Abstract
Studies of human cancer metastasis have been limited by a lack of experimental assays in which cancer cells from patients metastasize in vivo in a way that correlates with clinical outcome. This makes it impossible to study intrinsic differences in the metastatic properties of cancers from different patients. We recently developed an assay in which human melanomas readily engraft in NOD/SCID IL2Rγnull (NSG) mice (1, 2). Here we show that melanomas from 25 patients exhibited reproducible differences in the rate of spontaneous metastasis after transplantation into NSG mice and that these differences correlated with clinical outcome in the patients. Stage IIIB/C melanomas that formed distant metastases within 22 months in patients also formed tumors that metastasized widely in NSG mice, while stage IIIB/C melanomas that did not form distant metastases within 22–50 months in patients metastasized more slowly in NSG mice. These differences in the efficiency of metastasis correlated with the frequency of circulating melanoma cells in the blood of NSG mice, suggesting that the rate of entry into the blood is one factor that limits the rate of metastasis. NSG mice can therefore be used to study the metastasis of human melanomas in vivo, revealing intrinsic differences among stage III melanomas in their ability to circulate/survive in the blood and metastasize.
INTRODUCTION
Stage I and II cutaneous melanoma is confined to the skin, while stage III melanoma is characterized by regional metastasis, and stage IV melanoma by distant metastasis. Stage III melanoma patients exhibit a broad range of 5-year survival rates, from 23%–87%, depending on whether they have microscopic versus macroscopic disease in the lymph nodes, the number of tumor-bearing lymph nodes, and the ulceration status of the primary tumor (3). It is unclear whether intrinsic differences in metastatic potential among stage III melanomas contribute to this wide range of outcomes. This is due, in part, to a lack of experimental assays in which the metastasis of melanomas from patients can be compared in vivo in a way that correlates with clinical outcome. Absent such assays it is impossible to dissect the relative contributions of melanoma cell-intrinsic (e.g. different mutations) versus melanoma cell-extrinsic (e.g. different autologous immune responses) differences among patients to the range of outcomes observed.
A number of studies have identified gene expression profiles associated with melanoma progression or metastasis (4–12). Studies of breast cancers and adenocarcinomas have suggested intrinsic differences among tumors in their potential to progress or metastasize depending on their mutations and gene expression profile (13–15). Studies of melanoma are consistent with this possibility (4, 16) but it has not yet been possible to functionally compare the intrinsic metastatic potential of primary human melanomas in vivo. A number of genes or gene products have been implicated in melanoma progression or metastasis including Acid phosphatase 5 (16), MITF (17), Rho GTPases (18, 19), Growth arrest-specific 1 (20), the NEDD9 adaptor protein (21), KISS1 (22), BRMS1 (23), nm23 (24), β-catenin (25), and BRAF (26). Nonetheless, it would be desirable to study these mechanisms in vivo using melanomas obtained directly from patients.
We developed a xenotransplantation assay in which melanoma cells obtained directly from patients efficiently form tumors upon subcutaneous transplantation into highly immunocompromised NOD/SCID IL2Rγnull (NSG) mice (1, 2). These mice are more permissive for the engraftment of human melanoma cells than other immunocompromised mouse models, as all stage III and IV melanomas that we have tested have engrafted in NSG mice and engraftment requires many fewer melanoma cells than in less immunocompromised mice, such as NOD/SCID (1, 2). Approximately 30% of single, unselected melanoma cells from patients form tumors in NSG mice based on results from 32 primary cutaneous or metastatic melanomas obtained from stage II, III, and IV patients (1, 2). Melanoma cells with many surface marker phenotypes are capable of forming tumors, and we have been unable to identify any subpopulation of melanoma cells that lacks the ability to form a tumor (1, 2). In the current study we show that there are intrinsic differences among melanomas in the capacity to metastasize in NSG mice and that these differences correlate with clinical outcome in patients.
RESULTS
Melanomas from 25 patients exhibited differences in metastasis in NSG mice
We compared the metastasis of 27 human melanomas in NSG mice (Fig. 1A). These melanomas were surgically removed from 25 patients with stage II (n=1), stage III (n=20), and stage IV (n=4) disease (see Fig. 1A and Table S1 for detailed information on tumor sites and patients). Each melanoma represented excess tissue from biopsy-proven or known melanomas, in which tumor banking did not impact prognosis or patient treatment. Most of the tumors we studied were small samples from palpable regional lymph node metastases in stage IIIB/C patients. Two tumors were surgically removed concurrently from each of two patients (633/634 and 498/499). Consistent with our previously published results (1, 2), small numbers of cells from each of these melanomas readily formed tumors after subcutaneous injection into NSG mice. Mice were necropsied when they became ill or when subcutaneous tumors approached end-point diameter.
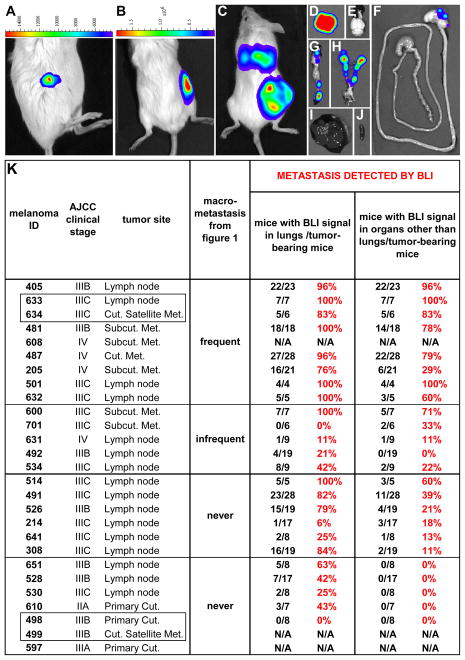
Figure 1. Human melanomas exhibit intrinsic differences in their metastatic efficiency in NSG mice.

