Abstract
Artificial trans fatty acids promote atherosclerosis by blocking macrophage clearance of cell debris. Classical fatty-acid response mechanisms include TLR4-NF-κB activation, and Erk1/2 phosphorylation, but these may not indicate long-term mechanisms. Indeed, nuclear NF-κB was increased by 60 minute treatment by 30 μM of the 18 carbon trans unsaturated fatty acid elaidic acid (elaidate), the physiological cis-unsaturated fatty acid oleic acid (oleate), and the 18 or 16 carbon saturated fatty acids stearic and palmitic acid (stearate or palmitate). However, except for stearate, effects on related pathways were minimal at 44 hours. To determine longer term effects of trans fatty acids, we compared whole exome mRNA expression of (trans) elaidate to (cis) oleate, 30 μM, at 44 hours in human macrophages. We found that elaidate changed Zn2+-homeostasis gene mRNAs markedly. This might be important because Zn2+ is a major regulator of macrophage activity. Messenger RNAs of seven Zn2+-binding metallothioneins decreased 2–4 fold; the zinc importer SLC39A10 increased 2-fold, in elaidate relative to oleate-treated cells. Results were followed by quantitative PCR comparing cis, trans, and saturated fatty acid effects on Zn2+-homeostasis gene mRNAs. This confirmed that elaidate uniquely decreased metallothionein expression and increased SLC39A10 at 44 hours. Further, intracellular Zn2+ was measured using N-(carboxymethyl)-N-[2-[2-[2(carboxymethyl)amino]-5-(2,7,-difluoro-6-hydroxy-3-oxo-3H-xanthen-9-yl)-phenoxy]-ethoxy]-4-methoxyphenyl]glycine, acetoxymethyl ester (FluoZin-3-AM). This showed that, at 44 hours, only cells treated with elaidate had increased Zn2+. The durable effect of elaidate on Zn2+ activation is a novel and specific effect of trans fatty acids on peripheral macrophage metabolism.
Keywords: Trans fat, Intracellular zinc, Atherosclerosis, Macrophage metabolism
Introduction
Small quantities of artificial trans-monounsaturated fat or large quantities of saturated fat promote atherosclerotic disease and disorders of energy metabolism (Kummerow, 2009; Micha and Mozaffarian, 2009). Lipid metabolism is altered by trans-fatty acids; consequences include increased unsaturation of fatty acids in cell membranes (Sepulveda et al., 2010; Zacherl et al., 2014). Fatty acids, especially trans- and saturated fatty acids, also mediate receptor-mediated cell signaling, which might in turn influence inflammatory cytokines that may modify many disease processes (Hotamisligil 2006; Wymann et al. 2003; Wymann and Schneiter, 2008). Because lipids have many metabolic and signaling effects, isolating cause and effect is difficult (Wymann and Schneiter, 2008). In health, macrophages protect the vascular endothelium by removing cellular debris including cell membrane components, oxidized phospholipids and cholesterol, from the vascular intima. This should preclude accumulation of extracellular membrane debris, as well as related inflammation, cell proliferation, and atherosclerosis. Progressive atherosclerosis involves cycles of cell death and recruitment of inflammatory cells by cytokines, with growth of atheromas until failure of vascular function occurs. How macrophage removal of cell membrane debris gets out of control is not fully understood, but it is exacerbated by saturated- and trans-monounsaturated fatty acids (Choi et al. 2008; Boullier et al. 2001).
We evaluated short- and long-term effects of fatty-acid on human macrophages. Classical response pathways were activated at ~60 minutes by all fatty acids at 30 μM, but only saturated fatty acids had extended (44 hour) effects, particularly octadecanoic acid (stearate). Since trans fatty acids definitely affect continuing peripheral lipid metabolism, we followed these studies by exploratory whole-exome mRNA expression arrays. We compared stimulation of human macrophages at 44 hours of exposure to 30 μM (E)-octadec-9-enoate, C18:Δ9-10 trans, commonly called elaidic acid (elaidate) or (Z)-octadec-9-enoate, C18: Δ9-10 trans, commonly called oleic acid (oleate). Effects on previously studied pathways were confirmed; elaidate partially blocks β-oxidation, with consequent effects on fatty acid unsaturation and other processes (Zacherl et al., 2014). However, there was also reduced mRNA expression of zinc-binding metallothioneins and increased expression of a zinc importer in elaidate treated cells. This was an unexpected finding. Zinc regulates expression of inflammatory cytokines and innate immunity receptors in macrophages (Cousins et al. 2003; Haase and Rink, 2007). The primary function of the metallothioneins is zinc storage. Zinc is essential to many biological processes, and interacts with thousands of proteins (Hambidge and Krebs, 2007). Metallothionein-null mice survive, they cannot store zinc normally, and zinc supplementation is required for their survival (Davis and Cousins, 2000; Tran et al. 1998). We confirmed the results of the array study by Quantitative PCR, and performed analysis of intracellular Zn2+.
Our data indicate that effects of elaidate on cellular Zn2+ involve a durable increase in Zn2+ activity that cannot be attributed to fatty acid signaling by classical pathways. Large changes in transcription of lipid regulatory enzymes, including steroyl CoA desaturase, the ABCG1 fatty acid/cholesterol transporter, and hydroxymethylglutaryl CoA synthase, also occur selectively with long-term elaidate stimulation, suggesting that alternate intracellular metabolic regulation may lead to elevated Zn2+ and other pathological cellular responses.
