Abstract
The intensity and submodality of pain are widely attributed to stimulus encoding by peripheral and subcortical spinal/trigeminal portions of the somatosensory nervous system. Consistent with this interpretation are studies of surgically anesthetized animals, showing that relationships between nociceptive stimulation and activation of neurons are similar at subcortical levels of somatosensory projection and within the primary somatosensory cortex (in cytoarchitectural areas 3b and 1 of SI). Such findings have led to characterizations of SI as a network which preserves, rather than transforms, the excitatory drive it receives from subcortical levels. Inconsistent with this perspective are images and neurophysiological recordings of SI neurons in lightly anesthetized primates. These studies show that an extreme anterior position within SI (area 3a) receives input originating predominantly from unmyelinated nociceptors, distinguishing it from posterior SI (areas 3b and 1), long recognized as receiving input predominantly from myelinated afferents, including nociceptors. Of particular importance, interactions between these subregions during maintained nociceptive stimulation are accompanied by an altered SI response to myelinated and unmyelinated nociceptors. A revised view of pain coding within SI cortex is discussed, and potentially significant clinical implications are emphasized.
Keywords: Primary Somatosensory Cortex; SI; Cortical areas 3a,3b,1; Pain Intensity
1. Introduction
Wide agreement exists about the spinal dorsal horn, thalamic, and cerebral cortical targets of afferent drive triggered by noxious environmental stimuli. Pioneering studies established that dorsal horn neurons with thalamic projections maintain or combine the stimulus preferences and response characteristics of distinguishable classes of peripheral nociceptors [136-138]. Those studies also showed that the response properties of nociresponsive ventrobasal thalamic and primary somatosensory cortical (SI) neurons correspond to those of dorsal horn neurons [33;51;142]. These findings do not, however, permit rejection of the possibility that nociresponsive neuronal activity undergoes functionally significant alteration in its projection from spinal dorsal horn to SI. Most observations from CNS nociresponsive neurons have been obtained under surgical levels of anesthesia known to: (i) decrease the number of stimulus-activated neurons at both spinal and supraspinal levels of the somatosensory nervous system; and (ii) not only reduce, but fundamentally alter a neuron's response to stimulus-evoked excitatory drive (e.g., convert the response from tonic to phasic – from slowly to rapidly adapting) [42].
Recent studies using vibrotactile [103; 111; 133] or skin brushing [111] stimulation have shown that neurons in SI of lightly anesthetized animals respond to stimulation of a skin region far more extensive than the strict somatotopy of functionally segregated inputs observed under surgical levels of general anesthesia [99-101]. Moreover, in lightly anesthetized or unanesthetized subjects, subpopulations of SI mechanoresponsive neurons are distinguishable on the basis of patterns of spike firing that signal/encode behaviorally relevant sensory attributes [111]. While existing information does not allow unambiguous identification of subpopulations of SI neurons [18;112;113] which signal/encode perceptually distinguishable attributes of nociceptive sensations, SI is parcellated into anterior and posterior regions preferentially activated by functionally distinct nociceptors, as detailed below.
SI is known to receive abundant projections from spinal and brain stem neurons that receive their input from myelinated afferents. The spinal terminals of myelinated (Aδ) nociceptors do not ramify extensively, and the pain percept that accompanies their activation is experienced as sharp and well localized (as first/fast/discriminative pain). Reflex adjustments to nociceptive stimulation depend upon myelinated afferent input to the spinal cord and occur before a conscious reaction is initiated [128]. Collaterals of spinothalamic axons that terminate in brain stem nuclei can initiate a stereotypic fight or flight response that includes orientation to the stimulus [77]. The noxious afferent drive to cell columns in areas 3b and 1of SI is interspersed with a substantially more dense input from non-nociresponsive thalamic neurons whose activity encodes features of the stimulus such as the location and extent of skin contact, the direction of stimulus motion across the skin, the roughness, softness or texture of the stimulus object, or the frequency of vibrotactile stimulation [111]. Considered collectively, the dorsal horn projection of myelinated afferent input to areas 3b and 1 appears to support abilities of normal subjects to localize skin stimuli and discriminate between sensory attributes that include pain intensity. Associated activation of other cortical regions is requisite not only for attention to a stimulus [84;92], but also for determination of whether the stimulus is behaviorally salient [71].
In contrast to widespread agreement about the SI processing of afferent drive arising in myelinated afferents, no consensus exists about the cortical mechanisms responsible for sensations evoked by stimuli that activate unmyelinated (C) nociceptive afferents. In multiple respects the pain experiences evoked by input from C-nociceptive afferents (i.e., the “slow”, “2nd”, or “burning” pain percept) differ strikingly from those elicited by activation of myelinated nociceptive afferents. The slow conduction velocity of peripheral C nociceptive afferents renders information provided to the CNS useless for rapid reactions to the onset of a painful stimulus; selective C nociceptor activation does not elicit behavioral reflex responses; conscious reactions to nociceptor activation can occur before input from C nociceptors reaches the cerebral cortex; not only do the peripheral terminals of C nociceptors ramify extensively, but their central terminations within the substantia gelatinosa access the diffusely conducting propriospinal system [146]; C nociceptor activity occurs at a slow rate and does not adapt rapidly; and C nociceptors do not track rapid changes in stimulus intensity. These attributes of C nociceptors are incompatible with the fast detection and localization of a nociceptive stimulus. Consistent with the slow progression and poor localization of second pain, they appear ideal for detection of inflammatory injury [65] and for tracking a slowly progressing insult such as protein denaturation of the skin during prolonged or repetitive heat stimulation.
The perspectives of this review are that (i) posterior and anterior areas within SI (areas 3b/1 and 3a) are the sites of the initial cortical activations attributable to Aδ vs. C nociceptive afferent drive, respectively; and (ii) when a nociceptive stimulus is maintained, neuronal activation within SI switches from predominantly Aδ to C nociceptor mediated. Accordingly, during maintained cutaneous heat stimulation the initial sensation of sharp pain that accurately reflects the increase in stimulus temperature at the site of stimulus contact converts to an aching, poorly localized pain that increases in magnitude with continuing stimulation (i.e., temporally summates; C. Vierck, A. Mauderli and J. Riley, unpublished observations). More generally, SI nociceptive cortical processing of different combinations, magnitudes and durations of myelinated and unmyelinated input could account for many of the subjective characteristics (qualitative features) of pain [83;144], such as those categorized by the McGill pain questionnaire [45]. Also, interactions between those SI neurons that receive input from specific Aδ and C nociceptors may determine the submodality of a variety of aversive sensations (e.g., cold, heat or mechanical pain and itch).
The following sections delineate what the authors regard as the neuromechanistic bases for such interactions. We attempt to do this by comparing and contrasting the SI neuronal activation triggered by precisely controlled environmental stimulation of myelinated vs. unmyelinated cutaneous afferents. Experimental findings obtained using high-resolution imaging approaches and neurophysiological recordings in lightly anesthetized or awake subjects are emphasized.
2. Sources of nociceptive input to SI
Myelinated nociceptive input to SI involves transmission of the activity of laminae I and V neurons in the spinal dorsal horn via the spinothalamic tract and subsequently via the contralateral thalamus (nucleus ventralis posterolateralis; VPL) to areas 3b/1 [12;16;18;22;57;69;91;109]. Thalamically-projecting nociceptive-specific (NS) neurons in lamina I of the dorsal horn respond to input from myelinated nociceptive afferents [27;31]. Responses of lamina V wide dynamic range (WDR) neurons are graded in magnitude by input from myelinated non-nociceptive (Aβ) and nociceptive Aδ and C afferents, exhibiting a low rate of firing to non-nociceptive input and a progressively higher rate as stimulus intensity increases over the range experienced as painful [67]. Both the NS lamina I and WDR lamina V neurons respond to nociceptive input with short latencies and an initial discharge rate that grades with the intensity of stimulation - attributes consistent with characteristics of the first pain experience.
Conceptualization of the role of SI cortex in somatosensory coding has long been based on the conviction that the projection of nociceptive, thermal and tactile information to SI requires a synaptic relay in nucleus ventralis posterolateralis (VPL) [139]. Anterograde neuroanatomical tracing and neurophysiological recordings have established that the afferent drive triggered by non-noxious mechanical skin contact is conveyed to the thalamus in the dorsal column–medial lemniscal (DC–ML) pathway and spinocervicothalamic pathway, whereas information about noxious and thermal stimulation is conveyed via axons in the spinothalamic tract (STT). These projections terminate on neurons in the contralateral VPL [95] which, in turn, project to areas 3b/1 in SI (blue-colored path in Figure 1). As a consequence, areas 3b/1 contain many non-nociresponsive neurons and a sparse representation of nociresponsive neurons whose response properties closely resemble those of NS or WDR neurons in the spinal dorsal horn (as suggested by the blue-colored pathway in Figure 1; [59]). These findings constitute the basis for the widely-held belief that the thalamic nucleus VPL is both a necessary and sufficient source of the nociceptive afferent drive that reaches SI.
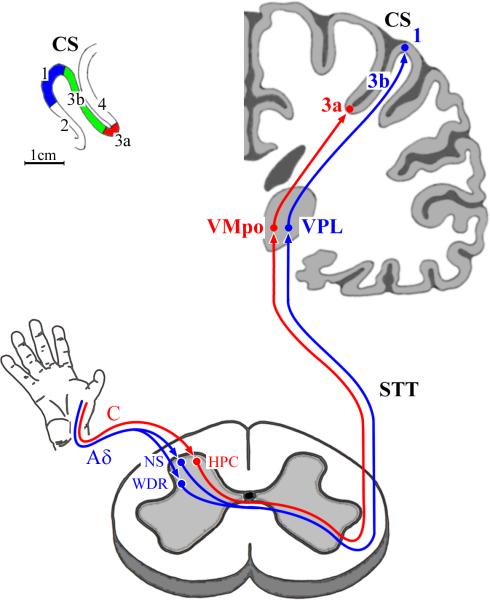
Figure 1. Neuroanatomical projection paths that convey nociceptive afferent drive from skin nociceptors to contralateral primary somatosensory cortex (SI).