(A) 27 melanomas from 25 patients were transplanted subcutaneously into NSG mice in doses between 1 and 50,000 cells per injection. Mice with subcutaneous tumors were monitored until the tumors grew to 1.4±0.9 cm (mean ± SD) in diameter, 21.8±10.2 weeks after transplantation. Upon necropsy, organs were visually examined for the presence of metastases. “AJCC clinical stage” is the American Joint Committee on Cancer stage of the patient at the time of melanoma removal. “Tumor site” reflects the location of the tumor in the patient. Melanomas 633/634 and 498/499 (boxed) were pairs of tumors obtained from different locations in the same patients at the same time. The metastatic behavior of each melanoma was examined in 2 to 22 independent experiments with 6 to 136 mice that formed subcutaneous tumors per melanoma (see Table S2 for more details). The rate of metastasis (%) is the percentage of mice with subcutaneous tumors that developed macrometastases. (B) Tumors that formed at the injection site as well as macrometastases in mice transplanted with cells from four patients. Metastases were confirmed as melanoma by a dermatopathologist after staining sections with hematoxylin and eosin (H&E) and S-100. Table S1 contains clinical details for each melanoma and patient. Table S2 has details regarding rates of metastasis at different cell doses for each melanoma. Table S3 shows the results for each independent experiment conducted on each melanoma. Table S4 shows the location where macrometastasis was detected for each melanoma.
Melanomas from some patients metastasized aggressively in the mice. For example, cells from melanoma 481 (a subcutaneous regional metastasis from a patient with stage IIIB disease) formed subcutaneous tumors at the injection site in 135 NSG mice in 18 independent experiments and 75 of these mice (56%) developed macrometastases in the lungs, liver, and other visceral organs (Fig. 1A; Tables S2 and S3). In our study, macrometastases were defined as tumors that were visible upon necropsy and not at the subcutaneous injection site (generally in visceral organs). Overall, 9 of the 27 melanomas we studied were frequent metastasizers, meaning that visceral macrometastases were observed in 14% to 70% of the mice with subcutaneous tumors. These included 3 of 4 metastatic melanomas from stage IV patients as well as 6 of 20 regionally metastatic melanomas from stage IIIB/C patients.
Macrometastases were most commonly found in the liver, lungs, kidneys, and retroperitoneum (Fig. 1B and Table S4). The metastatic tumors were histologically similar to their antecedent subcutaneous tumors in mice as well as in the parent tumors obtained from patients, with similar degrees of pigmentation and characteristic morphological and immunohistochemical features (Fig. 1B).
Another 5 of the 27 melanomas we studied formed infrequent macrometastases in NSG mice. For example, cells from melanoma 492 (a regional lymph node metastasis from a patient with stage IIIB disease) formed subcutaneous tumors at the injection site in 81 NSG mice in 8 independent experiments and only 2 of these mice had macrometastases (Fig. 1A; Tables S2 and S3). The other four “infrequent” macrometastasizers were also from regionally metastatic melanomas in patients with stage IIIB/C or IV disease, and gave rise to macrometastases (mainly in the liver, Table S4) in 1% to 6% of NSG mice with subcutaneous tumors (Fig. 1A).
The remaining 13 melanomas did not form visible macrometastases in any mice. For example, cells from melanomas 491 and 526 (regional lymph node metastases from patients with stage IIIB/C disease) formed subcutaneous tumors in 117 and 136 NSG mice in 14 or 22 independent experiments, respectively, yet macrometastases were never detected (Fig. 1a; Tables S2 and S3). The other 11 melanomas that never generated macrometastases included one primary cutaneous melanoma from a patient with stage IIA disease as well as 7 regional metastases from patients with stage IIIB/C disease, and three cutaneous melanomas from patients with stage IIIA/B disease (Fig. 1A).
The differences in the metastasis of the melanomas we studied were observed in multiple independent experiments, irrespective of the number of cells injected subcutaneously (Table S2) or the number of times the melanomas were passaged in mice (Table S3). For example, melanomas 491, 214, and 308 (regional lymph node metastases from patients with stage IIIC disease; Fig. 1A) never formed macrometastases in NSG mice despite being studied in 8–14 independent experiments that involved tumors obtained directly from patients or after up to 7–11 passages of subcutaneous tumors in mice. In contrast, melanomas 405 and 481 (regional metastases from patients with stage IIIB disease; Fig. 1A) were each studied in 8–18 independent experiments performed after 1–7 passages in mice, forming macrometastases in 13% to 88% of the mice in every experiment.
As evaluation of visible macrometastases at necropsy may underestimate the extent of metastasis, we reassessed the metastatic potential of 24 melanomas using bioluminescence imaging (BLI) (27). To minimize ex vivo manipulation, melanoma cells were rapidly (4 hours) infected in culture with a lentivirus bearing luciferase-Gfp under the control of the ubiquitin promoter, then injected into mice. The resulting subcutaneous tumors were dissociated and sorted to select luciferase-GFP+ cells. For each melanoma, 4 to 28 NSG mice were injected subcutaneously on the flank with 100 luciferase-GFP+ cells. BLI of the mice two days after injection detected the melanoma cells at the injection site (Fig. 2A), highlighting the sensitivity of this method. The experiment in Fig. 2A was performed with a heavily pigmented melanoma (205), demonstrating that pigmentation did not prevent us from detecting small numbers of melanoma cells by BLI. Virtually all mice developed subcutaneous tumors, irrespective of which melanoma was transplanted. The mice were monitored until subcutaneous tumor diameters approached 20 mm and then the mice and their organs underwent BLI to assess the presence of disseminated melanoma cells (Fig. 2A–J).
Figure 2. Bioluminescence imaging (BLI) confirms intrinsic differences in metastatic efficiency among human melanomas in NSG mice.
