Abstract
Diaphragm pacing allows certain quadriplegic patients to be weaned from mechanical ventilation. Pacing failure can result from device dysfunction, neurotransmission failure, or degraded lung mechanics (such as atelectasis). We report two cases where progressive pacing failure was attributed to deteriorated chest wall mechanics. The first patient suffered from cervical spinal cord injury at age 45, was implanted with a phrenic stimulator (intrathoracic), successfully weaned from ventilation, and permanently paced for 7 years. Pacing effectiveness then slowly declined, finally attributed to rib cage stiffening due to ankylosing spondylitis. The second patient became quadriplegic after meningitis at age 15, was implanted with a phrenic stimulator (intradiaphragmatic) and weaned. After a year hypoventilation developed without obvious cause. In relationship with complex endocrine disorders, the patient had gained 31 kg. Pacing failure was attributed to excessive mechanical inspiratory load. Rib cage mechanics abnormalities should be listed among causes of diaphragm pacing failure and it should be kept in mind that a “good diaphragm” is not sufficient to produce a “good inspiration”.
Keywords: Chronic respiratory insufficiency, Mechanical ventilation, Quadriplegia, Diaphragm pacing, Ankylosing spondylarthritis, Obesity
Introduction
Diaphragm pacing allows patients suffering from high cervical spinal cord lesions who are quadriplegic and ventilator-dependent to be separated from their mechanical ventilator. Diaphragm pacing has clinical benefits and reduces health costs [1–5]. All of the currently available diaphragm pacing techniques rely on a similar principle: electrodes are implanted in the vicinity of the phrenic nerve (either intrathoracically during a video-assisted mini-thoracotomy [6] -cuff electrodes-, or intradiaphragmatically during laparoscopy [6] -hook wire electrodes-) and connected to an external stimulator that provides a modular stimulation current. After a post-implantation reconditioning period aimed at correcting diaphragmatic disuse atrophy [7], successful diaphragm pacing generally results in stable tidal volumes that are obtained with stable stimulation intensities [6]. Deteriorated ventilation can result from device dysfunction, neuromuscular issues including neurotransmission failure, or degraded respiratory mechanics (e.g. airway obstruction, atelectasis, etc.). These events generally translate in rapid onset clinical, spirometric and blood gases abnormalities. We report two observations of quadriplegic patients in whom a previously fully efficient diaphragm pacing slowly lost efficacy, resulting in full time or part time return to mechanical ventilation, without any of the usual mechanisms of diaphragm pacing failure being identified. In both cases, major changes in the mechanical properties of the chest wall appeared as plausible explanations of diaphragm pacing failure.
Case reports
Context
In France, diaphragm pacing is authorized and reimbursed by the social security system in quadriplegia-related ventilator dependency and central hypoventilation. Our multidisciplinary centre is the only centre for diaphragm pacing nationwide. Over 15 years, approximately 50 patients have been implanted with phrenic stimulators, mostly via the intrathoracic approach.
Case #1
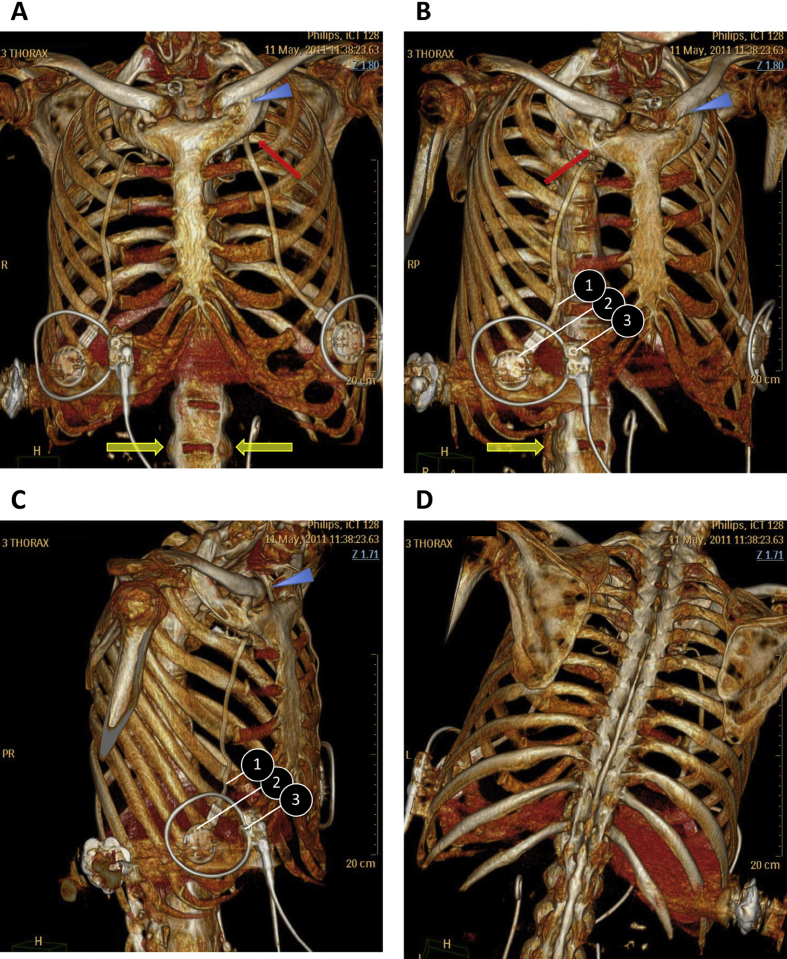