Shown in red – projection from unmyelinated C nociceptors to: (1) HPC cells in spinal cord lamina I: (2) neurons in the posterior part of ventromedial nucleus of thalamus (VMpo): and (3) area 3a neurons in SI cortex. Shown in blue - projection from myelinated Aδ-nociceptors to: (1) NS neurons in lamina I and WDR neurons in lamina V of the spinal cord dorsal horn; (2) neurons in the ventral posterior lateral nucleus of thalamus (VPL); and (3) SI neurons in 3b/1. Inset at top-left shows: (1) cytoarchitectonic areas which comprise SI (3a, 3b, 1, and 2); and (2) the location of each area in the postcentral gyrus in human cerebral cortex; CS = central sulcus; cytoarchitectonic area 4 = primary motor cortex. Note the position of area 3a (ca. 2cm below the cortical surface) at the fundus of the CS, as well as its narrow (<6mm) rostro-caudal extent relative to that of either area 3b or area 1.
Neuroanatomical tracing studies have identified an alternative thalamic projection to SI (see red-colored path in Figure 1) which conveys nociceptive information very different from that conveyed by the VPL projection to areas 3b/1. Anterograde and retrograde tracing methods have demonstrated axonal projections of a unique class of dorsal horn lamina I neurons to nucleus VMpo (the posterior part of the ventral medial nucleus) [29;32;97]. These VMpo neurons project to insular cortex and to area 3a within SI [33]. While area 3a has been recognized as a recipient of information contributing to fine control of skeletomotor functions via corticocortical connections with motor cortex [53;56], the possibility that area 3a contributes to pain perception was raised by the demonstration that selective activation of unmyelinated (C) nociceptors is accompanied by a robust activation of area 3a neurons [134] (described below).
C-nociceptive afferents terminate directly on a distinct category of lamina I dorsal horn neurons whose axons enter the spinothalamic tract and project to VMpo [30;31;78]. These lamina I neurons: (i) receive abundant input from neurons in the substantia gelatinosa (laminae II and III) – a dorsal horn region comprised of densely packed interneurons that receive peptidergic nerve terminals and respond to inflammatory mediators; (ii) respond to cold (35°C to <10°C), pinch and heat (35°C to 53°C) and are referred to as HPC or polymodal neurons; and (iii) like the C-nociceptive afferents that provide their input, are exquisitely responsive to prolonged thermal stimulation with prominent after-discharge following stimulus termination. The lamina I neurons that receive input from unmyelinated nociceptors have been shown to be relevant not only to normal nociceptive transmission, but also to the development of chronic pain [6;74;110].
A CNS neuron that receives cutaneous C nociceptor input typically exhibits slow temporal summation of spike firing in response to repetitive heating of its receptive field [93]. Similarly, brief cutaneous contact with a preheated thermode is accompanied by a late (post-stimulus) sensation that progresses from warmth to strong pain when the contacts occur repetitively [121]. Important features of slow temporal summation of second (“slow”) pain are that it: (i) is dependent upon activation of C-nociceptors, (ii) requires repetition of the contact stimulus at intervals of 3 sec or less, and (iii) “resets” (i.e., ratings are restored to low values observed at the onset of repetitive stimulation) if an interval greater than 3 sec is interposed in a series of stimuli delivered at intervals of 3sec or less. Each of these features of the second/slow pain percept is characteristic of lamina I HPC neurons [27].
3. Nociresponsive SI neurons
Demonstrations of the projection that synaptically links NS and WDR neurons in the spinal dorsal horn with thalamic nucleus VPL have led to the expectation that neurons in areas 3b/1 of SI would be responsive to noxious environmental stimulation. Subsequent neurophysiological recordings obtained in anesthetized monkeys [60] revealed a sparse distribution of nociresponsive 3b/1 neurons confined to the middle layers and interspersed among pyramidal neurons that respond solely to non-noxious mechanical stimulation of a spatially restricted skin region. The functional properties of these nociresponsive 3b/1 neurons are as follows: (i) short response latencies consistent with afferent drive arising in myelinated peripheral afferents; (ii) little or no tendency for after-discharge; and (iii) faithful signaling, via a mean rate firing code, of the intensity of noxious skin heating stimulation (43°C to 50°C). These area 3b/1 neurons are widely regarded as an initial neocortical stage in the encoding of first pain (i.e., the well localized, stimulus-locked sensation evoked by activation of Aδ nociceptors) [84].
The functional properties of area 3a nociresponsive neurons differ considerably from those of the nociresponsive neurons in areas 3b/1. Single-neuron recordings and high-resolution near-infrared optical intrinsic signal imaging of area 3a during noxious skin stimulation have revealed activity consistent with the response of lamina I HPC neurons. Specifically, delivery of multisecond-duration noxious skin heating is accompanied by a gradually increasing activation within the topographically appropriate region of area 3a. Simultaneously, activity in areas 3b/1 undergoes a profound suppression that persists following removal of the stimulus [113]. Activation of area 3a neurons by stimulation of unmyelinated nociceptors is particularly robust, and direct interactions between activities of nociresponsive neurons in area 3a and mechanoresponsive neurons in 3b/1 parallel the sign and magnitude of interactions that occur between pain and touch in conscious subjects [3;4]. Optical intrinsic signal (OIS) images showing the status of SI activation during and after noxious skin heating are shown in Figure 2.
Figure 2. Optical response of SI to thermoneutral vs. thermonoxious tactile stimulation.
A: Low and higher-magnification views of somatosensory cortex in the left hemisphere of a squirrel monkey. Green rectangles in the higher-magnification view indicate regions of interest (ROI) in areas 3a and 3b/1. B: Site on contralateral hand exposed to skin flutter stimulation delivered via a 5mm-diameter contactor with precise control of temperature. The parameters of the flutter stimulus were: amplitude – 200μm; frequency – 25Hz; duration – 5s, probe temperature – either 38°C (neutral) or 52°C (noxious). C: Series of images showing the temporal development of the stimulus-evoked SI optical intrinsic signal. The optical response to flutter stimulation with a probe temperature of 38°C mainly occupies 3b/1 (top row of images), with a reduction over time in the activity evoked in area 3a. In striking contrast, the highest intensity and greatest spatial extent of the SI optical response to 52°C flutter occurs in area 3a, well after the stimulus is removed from the skin (bottom row). Furthermore, although 3b/1 initially respond to 52°C flutter, 3b/1 activation decreases and eventually disappears during the period when the optical response to 38°C flutter is maximal or near-maximal (between 6-10s after stimulus onset).
Consistent with the imaging observations illustrated in Figure 2, extracellular microelectrode recordings in lightly anesthetized squirrel monkeys (Figure 3) have revealed that brief (1-7 sec duration) contact of the skin with a preheated thermode (49-56° C) elicits vigorous, but delayed spike discharge activity in area 3a neurons. Importantly, this spike firing attains a maximum after the stimulator probe is retracted from the skin [134]. Also, in response to repetitive application of a noxious thermal stimulus to the skin, both the optical intrinsic signal and spike discharge responses of individual area 3a neurons temporally summate (“wind-up”). This prominent slow temporal summation of area 3a neuronal activity closely matches that of both lamina I HPC cells [27] and ratings of second pain sensation by humans in response to the same stimulus [121]. Additional support for the role of area 3a nociresponsive neurons in the signaling of slow/2nd/burning pain derives from demonstrations that intradermal injection of capsaicin is followed by: (i) prolonged activation of an extensive region within area 3a; (ii) concurrent suppression of neurons within the corresponding topographic region of areas 3b/1; and (iii) sensitization to mechanical skin stimulation of nociresponsive neurons within the activated region of area 3a [134]. Viewed collectively, these observations raise the possibility that area 3a participates in the encoding of slow/2nd burning pain and temporal summation of second pain. In addition to area 3a [7], cerebral cortical activation by peripheral stimulation of unmyelinated nociceptors includes SII, the insula, anterior cingulate cortex, prefrontal cortex and hippocampus [7] [91;94;118]. This review does not attempt to compare the nociceptive sensitivity of SI neurons with those of neurons in other cortical regions.
Figure 3. Response of area 3a neurons to thermoneutral vs. thermonoxious skin contact (from Whitsel et al., 2009).
A: Spike train data obtained from a neuron in area 3a using indent-and-hold stimulation – on each trial the skin was indented 0.5mm for 7s. For this neuron, 51°C (trials 7-12) evoked a significant response, but not 38°C (trials 1-6 and 13-18). B: Spike trains obtained from a neuron in area 3a, using a “wind-up” protocol which evokes slow/2nd/burning pain in humans. Brief (0.8 sec duration) skin indentations were delivered repetitively (1 stimulus every 2 sec). In contrast to this neuron's insensitivity to 38°C contact, a significant elevation of spike firing occurred during exposures to 55°C stimulation, and this elevation of spike firing persisted following the stimulus. Panels at top: raster-type displays of spike trains recorded during and following a series of successive contacts delivered to the skin by a probe maintained at a thermoneutral (38°C) or thermonoxious temperatures (51°C – neuron in A; 55°C – neuron in B). Vertical bar indicates time of probe retraction. Panels in second row: superimposed PST histograms showing mean firing rates (MFR) in response to 38°C (dark shading) vs. 51 ° C or 55°C (light shading) skin contact; horizontal arrow along the ordinate indicates spontaneous (no-stimulus) firing rate. Panels in third row: “difference PSTs” showing the difference between the mean firing rates (ΔMFR) recorded in noxious test trials vs. non-noxious control trials. Bottom panels: difference PSTs showing the difference between the mean firing rate recorded in non-noxious recovery trials (13-18) vs. non-noxious control trials (1-6).