Luciferase-GFP+ melanoma cells from different patients exhibited intrinsic differences in metastatic efficiency revealed by BLI in NSG mice. BLI of an NSG mouse 2 days (A) and 45 days (B) after subcutaneous transplantation of 100 luciferase-GFP+ cells from melanoma 205, a heavily pigmented melanoma (see Fig. 1B). (C) BLI of an NSG mouse 13 weeks after subcutaneous transplantation of 100 luciferase-GFP+ cells from melanoma 487. BLI of individual organs dissected from the mouse in (C) revealed metastases in the lungs (D), stomach (F), pancreas (G), kidneys, adrenal glands and ovaries (H), but not in the brain (E), liver (I) or spleen (J). The maximum luminescence shown in red in panels C and D is 31x106 photons/second/cm2/steradian and in panels E–J is 6.2x106 photons/second/cm2/steradian. (K) A summary for each melanoma of the percentage of mice with subcutaneous tumors that developed metastases detected by BLI. Supplementary Table 4 summarizes the locations in which metastases were detected by BLI. Melanomas 608, 499, and 597 did not undergo BLI.
Background BLI signals in control mice were up to 285,000 photons/sec/cm2/steradian in most organs. To assess the correlation between BLI signal and the presence of metastases, 317 sections from the organs of mice transplanted with melanomas were analysed by a dermatopathologist. Only 2% of sections from organs with BLI signals below 1x106 photons/sec/cm2/steradian contained melanoma nodules (clusters of S100+ melanoma cells). In contrast, 40% of sections from organs with BLI signals of 1–3x106 photons/sec/cm2/steradian and 70% of sections from organs with BLI signals above 3x106 photons/sec/cm2/steradian had melanoma nodules. Therefore, any organ containing BLI signals greater than 1x106 photons/sec/cm2/steradian above background was considered to contain metastases.
To histologically validate this standard we examined sections from the lungs of 62 mice with subcutaneous melanomas. Of 50 mice that appeared to have melanoma in their lungs by BLI, 44 were confirmed to contain melanoma cells by histology. This is likely an underestimate because we examined a limited number of sections from each lung. Of 12 mice that appeared not to contain melanoma in their lungs by BLI, none were found to contain melanoma cells by histology. Therefore, there was a strong correlation between the presence of melanoma cells by BLI and by histology.
BLI was a much more sensitive indicator of metastasis in the NSG mice than visual inspection for macrometastases at necropsy. Most mice with macrometastases had organs with very strong BLI signals, typically greater than 107 photons/sec/cm2/steradian in at least one organ (Fig. 2K and Table S4). Five of the melanomas that were studied by BLI were heavily pigmented (405, 487, 205, 514 and 214). In all of these cases we were able to observe BLI signals in subcutaneous tumors as well as in multiple organs (Fig. 2K and Table S4). The percentage of mice with metastases detected by BLI was always higher than the percentage with macrometastases at necropsy (Fig. 2k). Six of the melanomas that never had macrometastases at necropsy formed metastases detected by BLI in multiple organs (melanomas 514, 491, 526, 214, 641 and 308; Fig. 2k). However, the distribution of metastases by BLI was generally similar to the distribution of macrometastases at necropsy (Table S4).
There were also five melanomas that never formed macrometastases and which metastasized only to a limited extent by BLI, if at all (melanomas 530, 651, 528, 610, and 498; Fig. 2K and Table S4). Micrometastases were sometimes observed by BLI in the lungs of mice transplanted with these tumors (and confirmed by a dermatopathologist in lung sections) but no metastases were ever detected in any other organ. One of these melanomas (610) was from a primary cutaneous melanoma removed from a patient with stage IIA disease and another (498) was the primary cutaneous melanoma removed from a patient with documented concurrent metastatic stage IIIB disease (Fig. 2K). The other three melanomas were lymph node metastases obtained from patients with stage IIIB/C disease (651, 528, 530; Fig. 2K). Our results suggest that there are intrinsic differences in the metastatic potential of melanomas obtained from patients with stage IIIB/C disease. These differences did not reflect a difference in melanoma growth rate, as we observed no statistically significant correlation between the metastasis of melanomas and their growth rate as subcutaneous tumors in NSG mice (Fig. S1).
The behavior of human melanomas in NSG mice correlates with clinical outcome
To assess whether the differences in metastatic efficiency in NSG mice reflected biological differences among melanomas that were evident in the clinical outcome of patients we collected clinical data on 25 patients who donated melanomas for our study (Table S1). The patients who donated melanomas 610, 641, and 701 were excluded from this analysis because we were unable to obtain information on their clinical status after melanoma banking and they died from causes unrelated to melanoma 6–10 months later.
Four patients had stage IV disease when their tumors were banked and all died within 14 months of tumor banking (Fig. 3A, Table S1). Another 12 patients who had stage III disease at the time of tumor banking formed distant metastases and progressed to stage IV disease after tumor banking. Most of these patients died 3.4 to 40.6 months after tumor banking but one (600) remained alive with distant metastatic disease 33.2 months after tumor banking (Fig. 3A, Table S1). All 16 of the melanomas that formed distant metastases in patients also metastasized widely to multiple organs in mice (Fig. 3A).
Figure 3. Metastatic efficiency in NSG mice correlated with disease progression in patients.
(A) Side-by-side comparison of distant metastasis in patients versus metastasis in mice for all 22 patients from whom follow-up clinical data were available after tumor banking. Most of the melanomas were lymph node metastases from stage IIIB/C patients at the time of banking. The melanomas were ranked by clinicians in terms of the aggressiveness of disease in patients after banking, based primarily on the rate of metastasis, from 514 at the top (most aggressive) to 498/499 at the bottom (least aggressive). The latest AJCC stage (or stage at time of death) and the time (in months) of survival after tumor banking are indicated in the “most recent survival and staging data” column. The patients with melanomas 514 to 492 formed distant metastases (stage IV disease) and died 3.4 to 40.6 months after melanoma banking. The patient who donated melanoma 600 remained alive at last follow-up, 33.2 months after melanoma banking but had progressed to stage IV disease with brain metastasis. All of the patients who progressed to stage IV disease did so within 22 months of tumor banking. The patients with melanomas 651 to 498/499 did not form distant metastases or progress to stage IV disease. Patient 528 died 40.7 months after melanoma banking with lung cancer and advanced chronic obstructive pulmonary disease but no evidence of melanoma at the time of death. See Table S1 for clinical details. All 16 of the melanomas that formed distant metastases in patients (stage IV disease) also metastasized to multiple organs in NSG mice (see Fig. 2K). Of the 6 melanomas that did not form distant metastases in patients, 5 also did not metastasize widely in mice (no metastasis or only micrometastases in the lungs, no metastasis to other organs; see Fig. 2K). (B) Side by side comparison of brain metastasis in patients and in NSG mice injected subcutaneously with the same melanomas. (C) Kaplan-Meier survival curves for all patients with at least 30 months of follow-up after tumor banking. Patients with melanomas that did not metastasize widely in mice lived significantly longer than all patients or than patients whose melanomas metastasized widely in mice (log-rank test, P<0.05).