Materials and Methods
Cell Culture
Human peripheral blood monocytes were isolated from buffy coats as described (Robinson et al., 2009). The cells were transferred to AIM-V medium containing 20 μg/ml of human CSF-1 (Peprotech, Rocky Hill, NJ). For quantitative PCR and expression array analysis, monocytes were CD14 purified with magnetic beads from Miltenyi Biotech (San Diego, CA) as described (Robinson et al., 2009). Three days after isolation, CD14 purified cells or peripheral blood monocytes were treated with 30 μM oleate, elaidate, stearate, or palmitate, all carried by lipid-free bovine serum albumin (BSA) from Sigma Aldrich, St. Louis, MO, with 20 μg/ml human CSF-1 in Dulbecco’s minimal essential medium (DMEM). Controls included a 10% FBS and a BSA only incubation. Fatty acid BSA preparation was as described (Watkins et al., 1991). For the expression arrays, quantitative PCR and Zn2+ measurement, macrophages were treated for 15 or 44 hours with fatty acids. For the NF-κB studies, macrophages were treated with fatty acids for 1 hour.
Nuclear Localization of Nuclear factor-κB
Human primary macrophages were cultured as for Zn2+ measurement. Cells were treated one hour with or without the Zn2+ chelator 5 μM N,N,N′,N-Tetrakis (2-pyridylmethyl)ethylenediamine (TPEN) at 37 °C, with fatty acids and controls as indicated in results. Subsequent steps were performed at room temperature. Cultures were rinsed with PBS with 0.2 mM EDTA and fixed in 3% formaldehyde for 15 minutes, and rinsed again with PBS. Cells were blocked with 1% BSA for 30 minutes and then incubated with 500 μl of 1:250 NF-κB polyclonal rabbit antibody SAB4502610 Anti-NF-κB p65 antibody raised to a human P65 peptide sequence, in rabbit ~1 mg/ml, affinity isolated antibody, from Sigma (St. Louis, MO). After rinsing, cultures were incubated with 1:50 Alexa Fluor 594 (red) donkey anti-rabbit for one hour (Life Technologies, Grand Island, NY) and rinsed again. Cultures were also labeled in Hoechst fluor (Life Technologies, Grand Island, NY) for one minute. Imaging used a Nikon TE2000 inverted phase-fluorescence microscope using a 14-bit 2000×2000 pixel monochrome CCD (Spot Instruments, Sterling Heights, MI). For red fluorescence, excitation was 536–556 nm with a 580 nm dichroic mirror and a 590 nm barrier. For blue fluorescence, excitation was 380–425 nm with a 430 nm dichroic filter and a 450 nm barrier. Fluorescent signal was photographed using 1.3 NA 40x or 100x oil objectives. Nuclei were defined by the blue Hoechst label to calculate the nuclear proportion of total cellular NF-κB label.
Western blots and ELISA
Cells were lysed on ice in 150 mM NaCl, 50 mM Tris pH 7.5, 0.1% SDS, and 1% Triton-X-100, with phosphatase and proteinase inhibitors. Lysates were centrifuged to remove insoluble material. Protein concentrations were determined by Coomassie blue dye binding in acid (Bio-Rad Protein Assay, Bio-Rad, Hercules, CA). Western Blots were essentially as described (Liu et al., 2012); 16 μg of protein were run in sample buffer denatured by 10 min at 75 °C and separated on SDS-PAGE in pre-cast 4% – 12% gradient bis-tris-polyacrylamide gels (Life Technologies, Grand Island, NY). Proteins were transferred to PVDF membranes; 5% casein from bovine milk (blotting-grade blocker, Bio-Rad Laboratories) was used to block unreacted groups, followed by incubation with primary antibodies at 4 °C overnight. Blots were washed and incubated with horseradish peroxidase-labeled anti-rabbit IgG and anti-mouse IgG at 1:40,000 (Jackson Immuno Research, West Grove, PA). Proteins were detected by enhanced chemiluminescence (SuperSignal, ThermoScientific). Blots were re-probed for β-actin after the membrane was stripped for 20 min in 2% SDS in tris buffer with mercaptoethanol (Restore Plus Stripping Buffer, ThermoScientific). Mouse monoclonal anti-β-actin (clone AC-15, ascites fluid, Sigma) was used at 1:10,000. Phospho-p44/42 MAPK (Erk1/2) anti-Thr202/Tyr204 rabbit monoclonal antibodies, D13.14.4E (Cell Signaling, Danvers, MA) were used at 1:2,000; purified p44/42 MAPK (Erk1/2) rabbit polyclonal antibody was used at 1:1,000 (cell signaling). Rabbit polyclonal anti-ZNF816A was used at 1:1,000 (Abcam, Cambridge, MA), to a synthetic peptide of N terminal amino acids 71–120 of Human ZNF816A. Mouse monoclonal anti-TLR4 (Clone# 285227) was used at 1 μg/ml (R&D Systems, Minneapolis, MN). Solid phase ELISA for TNFα used antibodies to recombinant human TNFα (Quantikine TNFα ELISA, R&D Systems).
cDNA Array
Cells were CD14 magnetic bead purified (Robinson et al., 2009) and plated. After a 44 hour incubation with 30 μM elaidate or oleate, media were removed and cells were washed twice in PBS. Macrophages were treated with 0.25% trypsin EDTA at 37 °C to loosen cells. Trypsin action was stopped with an equal volume of DMEM with 10% FBS. Cells were scraped from the plate and washed twice with PBS. Messenger RNA was extracted, reverse transcribed, and used to produce cDNA for analysis using the human U133 Plus 2.0 array (Affymetrix, Santa Clara, CA). This array contains the genome-wide human genome U133 set and an additional 6,500 genes for analysis of over 47,000 human transcripts. The complete array probe-set and descriptions are available from the Affymetrix corporation on request.