4. Experimental bias in published studies of the nociceptive representation in SI
The concept that nociceptive processing within SI occurs solely in areas 3b/1 has existed for decades and continues to dominate the literature. This focus has been sustained by constraints imposed by SI morphological features on observations obtained in human cortical imaging studies. As shown in Figure 1, area 3a is relatively inaccessible at most mediolateral levels of human cerebral cortex, occupying a narrow 5-6mm wide region deep in the fundus of the central sulcus. This location is highly problematic, given the limited spatial resolution achievable using currently available human imaging methods. Furthermore, in the cerebral cortex of most primates: (i) the fundus of the central sulcus is occupied by major blood vessels, making insertion of an electrode into this region not only difficult but risky; (ii) area 3a is much narrower in anteroposterior extent than either area 3b or area 1; and (iii) inter-individual variation in the size and position of area 3a within the central sulcus [43;44;96;131] makes the use of a “standard atlas” difficult, if not misleading.
An additional barrier to understanding the contributions of SI to somatosensory perception derives from the fact that surgically anesthetized animals provide estimates of neuronal responsivity that differ from those obtained from lightly anesthetized laboratory animals or awake humans. Recordings from SI of anesthetized animals typically isolate responses only from middle-layer neurons which reflect the properties of thalamocortical axons [42;114]. In addition, although the axonal projections of NS and WDR dorsal horn neurons to VPL are dense [140], few neurons in the middle layers of areas 3b/1 respond to noxious stimulation [57;59]. This apparent contradiction is resolved by the demonstration that the STT projects to neurons in a fringe region of VPL [98;145] with axonal terminations primarily in the superficial layers of 3b/1, on the distal dendrites of pyramidal neurons. Neurons in a core region of VPL receive non-nociceptive input from the dorsal column–medial lemniscal (DC–ML) pathway and project, mostly to the middle layers of area 3b/1[10]. Thus, pyramidal neurons in area 3b/1 of anesthetized animals respond primarily to non-nociceptive input conveyed via the DC–ML path and much less robustly to the nociceptive drive conveyed to their distal dendrites. However, recordings from each of the six cortical layers in lightly anesthetized animals reveal novel representations of stimulus features presumed to reflect between-layer and intercolumnar interactions [132].
5. Intracortical interactions: the SI encoding of nociception involves both area 3a and areas 3b/1
Horizontal intercolumnar interactions within SI accompany both non-noxious and noxious stimulation, but published imaging and neurophysiological studies have primarily revealed the contributions of these interactions to the SI encoding of non-noxious sensations. A spatially extensive region in SI becomes activated within a very short time (~15-30 msec) following the onset of a gentle tactile stimulus. However, if the stimulus is maintained or applied repetitively, the activated SI region shrinks (beginning within <50 msec after stimulus onset) to multiple, radially-oriented foci of activity uniquely determined by the physical characteristics of the stimulus [19;103;114] . The changing activation pattern over time is attributable to stimulus-specific dynamic intracortical excitatory and inhibitory processes [66;132]. Within each focus, features not explicitly represented in the thalamocortical input are dynamically constructed by intracortical influences. For example, information about direction of tactile motion is extracted from precisely timed sequential inputs to a spatial array of 3b/1 cell columns. These sequential inputs derive both from the thalamus and from neurons in the multiple cortical columns whose receptive fields are traversed by a moving skin stimulus.
Following surgical section of one dorsal column (DC) the usually robust ability of primates to perceive direction of tactile motion is lost contralaterally, as is their ability to discriminate between frequencies or durations of repetitive tactile stimulation [119;120;123]. In contrast, spatiotactile localization and discrimination remain normal [122;124]. This sparing of spatial discrimination, coupled with the loss of cortically derived functions dependent upon stimulus movement, repetition or duration is compatible with high resolution 2-deoxyglucose metabolic maps of SI responses to tactile stimulation [113]. For example, a repetitive tactile stimulus normally triggers maximal neuronal activity within areas 3b/1, whereas activity in the bordering regions of 3a and 2 is suppressed. Following DC transection, repetitive tactile stimulation now evokes activity within areas 3a and 2, accompanied by powerful suppression/inhibition of activity within the 3b/1 region that is particularly deprived of afferent drive by the DC lesion. These abnormalities of the SI response account for the inability of a subject with a DC lesion to discriminate between opposing directions of tactile motion, and also for a progressive loss of sensitivity to repeated presentation of a tactile stimulus [9;35;82] – functions dependent upon the activation of areas 3b/1.
Functionally meaningful intracortical interactions between areas 3a and 3b/1 have been demonstrated with imaging of SI during combined vibrotactile and thermonoxious skin stimulation [112;113]. As shown in Figure 2, a vibrotactile stimulus leads to vigorous contralateral activation of areas 3b/1, but not of area 3a. When the temperature of the vibrating probe is 52°C, however, area 3a is activated, and the topographically corresponding region of areas 3b/1 is progressively inhibited as stimulation continues. These observations indicate that full appreciation of the contributions of SI to somatosensory perception requires neurophysiological recording and/or imaging methods sensitive to interactions that occur between the different layers and cytoarchitectural fields that comprise the responding cortical territory.
Electrophysiological recordings of neuronal responses to simultaneous 25 Hz flutter and 47°C-51°C stimulation of the skin have supported the results obtained in imaging studies of SI [133]. As illustrated (Figure 4A), when a flutter stimulus is near-threshold for activation of rapidly adapting (RA) neurons in 3b/1, simultaneous application of noxious heat to the same skin site suppresses the 3b/1 responses to flutter. Similarly, human psychophysical tests demonstrate that reduced sensitivity to flutter stimulation occurs during concurrent nociceptive thermal stimulation [3] – an effect accompanied by suppression of blood flow in the responding sectors of 3b/1 [4]. However, concurrent application of noxious heat does not suppress activation of 3b/1 neurons by a suprathreshold flutter stimulus [133]. Surprisingly, the flutter-evoked response under this condition can be substantially larger than when the stimulus is applied with the probe at a non-noxious temperature. These outcomes appear to account for otherwise difficult-to-explain reports that experimental or clinical pain is accompanied by increased sensitivity (hyperesthesia) to strong cutaneous stimulation, but hypoesthesia occurs when the cutaneous stimulus is near-threshold [5;40;107;133].
Figure 4. Response of an exemplary rapidly adapting (RA-type) area 3b neuron to thermoneutral and thermonoxious 25Hz skin flutter stimulation (from Whitsel et al., 2010).
Format as in Figure 3. The first and the last 6 trials were delivered with the probe at a neutral (38°C) temperature; trials 7-12 were delivered with the probe at a noxious (48°C) temperature. A: The response to near-threshold (50μm peak-to-peak amplitude) skin flutter stimulation. The presence of noxious input in trials 7-12 significantly suppressed this neuron's response to 50μm flutter. B: The response to supra-threshold (200μm peak-to-peak amplitude) skin flutter stimulation. The presence of noxious input in trials 7-12 failed to suppress the spike firing evoked by suprathreshold skin flutter.
According to Whitsel et al. [132], interactions between input to areas 3b/1 and C-nociceptive input to area 3a are dependent upon interareal connections provided by long-distance collaterals of pyramidal neurons (Figure 5) [15]. These interareal connections are glutamatergic, but their overall effect on target neuronal populations is balanced between influences on local excitatory and inhibitory neurons [50]. Because an area 3a response to activation of C nociceptors is delayed relative to the 3b/1 response to Aβ/Aδ input, the 3b/1 response to a stimulus that simultaneously activates Aβ, Aδ and C afferents develops rapidly and without restraint at the onset of stimulation. When such a stimulus remains in contact with the skin or is applied repetitively, however, the nociresponsive area 3a neurons become increasingly activated and convey a progressively increasing inhibitory influence to neurons in areas 3b/1. Thus, nociceptive heat applied to the skin by a thermode or water bath activates areas 3b/1 within a few seconds of the onset of such a stimulus [17;18;23;80], but no activation is detected in the same regions of 3b/1 after prolonged exposure to noxious skin heating [4;37;39;54;84;88]. Aδ afferent inhibition of responses to C afferent stimulation has been demonstrated with cortical recordings [14;81;116] and also with spinal recordings [47;68;76;104]. Inhibitory interactions that are reciprocal for Aδ and C afferent input [116] may be unique to the cortical processing of nociception.
Figure 5. Diagram of interareal connections subserving interactions between areas 3a and 3b/1.
The regions in areas 3a and 3b/1 that process input from the same body region are linked by glutamatergic (excitatory) connections arising from pyramidal neurons of each area. These axonal connections terminate synaptically on both excitatory and inhibitory interneurons. Due to greater responsivity of inhibitory interneurons relative to excitatory interneurons, the overall effect of the interareal connections on the target neuronal population typically is inhibitory, as in the competitive interactions that occur frequently between areas 3a and 3b/1. However, in vigorously activated SI cell columns, the normally inhibitory synaptic action of GABA has been postulated to diminish due to activity-dependence of neuronal [Cl−]I [133]. When this occurs, GABA's action switches to excitation, and the normally inhibitory interaction between areas becomes facilitatory. Such a transformation of the cortical action of GABA would be accompanied by an alteration of perception. For example, a tactile stimulus could be experienced as painful if vigorous activation of 3b/1 triggers activity in area 3a nociresponsive neurons proposed to underlie slow/2nd/burning pain. Such a modification of the action of GABA would be accompanied by allodynia – a common clinical malady.