Six patients (651, 597, 528, 530, 526, and 498/499) did not develop distant metastases and nearly all remained alive with resected stage III disease 22.0 to 50.8 months after melanoma banking (Fig. 3A, Table S1). Most of these patients had no evidence of ongoing disease, with the exception of a regional recurrence that was resected from the patient who donated melanoma 530. The patient who donated melanoma 528 died from a non-melanoma cause (lung cancer with advanced chronic obstructive pulmonary disease) 40.7 months after tumor banking with no evidence of melanoma (Fig. 3A; Table S1). Melanomas from five of these patients (530, 651, 597, 528, and 498/499) never formed macrometastases in NSG mice and by BLI formed micrometastases only in the lungs (Fig. 3A and 2K). The melanoma from the sixth patient (526) never formed macrometastases in NSG mice but did metastasize to multiple organs by BLI (Fig. 3A and 2K).
We also wondered whether melanomas that metastasized to the brain in patients were more likely to metastasize to the brain in NSG mice. Sixteen of the patients who donated melanomas for our study had received brain scans. Ten of these 16 patients developed brain metastases (Fig. 3B, Table S1). The melanomas from 7 of these 10 patients also metastasized to the brain in NSG mice (Fig. 3B). In contrast, 6 of 16 patients did not develop brain metastases, and only one melanoma from these patients formed brain metastases in NSG mice (Fig. 3B). This suggests that melanomas that form brain metastases in patients are also more likely to form brain metastases in NSG mice.
Thus all of the patients who developed stage IV disease had melanomas that metastasized widely in NSG mice (16/16; Fig. 3A). All of these patients progressed to stage IV disease within 22 months of tumor banking. In contrast, 5 of 6 patients who did not progress to stage IV disease had melanomas that did not metastasize widely in NSG mice, either not at all or forming only micrometastases in the lungs (Fig. 3A). This demonstrates a strong correlation in which melanomas that would form distant metastases in patients (within 22 months of tumor banking) always widely metastasized in NSG mice, while melanomas that did not form distant metastases in patients (within 22.0–50.8 months of banking) metastasized only to a limited extent in NSG mice. This suggests that melanomas have intrinsic differences in metastatic efficiency that contribute to disease progression in patients and metastasis in NSG mice.
At the 30 month time point after melanoma banking, median survival for the patients with at least 30 months of follow-up (n=21) was 19.2 months (Fig. 3C); however, those whose melanomas metastasized widely in NSG mice (n=17) had a median survival of only 14.1 months, and only three of the patients (492, 600 and 526) remained alive after 30 months (Fig. 3c). Patients whose melanomas did not metastasize widely in NSG mice (n=4) all remained alive and free of disease at 30 months after melanoma banking (Fig. 3C). The patient who donated melanoma 651 (an inefficient metastasizer) was excluded from this analysis because we had only 22 months of follow-up data, though this patient also remained alive and free of disease at this time point. Patients whose melanomas did not metastasize widely in NSG mice lived significantly (p=0.0121) longer than those whose melanomas did metastasize widely in NSG mice (Fig. 3C). The behavior of human melanomas in NSG mice is therefore predictive of clinical outcome.
Differences in metastasis correlate with the frequency of circulating melanoma cells
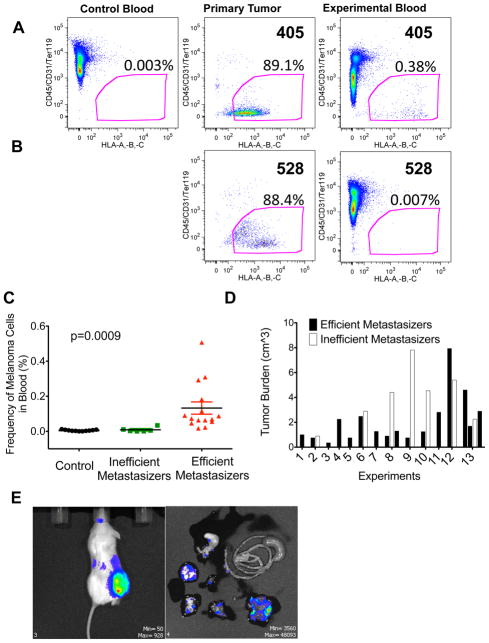
We developed a method for identifying and quantifying circulating human melanoma cells in NSG mice (Fig. 4A). We distinguished these cells from mouse cells using an antibody against human major histocompatibility complex proteins, HLA-A, B, and C. In negative control mice that were not transplanted with human cells, fewer than 0.01% of mouse blood cells were HLA+ (0.005±0.004%, mean±SD; Fig. 4A); however, most cells dissociated from the melanomas that grew subcutaneously in mice were HLA+, and we detected HLA+ cells in the blood of some mice (Fig. 4A, C). When HLA+ cells were sorted by flow cytometry from the blood of mice bearing subcutaneous tumors and transplanted subcutaneously into NSG mice, they usually formed melanomas (5 out of 9 injections of 5–500 cells) that metastasized widely, just as the primary tumors from which they derived (Figure 4E).
