSIP] pattern) (EVG stain). B: The alveolar septal thickening mainly consists of collagen deposition with mild inflammatory cell infiltration and lymphoid hyperplasia (HE stain).
Fig. 3.
Lung biopsy specimens from the right S8 showing subpleural and perilobular fibrosis adjacent to relatively normal alveoli (usual interstitial pneumonia [UIP] pattern) (A), as well as fibroblastic foci with dense collagen fibrosis (B) and smooth muscle cell proliferation in areas of dense collagen fibrosis (C).
Fig. 4.
Numerous macrophages with a few eosinophils were present in the alveolar lumen (DIP-like lesion) (A), and alveolar wall destruction with enlargement was seen in lung parenchyma (B). Patchy fibrotic lesions were also scattered around the terminal and respiratory bronchioles (C; arrow).
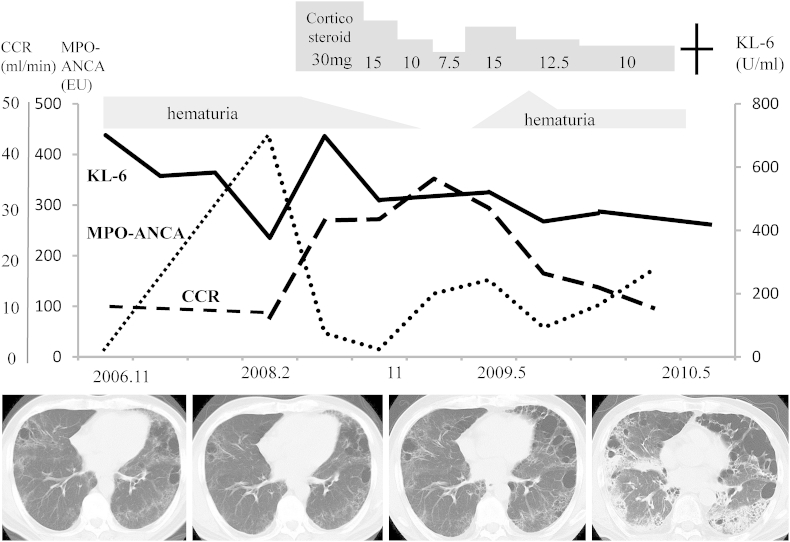
Two years after initial admission, he developed progressive renal dysfunction with elevated MPO-ANCA level (428 EU). Renal biopsy specimens revealed interstitial nephritis and glomerulonephritis. Ultimately, MPA preceded by CPFE was diagnosed based on his clinical course and pathologic findings. He was treated with prednisolone 30 mg/day, and the dose was tapered based on his respiratory condition. After beginning treatment, serum MPO-ANCA titer decreased and hematuria disappeared. When prednisolone was tapered to 7.5 mg/day at 1 year after the start of treatment, his serum MPO-ANCA titer increased to 152 EU and he developed hematuria. Prednisolone dosage was increased to 15 mg/day, after which serum MPO-ANCA titer normalized but hematuria continued. During the 4-year treatment period, CPFE had progressed slowly, with enlargement of paraseptal emphysema in the upper lobes and prominent reticular shadows without honeycombing in the lower lobes. Diffusing capacity for carbon monoxide had also decreased as emphysema and fibrosis progressed. One year later, he died of respiratory failure caused by severe pneumococcal pneumonia (Fig. 5).
Fig. 5.
Clinical course of the patient. MPO-ANCA: myeloperoxidase-antineutrophil cytoplasmic antibody; KL-6: Krebs von den Lungen-6; CCR: creatinine clearance.
3. Discussion
The prognosis for MPA is poor even if corticosteroids and immunosuppressant therapy are administered. Lung involvement, such as diffuse alveolar hemorrhage and pulmonary fibrosis (PF), is an important prognostic factor. In a recent study of patients with antineutrophil cytoplasmic antibody–associated vasculitis in Japan, 47.4% and 11.5% of MPA cases were complicated with PF and diffuse alveolar hemorrhage, respectively [1]. More recently, preceding idiopathic interstitial pneumonia was reported in MPA patients [2,3]. Initially, our patient had presented only with CPFE comprising unclassified interstitial pneumonia and paraseptal emphysema without vasculitis. Later, MPA was diagnosed based on the presence of glomerulonephritis and an elevated MPO-ANCA titer.
In PF with MPA the most common findings on chest CT are honeycombing, reticulonodular shadows, ground glass opacities, traction bronchiectasis, and a usual interstitial pneumonia pattern [2,4,5]. In the present case, chest CT showed paraseptal emphysema in the bilateral upper lobes and reticular lesions with enlarged cysts in the bilateral lower lobes, which had walls thinner than those typical of honeycombing. Tzouvelekis et al. reported that 7 of 40 CPFE patients (17.5%) were positive for MPO-ANCA and that MPA was diagnosed in 3 of these 7 patients [6].