6. Cortical encoding of sensation intensity
The attributes of neurons within the spinothalamocortical projection to areas 3b/1 are widely presumed to underlie the capacities of normal individuals to detect and perceive the intensity of a noxious skin stimulus [12;20;57;59;80]. However, there has been a long-standing debate as to whether NS or WDR neurons with axons in the spinothalamic projection pathway encode the intensity of noxious stimulation, based on the assumption that pain intensity is represented by the discharge of a single category of dorsal horn neuron with little or no cortical elaboration of this information. This discussion has been framed in terms of specificity (NS) versus pattern (WDR) theories [21;33;79], neither of which considers how or why sensory cortex would ignore input conveyed to it via the central projections of any category of peripheral receptor .
It is a virtual certainty that sensation intensity is encoded by an across-areal integration of SI neuronal responses to a nociceptive stimulus [24;95;111]. The stimulus-response function that spans the full range of painful intensities evoked by a brief thermal stimulus depends upon integration of the activity of area 3b/1 NS and WDR neurons that respond to overlapping (but different) ranges of stimulus intensities. When the stimulus is maintained, activating C nociceptors and, as a result, nociresponsive neurons in area 3a, nociresponsive neurons in 3b/1 would make little or no contribution to the coding of pain intensity. This outcome is expected because thermal stimuli that trigger second pain [121] not only activate area 3a but also to suppress activity in areas 3b/1 [113].
7. Spinal, thalamic and cortical lesions affecting pain processing by SI
Early clinical reports indicated that pain sensibility is retained by patients with cortical infarcts involving somatosensory cortex [48]. However, subsequent clinical literature contains numerous reports of patients who, after anterior parietal ablations, lost the ability to experience pain in response to noxious stimulation, particularly if the ablations included the posterior wall of the central sulcus [36;41;46;52; 72;73;87;90;105;106;117;143]. While some of the reported ablations were accidental and not well defined in spatial extent, others were deliberate surgical excisions of defined portions of SI cortex performed in attempts to treat epilepsy or chronic pain. These observations were complicated by chronic conditions that alter central neuronal activity patterns, but they suggest that pain perception requires participation of a cortical region that either is a component of SI, or is in close proximity to SI. Given current knowledge, a parsimonious interpretation of these clinical reports is that: (i) this region corresponds to areas 3a and 3b/1; (ii) when both area 3a and areas 3b/1 are removed, noxious stimulation of the affected body regions ceases to evoke pain; and (iii) if a postcentral ablation fails to extend deep enough into the central sulcus to remove area 3a, the loss of pain will at most be transient – until area 3a recovers from indirectly induced trauma.
SI cortical lesions in humans validate the idea that area 3a contributes to the coding of pain sensations and support a critical role for intracortical interactions between areas 3b/1 and 3a. For example, hyperpathia (increased sensitivity to suprathreshold nociceptive stimulation) has been observed with large parietal cortical lesions that spare the posterior bank of the central sulcus (thus sparing area 3a) [13]. Hyperpathia has also been observed following parietal cortical lesions of monkeys that spare area 3a [86]. In contrast, latencies for detection of a small increment in nociceptive thermal stimulation that optimally activates Aδ nociceptors are elevated following parietal cortical lesions [61]. Such findings are compatible with reciprocal and predominantly inhibitory intracortical interactions between areas 3a and 3b/1 as proposed by Whitsel et al. [118] and illustrated in Figure 5. A lesion that destroys a sector of 3b/1 would attenuate pain from selective activation of Aδ nociceptors but would release the neighboring region in area 3a from interareal inhibition, enhancing pain from C-nociceptor input.
In addition to evidence suggesting that area 3a activation might be necessary for evocation of 2nd/slow pain by nociceptive stimulation, experimental lesions and/or clinical conditions that differentially deafferent or deactivate 3b/1 support the importance for pain perception of intracortical interactions within SI. Following unilateral DC section, activity within contralateral 3b/1 undergoes a substantial suppression in response to tactile stimulation that vigorously activates myelinated afferents [113]. Accordingly, escape responses of monkeys to electrocutaneous stimulation that activates only myelinated afferents are reduced after ipsilateral DC section [127]. In contrast, thermal stimulation of myelinated and unmyelinated nociceptors (using a 51°C contact thermode) generates intense optical activation of area 3a following interruption of the DC [132]. Without input to 3b/1 via the DC–ML path, the inhibition of area 3a that normally results from activation of 3b/1 neurons by myelinated afferent input is reduced/eliminated, leading to an enhanced response of area 3a neurons to activity arising from unmyelinated nociceptors. Similarly, human patients with large spinal lesions that include the DCs report substantially increased sensitivity/responsivity to noxious stimulation [82], and chronic pain from spinal cord injury is more prevalent among patients with substantial damage the DC-ML pathway [34].
Although the STT is the major ascending spinal source of nociceptive input to VPL, VMpo, and SI, pain sensibility can return following surgical interruption of the STT [130], and ischemic infarcts involving the STT can result in chronic pain [11]. There are routes of nociceptive spinothalamocortical projection other than the STT [126;136], and one or more of these sources may contribute to development of abnormal pain sensitivity over time following STT damage. Neuroplastic adaptations to interruption of STT projections to the thalamus and cortex can result in abnormal patterns of thalamocortical activity [97;129]. Especially relevant to the role(s) of SI cortex in pain perception is the possibility that chronic pain results from lesions along the course of the STT that disrupt the normal balance between the activities of cortical areas 3a and 3b/1. Because STT axons from cells in contralateral lamina I are dorsally shifted relative to axons originating from the deep dorsal horn at some levels of the spinal cord [2], anterolateral chordotomy at such a level for the relief of lower body pain could preferentially deafferent VPL and areas 3b/1, leaving intact nociceptive input from dorsally shifted STT axons to VMpo. Exaggerated responses in area 3a and increased pain sensitivity would be expected from a selective deafferentation of 3b/1 that spares the projection of nociceptive input to area 3a. Similarly, severe dysthesthesia has been reported following surgical attempts to interrupt the STT laterally in the midbrain [38] - an outcome perhaps due to the sparing of medially shifted projections to VMpo.
A possible alternative to chordotomy for reducing nociceptive STT input to the thalamus involves the selective ablation of lamina I cells whose input derives from dermatomal regions in which chronic pain is experienced. Intrathecal injection of the neurotoxin SP-saporin destroys lamina I cells containing NK-1 receptors for substance P released from the terminals of unmyelinated nociceptors. This procedure reduces nociceptive sensitivity of rats to thermal stimulation of unmyelinated nociceptors [125;135]. Intrathecal injection of SP-saporin produces less rostral deafferentation than that produced by surgical section of the STT, and it would preferentially reduce C nociceptive input to area 3a.
Chronic pain can occur not only following interruption of the STT in the spinal cord or brain stem but also after ischemic or hemorrhagic lesions of the thalamus that involve VPL [64]. Although variability in the location and extent of lesions makes it difficult to identify which thalamic structures are necessarily involved and spared in patients with thalamic pain, an analysis of 4 patients with thalamic lesions and chronic pain has been instructive [63]. This study evaluated, with MRI imaging, whether damage to the ventral caudal nucleus (Vc; homologous in humans to VPL) or to VMpo, or both, was associated with chronic pain experienced by the patients. Although this analysis set out to evaluate the hypothesis that pain can be disinhibited by interruption of projections from VMpo to the insula and the anterior cingulate cortex [26], it turned out that Vc was damaged in each case with chronic pain, but VMpo was spared. Such observations are in accord with the possibility that chronic pain following thalamic lesions can occur as a result of deafferentation of areas 3b/1, if nociceptive input to area 3a is spared.
Repetitive transcranial magnetic stimulation (rTMS) or direct electrical stimulation of the cerebral cortex is increasingly utilized as a functional equivalent of a destructive cortical lesion in human patients [102]. Surprisingly, rTMS stimulation anterior to the central sulcus (over “motor cortex”) has been found to reduce chronic pain [75]. Evidence that “motor cortical” rTMS (mc rTMS) can inactivate area 3a includes: (i) mc rTMS significantly reduces the pain evoked in a normal subject by capsaicin – a selective activator of C-nociceptors and of area 3a nociresponsive neurons [108;133]; (ii) mc rTMS preferentially reduces the pain associated with C nociception relative to first pain [85], and (iii) although downstream influences must be considered, the parameters of mc rTMS stimulation which most effectively reduce pain are those associated with suppression of cortical neuron responsivity [62]. Transcranial magnetic stimulation has been reported to be more effective for facial pain than for other body regions [70]. A possible reason for this preferential effect on facial pain is that in humans the sector of area 3a that represents the face lies close to the cortical surface and is more accessible to rTMS than are the remaining sectors of area 3a that occupy the fundus of the central sulcus.
8. Summary and functional implications of SI encoding of pain
Early neurophysiological evaluations of nociceptive projections to SI utilized fast onset, short duration stimuli that preferentially activate myelinated cutaneous nociceptors. Accordingly, the focus of such studies was 3b/1 cortex which receives myelinated afferent drive via VPL -- the principal thalamic target of ascending projections from spinal NS and WDR neurons. A projection which does not fit this pattern , from HPC lamina I cells to thalamic nucleus VMpo [28], has been challenged on technical grounds [55;141]. Even though subsequent anatomical studies established the validity of a lamina I projection to VMpo [8;32;97], the classical view persists – i.e., that SI processing of pain occurs in areas 3b and 1. Craig and Blomqvist (2002), for example, viewed the projection from VMpo to area 3a as a “corollary track” subserving sensorimotor integration.