Figure 4. The frequency of circulating melanoma cells in the blood of xenografted NSG mice correlates with metastatic efficiency.
Blood was collected from untransplanted control or melanoma-transplanted experimental NSG mice by cardiac puncture. Live nucleated blood cells were analysed for HLA+ melanoma cells by flow cytometry. Blood from mice bearing an efficiently metastasizing melanoma (A; melanoma 405) and an inefficiently metastasizing melanoma (B; melanoma 528). HLA+ cells were not detected in the blood of untransplanted control mice (A, <0.01% background staining) or in the blood of mice bearing inefficiently metastasizing melanomas (B, <0.01% background staining) but were detected in the blood of mice bearing efficiently metastasizing melanomas (A, 0.02–0.51%). Note that melanoma 528 (B) was analysed side-by-side with melanoma 405 (A) such that the negative control in panel (A) applied to both analyses. (C) The frequency of HLA+ melanoma cells in the blood of all mice. The difference between mice bearing efficiently-metastasizing (n=15 mice transplanted with melanomas 405, 481, and 633) and inefficiently metastasizing melanomas (n=7 mice transplanted with melanomas 528 and 651) was statistically significant (p=0.0023 by Anova comparing efficient metastasizers to both other treatments; p=0.0004 by post-hoc pairwise Mann-Whitney t-tests). (D) Several of the mice with inefficiently metastasizing melanomas had large tumor burdens and yet still did not have detectable levels of circulating melanoma cells in their blood (see C). (E) NSG mice were injected subcutaneously with 100 HLA+/GFP+ cells from the blood of NSG mice with subcutaneous melanomas. Palpable subcutaneous tumors developed within 2 months after injection. Mice were analysed by BLI for metastases when the subcutaneous tumor reached 2cm in diameter. Widespread metastasis was observed, just as in the primary melanomas from which the circulating melanoma cells derived.
To determine whether the frequency of circulating melanoma cells correlated with the metastatic efficiency of melanomas in mice, NSG mice were transplanted subcutaneously with melanoma cells obtained from efficiently metastasizing (n=15 mice transplanted with melanomas 405, 481, or 633) or inefficiently metastasizing melanomas (n=6 mice transplanted with melanomas 528 or 651). Mice that had inefficiently metastasizing melanomas had only rare HLA+ cells in their blood, at levels not significantly different from background in control mice (0.009±0.01% of blood cells; Fig. 4B). In contrast, all of the mice that had efficiently metastasizing subcutaneous melanomas also had human melanoma cells in their blood, representing 0.02–0.51% of nucleated blood cells (0.13±0.14% of blood cells; Fig. 4A), significantly (p=0.0004) more than mice with inefficiently metastasizing melanomas (Fig. 4C). No melanoma cells were detected in the blood of mice with inefficiently metastasizing melanomas even when mice with higher tumor burdens were analysed (Fig. 4C, D). Intrinsic differences in the rate at which melanoma cells from patients with stage IIIB/C disease enter circulation appear to be one factor that limits the rate of metastasis.
DISCUSSION
Studies that compare clinical outcomes in cancer patients to the behavior of their tumors in xenograft assays are rare. Three recent studies observed a correlation between clinical outcomes and the ability of tumors to engraft in immunocompromised mice: 40% of non–small cell lung cancers (28), 28% of uveal melanomas (29), and 37% of breast cancers (30) formed tumors upon transplantation into NOD/SCID or SCID mice, and the patients whose tumors engrafted exhibited a worse clinical outcome. However, these studies did not assess whether there is a correlation between the rate of metastasis in mice and in patients. It is likely that the more highly immunocompromised environment within NSG mice, as compared to NOD/SCID or SCID mice, facilitates the modelling of human melanoma metastasis by attenuating the xenogeneic immune response to human cells. We have not yet encountered a melanoma that could not engraft in NSG mice and many more melanoma cells form subcutaneous tumors in NSG as compared to NOD/SCID mice (1, 2). Xenotransplantation of human melanomas into NSG mice offers an opportunity to study the mechanisms that regulate the metastasis of human melanomas in vivo and to study circulating melanoma cells.
The NSG mouse assay makes it possible to study intrinsic differences in the metastatic efficiency of melanomas from patients, within genetically identical mice, in a way that is not confounded by differences among patients in immune activity, therapy, and other variables extrinsic to the melanoma cells. The correlation we observed between metastasis in NSG mice and in patients (Fig. 3A) indicates that intrinsic differences in metastatic efficiency among stage III melanomas are an important determinant of clinical outcome in patients; however, our results do not exclude important roles for melanoma-extrinsic mechanisms that cannot be modelled in NSG mice, such as autologous immune responses in patients. We believe the NSG xenograft assay will make it possible to identify new biomarkers of metastatic propensity in melanoma and to study the effects of certain systemic therapies on metastasis.
MATERIALS AND METHODS
Tissue banking
The tissue bank protocol used for this study was developed and approved jointly by the clinical director of the University of Michigan (UM) melanoma program, the UM Cancer Center director of tissue procurement, the UM chief of anatomic pathology, and UM director of the section of dermatopathology. The protocol was developed to avoid any compromise in patient care, pathologic diagnosis, tumor staging or treatment. Patient confidentiality was maintained by password and firewall protected access to all pertinent databases. Melanoma specimens were obtained with informed consent from all patients according to protocols approved by the Institutional Review Board of the UM Medical School (IRBMED approvals HUM00050754 and HUM00050085). All patients included in this study had clinically apparent melanoma disease (biopsy-proven stage II, III, or IV, or obvious clinical stage IV) from which a small (typically 2–5 mm) tissue sample not required for standard-of-care pathology assessment was obtained. Most of the melanomas in this study were regional stage III lymph node or skin/soft tissue disease with palpable, clinically enlarged node(s) or soft tissues, undergoing definitive surgical resection, with biopsy-proven (most often needle core) diagnosis confirmed before surgery.