This patient suffered from a high cervical spinal injury (C1/C2 fracture, traffic accident) resulting in flaccid quadriplegia and full ventilator dependency in May 2000, aged 45. Diagnostic phrenic nerve stimulation demonstrated bilateral diaphragm responses, allowing diaphragm pacing. In May 2001, the patient was implanted with intrathoracic phrenic stimulators (Atrostim™, Atrotech, Finland). After 4 weeks of diaphragm reconditioning, complete ventilator weaning was obtained, the patient rapidly using diaphragm pacing 24 h a day and then doing so for several years. Decreased pacing efficiency was first noted in 2007, with the need to increase stimulation intensities to maintain tidal volume at the level needed to keep alveolar ventilation adequate (650–700 mL). The situation slowly deteriorated over the following years, with the appearance of dyspnoea in the sitting position, decreased tidal volumes, and partial followed by total loss of ventilatory autonomy leading to nocturnal, and then permanent ventilator dependency in 2012 (tidal volume 200–250 mL). Phrenic nerve function was verified several times, always with normal phrenic nerve conduction times and ample, and symmetrical diaphragm action potentials. A progressive decrease in tracheal pressure and abdominal movements following phrenic stimulation was noted. During this course, device replacement, aggressive correction of malnutrition and aggressive urologic management of chronic urinary tract infection (both liable to be responsible of muscle weakness and/or neuromuscular transmission impairment) were conducted without modifying the course of diaphragm pacing deterioration. In May 2011, high-resolution chest computed tomography revealed widespread vertebral fusion suggestive of ankylosing spondylitis (Fig. 1). This diagnostic had indeed been established prior to the accident having led to quadriplegia (HLA B-27 positive), the patient reporting the occasional use of non-steroidal anti-inflammatory drugs to control intermittent back pain before he became quadriplegic. It was concluded that the disease had evolved over years, the patient not perceiving pain because of the spinal lesion, and diaphragm pacing failure was attributed to thoracic ankylosis preventing diaphragm contractions to adequately inflate the rib cage. The patient died in May 2012 of severe pneumonia.
Fig. 1.
Volume rendering coronal oblique reformations obtained after multidetector computed tomography of the chest. Complete fusion of the vertebral bodies and ossification of the intervertebral disks result in a “bamboo spine” aspect (d) and the formation of marginal syndesmophytes between adjoining vertebrae (yellow arrows). Both sternoclavicular (blue arrowheads) and costosternal joints (red arrows) are fused. (1: wire connecting the phrenic electrode to the subcutaneous receiver; 2: subcutaneous receiver; 3: external surface electrode transmitting energy and settings from the stimulator -not shown- to the subcutaneous receiver).
Case #2
At age 15, this patient suffered from meningococcal meningitis complicated by acute cerebral oedema leading to compressive spinal cord ischaemia. Magnetic resonance imaging did not show spinal cord lesions below C3. Diagnostic phrenic nerve stimulation demonstrated bilateral diaphragm response, allowing diaphragm pacing. In September 2009, the patient was implanted with intradiaphragmatic phrenic stimulators (NeuRXDPS™, Synapse Biomedical, Oberlin, OH, USA). After nine months (July 2010), diaphragm pacing was considered efficient, producing tidal volumes of 500 mL on average and achieving normocapnia (PaCO2 39 mmHg). One year later, tidal volumes had fallen to less than 250 mL and the patient was hypercapnic (PaCO2 51 mmHg) in spite of maximal stimulation intensities (25 mA). Phrenic stimulation induced-thoracic expansion had become almost undetectable. No device dysfunction was identified. Phrenic nerve conduction was normal, as was tracheal twitch pressure in response to bilateral phrenic stimulation. Over the same time frame, complex hormonal disorders led to massive weight gain (from 67 to 104 kg, body mass index 22.6–35.1 kg/m2), with an ulterior diagnostic of Klinefelter syndrome. Diaphragm pacing failure was attributed to excessive chest wall and abdominal weight preventing diaphragm contractions to adequately inflate the rib cage.