Because activation in the region designated as “SI” in human imaging studies (i.e., area 3b and 1) can be poorly correlated with subjects’ reports of clinical/pathological pain [89], the focus of human imaging research has turned to cortical regions more readily imaged and believed responsible for the emotional/motivational accompaniments of pain. Cortical imaging studies, therefore, have emphasized: (i) affective processing by the insula, cingulate gyrus and prefrontal cortex; and (ii) nociceptive modulation via descending connections such “higher-order” cortical areas issue to nuclei in the rostral brainstem [49;115;147]. Pain commonly is viewed as established by processing in the spinal cord, shaped by the balance of excitatory and inhibitory influences from the brain stem. However, evidence implicates SI as a site for integration of input from different afferent sources, leading to perceptual recognition of the presence, location, intensity, submodality and quality of touch, innocuous thermal sensibility, and pain. An example of interactive processing within SI is provided by repetitive or long duration noxious stimulation, which securely drives area 3a neurons and suppresses neuronal activity in 3b/1. These findings and the sparse distribution of nociceptive neurons in 3b/1 help explain the common failure of human imaging studies to detect a robust or reliable response of 3b/1 to painful stimulation or during chronic pain [4;58].
Cutaneous first pain, as coded by neurons in 3b/1 supports recognition of the location and intensity of stimulation, providing an early warning to assist reflexive actions with prompt attention to the stimulus and initiation of a conscious reaction. In contrast, the second/slow/burning pain signaled by area 3a neurons is better suited to monitor the slow progression of nociceptive stimulus intensity which, in the case of nociceptive heat, accompanies protein denaturation and tissue damage. Stimulus location and the precise time of application are not well specified by the activity of unmyelinated nociceptors that signal tissue damage and release of inflammatory mediators. A notable feature of the projection system that conveys information to the CNS about the status of the tissues innervated by C-fibers is the perceptual sensitization that accompanies long-duration or repetitive nociceptive stimulation [121]. This feature makes the nociceptive projection to area 3a especially relevant to many forms of clinical/pathological pain. The high relevance of C-nociceptor input to clinical pain is consistent with the powerful and selective effects of morphine on input to the CNS from unmyelinated nociceptors [25].
It will be important to utilize different forms and paradigms of nociceptive stimulation in order to appreciate the distinctive functions of nociresponsive neurons in areas 3a and 3b/1and understand the importance of interactions between these neuronal populations. Area 3a long has been characterized as a region devoted to processing of proprioceptive input. If, as the authors predict, area 3a contributes to the processing of pain referred to deep somatic tissues, stimuli that trigger deep muscular pain and fatigue should activate area 3a neurons. Other predictions about the functional characteristics of area 3a neurons are provided by the unique sensitivities of the lamina I HPC neurons that project to thalamic nucleus VMpo. This set of dorsal horn neurons responds to noxious heat, noxious cold and skin pinch [31]. Also, sensitivity to puretic stimulation has been demonstrated for HPC lamina I cells [1]. Appreciation of the cortical processing of aversive submodalities will benefit from imaging and neurophysiological recordings that include observations of the responsivity of areas 3a and 3b/1 neurons to different forms of deep and superficial stimulation.
Acknowledgments
This work was supported, in part, by NIH grants 1-R21-NS072811-01A1, ARO W911NF-08-1-0308 and R21 NS078619-01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no conflict of interest with the information reviewed in this manuscript.
References
- 1.Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- 2.Apkarian AV, Hodge CJ. Primate spinothalamic pathways: II. The cells of origin of the dorsolateral and ventral spinothalamic pathways. J Comp Neurol. 1989;288:474–492. doi: 10.1002/cne.902880308. [DOI] [PubMed] [Google Scholar]
- 3.Apkarian AV, Stea RA, Bolanowski SJ. Heat-induced pain diminishes vibrotactile perception: a touch gate. Somatosens Mot Res. 1994;11:259–267. doi: 10.3109/08990229409051393. [DOI] [PubMed] [Google Scholar]
- 4.Apkarian AV, Stea RA, Manglos SH, Szeverenyi NM, King RB, Thomas FD. Persistent pain inhibits contralateral somatosensory cortical activity in humans. Neurosci Lett. 1992;140:141–147. doi: 10.1016/0304-3940(92)90088-o. [DOI] [PubMed] [Google Scholar]
- 5.Ayesh EE, Jensen TS, Svensson P. Hypersensitivity to mechanical and intra-articular electrical stimuli in persons with painful temporomandibular joints. J Dent Res. 2007;86:1187–1192. doi: 10.1177/154405910708601209. [DOI] [PubMed] [Google Scholar]
- 6.Basbaum AI. Distinct neurochemical features of acute and persistent pain. Proc Natl Acad Sci U S A. 1999;96:7739–7743. doi: 10.1073/pnas.96.14.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumgartner U, Tiede W, Treede RD, Craig AD. Laser-evoked potentials are graded and somatotopically organized anteroposteriorly in the operculoinsular cortex of anesthetized monkeys. J Neurophysiol. 2006;96:2802–2808. doi: 10.1152/jn.00512.2006. [DOI] [PubMed] [Google Scholar]
- 8.Beggs J, Jordan S, Ericson AC, Blomqvist A, Craig AD. Synaptology of trigemino- and spinothalamic lamina I terminations in the posterior ventral medial nucleus of the macaque. J Comp Neurol. 2003;459:334–354. doi: 10.1002/cne.10613. [DOI] [PubMed] [Google Scholar]
- 9.Bender MB, Stacy C, Cohen J. Agraphesthesia. A disorder of directional cutaneous kinesthesia or a disorientation in cutaneous space. J Neurol Sci. 1982;53:531–555. doi: 10.1016/0022-510x(82)90249-0. [DOI] [PubMed] [Google Scholar]
- 10.Berkley KJ. Spatial relationships between the terminations of somatic sensory and motor pathways in the rostral brainstem of cats and monkeys. I. Ascending somatic sensory inputs to lateral diencephalon. J Comp Neurol. 1980;193:283–317. doi: 10.1002/cne.901930119. [DOI] [PubMed] [Google Scholar]
- 11.Boivie J, Leijon G, Johansson I. Central post-stroke pain--a study of the mechanisms through analyses of the sensory abnormalities. Pain. 1989;37:173–185. doi: 10.1016/0304-3959(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 12.Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain. 2002;125:1326–1336. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- 13.Breuer AC, Cuervo H, Selkoe DJ. Hyperpathia and sensory level due to parietal lobe arteriovenous malformation. Arch Neurol. 1981;38:722–724. doi: 10.1001/archneur.1981.00510110082015. [DOI] [PubMed] [Google Scholar]
- 14.Bromm B, Treede RD. Nerve fibre discharges, cerebral potentials and sensations induced by CO2 laser stimulation. Hum Neurobiol. 1984;3:33–40. [PubMed] [Google Scholar]
- 15.Burton H, Fabri M. Ipsilateral intracortical connections of physiologically defined cutaneous representations in areas 3b and 1 of macaque monkeys: projections in the vicinity of the central sulcus. J Comp Neurol. 1995;355:508–538. doi: 10.1002/cne.903550404. [DOI] [PubMed] [Google Scholar]
- 16.Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci U S A. 1999;96:7705–7709. doi: 10.1073/pnas.96.14.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ, Frey KA. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol. 1994;71:802–807. doi: 10.1152/jn.1994.71.2.802. [DOI] [PubMed] [Google Scholar]
- 18.Chen LM, Friedman RM, Roe AW. Area-specific representation of mechanical nociceptive stimuli within SI cortex of squirrel monkeys. Pain. 2009;141:258–268. doi: 10.1016/j.pain.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu JS, Tommerdahl M, Whitsel BL, Favorov OV. Stimulus-dependent spatial patterns of response in SI cortex. BMC Neurosci. 2005;6:47. doi: 10.1186/1471-2202-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chudler EH, Anton F, Dubner R, Kenshalo DR. Responses of nociceptive SI neurons in monkeys and pain sensation in humans elicited by noxious thermal stimulation: effect of interstimulus interval. J Neuroophysiol. 1990;63:569. doi: 10.1152/jn.1990.63.3.559. [DOI] [PubMed] [Google Scholar]
- 21.Coghill RC, Mayer DJ, Price DD. Wide dynamic range but not nociceptive-specific neurons encode multidimensional features of prolonged repetitive heat pain. J Neurophysiol. 1993;69:703–716. doi: 10.1152/jn.1993.69.3.703. [DOI] [PubMed] [Google Scholar]