Quantitative PCR
Peripheral blood monocytes were incubated for 15 or 44 h in 30 μM fatty acids, and quantitative PCR was performed as in our recent work (Zacherl et al., 2014). In most cases QuantiTect (Qiagen) assays were used. These are pre-tested primer sets specified by GeneBank sequence, amplicon size, and approximate position. All extend across one or more introns. Products were verified by amplicon size on agarose gels (not shown). For human metallothionein-1X (MT1X) NM_005952 (468 bp), the QuantiTect amplicon extends across the first three exons and has an amplicon size of 157 bp. For human metallothionein-2A (MT2A) NM_005953 (466 bp), the QuantiTect amplicon extends across several mid-molecule exons and has an amplicon size of 139 bp. For human solute carrier family 39, member 10 (SLC39A10) NM_020342 (5386 bp) the QuantiTect amplicon extended from exons 4–5 and is 138 bp. Results were normalized as a percentage of GAPDH expression, in this case using primers from NM_002046.3, F 5′-ACAGTCAGCCGCATCTTCTT, R 5′-GACAAGCTTCCCGTTCTCAG, product 259 bp.
Intracellular Zn2+ activity
Human primary macrophages were cultured on 35 mm MatTek (Ashland, MD) dishes containing 10 mm glass micro-wells. Cultures included the fatty acid treatments oleate, elaidate, stearate, and palmitate, 30 μM, and two controls, 10% FBS and BSA alone, in DMEM with 20 μg/ml human CSF-1. Cultures were incubated with all six combinations for 15 or 44 hours. Cells were then rinsed with fat-free DMEM and 5 μl of 5 mM N-(carboxymethyl)-N-[2-[2-[2(carboxymethyl)amino]-5-(2,7,-difluoro-6-hydroxy-3-oxo-3H-xanthen-9-yl)phenoxy]ethoxy]-4-methoxyphenyl]glycine, acetoxymethyl ester (FluoZin-3-AM), Life Technologies, Grand Island, NY, was added in 1 ml of Dubelco’s modified essential medium. After 30 minute incubation, excess FlouZin-3 was removed by rinsing in fat-free media. Cells were photographed within 20 minutes in differential interference contrast and in green fluorescence to measure the Zn2+ activity, in fresh fat-free media using an AndorZyla VSC-00073 camera with 40x oil DIC H N2 optics; for fluorescence a 200 ms exposure and a mono 16 bit image was used. To quantify the intensity of signal, 11–21 images were measured per condition, with ~ 100 cells in each image.
Results
Nuclear Factor-κB translocation
We hypothesized that the Zn2+ response to fatty acids might be associated with inflammation-related signaling, much of which operates through NF-κB activation. Since NF-κB translocation is rapid (Renard et al., 1997), one hour fatty acids treatment was studied (Fig 1). Elaidate, stearate, and palmitate-treated macrophages showed increased intranuclear NF-κB, p< 0.0001 versus controls (Fig 1B). Oleate did not increase nuclear localization of NF-κB relative to controls. The increased intranuclear NF-κB in the trans- and saturated-fatty acid treated macrophages suggests common signaling at this short time point. As a control for specificity, adding the chelator, TPEN, 5 μM to the incubation media abolished differences in nuclear localization between groups (Fig 1C). TPEN chelates Zn2+, an established regulator of NF-κB activation. This is consistent with receptor activation of NF-κB translocation mediated by the fatty acids, at one hour.
Figure 1. Effects of fatty acid treatment on NF-κB nuclear localization.

Macrophages were treated with 30 μM fatty acids for 1 hour with and without 5 μM of the chelator TPEN. Immunofluorescence was conducted on the fixed cells and photographed.
A. Images of cells showing with low and high response. Oleate-treated cells had very little nuclear localization of NF-κB. Dark holes can be seen where the nuclei should be. In the elaidate-treated cells, the red NF-κB signal is largely co-localized with the blue nuclear label.
B. The proportion of NF-κB localized to the nucleus with each treatment at 1 hour. Thirty μM elaidate-, stearate-, and palmitate- treated cells had significantly more nuclear localization than oleate- or control-treated cells; ***p<0.0001.
C. The same experiment after the addition of 5 μM of the zinc chelator TPEN to the incubation medium. This treatment abolished differences, reflecting that components of the NF-κB activation cascade downstream of receptors require zinc for proper function.
Fatty acid receptor-mediated responses at 44 hours
Short-term cellular response to fatty acids is known to be mediated, in major part, by the innate immune system, particularly via toll-like receptor-4 (TLR4) (Cullberg et al., 2014); the activation of NF-κB by elaidate, stearate, and palmitate, is consistent with this (Fig 1). Whether durable activation of macrophages by fatty acids reflects longer-term effects on similar mechanisms, was uncertain. To study this, effects of fatty acids on TLR4-related signaling mechanisms were studied at 44 hours (Fig 2). We included macrophages without added fatty acids, and non-activated lymphocytes, which have similar receptors, as additional controls. Quantity of TLR4 was reduced at 44 hours by 30 μM fatty acids on albumin carriers, although TLR4 expression in stearate was much higher than in oleate, elaidate, or palmitate. Phospho-Erk1/2 was highest in controls and in stearate; total Erk1/2 was markedly reduced in the unsaturated fatty acids oleate and elaidate. These results indicate that the signaling pathway activity varies between the fatty acids at 44 hours.