Discussion
These two observations point at alterations of chest wall compliance as a mechanism of diaphragm pacing failure that has seemingly not been described before. In both cases, extensive workup failed to find any technical dysfunction of the stimulators, and any patent abnormality of the phrenic nerves. In the first case, malnutrition of chronic infection could have been responsible for diaphragmatic dysfunction, but their aggressive correction did not improve the situation. Chronic diaphragm fatigue could also be discussed, but the patient had been paced continuously between 2000 and 2007 without any reduction in ventilation. In the hypothesis of diaphragm fatigue, improvement should have been observed when the patient was returned to nocturnal mechanical ventilation.
In ankylosing spondylitis, vertebral fusion (Fig. 1), responsible for the typical “bamboo spine” aspect, is associated with sternoclavicular and sternocostal ankylosis (present in our patient), and at times with costovertebral joints abnormalities [8]. These abnormalities are naturally bound to deteriorate the transformation of diaphragm strength into rib cage movement. In addition, the coordinated action of the diaphragm and abdominal muscles is an important determinant of the compensation of rib cage stiffness in ankylosing spondylitis [9]. In our patient, the lack of any abdominal muscle activity probably aggravated the impact of ankylosis on tidal volume production.
Massive obesity is associated with a restrictive ventilatory defect and decreased rib cage compliance [10]. This corresponds to an elastic load which, to be overcome, requires the respiratory muscles to increase the work that they develop [11] in response to an augmented neural drive [12]. Although this has apparently not been specifically studied, the conjunction of obesity and respiratory muscle weakness is bound to aggravate the deleterious effects of rib cage mechanical deterioration on the production of ventilation. In spite of diaphragm pacing, our second patient represents an extreme case of respiratory weakness, because of the absence of co-contraction of thoracic muscles with the diaphragm and of the absence of any possibility to compensate the inspiratory load by active expiration. Diaphragm pacing also prevent the extension of diaphragm activity over expiration that has been described as a feature of respiratory load compensation in obesity [13].
Conclusion
In conclusion, our observations are practical reminders that the inspiratory action of the diaphragm does not only depend on diaphragm contractile properties, but also of a diaphragm “contractile environment” that includes diaphragm geometry, abdominal compliance [14,15], and rib cage compliance. It is also a reminder that breathing is intimately dependent on, and interferes with, spinal cord and costovertebral joints mechanics [16]. That a “good diaphragm” is not sufficient to produce a “good inspiration” must be kept in mind when managing patients with diaphragm pacing.
Funding and role of the funding source
This work was supported by the program “Investissement d'Avenir ANR-10-AIHU 06” of the French Government and the non-profit research association “ADOREPS”, Paris, France. One of the patients described was included in a study of diaphragm pacing supported by grant DRC98075 from the Programme Hospitalier de Recherche Clinique National of the French Ministry of Health and of which the sponsor was the Direction de la Recherche Clinique, Assistance Publique-Hôpitaux de Paris, Paris, France. The sponsor had no responsibility in the writing of the present manuscript.
Patients consent
The patients admitted to the department of respiratory and critical care of the Pitié-Salpêtrière hospital are systematically informed that their medical data can be used, anonymously, for research and publication purposes and are given the option to agree or refuse. The two patients described in this case report agreed to this use.
Acknowledgements
The authors are grateful to Paul Robinson for editing English style and grammar.