Tumor cell preparation
Tumors were mechanically dissociated with a McIlwain tissue chopper (Mickle Laboratory Engineering) before sequential enzymatic digestion in 200 U/ml collagenase IV (Worthington) for 20 min followed by 0.05% trypsin-EGTA for 2 min, both at 37°C. DNase (50–100 U/mL) was added to reduce clumping of cells during digestion. Cells were filtered (40 μm cell strainer) to obtain a single cell suspension. Dead cells and debris were depleted by density centrifugation (1.1 g/ml Optiprep; Sigma) when necessary. Cells were always passaged in vivo (in immunocompromised mice as detailed below), not in vitro.
For analysis of circulating melanoma cells, blood was collected from each mouse by cardiac puncture, using a syringe pre-treated with citrate-dextrose solution (Sigma). Red blood cells were precipitated by Ficoll sedimentation following the manufacturer’s instructions (Ficoll Paque Plus, GE Healthcare). Remaining cells were washed with HBSS (Invitrogen) prior to antibody staining and flow cytometric analysis.
All antibody staining was carried out for 20 min on ice, followed by washing and centrifugation. In order to select live human melanoma cells and to exclude endothelial and hematopoietic cells, tumors obtained directly from patients were stained with directly conjugated antibodies to human CD45 (HI30-APC; BD Biosciences), human CD31 (WM59-APC; eBiosciences), Glycophorin A (HIR2-APC; Biolegend) and HLA-A,B,C (G46-2.6-PE; BD Biosciences). Tumors or blood obtained from mice were stained with antibodies against mouse CD45 (30-F11-APC; eBiosciences), mouse CD31 (390-APC; Biolegend), mouse Ter119 (TER-119-APC; eBiosciences) and human HLA-A,B,C (G46-2.6-PE; BD Biosciences). Cells were resuspended in 10 μg/ml DAPI (Sigma) and sorted on a FACSAria (Becton Dickinson).
Transplanting melanoma cells
After sorting, cells were counted and resuspended in staining medium (L15 medium containing 1 mg/ml BSA, 1% penicillin/streptomycin and 10 mM HEPES [pH7.4]) with 25% high protein Matrigel (product 354248; BD Biosciences). Subcutaneous injections of human melanoma cells were performed in each flank and the interscapular region of NOD.CB17-Prkdcscid Il2rgtm1Wjl/SzJ (NOD/SCID IL2R null, NSG) mice (Jackson Laboratories) in a final volume of 50μl. These experiments were performed according to protocols approved by the animal use committees at the University of Michigan (protocol 9055) and the University of Texas Southwestern Medical Center (protocol 2011-0118).
Lentiviral transduction of human melanoma cells
Replication incompetent lentiviruses were generated by the cotransfection of 293T cells with three plasmids (fuw, vsv and delta), kindly provided by Dr. Alnawaz Rehemtulla (University of Michigan). Forty-eight hours after transfection, supernatant with virus was collected and passed through a 0.45 μm low binding filter. Virus was concentrated by ultracentrifugation and aliquots were frozen at −80°C until use. Around 150,000 sorted human melanoma cells (obtained directly from the patients or from xenografts) were incubated for 4h at 37°C, 6.5% CO2 in a well of an ultra-low attachment 24-well plate (Corning) containing 300 μl of cell culture medium (50% DMEM-low glucose, 30% Neurobasal (Invitrogen), 15% chick embryo extract, 1% Penicillin/Streptomycin, 1% non-essential amino acids (Gibco), 117 nM retinoic acid, 50 μM 2-mercaptoethanol (Sigma), 1% N2 supplement, 2% B27 supplement (Gibco), 20 ng/mL recombinant human bFGF, 20 ng/mL IGF-1 (R&D Systems)). 20 μl lentivirus and 10 μg/ml polybrene (Sigma) were added to the cultures. Cells were then washed twice with staining medium and approximately 25,000 cells (a mixture of infected and non-infected cells) were injected into NSG mice. After growing to 1–2 cm in diameter, tumors were excised, dissociated into a single cell suspension and luciferase-GFP+ cells were collected by flow cytometry for injections into secondary recipients. Metastasis was monitored by BLI in these secondary recipients.
Bioluminescence imaging (BLI)
Mice were injected with 100 luciferase-GFP+ cells on the right flank and monitored until tumor diameters approached 20 mm, at which point they were imaged along with an uninjected control mouse on an IVIS Imaging System 200 Series (Calliper Life Sciences) using Living Image software. Mice were injected intraperitoneally with 100 μl of PBS containing 40 mg/ml D-luciferin monopotassium salt (Biosynth) ten minutes before imaging, followed by general anesthesia two minutes before imaging. Following imaging of the whole mouse, the mice were euthanized, and individual organs were quickly imaged. The exposure time of images ranged from 10 to 60 seconds. The bioluminescence signal was quantified using “region of interest” measurement tools in Living Image software. After imaging, tumors and organs were fixed in 10% neutral buffered formalin for histopathology.
Histopathology and immunostaining
Melanomas and mouse organs were fixed in 10% neutral buffered formalin, paraffin embedded, sectioned, and stained with hematoxylin and eosin for histopathology. Sections from paraffin-embedded tumors were stained for S100 and analysed by a dermatopathologist to confirm they were melanomas. Binding of anti-S100 antibody (DAKO) was carried out for 30 min at room temperature, detected by anti-rabbit secondary antibody (30 min at room temperature) and revealed using DAB chromagen after quenching endogenous peroxidases. Slides were counterstained with hematoxylin.
Statistics
The frequency of mice with metastases was determined by dividing the total number of mice with metastases by the total number of mice with subcutaneous tumors. Photon flux was calculated by subtracting the bioluminescence signal from organs obtained from a negative control mouse imaged after luciferin injection from the signal detected in the organs of mice transplanted with luciferase-GFP+ cells. Tumor growth rates were determined by maximum tumor diameter (in mm) at euthanasia divided by elapsed time (in weeks) from injection. Differences in mean growth rates were compared using unpaired Student’s t tests. Differences in the survival of different groups of patients were evaluated using a log-rank test. In all cases results are expressed as mean ± standard deviation. Correlations between metastatic potential and tumor growth rates were assessed by linear regression analysis using GraphPad Prism 3.0 software. Confidence intervals for contingency table analysis were calculated using the binomial method.