Figure 2. TLR4, ZNF816, and Erk1/2 expression, Erk phosphorylation in 30 μM fatty acids and in control macrophages and lymphocytes, and TNFα production by ELISA all at 44 hours.
Westerns shown are single blots; irrelevant lanes between untreated macrophages and lymphocyte controls are deleted. Duplicate blots (not shown) for β-actin, phospho-Erk1/2, and Erk1/2 were used for densitometry.
A. TLR4 expression in 30 μM fatty acids, 44 hours. All fatty acids reduced TLR4 relative to untreated macrophages, but the loss of TLR4 in stearate was much less than with oleate, elaidate, or palmitate treatment.
B. Phospho-Erk1/2 decreased with fatty acid treatment except in stearate. For densitometry, see (G)
C. An inflammation-related transcriptional repressor, ZNF816, was increased in fatty acid-treated macrophages. A lower MW form present in controls was lost in fatty acid-treated cells.
D. Actin controls for loading, used in densitometry.
E. Blots for total Erk1/2; compare with B.
F. Effect of fatty acid treatment on TNFα expression in cell supernatants at 44 hours, by ELISA. Stearate uniquely stimulates TNFα production at this time point. N=4, mean ± range, p<0.01
G. Phospho-Erk1/2 relative to actin in two blots. Stearate is increased relative to all other conditions; differences are significant relative to oleate and palmitate, p<0.05. N=2, mean ± range.
Since stearate has been shown to have distinct effects on TLR4 and phospho-Erk signaling compared to oleate, palmitate, and elaidate, we examined how the fatty acids affect long-term production of the important downstream inflammatory cytokine, TNFα, in cell supernatants at 44 hours (Fig 2F). This assay showed that stearate caused much greater production of TNFα at 44 hours (Fig 2F), a result highly significant relative to all other groups. This pattern matched greater phospho-Erk1/2 in stearate treated cells by densitometry (Fig 2G), and might reflect maintenance of TLR4 in stearate treated cells (Fig 2A). However, the ELISA for TNFα gave much clearer differences. We did additional blots for a nuclear transcription repressor, ZNF816 (Fig 2C, right side). ZNF816 is widely expressed, including in macrophages and lymphoctyes, and has been associated with psoriasis (Sun et al. 2010). Previous screening showed correlation of ZNF816 single nucleotide polymorphism isoforms with susceptibility to infection (Peck-Palmer et al., 2011), so ZNF816 might regulate macrophage inflammatory response. This transcription inhibitor was up-regulated by all fatty acid treatments at 44 hours (Fig 2C). However, a major fractional-size immunoreactive product seen in both untreated macrophages and lymphocytes was absent in fatty-acid treated cells (see Discussion).
Genome-wide mRNA expression in elaidate and oleate treated macrophages
Since elaidate is strongly implicated in atherosclerosis, but did not show effects consistent with long-term receptor-mediated response that was seen in stearate (Fig 2), we did genome-wide analysis of macrophage mRNA expression in elaidate versus oleate treated cells. Macrophages incubated for 44 hours in 30 μM of the trans fatty acid, elaidate had decreased expression of six metallothionein genes relative to cells incubated with its natural cis isomer, oleate (Table 1). In addition, the mRNA for the zinc transporter, SLC39A10 had enhanced expression. Other pathways where several mRNAs changed included in lipid metabolism; three key rate-limiting enzymes (Table 1). Many other mRNAs changed significantly, including mRNAs for macrophage differentiation, immune function or stress response, and EGF family proteins (not shown). These pathways have precedents in macrophage differentiation studies. However, the zinc pathway was novel, and therefore it was studied further.
Table 1. Zinc metabolism genes with highly altered expression and selected lipid metabolism genes.
Gene: human gene name; Probes: number of separate probes are present on the array; if two, results are averaged. Expression for oleate (OL) or Elaidate (EL) treated cells are averages of 20 measurements for each probe, using the Affymetrix human U133 Plus 2.0 array, containing over 50,000 DNA oligomers. The log2 ratio indicates decreased (negative) or increased (positive) expression of elaidate relative to oleate. The p value is from a paired T test for difference between OL and EL.