- 22.Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- 23.Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, Duncan GH. Distributed processing of pain and vibration by the human brain. J Neurosci. 1994;14:4095–4108. doi: 10.1523/JNEUROSCI.14-07-04095.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen RH, Vierck CJ., Jr Relationships between touch sensations and estimated population responses of peripheral afferent mechanoreceptors. Exp Brain Res. 1993;94:120–130. doi: 10.1007/BF00230475. [DOI] [PubMed] [Google Scholar]
- 25.Cooper BY, Vierck CJ, Jr., Yeomans DC. Selective reduction of second pain sensations by systemic morphine in humans. Pain. 1986;24:93–116. doi: 10.1016/0304-3959(86)90030-8. [DOI] [PubMed] [Google Scholar]
- 26.Craig AD. A new version of the thalamic disinhibition hypothesis of central pain. Pain Forum. 1998;7:1–14. [Google Scholar]
- 27.Craig AD, Andrew D. Responses of spinothalamic lamina I neurons to repeated brief contact heat stimulation in the cat. J Neurophysiol. 2002;87:1902–1914. doi: 10.1152/jn.00578.2001. [DOI] [PubMed] [Google Scholar]
- 28.Craig AD, Blomqvist A. Is there a specific lamina I spinothalamocortical pathway for pain and temperature sensations in primates? J Pain. 2002;3:95–101. doi: 10.1054/jpai.2002.122953. [DOI] [PubMed] [Google Scholar]
- 29.Craig AD, Bushnell MC, Zhang ET, Blomqvist A. A thalamic nucleus specific for pain and temperature sensation. Nature. 1994;372:770–773. doi: 10.1038/372770a0. [DOI] [PubMed] [Google Scholar]
- 30.Craig AD, Kniffki KD. Spinothalamic lumbosacral lamina I cells responsive to skin and muscle stimulation in the cat. J Physiol. 1985;365:197–221. doi: 10.1113/jphysiol.1985.sp015767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Craig AD, Krout K, Andrew D. Quantitative response characteristics of thermoreceptive and nociceptive lamina I spinothalamic neurons in the cat. J Neurophysiol. 2001;86:1459–1480. doi: 10.1152/jn.2001.86.3.1459. [DOI] [PubMed] [Google Scholar]
- 32.Craig AD, Zhang ET. Retrograde analyses of spinothalamic projections in the macaque monkey: input to posterolateral thalamus. J Comp Neurol. 2006;499:953–964. doi: 10.1002/cne.21155. [DOI] [PubMed] [Google Scholar]
- 33.Craig A. Pain mechanisms: labeled lines versus convergence in central processing. Annual Review of Neuroscience. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- 34.Cruz-Almeida Y, Felix ER, Martinez-Arizala A, Widerstrom-Noga EG. Decreased Spinothalamic and Dorsal Column-Medial Lemniscus-Mediated Function Is Associated with Neuropathic Pain after Spinal Cord Injury. J Neurotrauma. 2012 doi: 10.1089/neu.2012.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davidoff RA. The dorsal columns. Neurology. 1989;39:1377–1385. doi: 10.1212/wnl.39.10.1377. [DOI] [PubMed] [Google Scholar]
- 36.de Gutierrez Mahoney C. The treatment of painful hantom limb by removal of post-central cortex. J Neurosurg. 1944;1:156–162. [Google Scholar]
- 37.Derbyshire SW, Jones AK. Cerebral responses to a continual tonic pain stimulus measured using positron emission tomography. Pain. 1998;76:127–135. doi: 10.1016/s0304-3959(98)00034-7. [DOI] [PubMed] [Google Scholar]
- 38.Drake C, McKenzie K. Mesencephalic tractotomy for pain: experience with six cases. J Neurosurg. 1953;10:457–462. doi: 10.3171/jns.1953.10.5.0457. [DOI] [PubMed] [Google Scholar]
- 39.Dube AA, Duquette M, Roy M, Lepore F, Duncan G, Rainville P. Brain activity associated with the electrodermal reactivity to acute heat pain. Neuroimage. 2009;45:169–180. doi: 10.1016/j.neuroimage.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 40.Eliav E, Gracely RH. Sensory changes in the territory of the lingual and inferior alveolar nerves following lower third molar extraction. Pain. 1998;77:191–199. doi: 10.1016/S0304-3959(98)00100-6. [DOI] [PubMed] [Google Scholar]
- 41.Erickson T, Bleckwenn W, Woolsey C. Observations on the post central gyrus in relation to pain. Trans Am Neurol Assoc. 1952;56:57–59. [PubMed] [Google Scholar]
- 42.Favorov OV, Diamond ME, Whitsel BL. Evidence for a mosaic representation of the body surface in area 3b of the somatic cortex of cat. Proc Natl Acad Sci U S A. 1987;84:6606–6610. doi: 10.1073/pnas.84.18.6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geyer S, Schleicher A, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Neuroimage. 1999;10:63–83. doi: 10.1006/nimg.1999.0440. [DOI] [PubMed] [Google Scholar]
- 44.Geyer S, Schormann T, Mohlberg H, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Part 2. Spatial normalization to standard anatomical space. Neuroimage. 2000;11:684–696. doi: 10.1006/nimg.2000.0548. [DOI] [PubMed] [Google Scholar]
- 45.Gilron I, Tu D, Holden RR. Sensory and Affective Pain Descriptors Respond Differentially to Pharmacological Interventions in Neuropathic Conditions. Clin J Pain. 2012 doi: 10.1097/AJP.0b013e31824ce65c. [DOI] [PubMed] [Google Scholar]
- 46.Hamby WB. Reversible central pain. Arch Neurol. 1961;5:528–532. doi: 10.1001/archneur.1961.00450170066008. [DOI] [PubMed] [Google Scholar]
- 47.Hanai F. Effect of electrical stimulation of peripheral nerves on neuropathic pain. Spine (Phila Pa 1976 ) 2000;25:1886–1892. doi: 10.1097/00007632-200008010-00005. [DOI] [PubMed] [Google Scholar]
- 48.Head H, Holves G. Sensory disturbances from cerebral lesions. Brain. 1911;34:102–254. [Google Scholar]
- 49.Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirsch JA, Gilbert CD. Synaptic physiology of horizontal connections in the cat's visual cortex. J Neurosci. 1991;11:1800–1809. doi: 10.1523/JNEUROSCI.11-06-01800.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hodge CJ, Jr., Apkarian AV. The spinothalamic tract. Crit Rev Neurobiol. 1990;5:363–397. [PubMed] [Google Scholar]
- 52.Horrax G. Experiences with cortical excisions for the relief of intractable pain in the extremities. Surgery. 1946;20:593–602. [PubMed] [Google Scholar]
- 53.Huffman KJ, Krubitzer L. Area 3a: topographic organization and cortical connections in marmoset monkeys. Cereb Cortex. 2001;11:849–867. doi: 10.1093/cercor/11.9.849. [DOI] [PubMed] [Google Scholar]
- 54.Jones AK, Brown WD, Friston KJ, Qi LY, Frackowiak RS. Cortical and subcortical localization of response to pain in man using positron emission tomography. Proc Biol Sci. 1991;244:39–44. doi: 10.1098/rspb.1991.0048. [DOI] [PubMed] [Google Scholar]
- 55.Jones EG. A pain in the thalamus. J Pain. 2002;3:102–104. doi: 10.1054/jpai.2002.122952. [DOI] [PubMed] [Google Scholar]
- 56.Jones EG, Porter R. What is area 3a? Brain Res. 1980;203:1–43. doi: 10.1016/0165-0173(80)90002-8. [DOI] [PubMed] [Google Scholar]
- 57.Kenshalo DR, Chudler EH, Anton F, Dubner R. SI nociceptive neurons participate in the encoding process by which monkeys perceive the intensity of noxious thermal stimulation. Brain Res. 1988;454:378–382. doi: 10.1016/0006-8993(88)90841-4. [DOI] [PubMed] [Google Scholar]
- 58.Kenshalo D, Willis W. The role of the cerebral cortex in pain sensation. In: Peters A, editor. Cerebral Cortex. Plenum; New York: 1991. pp. 153–212. [Google Scholar]
- 59.Kenshalo DR, Iwata K, Sholas M, Thomas DA. Response properties and organization of nociceptive neurons in area 1 of monkey primary somatosensory cortex. J Neurophysiol. 2000;84:719–729. doi: 10.1152/jn.2000.84.2.719. [DOI] [PubMed] [Google Scholar]
- 60.Kenshalo D, Iwata K, Sholas M, Thomas D. Response properties and organization of nociceptive neurons in area 1 of monkey primary somatosensory cortex. J Neurophysiol. 2000;84:719–729. doi: 10.1152/jn.2000.84.2.719. [DOI] [PubMed] [Google Scholar]
- 61.Kenshalo DJ, Willis WJ. The role of the cerebral cortex in pain sensation. In: Peters A, editor. Cerebral Cortex. Plenum; New York: 1991. pp. 153–212. [Google Scholar]
- 62.Khedr E, Koth H, Kamel N, Ahmed M, Sadek R, Rothwell J. Long-lasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiat. 2005;76:833–838. doi: 10.1136/jnnp.2004.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim JH, Greenspan JD, Coghill RC, Ohara S, Lenz FA. Lesions limited to the human thalamic principal somatosensory nucleus (ventral caudal) are associated with loss of cold sensations and central pain. J Neurosci. 2007;27:4995–5004. doi: 10.1523/JNEUROSCI.0716-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol. 2009;8:857–868. doi: 10.1016/S1474-4422(09)70176-0. [DOI] [PubMed] [Google Scholar]
- 65.Koerber HR, McIlwrath SL, Lawson JJ, Malin SA, Anderson CE, Jankowski MP, Davis BM. Cutaneous C-polymodal fibers lacking TRPV1 are sensitized to heat following inflammation, but fail to drive heat hyperalgesia in the absence of TPV1 containing C-heat fibers. Mol Pain. 2010;6:58. doi: 10.1186/1744-8069-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kohn A, Pinheiro A, Tommerdahl MA, Whitsel BL. Optical imaging in vitro provides evidence for the minicolumnar nature of cortical response. Neuroreport. 1997;8:3513–3518. doi: 10.1097/00001756-199711100-00019. [DOI] [PubMed] [Google Scholar]
- 67.Le BD, Chitour D. Do convergent neurones in the spinal dorsal horn discriminate nociceptive from non-nociceptive information? Pain. 1983;17:1–19. doi: 10.1016/0304-3959(83)90123-9. [DOI] [PubMed] [Google Scholar]