CPFE was recently classified within the spectrum of smoking-induced chronic lung diseases and is identified by chest CT findings of both upper lobe emphysema and lower lobe fibrosis [8]. Cottin et al. studied patients with connective tissue disease who also had CPFE. Some had rheumatoid arthritis or systemic sclerosis, but none had MPA [9]. Occupational exposure to agents such as silica [10], diffuse panbronchiolitis [11], and cigarette smoke may be related to MPA development. In addition, Guilpain reported that oxidative stress—especially MPO-induced hypochlorous acid—could trigger fibroblast proliferation, resulting in PF [12].
Regarding the histologic features of surgical lung biopsy specimens, PF with MPA is classified as having UIP or NSIP pattern with lymphoid hyperplasia, bronchiolitis, and fibrosis scattered around the terminal and respiratory bronchioli [3,4,7]. However, these findings are common in patients with connective tissue disease. Thus, it is difficult to differentiate MPA from idiopathic interstitial pneumonia on the basis of radiologic and histologic findings.
In summary, some MPA cases are associated with CPFE. However, the pathogenesis of pulmonary fibrosis in MPA remains unclear. Therefore, future studies should focus on the role of MPO-ANCA to improve understanding of the clinicopathologic features of CPFE with MPA.
Acknowledgments
We would to thank Dr K. Shibuya for analysis of pathological findings (Department of Pathology, Toho University Omori Medical Center, Tokyo, Japan).
References
- 1.Sada K.E., Yamamura M., Harigai M. Classification and characteristics of Japanese patients with antineutrophil cytoplasmic antibody-associated vasculitis in a nationwide, prospective, inception cohort study. Arthritis Res. Ther. 2014;16:R101. doi: 10.1186/ar4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando M., Miyazaki E., Ishii T. Incidence of myeloperoxidase anti-neutrophil cytoplasmic antibody positivity and microscopic polyangitis in the course of idiopathic pulmonary fibrosis. Respir. Med. 2013;107:608–615. doi: 10.1016/j.rmed.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Hervier B., Pagnoux C., Agard C. Pulmonary fibrosis associated with ANCA-positive vasculitides. Retrospective study of 12 cases and review of the literature. Ann. Rheum. Dis. 2009;68:404–407. doi: 10.1136/ard.2008.096131. [DOI] [PubMed] [Google Scholar]
- 4.Homma S., Matsushita H., Nakata K. Pulmonary fibrosis in myeloperoxidase antineutrophil cytoplastic antibody associated vasculitides. Respirology. 2004;9:190–196. doi: 10.1111/j.1440-1843.2004.00581.x. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka T., Otani K., Egashira R. Interstitial pneumonia associated with MPO-ANCA: clinicopathological features of nine patients. Respir. Med. 2012;106:1765–1770. doi: 10.1016/j.rmed.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Tzouvelekis A., Zacharis G., Oikonomou A. Increased incidence of autoimmune markers in patients with combined pulmonary fibrosis and emphysema. Pulm. Med. 2013;13:31. doi: 10.1186/1471-2466-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hebisawa A., Kuramochi S., Ogura T. Nihon Kyobu Rinsyou. 2008;67:210–219. (in Japanese) [Google Scholar]
- 8.Cottin V., Nunes H., Brillet P.Y. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur. Respir. J. 2005;26:586–593. doi: 10.1183/09031936.05.00021005. [DOI] [PubMed] [Google Scholar]
- 9.Cottin V., Nunes H., Mouthon L. Combined pulmonary fibrosis and emphysema syndrome in connective tissue disease. Arthritis Rheum. 2010;63:295–304. doi: 10.1002/art.30077. [DOI] [PubMed] [Google Scholar]
- 10.Rihova Z., Maixnerova D., Jancova E. Silica and asbestos exposure in ANCA-associated vasculitis with pulmonary involvement. Ren. Fail. 2005;27:605–608. doi: 10.1080/08860220500200395. [DOI] [PubMed] [Google Scholar]
- 11.Sitara D., Hoffbrand B.I. Chronic bronchial suppuration and antineutrophil cytoplasmic antibody (ANCA) positive systemic vasculitis. Postgrad. Med. J. 1990;66:669–671. doi: 10.1136/pgmj.66.778.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guilpain P., Chereau C., Goulvestre C. The oxidation induced by antimyeloperoxidase antibodies triggers fibrosis in microscopic polyangiitis. Eur. Respir. J. 2011;37:1503–1513. doi: 10.1183/09031936.00148409. [DOI] [PubMed] [Google Scholar]