Acknowledgments
Thanks to David Adams and the UM Core Facility for flow cytometry as well as Melissa Voutsalath and Nisha Meireles for tissue and clinical data collection. Thanks to Hannah Foster for technical assistance and to Dr. Alnawaz Rehemtulla for providing the luciferase-GFP plasmid.
Funding: This work was supported by the Howard Hughes Medical Institute, the Melanoma Research Foundation, the Allen H. Blondy Research Fellowship at the University of Michigan (UM), and the Cancer Prevention and Research Institute of Texas. The UM Melanoma Bank was supported by gifts from Lewis and Lillian Becker, and from Howard Cooper. Flow cytometry was partially supported by the UM-Comprehensive Cancer NIH CA46592. Elsa Quintana was supported by the Marie Curie Outgoing International Fellowship from the European Commission. Mark Shackleton was supported by the Australian National Heath and Medical Research Council, the Human Frontiers Science Program, and Australia Post. This work was initiated at the University of Michigan and completed at UT Southwestern Medical Center.
Footnotes
Author contributions: E.Q., E.P., and S.J.M. conceived the project. E.Q., E.P., M.S., U.E., and D.W. performed the experiments. E.Q. and D.W. analysed the data on melanoma metastasis with S.J.M. E.P. performed the experiments on circulating melanoma cells and analysed the data with S.J.M. T.M.J. consented the patients, banked the melanoma specimens and provided clinical information on patients. D.R.F performed all pathology and diagnosed the tumors with T.M.J. The manuscript was written by E.Q., E.P. and S.J.M.
Competing interests: S.J.M. is a founder and stockholder in OncoMed Pharmaceuticals. E.Q. was an employee of the University of Michigan while performing this study, but is now an employee of OncoMed Pharmaceuticals, a company that develops cancer therapeutics using xenograft assays.
References
- 1.Quintana E, Shackleton M, Foster HR, Fullen DR, Sabel MS, Johnson TM, Morrison SJ. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer cell. 2010 Nov 16;18:510–523. doi: 10.1016/j.ccr.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008 Dec 4;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC, Jr, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009 Dec 20;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeffs AR, Glover AC, Slobbe LJ, Wang L, He S, Hazlett JA, Awasthi A, Woolley AG, Marshall ES, Joseph WR, Print CG, Baguley BC, Eccles MR. A gene expression signature of invasive potential in metastatic melanoma cells. PLoS One. 2009;4:e8461. doi: 10.1371/journal.pone.0008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.John T, Black MA, Toro TT, Leader D, Gedye CA, Davis ID, Guilford PJ, Cebon JS. Predicting clinical outcome through molecular profiling in stage III melanoma. Clin Cancer Res. 2008 Aug 15;14:5173–5180. doi: 10.1158/1078-0432.CCR-07-4170. [DOI] [PubMed] [Google Scholar]
- 6.Haqq C, Nosrati M, Sudilovsky D, Crothers J, Khodabakhsh D, Pulliam BL, Federman S, Miller JR, 3rd, Allen RE, Singer MI, Leong SP, Ljung BM, Sagebiel RW, Kashani-Sabet M. The gene expression signatures of melanoma progression. Proc Natl Acad Sci U S A. 2005 Apr 26;102:6092–6097. doi: 10.1073/pnas.0501564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kabbarah O, Nogueira C, Feng B, Nazarian RM, Bosenberg M, Wu M, Scott KL, Kwong LN, Xiao Y, Cordon-Cardo C, Granter SR, Ramaswamy S, Golub T, Duncan LM, Wagner SN, Brennan C, Chin L. Integrative genome comparison of primary and metastatic melanomas. PLoS One. 2010;5:e10770. doi: 10.1371/journal.pone.0010770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaeger J, Koczan D, Thiesen HJ, Ibrahim SM, Gross G, Spang R, Kunz M. Gene expression signatures for tumor progression, tumor subtype, and tumor thickness in laser-microdissected melanoma tissues. Clin Cancer Res. 2007 Feb 1;13:806–815. doi: 10.1158/1078-0432.CCR-06-1820. [DOI] [PubMed] [Google Scholar]
- 9.Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, Radmacher M, Simon R, Yakhini Z, Ben-Dor A, Sampas N, Dougherty E, Wang E, Marincola F, Gooden C, Lueders J, Glatfelter A, Pollock P, Carpten J, Gillanders E, Leja D, Dietrich K, Beaudry C, Berens M, Alberts D, Sondak V. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000 Aug 3;406:536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- 10.Jonsson G, Busch C, Knappskog S, Geisler J, Miletic H, Ringner M, Lillehaug JR, Borg A, Lonning PE. Gene expression profiling-based identification of molecular subtypes in stage IV melanomas with different clinical outcome. Clin Cancer Res. 2010 Jul 1;16:3356–3367. doi: 10.1158/1078-0432.CCR-09-2509. [DOI] [PubMed] [Google Scholar]