References
- 1.Adler D., Gonzalez-Bermejo J., Duguet A., Demoule A., Le Pimpec-Barthes F., Hurbault A. Diaphragm pacing restores olfaction in tetraplegia. Eur Respir J. 2009;34:365–370. doi: 10.1183/09031936.00177708. [DOI] [PubMed] [Google Scholar]
- 2.Esclarin A., Bravo P., Arroyo O., Mazaira J., Garrido H., Alcaraz M.A. Tracheostomy ventilation versus diaphragmatic pacemaker ventilation in high spinal cord injury. Paraplegia. 1994;32:687–693. doi: 10.1038/sc.1994.111. [DOI] [PubMed] [Google Scholar]
- 3.Hirschfeld S., Exner G., Luukkaala T., Baer G.A. Mechanical ventilation or phrenic nerve stimulation for treatment of spinal cord injury-induced respiratory insufficiency. Spinal Cord. 2008;46:738–742. doi: 10.1038/sc.2008.43. [DOI] [PubMed] [Google Scholar]
- 4.Le Pimpec-Barthes F., Gonzalez-Bermejo J., Hubsch J.P., Duguet A., Morelot-Panzini C., Riquet M. Intrathoracic phrenic pacing: a 10-year experience in france. J Thorac Cardiovasc Surg. 2011;142:378–383. doi: 10.1016/j.jtcvs.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 5.Romero F.J., Gambarrutta C., Garcia-Forcada A., Marin M.A., Diaz de la Lastra E., Paz F. Long-term evaluation of phrenic nerve pacing for respiratory failure due to high cervical spinal cord injury. Spinal Cord. 2012;50:895–898. doi: 10.1038/sc.2012.74. [DOI] [PubMed] [Google Scholar]
- 6.DiMarco A.F. Phrenic nerve stimulation in patients with spinal cord injury. Respir Physiolo Neurobiol. 2009;169:200–209. doi: 10.1016/j.resp.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Nochomovitz M.L., Hopkins M., Brodkey J., Montenegro H., Mortimer J.T., Cherniack N.S. Conditioning of the diaphragm with phrenic nerve stimulation after prolonged disuse. Am Rev Respir Dis. 1984;130:685–688. doi: 10.1164/arrd.1984.130.4.685. [DOI] [PubMed] [Google Scholar]
- 8.Pascual E., Castellano J.A., Lopez E. Costovertebral joint changes in ankylosing spondylitis with thoracic pain. Br J Rheumatol. 1992;31:413–415. doi: 10.1093/rheumatology/31.6.413. [DOI] [PubMed] [Google Scholar]
- 9.Romagnoli I., Gigliotti F., Galarducci A., Lanini B., Bianchi R., Cammelli D. Chest wall kinematics and respiratory muscle action in ankylosing spondylitis patients. Eur Respir J. 2004;24:453–460. doi: 10.1183/09031936.04.00123903. [DOI] [PubMed] [Google Scholar]
- 10.Naimark A., Cherniack R.M. Compliance of the respiratory system and its components in health and obesity. J Appl Phys. 1960;15:377–382. doi: 10.1152/jappl.1960.15.3.377. [DOI] [PubMed] [Google Scholar]
- 11.Kress J.P., Pohlman A.S., Alverdy J., Hall J.B. The impact of morbid obesity on oxygen cost of breathing (vo(2resp)) at rest. Am J Respir Crit Care Med. 1999;160:883–886. doi: 10.1164/ajrccm.160.3.9902058. [DOI] [PubMed] [Google Scholar]
- 12.Steier J., Jolley C.J., Seymour J., Roughton M., Polkey M.I., Moxham J. Neural respiratory drive in obesity. Thorax. 2009;64:719–725. doi: 10.1136/thx.2008.109728. [DOI] [PubMed] [Google Scholar]
- 13.Sampson M.G., Grassino A.E. Load compensation in obese patients during quiet tidal breathing. J Appl Phys. 1983;55:1269–1276. doi: 10.1152/jappl.1983.55.4.1269. [DOI] [PubMed] [Google Scholar]
- 14.McCool F.D., Pichurko B.M., Slutsky A.S., Sarkarati M., Rossier A., Brown R. Changes in lung volume and rib cage configuration with abdominal binding in quadriplegia. J Appl Phys. 1986;60:1198–1202. doi: 10.1152/jappl.1986.60.4.1198. [DOI] [PubMed] [Google Scholar]
- 15.Estenne M., De Troyer A. Mechanism of the postural dependence of vital capacity in tetraplegic subjects. Am Rev Respir Dis. 1987;135:367–371. doi: 10.1164/arrd.1987.135.2.367. [DOI] [PubMed] [Google Scholar]
- 16.Shirley D., Hodges P.W., Eriksson A.E., Gandevia S.C. Spinal stiffness changes throughout the respiratory cycle. J Appl Phys. 2003;95:1467–1475. doi: 10.1152/japplphysiol.00939.2002. [DOI] [PubMed] [Google Scholar]