- 68.Lee KH, Chung JM, Willis WD., Jr Inhibition of primate spinothalamic tract cells by TENS. J Neurosurg. 1985;62:276–287. doi: 10.3171/jns.1985.62.2.0276. [DOI] [PubMed] [Google Scholar]
- 69.Lee MC, Zambreanu L, Menon DK, Tracey I. Identifying brain activity specifically related to the maintenance and perceptual consequence of central sensitization in humans. J Neurosci. 2008;28:11642–11649. doi: 10.1523/JNEUROSCI.2638-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lefaucheur JP, Drouot X, Menard-Lefaucheur I, Zerah F, Bendib B, Cesaro P, Keravel Y, Nguyen JP. Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. J Neurol Neurosurg Psychiatry. 2004;75:612–616. doi: 10.1136/jnnp.2003.022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol. 2011;93:111–124. doi: 10.1016/j.pneurobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 72.Lende RA, Kirsch WM, Druckman R. Relief of facial pain after combined removal of precentral and postcentral cortex. J Neurosurg. 1971;34:537–543. doi: 10.3171/jns.1971.34.4.0537. [DOI] [PubMed] [Google Scholar]
- 73.Lewin W, Phillips C. Observations on partial removal of the post-central gyrus for pain. J Neurol Neurosurg Psychiatry. 1952;15:143–147. doi: 10.1136/jnnp.15.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li JL, Li YQ, Nomura S, Kaneko T, Mizuno N. Protein kinase C gamma-like immunoreactivity in the substantia gelatinosa of the medullary dorsal horn of the rat. Neurosci Lett. 2001;311:185–188. doi: 10.1016/s0304-3940(01)02171-1. [DOI] [PubMed] [Google Scholar]
- 75.Lima MC, Fregni F. Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology. 2008;70:2329–2337. doi: 10.1212/01.wnl.0000314649.38527.93. [DOI] [PubMed] [Google Scholar]
- 76.Liu XG, Morton CR, Azkue JJ, Zimmermann M, Sandkuhler J. Long-term depression of C-fibre-evoked spinal field potentials by stimulation of primary afferent A delta-fibres in the adult rat. Eur J Neurosci. 1998;10:3069–3075. doi: 10.1046/j.1460-9568.1998.00310.x. [DOI] [PubMed] [Google Scholar]
- 77.Lovick TA. Integrated activity of cardiovascular and pain regulatory systems: role in adaptive behavioural responses. Prog Neurobiol. 1993;40:631–644. doi: 10.1016/0301-0082(93)90036-r. [DOI] [PubMed] [Google Scholar]
- 78.Lu Y, Perl ER. Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (laminae I and II). J Neurosci. 2005;25:3900–3907. doi: 10.1523/JNEUROSCI.0102-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maixner W, Dubner R, Bushnell MC, Kenshalo DR, Jr., Oliveras JL. Wide-dynamic-range dorsal horn neurons participate in the encoding process by which monkeys perceive the intensity of noxious heat stimuli. Brain Res. 1986;374:385–388. doi: 10.1016/0006-8993(86)90435-x. [DOI] [PubMed] [Google Scholar]
- 80.Moulton EA, Keaser ML, Gullapalli RP, Greenspan JD. Regional intensive and temporal patterns of functional MRI activation distinguishing noxious and innocuous contact heat. J Neurophysiol. 2005;93:2183–2193. doi: 10.1152/jn.01025.2004. [DOI] [PubMed] [Google Scholar]
- 81.Mouraux A, Guerit JM, Plaghki L. Non-phase locked electroencephalogram (EEG) responses to CO2 laser skin stimulations may reflect central interactions between A partial partial differential- and C-fibre afferent volleys. Clin Neurophysiol. 2003;114:710–722. doi: 10.1016/s1388-2457(03)00027-0. [DOI] [PubMed] [Google Scholar]
- 82.Nathan PW, Smith MC, Cook AW. Sensory effects in man of lesions of the posterior columns and of some other afferent pathways. Brain. 1986;109(Pt 5):1003–1041. doi: 10.1093/brain/109.5.1003. [DOI] [PubMed] [Google Scholar]
- 83.Nielsen J, rendt-Nielsen L. The influence of rate of temperature change and peak stimulus duration on pain intensity and quality. Somatosens Mot Res. 1998;15:220–229. doi: 10.1080/08990229870781. [DOI] [PubMed] [Google Scholar]
- 84.Oshiro Y, Quevedo AS, McHaffie JG, Kraft RA, Coghill RC. Brain mechanisms supporting discrimination of sensory features of pain: a new model. J Neurosci. 2009;29:14924–14931. doi: 10.1523/JNEUROSCI.5538-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Passard A, Attal N, Benadhira R, Brasseur L, Saba G, Sichere P, Perrot S, Januel D, Bouhassira D. Effects of unilateral repetitive transcranial magnetic stimulation of the motor cortex on chronic widespread pain in fibromyalgia. Brain. 2007;130:2661–2670. doi: 10.1093/brain/awm189. [DOI] [PubMed] [Google Scholar]
- 86.Peele T. Acute and chronic parietal lobe ablations in monkeys. J Neurophysiol. 1944;7:269–286. [Google Scholar]
- 87.Perl E. Pain and nociception. Handboook of Physiology. The Nervous System. Sensory Processes American Physiological Society. 1984:915–975. [Google Scholar]
- 88.Peyron R, Garcia-Larrea L, Gregoire MC, Costes N, Convers P, Lavenne F, Mauguiere F, Michel D, Laurent B. Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain. 1999;122(Pt 9):1765–1780. doi: 10.1093/brain/122.9.1765. [DOI] [PubMed] [Google Scholar]
- 89.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 90.Ploner M, Freund HJ, Schnitzler A. Pain affect without pain sensation in a patient with a postcentral lesion. Pain. 1999;81:211–214. doi: 10.1016/s0304-3959(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 91.Ploner M, Gross J, Timmermann L, Schnitzler A. Cortical representation of first and second pain sensation in humans. Proc Natl Acad Sci U S A. 2002;99:12444–12448. doi: 10.1073/pnas.182272899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 93.Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 94.Qiu Y, Noguchi Y, Honda M, Nakata H, Tamura Y, Tanaka S, Sadato N, Wang X, Inui K, Kakigi R. Brain processing of the signals ascending through unmyelinated C fibers in humans: an event-related functional magnetic resonance imaging study. Cereb Cortex. 2006;16:1289–1295. doi: 10.1093/cercor/bhj071. [DOI] [PubMed] [Google Scholar]
- 95.Quevedo AS, Coghill RC. Filling-in, spatial summation, and radiation of pain: evidence for a neural population code in the nociceptive system. J Neurophysiol. 2009;102:3544–3553. doi: 10.1152/jn.91350.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rademacher J, Caviness VS, Jr., Steinmetz H, Galaburda AM. Topographical variation of the human primary cortices: implications for neuroimaging, brain mapping, and neurobiology. Cereb Cortex. 1993;3:313–329. doi: 10.1093/cercor/3.4.313. [DOI] [PubMed] [Google Scholar]
- 97.Ralston HJ., III. Pain and the primate thalamus. Prog Brain Res. 2005;149:1–10. doi: 10.1016/S0079-6123(05)49001-9. [DOI] [PubMed] [Google Scholar]
- 98.Rausell E, Jones EG. Chemically distinct compartments of the thalamic VPM nucleus in monkeys relay principal and spinal trigeminal pathways to different layers of the somatosensory cortex. J Neurosci. 1991;11:226–237. doi: 10.1523/JNEUROSCI.11-01-00226.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reed JL, Pouget P, Qi HX, Zhou Z, Bernard MR, Burish MJ, Haitas J, Bonds AB, Kaas JH. Widespread spatial integration in primary somatosensory cortex. Proc Natl Acad Sci U S A. 2008;105:10233–10237. doi: 10.1073/pnas.0803800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reed JL, Qi HX, Pouget P, Burish MJ, Bonds AB, Kaas JH. Modular processing in the hand representation of primate primary somatosensory cortex coexists with widespread activation. J Neurophysiol. 2010;104:3136–3145. doi: 10.1152/jn.00566.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reed JL, Qi HX, Zhou Z, Bernard MR, Burish MJ, Bonds AB, Kaas JH. Response properties of neurons in primary somatosensory cortex of owl monkeys reflect widespread spatiotemporal integration. J Neurophysiol. 2010;103:2139–2157. doi: 10.1152/jn.00709.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci. 2007;8:559–567. doi: 10.1038/nrn2169. [DOI] [PubMed] [Google Scholar]
- 103.Simons SB, Chiu J, Favorov OV, Whitsel BL, Tommerdahl M. Duration-dependent response of SI to vibrotactile stimulation in squirrel monkey. J Neurophysiol. 2007;97:2121–2129. doi: 10.1152/jn.00513.2006. [DOI] [PubMed] [Google Scholar]
- 104.Sjolund BH. Peripheral nerve stimulation suppression of C-fiber-evoked flexion reflex in rats. Part 1: Parameters of continuous stimulation. J Neurosurg. 1985;63:612–616. doi: 10.3171/jns.1985.63.4.0612. [DOI] [PubMed] [Google Scholar]
- 105.Stone T. Phantom limb pain and central pain; relief by ablation of a portion of posterior central cerebral convolution. Arch Neurol Psychiat. 1950;63:739–748. [PubMed] [Google Scholar]
- 106.Sugar O, Bucy P. Postherpetic trigeminal neuralgia. Arch Neurol Neurosurg Psychiat. 1951;63:739–748. doi: 10.1001/archneurpsyc.1951.02320020003001. [DOI] [PubMed] [Google Scholar]
- 107.Svensson P, Graven-Nielsen T, rendt-Nielsen L. Mechanical hyperesthesia of human facial skin induced by tonic painful stimulation of jaw muscles. Pain. 1998;74:93–100. doi: 10.1016/S0304-3959(97)00156-5. [DOI] [PubMed] [Google Scholar]