| Zn-management Genes | # Probes | OL | EL | log2 ratio | p value |
|---|---|---|---|---|---|
| MT2A | 1 | 6935 | 2317 | −1.3 | 0.00002 |
| MT1F | 2 | 2753 | 847 | −1.7 | 0.00002 |
| MT1X | 2 | 3839 | 1517 | −1.6 | 0.00002 |
| MT1G | 2 | 4207 | 1618 | −1.3 | 0.00002 |
| MT1H | 1 | 3566 | 1329 | −1.3 | 0.00007 |
| MT1E | 2 | 2893 | 1035 | −1.1 | 0.00003 |
| SLC39A10 | 1 | 930 | 1739 | 0.8 | 0.00002 |
| Key Lipid Metabolism Genes | |||||
| ABCG1 | 1 | 290 | 646 | 1.5 | 0.00002 |
| SCD | 2 | 1376 | 2447 | 0.8 | 0.00002 |
| HMGCS1 | 1 | 2250 | 3963 | 0.8 | 0.00005 |
Metalothionein and zinc transporter expression in four fatty acids
We assayed MT1X, MT2A, and SLC39A10 mRNAs in CD14 purified macrophages after 44 hours in fatty acids palmitate, stearate, oleate, or elaidate, a carrier control (bovine serum albumin, BSA), and a standard medium control including 10% fetal bovine serum (Fig 3A). Results at 44 hours were consistent with the expression array, and showed that the long-term effect on zinc regulating proteins were specific for elaidate. Elaidate-treated cells had the lowest expression for both metallothioneins, p< 0.01 compared to oleate; p< 0.001 compared to stearate and palmitate. The oleate-treated cells were intermediate in expression, p<0.01 compared to all other treatments. The saturated fatty acid-treated cells, stearate and palmitate, had the highest metallothionein expression, p< 0.001 compared to all other treatments. Elaidate-treated cells showed the highest levels of SLC39A10 expression, p< 0.05 compared to palmitate, and p< 0.01 compared to oleate and stearate. A 15 hour time point was also studied, and showed contrasting results (Fig 3B). Changes in mRNAs from 15 to 44 hours are indicated by the dashed lines between Fig 3A–B. At 15 hours, expression metallothioneins in oleate- treated and controls was high compared to palmitate (p<0.05), and to stearate and elaidate (p<0.01). The expression of SLC39A10 was highest in controls, and lower but similar in all fatty acid treatments at 15 hours. The zinc transporter expression varied significantly between fatty acids only at 44 hours. Oleate and elaidate treatment caused decreases (p<0.001, p< 0.01, respectively) in metallothionein expression from 15–44 hours. In saturated fatty acids, stearate and palmitate, changes in metallothionein and SLC39A10 expression were minor and not significant. These results point, again, to differences in acute and chronic response to fatty acids in macrophages (see Discussion).
Figure 3. Effects of fatty acid treatment on metallothioneins and on the zinc importer SLC39A10.
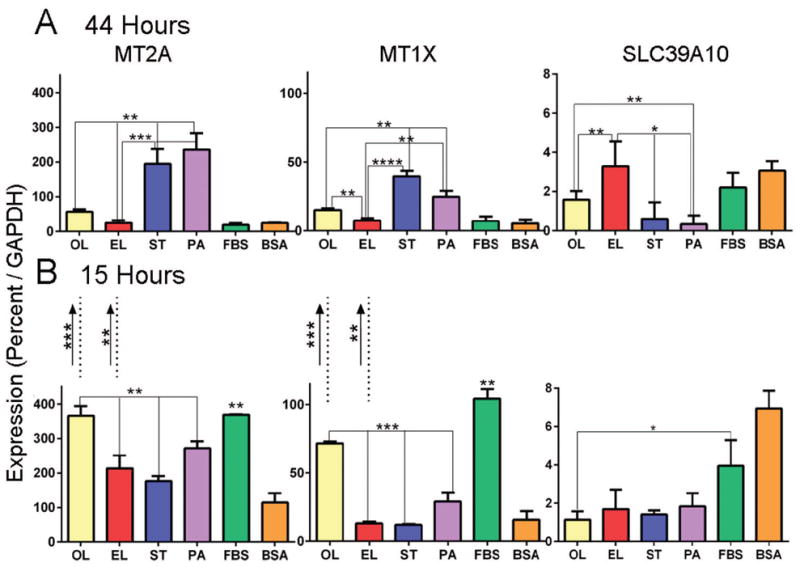
Macrophages (n = 3–5) were treated for 15 or 44 hours with 30 μM fatty acids on 0.1 mM BSA. Oleate (OL), elaidate (EL), stearate (ST), palmitate (PA), DMEM with 10% fetal bovine serum (FBS) and bovine serum albumin carrier without fatty acid (BSA). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
A. Quantitative PCR as % GAPDH at 44 hours. Elaidate had the lowest metallothionein expression and the highest SLC9A10 expression, significant at p<0.01 or better relative to all other fatty acids. In contrast, the saturated fatty acids, stearate and palmitate, had highest metallothionein mRNAs and lowest SLC9A10 relative to elaidate, oleate, and the controls.
B. Quantitative PCR measurement of mRNA expression after 15 hours. Metallothionein mRNAs were in elevated at this shorter time in oleate and complete medium with FBS. The zinc importer SLC39A10 was significantly higher in controls. Changes over time are indicated by the arrows between the graphs: Decreases in metallothionein mRNA in oleate and elaidate were the key changes with time.
Intracellular Zn2+ activity
Significant reductions in zinc-binding metallothioneins and increased expression of a zinc importer suggested that long-term response to elaidate might reflect chronic changes in intracellular Zn2+. To determine how fatty acids affected Zn2+, we used the fluorescent indicator FluoZin-3-AM (Fig 4). Cells were treated with the membrane permeable acetoxymethyl ester of the dye, the zinc-binding fluor being released by cellular esterases. FluoZin-3 is quite specific for Zn2+ ions and is currently the most specific indicator for low concentrations of intracellular Zn2+. At 44 hours, the elaidate-treated cells had distinctly elevated Zn2+ activity, p<0.001 compared to all other treatments, corresponding to the lowest expression of Zn2+ binding metallothioneins and elevated SLC39A10 (Table 1, Fig 3). None of the other fatty acid or control treatments had persistent low metallothionein expression or increased SLC39A10, and none had marked changes in Zn2+ at 44 hours. At 15 hours (Fig 4B) elaidate, stearate, and palmitate had high and similar Zn2+, but by 44 hours, Zn2+ in saturated fatty acid-treated macrophages returned to control levels, while in elaidate-treated cells, Zn2+ increased from 15 to 44 hours, p<0.05. Intracellular Zn2+ did not vary in oleate-treated macrophages.