- 11.Conway C, Mitra A, Jewell R, Randerson-Moor J, Lobo S, Nsengimana J, Edward S, Sanders DS, Cook M, Powell B, Boon A, Elliott F, de Kort F, Knowles MA, Bishop DT, Newton-Bishop J. Gene expression profiling of paraffin-embedded primary melanoma using the DASL assay identifies increased osteopontin expression as predictive of reduced relapse-free survival. Clin Cancer Res. 2009 Nov 15;15:6939–6946. doi: 10.1158/1078-0432.CCR-09-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryu B, Kim DS, Deluca AM, Alani RM. Comprehensive expression profiling of tumor cell lines identifies molecular signatures of melanoma progression. PLoS One. 2007;2:e594. doi: 10.1371/journal.pone.0000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003 Jan;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 14.van ‘t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002 Jan 31;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 15.van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002 Dec 19;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 16.Scott KL, Nogueira C, Heffernan TP, van Doorn R, Dhakal S, Hanna JA, Min C, Jaskelioff M, Xiao Y, Wu CJ, Cameron LA, Perry SR, Zeid R, Feinberg T, Kim M, Vande Woude G, Granter SR, Bosenberg M, Chu GC, DePinho RA, Rimm DL, Chin L. Proinvasion metastasis drivers in early-stage melanoma are oncogenes. Cancer cell. 2011 Jul 12;20:92–103. doi: 10.1016/j.ccr.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garraway LA, Widlund HR, Rubin MA, Getz G, Berger AJ, Ramaswamy S, Beroukhim R, Milner DA, Granter SR, Du J, Lee C, Wagner SN, Li C, Golub TR, Rimm DL, Meyerson ML, Fisher DE, Sellers WR. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005 Jul 7;436:117–122. doi: 10.1038/nature03664. [DOI] [PubMed] [Google Scholar]
- 18.Lindsay CR, Lawn S, Campbell AD, Faller WJ, Rambow F, Mort RL, Timpson P, Li A, Cammareri P, Ridgway RA, Morton JP, Doyle B, Hegarty S, Rafferty M, Murphy IG, McDermott EW, Sheahan K, Pedone K, Finn AJ, Groben PA, Thomas NE, Hao H, Carson C, Norman JC, Machesky LM, Gallagher WM, Jackson IJ, Van Kempen L, Beermann F, Der C, Larue L, Welch HC, Ozanne BW, Sansom OJ. P-Rex1 is required for efficient melanoblast migration and melanoma metastasis. Nat Commun. 2011;2:555. doi: 10.1038/ncomms1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000 Aug 3;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 20.Gobeil S, Zhu X, Doillon CJ, Green MR. A genome-wide shRNA screen identifies GAS1 as a novel melanoma metastasis suppressor gene. Genes Dev. 2008 Nov 1;22:2932–2940. doi: 10.1101/gad.1714608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim M, Gans JD, Nogueira C, Wang A, Paik JH, Feng B, Brennan C, Hahn WC, Cordon-Cardo C, Wagner SN, Flotte TJ, Duncan LM, Granter SR, Chin L. Comparative oncogenomics identifies NEDD9 as a melanoma metastasis gene. Cell. 2006 Jun 30;125:1269–1281. doi: 10.1016/j.cell.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg SF, Miele ME, Hatta N, Takata M, Paquette-Straub C, Freedman LP, Welch DR. Melanoma metastasis suppression by chromosome 6: evidence for a pathway regulated by CRSP3 and TXNIP. Cancer Res. 2003 Jan 15;63:432–440. [PubMed] [Google Scholar]
- 23.Shevde LA, Samant RS, Goldberg SF, Sikaneta T, Alessandrini A, Donahue HJ, Mauger DT, Welch DR. Suppression of human melanoma metastasis by the metastasis suppressor gene, BRMS1. Exp Cell Res. 2002 Feb 15;273:229–239. doi: 10.1006/excr.2001.5452. [DOI] [PubMed] [Google Scholar]
- 24.Leone A, Flatow U, King CR, Sandeen MA, Margulies IM, Liotta LA, Steeg PS. Reduced tumor incidence, metastatic potential, and cytokine responsiveness of nm23-transfected melanoma cells. Cell. 1991 Apr 5;65:25–35. doi: 10.1016/0092-8674(91)90404-m. [DOI] [PubMed] [Google Scholar]
- 25.Damsky WE, Curley DP, Santhanakrishnan M, Rosenbaum LE, Platt JT, Gould Rothberg BE, Taketo MM, Dankort D, Rimm DL, McMahon M, Bosenberg M. beta-Catenin Signaling Controls Metastasis in Braf-Activated Pten-Deficient Melanomas. Cancer Cell. 2011 Dec 13;20:741–754. doi: 10.1016/j.ccr.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arozarena I, Sanchez-Laorden B, Packer L, Hidalgo-Carcedo C, Hayward R, Viros A, Sahai E, Marais R. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell. 2011 Jan 18;19:45–57. doi: 10.1016/j.ccr.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins DE, Oei Y, Hornig YS, Yu SF, Dusich J, Purchio T, Contag PR. Bioluminescent imaging (BLI) to improve and refine traditional murine models of tumor growth and metastasis. Clin Exp Metastasis. 2003;20:733–744. doi: 10.1023/b:clin.0000006815.49932.98. [DOI] [PubMed] [Google Scholar]
- 28.John T, Kohler D, Pintilie M, Yanagawa N, Pham NA, Li M, Panchal D, Hui F, Meng F, Shepherd FA, Tsao MS. The ability to form primary tumor xenografts is predictive of increased risk of disease recurrence in early-stage non-small cell lung cancer. Clin Cancer Res. 2011 Jan 1;17:134–141. doi: 10.1158/1078-0432.CCR-10-2224. [DOI] [PubMed] [Google Scholar]
- 29.Nemati F, Sastre-Garau X, Laurent C, Couturier J, Mariani P, Desjardins L, Piperno-Neumann S, Lantz O, Asselain B, Plancher C, Robert D, Peguillet I, Donnadieu MH, Dahmani A, Bessard MA, Gentien D, Reyes C, Saule S, Barillot E, Roman-Roman S, Decaudin D. Establishment and characterization of a panel of human uveal melanoma xenografts derived from primary and/or metastatic tumors. Clin Cancer Res. 2010 Apr 15;16:2352–2362. doi: 10.1158/1078-0432.CCR-09-3066. [DOI] [PubMed] [Google Scholar]
- 30.DeRose YS, Wang G, Lin YC, Bernard PS, Buys SS, Ebbert MT, Factor R, Matsen C, Milash BA, Nelson E, Neumayer L, Randall RL, Stijleman IJ, Welm BE, Welm AL. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med. 2011;17:1514–1520. doi: 10.1038/nm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]