- 108.Tamura Y, Okabe S, Ohnishi T, Saito N, Arai N, Mochio S, Inoue K, Ugawa Y. Effects of 1-Hz repetitive transcranial magnetic stimulation on acute pain induced by capsaicin. Pain. 2004;107:107–115. doi: 10.1016/j.pain.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 109.Timmermann L, Ploner M, Haucke K, Schmitz F, Baltissen R, Schnitzler A. Differential coding of pain intensity in the human primary and secondary somatosensory cortex. J Neurophysiol. 2001;86:1499–1503. doi: 10.1152/jn.2001.86.3.1499. [DOI] [PubMed] [Google Scholar]
- 110.Todd A, Koerber H. Neuroanatomical substrates of spinal nociception. In: McMahan S, Koltzenburgh M, editors. Wall and Melzzck's Textbook of Pain. Elsevier; Churchill Livingston; London: 2005. pp. 73–96. [Google Scholar]
- 111.Tommerdahl M, Favorov O, Whitsel B. Dynamic representations of the somatosensory cortex. Neurosci Biobehav Rev. 2010;34:160–170. doi: 10.1016/j.neubiorev.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 112.Tommerdahl M, Delemos KA, Favorov OV, Metz CB, Vierck CJ, Jr., Whitsel BL. Response of anterior parietal cortex to different modes of same-site skin stimulation. J Neurophysiol. 1998;80:3272–3283. doi: 10.1152/jn.1998.80.6.3272. [DOI] [PubMed] [Google Scholar]
- 113.Tommerdahl M, Delemos KA, Vierck CJ, Jr., Favorov OV, Whitsel BL. Anterior parietal cortical response to tactile and skin-heating stimuli applied to the same skin site. J Neurophysiol. 1996;75:2662–2670. doi: 10.1152/jn.1996.75.6.2662. [DOI] [PubMed] [Google Scholar]
- 114.Tommerdahl M, Favorov O, Whitsel BL, Nakhle B, Gonchar YA. Minicolumnar activation patterns in cat and monkey SI cortex. Cereb Cortex. 1993;3:399–411. doi: 10.1093/cercor/3.5.399. [DOI] [PubMed] [Google Scholar]
- 115.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 116.Tran TD, Matre D, Casey KL. An inhibitory interaction of human cortical responses to stimuli preferentially exciting Adelta or C fibers. Neuroscience. 2008;152:798–808. doi: 10.1016/j.neuroscience.2007.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Veldhuijzen DS, Greenspan JD, Kim JH, Lenz FA. Altered pain and thermal sensation in subjects with isolated parietal and insular cortical lesions. Eur J Pain. 2010;14:535–11. doi: 10.1016/j.ejpain.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Veldhuijzen DS, Nemenov MI, Keaser M, Zhuo J, Gullapalli RP, Greenspan JD. Differential brain activation associated with laser-evoked burning and pricking pain: An event-related fMRI study. Pain. 2009;141:104–113. doi: 10.1016/j.pain.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vierck CJ., Jr. Tactile movement detection and discrimination following dorsal column lesions in monkeys. Exp Brain Res. 1974;20:331–346. doi: 10.1007/BF00237379. [DOI] [PubMed] [Google Scholar]
- 120.Vierck CJ., Jr. Impaired detection of repetitive stimulation following interruption of the dorsal spinal column in primates. Somatosens Mot Res. 1998;15:157–163. doi: 10.1080/08990229870880. [DOI] [PubMed] [Google Scholar]
- 121.Vierck CJ, Jr., Cannon RL, Fry G, Maixner W, Whitsel BL. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol. 1997;78:992–1002. doi: 10.1152/jn.1997.78.2.992. [DOI] [PubMed] [Google Scholar]
- 122.Vierck CJ, Jr., Cohen RH, Cooper BY. Effects of spinal tractotomy on spatial sequence recognition in macaques. J Neurosci. 1983;3:280–290. doi: 10.1523/JNEUROSCI.03-02-00280.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vierck CJ, Jr., Cohen RH, Cooper BY. Effects of spinal lesions on temporal resolution of cutaneous sensations. Somatosens Res. 1985;3:45–56. doi: 10.3109/07367228509144576. [DOI] [PubMed] [Google Scholar]
- 124.Vierck CJ, Jr., Favorov O, Whitsel BL. Neural mechanisms of absolute tactile localization in monkeys. Somatosens Mot Res. 1988;6:41–61. doi: 10.3109/08990228809144640. [DOI] [PubMed] [Google Scholar]
- 125.Vierck CJ, Jr., Kline RH, Wiley RG. Intrathecal substance p-saporin attenuates operant escape from nociceptive thermal stimuli. Neuroscience. 2003;119:223–232. doi: 10.1016/s0306-4522(03)00125-8. [DOI] [PubMed] [Google Scholar]
- 126.Vierck CJ, Jr., Luck MM. Loss and recovery of reactivity to noxious stimuli in monkeys with primary spinothalamic cordotomies, followed by secondary and tertiary lesions of other cord sectors. Brain. 1979;102:233–248. doi: 10.1093/brain/102.2.233. [DOI] [PubMed] [Google Scholar]
- 127.Vierck C, Hamilton D, Thornby J. Pain reactivity of monkeys after lesions to the dorsal and lateral columns of the spinal cord. Exper Brain Res. 1971;13:140–158. doi: 10.1007/BF00234083. [DOI] [PubMed] [Google Scholar]
- 128.Vincler M, Maixner W, Vierck CJ, Jr., Light AR. Effects of systemic morphine on escape latency and a hindlimb reflex response in the rat. J Pain. 2001;2:83–90. doi: 10.1054/jpai.2001.19560. [DOI] [PubMed] [Google Scholar]
- 129.Weng HR, Lee JI, Lenz FA, Schwartz A, Vierck C, Rowland L, Dougherty PM. Functional plasticity in primate somatosensory thalamus following chronic lesion of the ventral lateral spinal cord. Neuroscience. 2000;101:393–401. doi: 10.1016/s0306-4522(00)00368-7. [DOI] [PubMed] [Google Scholar]
- 130.White J, Sweet W. Pain and the Neurosurgeon: A Forty-year Experience. Charles C Thomas; Springfield: 1969. [Google Scholar]
- 131.White LE, Andrews TJ, Hulette C, Richards A, Groelle M, Paydarfar J, Purves D. Structure of the human sensorimotor system. I: Morphology and cytoarchitecture of the central sulcus. Cereb Cortex. 1997;7:18–30. doi: 10.1093/cercor/7.1.18. [DOI] [PubMed] [Google Scholar]
- 132.Whitsel B, Tommerdahl M, Kohn A, Vierck C, Favorov O. The SI response to noxious skin heating as revealed by optical intrinsic signal (OIS) imaging. In: Bushnell M, Casey K, editors. Pain Imaging. IASP Press; Seattle: 2000. [Google Scholar]
- 133.Whitsel BL, Favorov OV, Li Y, Lee J, Quibrera PM, Tommerdahl M. Nociceptive afferent activity alters the SI RA neuron response to mechanical skin stimulation. Cereb Cortex. 2010;20:2900–2915. doi: 10.1093/cercor/bhq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Whitsel BL, Favorov OV, Li Y, Quibrera M, Tommerdahl M. Area 3a neuron response to skin nociceptor afferent drive. Cereb Cortex. 2009;19:349–366. doi: 10.1093/cercor/bhn086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wiley RG, Kline RH, Vierck CJ., Jr. Anti-nociceptive effects of selectively destroying substance P receptor-expressing dorsal horn neurons using [Sar9,Met(O2)11]-substance P-saporin: behavioral and anatomical analyses. Neuroscience. 2007;146:1333–1345. doi: 10.1016/j.neuroscience.2007.01.066. [DOI] [PubMed] [Google Scholar]
- 136.Willis WD, Kenshalo DR, Jr., Leonard RB. The cells of origin of the primate spinothalamic tract. J Comp Neurol. 1979;188:543–573. doi: 10.1002/cne.901880404. [DOI] [PubMed] [Google Scholar]
- 137.Willis WD, Leonard RB, Kenshalo DR., Jr. Spinothalamic tract neurons in the substantia gelatinosa. Science. 1978;202:986–988. doi: 10.1126/science.102034. [DOI] [PubMed] [Google Scholar]
- 138.Willis WD, Trevino DL, Coulter JD, Maunz RA. Responses of primate spinothalamic tract neurons to natural stimulation of hindlimb. J Neurophysiol. 1974;37:358–372. doi: 10.1152/jn.1974.37.2.358. [DOI] [PubMed] [Google Scholar]
- 139.Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14:2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Willis WD, Jr., Zhang X, Honda CN, Giesler GJ., Jr. Projections from the marginal zone and deep dorsal horn to the ventrobasal nuclei of the primate thalamus. Pain. 2001;92:267–276. doi: 10.1016/s0304-3959(01)00268-8. [DOI] [PubMed] [Google Scholar]
- 141.Willis WD, Jr., Zhang X, Honda CN, Giesler GJ., Jr. A critical review of the role of the proposed VMpo nucleus in pain. J Pain. 2002;3:79–94. doi: 10.1054/jpai.2002.122949. [DOI] [PubMed] [Google Scholar]
- 142.Willis W, Coggeshall R. Sensory mechanisms of the spinal cord. Plenum Press; New York: 1991. [Google Scholar]
- 143.Woolsey C, Erickson T, Gilson W. Localization in somatic sensory and motor areas of human cerebral cortex as determined by direct recording of evoked potentials and electrical stimulation. J Neurosurg. 1979;51:476–506. doi: 10.3171/jns.1979.51.4.0476. [DOI] [PubMed] [Google Scholar]
- 144.Yarnitsky D, Ochoa JL. Release of cold-induced burning pain by block of cold-specific afferent input. Brain. 1990;113(Pt 4):893–902. doi: 10.1093/brain/113.4.893. [DOI] [PubMed] [Google Scholar]
- 145.Yokota T. Thalamic mechanism of pain: Shell theory of thalamic nociception. Jap J Physiol. 1989;39:335–348. doi: 10.2170/jjphysiol.39.335. [DOI] [PubMed] [Google Scholar]
- 146.Zheng J, Lu Y, Perl ER. Inhibitory neurones of the spinal substantia gelatinosa mediate interaction of signals from primary afferents. J Physiol. 2010;588:2065–2075. doi: 10.1113/jphysiol.2010.188052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhuo M. Cortical excitation and chronic pain. Trends Neurosci. 2008;31:199–207. doi: 10.1016/j.tins.2008.01.003. [DOI] [PubMed] [Google Scholar]