Figure 4. Effects of fatty acid treatment on intracellular Zn2+ activity.
Intracellular zinc activity after fatty acid incubation as measured with FluoZin-3. Macrophages were treated with 30 μM fatty acids for 44 or 15 hours. FluoZin-3, AM was applied for 30 minutes. Cells were photographed in untreated media.
A. Images of cells at the 44 hour time point. Note that the elaidate-treated cells had the most Zn2+ signal with palmitate-treated cells showing the least.
B. Quantified intracellular Zn2+ activity after treatment with fatty acid for 44 hours. Elaidate-treated cells had the most labile zinc, while the palmitate-treated cells had the least. (*p< 0.01, **p<0.001, ***p< 0.0001)
C. Quantified intracellular Zn2+ activity after treatment with fatty acid for 15 hours. Elaidate-, stearate-, and palmitate-treated cells had significantly higher labile zinc levels than the other three conditions (*p< 0.01, **p<0.001, ***p< 0.0001). From 15 to 44 hours, Zn2+ activity increased only for EL-treated cells (p< 0.01), while it decreased or did not change significantly in all other treatments. Oleate or BSA did not significantly affect Zn2+ activity at 15 or 44 hours.
Discussion
Saturated and trans-fatty acids promote atherosclerosis (Choi et al. 2008; Boullier et al. 2001; Zhu et al. 2005), but the mechanisms causing this are not clear. We showed that saturated fatty acids promote macrophage activation over prolonged periods by classical mechanisms including TLR4 expression, phosphoErk1/2 activation, and TNFα production (Fig 2). These findings fit with numerous other studies. However, response to the trans-fatty acid elaidate did not occur by similar mechanisms (Fig 2). Our novel finding, by whole exome screening, was that gene products that affect zinc homeostasis are altered greatly by elaidate relative to oleate at 44 hours (Table 1). We validated these changes by PCR, with additional comparisons to saturated fatty acids (Fig 3). Elaidate uniquely caused a sustained increase in Zn2+, to at least 44 hours (Fig 4). This indicates a role for zinc signaling in trans-fat response, which previously was unknown.
Elaidate causes longer-term changes in cellular fat metabolism as well (Table 1, lower part). This is not surprising since elaidate is difficult for macrophages to degrade. It stalls fatty acid metabolism and causes accumulation of intermediates of β-oxidation (Zacherl et al., 2014). How these changes might be related to Zn2+-handling gene expression (Fig 3) is unknown. The change in zinc-managing proteins at 44 hours in elaidate is highly consistent and significant; no other fatty acids had parallel effects. The decreased metallothionein expression and increased intracellular Zn2+ suggest cause and effect, but further work will be needed to confirm this. Metallothioneins are a 10-isoform family with four major divisions (West et al. 1990). These small proteins are largely localized to the Golgi apparatus in many cells including macrophages (Palmiter 1987). Metallothioneins contain no aromatic side chains include ~30% cysteine, which enable the proteins to bind Zn2+ as well as heavy metals, nickel, copper, mercury, and silver (Palmiter 1987; et al., 1991).
The zinc transporter SLC39A10 was characterized in LLC-PK1 kidney proximal tubule cells (Kaler and Prasad 2007). It is a 40 kDa Zip family protein (Kambe et al., 2004), and increases the concentration of zinc in the cytosol in a saturable time and temperature sensitive manner (Kaler and Prasad 2007). The large increase in SLC39A10 in elaidate treated macrophages thus is consistent with a probable role in the long-term increase Zn2+ activity observed (Fig 2B). There is probably also secondary regulation of SLC39A10; this is unstudied.
Studies of lipid activation of receptor-mediated pathways at 44 hours (Fig 2) show no detectable elaidate activity. Response of all of the fatty acids faded at 44 hours, except for stearate where TLR4 protein, phosphoErk1/2, and TNFα production all were maintained with significant differences relative to the other fatty acids. The selective effect of stearate on TNFα production and of elaidate on Zn2+ indicate strongly that saturated- and trans-fatty acids affect macrophage function by largely distinct mechanisms. We included blots of an inflammation-associated transcription repressor, ZNF816 (Sun et al. 2010; Peck-Palmer et al., 2011). The high-molecular weight variant of this protein was uniformly increased in all fatty acid treatments, while fatty acid treatments eliminated a prominent lower MW band, of unknown functional significance, that is present in untreated macrophages or lymphocytes. This result is communicated solely to indicate that a long-term transcription suppression mechanism might regulate fatty acid response, while significant additional work will be needed to determine the significance of transcription repressor effects in long-term fatty acid pathology.
How elaidate downregulates metallothionein and upregulates SLC39A10 mRNAs is also unclear. It is unlikely, but possible, that this occurs through cell-surface fatty acid receptors (Fig 2). Other possibilities include cellular regulation via lipid-related metabolic regulatory genes that change in elaidate, presumably secondary to the strong inhibition of β-oxidation by elaidate (Zacherl et al., 2014). Three key regulatory enzymes that are significantly increased are shown in Table 1. These include the ATP-binding cassette sub-family G-1, ABCG1, which mediates cholesterol and phospholipid transport, and regulates cellular lipid homeostasis, and is a strong candidate for regulation of inflammation and atherosclerosis (Westerterp et al., 2014). Other regulatory genes in lipid related pathways whose expression doubled included steroyl CoA desaturase-1, SCD, and hydroxymethylglutaryl CoA synthase 1, HMGCS1. The protein encoded by SCD desaturates fatty acids, increasing membrane fluidity. The product of HMGCS1 is the statin target, the rate-limiting cholesterol synthesis enzyme. Both SCD and HMGCS1 are implicated in regulation of inflammation and in lipid-related metabolism and pathology (Liu et al., 2011; Rodriguez et al., 2012). How these or other proteins might be linked atherosclerosis-inducing pathways is, as yet, uncertain.
Many inflammatory pathways are induced by fatty acids (Hotamisligil, 2006). Toll-like receptor-4 is a key receptor responsible for innate immune response in atherosclerosis (Pasterkamp et al., 2004). Recently, fatty acid signaling to toll-like receptors, particularly TLR4 in macrophage membranes, was shown to operate through fatty acids bound to the glycoprotein fetuin-A (Pal et al., 2012), increasing pro-inflammatory cytokines and oxidative stress. A common pathway stimulated by TLR4 is activation of nuclear factor-κB (NF-κB) through the IκB kinase (IKK) complex in macrophages (Adamson et al., 2011; Karin and Ben-Neriah, 2000). NF-κB is important in the induction of adhesion is cell proliferation surrounding atherosclerosis (De Martin et al., 2000). As short term effects, all of the fatty acids activate this pathway (Fig 1), but in longer term assays, our results are consistent with TLR4 activation as a major pathway mediating stearate effects, but not effects of elaidate (Fig 2).
In conclusion, our work suggests that the toxic trans fatty acid elaidate has long-term (44 hour) effects driven, at least in major part, by tonically increased intracellular Zn2+. Short-term NF-κB-related and intracellular Zn2+ signaling are induced by several fatty acids. However, only the 18-carbon saturated fatty acid stearate generated long term cytokine signals including TNFα, and it did not affect Zn2+ at 44 hours. Thus stearate might mediate toxic inflammatory effects mainly, at longer times, by receptor mediated mechanisms. Overall, toxic fatty acid effects can initiate pathological function in macrophages by at least two largely distinct mechanisms: receptor mediated effects of fatty acids, and trans-fatty acid-mediated changes in cellular regulatory gene expression, including Zn2+ regulating proteins.
Acknowledgments
Contract grant sponsor: NIH (USA) R055208; Contract grant sponsor: NIH (USA)AR055208; Contract grant sponsor: Department of Veterans Affairs (USA) BX002490; NIH KL2 TR000146.
This work was supported by the Department of Veterans Affairs grant BX002490 and by NIH grants R055208, AR055208, and KL2 TR000146. Opinions expressed are not those of the Department of Veterans Affairs.
References
- Adamson S, Leitinger N. Phenotypic modulation of macrophages in response to plaque lipids. Curr Opin Lipidol. 2011;22:335–342. doi: 10.1097/MOL.0b013e32834a97e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman JW, Liu J, Liu YP, Klaassen CD. Increase in metallothionein produced by chemicals that induce oxidative stress. Toxicol Appl Pharmacol. 1991;110:347–354. doi: 10.1016/s0041-008x(05)80017-1. [DOI] [PubMed] [Google Scholar]
- Boullier A, Bird DA, Chang MK, et al. Scavenger receptors, oxidized LDL, and atherosclerosis. Ann N Y Acad Sci. 2001;947:214–222. doi: 10.1111/j.1749-6632.2001.tb03943.x. [DOI] [PubMed] [Google Scholar]
- Choi SH, Chae A, Miller E, et al. Relationship between biomarkers of oxidized low-density lipoprotein, statin therapy, quantitative coronary angiography, and atheroma. J Am Coll Cardiol. 2008;52:24–32. doi: 10.1016/j.jacc.2008.02.066. [DOI] [PubMed] [Google Scholar]
- Cousins RJ, Blanchard RK, Popp MP, et al. A global view of the selectivity of zinc deprivation and excess on genes expressed in human THP-1 mononuclear cells. Proc Natl Acad Sci USA. 2003;100:6952–6957. doi: 10.1073/pnas.0732111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullberg KB, Larsen JO, Pedersen SB, Richelsen B. Effects of LPS and dietary free fatty acids on MCP-1 in 3T3-L1 adipocytes and macrophages in vitro. Nutr Diabetes. 2014;4:e113. doi: 10.1038/nutd.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SR, Cousins RJ. Metallothionein expression in animals: a physiological perspective on function. J Nutr. 2000;130:1085–1088. doi: 10.1093/jn/130.5.1085. [DOI] [PubMed] [Google Scholar]
- De Martin R, Hoeth M, Hofer-Warbinek R, Schmid JA. The transcription factor NF-κB and the regulation of vascular cell function. Arterioscler Thromb Vasc Biol. 2000;20:E83–E88. doi: 10.1161/01.atv.20.11.e83. [DOI] [PubMed] [Google Scholar]
- Haase H, Rink L. Signal transduction in monocytes: the role of zinc ions. Biometals. 2007;20:579–85. doi: 10.1007/s10534-006-9029-8. [DOI] [PubMed] [Google Scholar]
- Hambidge KM, Krebs NF. Zinc deficiency: a special challenge. J Nutr. 2007;137:1101–1105. doi: 10.1093/jn/137.4.1101. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Kaler P, Prasad R. Molecular cloning and functional characterization of novel zinc transporter rZip10 (Slc39a10) involved in zinc uptake across rat renal brush-border membrane. Am J Physiol Renal Physiol. 2007;292:F217–F229. doi: 10.1152/ajprenal.00014.2006. [DOI] [PubMed] [Google Scholar]
- Kambe T, Yamaguchi-Iwai Y, Sasaki R, Nagao M. Overview of mammalian zinc transporters. Cell Mol Life Sci. 2004;61:49–68. doi: 10.1007/s00018-003-3148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Kummerow FA. The negative effects of hydrogenated trans fats and what to do about them. Atherosclerosis. 2009;205:458–465. doi: 10.1016/j.atherosclerosis.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Liu L, Alonso V, Guo LT, et al. Na+/H+ exchanger regulatory factor 1 (NHERF1) directly regulates osteogenesis. J Biol Chem. 2012;287:43312–43321. doi: 10.1074/jbc.M112.422766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Strable MS, Ntambi JM. Stearoyl CoA desaturase 1: role in cellular inflammation and stress. Adv Nutr. 2011;2:15–22. doi: 10.3945/an.110.000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micha R, Mozaffarian D. Trans fatty acids: effects on metabolic syndrome, heart disease and diabetes. Nat Rev Endocrinol. 2009;5:335–344. doi: 10.1038/nrendo.2009.79. [DOI] [PubMed] [Google Scholar]
- Pal D, Dasgupta S, Kundu R, Maitra S, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18:1279–1285. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. Molecular biology of metallothionein gene expression. Experientia Suppl. 1987;52:63–80. doi: 10.1007/978-3-0348-6784-9_4. [DOI] [PubMed] [Google Scholar]
- Pasterkamp G, Van Keulen JK, De Kleijn DP. Role of Toll-like receptor 4 in the initiation and progression of atherosclerotic disease. Eur J Clin Invest. 2004;34:328–334. doi: 10.1111/j.1365-2362.2004.01338.x. [DOI] [PubMed] [Google Scholar]
- Peck-Palmer OM, Yende S, Song C, et al. Identification of host transcriptomic differences between black and white individuals with community acquired pneumonia and severe sepsis. Clin Chem. 2011;57(10S):A70. [Google Scholar]
- Renard P, Zachary MD, Bougelet C, et al. Effects of antioxidant enzyme modulations on interleukin-1-induced nuclear factor-κB activation. Biochem Pharmacol. 1997;53:149–160. doi: 10.1016/s0006-2952(96)00645-4. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Yaroslavskiy BB, Griswold RD, et al. Estrogen inhibits RANKL-stimulated osteoclastic differentiation of human monocytes through estrogen and RANKL-regulated interaction of estrogen receptor-alpha with BCAR1 and Traf6. Exp Cell Res. 2009;315:1287–1301. doi: 10.1016/j.yexcr.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AL, Wojcik BM, Wrobleski SK, et al. Statins, inflammation and deep vein thrombosis: a systematic review. J Thromb Thrombolysis. 2012;33:371–82. doi: 10.1007/s11239-012-0687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda JL, Tanhehco YC, Frey M, et al. Variation in human erythrocyte membrane unsaturated Fatty acids: correlation with cardiovascular disease. Arch Pathol Lab Med. 2010;134:73–80. doi: 10.5858/2008-0795-OAR1.1. [DOI] [PubMed] [Google Scholar]
- Sun LD, Cheng H, Wang ZX, et al. Association analyses identify six new psoriasis susceptibility loci in the Chinese population. Nat Genet. 2010;42:1005–9. doi: 10.1038/ng.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran CD, Butler RN, Philcox JC, et al. Regional distribution of metallothionein and zinc in the mouse gut: comparison with metallothionien-null mice. Biol Trace Elem Res. 1998;63:239–251. doi: 10.1007/BF02778942. [DOI] [PubMed] [Google Scholar]
- Watkins PA, Ferrell EV, Jr, Pedersen JI, Hoefler G. Peroxisomal fatty acid beta-oxidation in HepG2 cells. Arch Biochem Biophys. 1991;289:329–336. doi: 10.1016/0003-9861(91)90419-j. [DOI] [PubMed] [Google Scholar]
- West AK, Stallings R, Hildebrand CE, et al. Human metallothionein genes: structure of the functional locus at 16q13. Genomics. 1990;8:513–518. doi: 10.1016/0888-7543(90)90038-v. [DOI] [PubMed] [Google Scholar]
- Westerterp M, Bochem AE, Yvan-Charvet L, et al. ATP-binding cassette transporters, atherosclerosis, and inflammation. Circ Res. 2014;114:157–70. doi: 10.1161/CIRCRESAHA.114.300738. [DOI] [PubMed] [Google Scholar]
- Wymann MP, Bjorklof K, Calvez R, et al. Phosphoinositide 3-kinase gamma: a key modulator in inflammation and allergy. Biochem Soc Trans. 2003;31:275–280. doi: 10.1042/bst0310275. [DOI] [PubMed] [Google Scholar]
- Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- Zacherl JR, Chace DH, Christensen TC, et al. Elaidate, an 18-Carbon trans-Monoenoic Fatty Acid, Inhibits β-Oxidation in Human Peripheral Blood Macrophages. J Cell Biochem. 2014;115:62–70. doi: 10.1002/jcb.24633. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Liao H, Xie X, et al. Oxidized LDL downregulates ATP-binding cassette transporter-1 in human vascular endothelial cells via inhibiting liver X receptor (LXR) Cardiovasc Res. 2005;68:425–432. doi: 10.1016/j.cardiores.2005.07.003. [DOI] [PubMed] [Google Scholar]




