Abstract
Introduction
In the present study, we observed differences in the clinical and haematological parameters in patients of MRSA and non MRSA acute osteoarticular infection.
Methodology
For the patients of acute haematogenous osteomyelitis/septic arthritis, clinical features, haematological parameters and blood & aspirate cultures were recorded.
Results
Of 81 patients enrolled in the study, 61 were culture positive (22% MRSA). Statistically significant difference was found only in CRP (P < 0.001). ROC curve analysis shows that CRP levels of >13.9 mg/L, MRSA bone and joint infection could be predicted with 92.9% sensitivity and 79.1% specificity (AUC = 89.1).
Conclusion
Estimation of serum CRP levels at the time of presentation can aid in distinguishing MRSA osteomyelitis from non MRSA one.
Keywords: Osteomyelitis, Septic, Staphylococcus, Methicillin, Resistance
1. Introduction
Recent increase in the incidence of osteomyelitis and septic arthritis due to a rise in predisposing factors like diabetes mellitus and peripheral vascular disease, is a matter of great concern. Moreover, the increasing incidence of methicillin resistant staphylococcus aureus (MRSA), in the last two decades, has further complicated this health issue.1–3
MRSA is a common nosocomial infective agent and accounted for more than 50% of S. aureus isolates from intensive care units in the Western countries.4 In India, a recent multicentre study from 15 tertiary care hospitals, including our hospital and others scattered throughout the country found the prevalence of MRSA ranging from 22 to 60%.5 The prevalence of MRSA among S. aureus infections in the community was highest in Taiwan (40.5%) followed by Sri Lanka (38.8%), Philippines (30.1%), Vietnam (28.2%) Korea (20.5%) and India (4.3%).6
This data is of alarming significance as musculoskeletal infections associated with MRSA tend to be more virulent, deeply invasive, requiring longer hospital stays, associated with greater number of surgical interventions with higher complication rates and mortality.1,7 Studies have also shown that musculoskeletal infections by MRSA have a higher incidence of deep vein thrombosis (DVT) and septic pulmonary emboli.8,9
To mitigate morbidity and mortality due to MRSA, it is imperative to establish the diagnosis by high index of clinical suspicion along with imaging and growing the infecting organism in culture, which promotes the prompt institution of appropriate antibiotic therapy early in the course of the disease. However, numerous studies have quoted a culture negativity rate ranging from 30 to 50 percent.10,11 Even in culture positive cases it takes several days to isolate the causative organism and the start of appropriate antibiotic usually gets delayed.
It has been observed in various studies that clinical features like fever, severity of loss of function of affected extremity, multifocality and haematological features like total WBC count, neutrophil count, ESR, CRP, platelet counts vary significantly among MRSA and non MRSA acute musculoskeletal infections.12
Thus, in the present study, we propose to study these clinical and haematological parameters in patients of MRSA and non MRSA acute osteoarticular infection at the time of their presentation to our hospital and establish whether MRSA infections can be predicted on the basis of these parameters before culture of the organisms for early initiation of appropriate treatment.
2. Materials and methods
The present study was conducted by the Department of Orthopaedics, JIPMER, Puducherry, India from September 2011 to August 2013. Approval from Institute Research Council and Human Ethics Committee was obtained prior to starting the study. Written informed consent was obtained from all the participating subjects. A retrospective analysis of cases treated from January 2007 to August 2011 was also carried out from the hospital case records.
-
a.
Study subjects: All the patients of acute haematogenous osteomyelitis or septic arthritis treated during the study period were enrolled for the study. A total of 130 patients were enrolled, out of which 49 were excluded as 10 patients were already on antibiotics and 39 patients did not have complete case records. Cases with implant related infections, perioperative and postoperative infections were also excluded from study. After exclusion, we enrolled 81 patients in this study (57 prospective and 24 retrospective). After taking written informed consent from patient or their parent (if patient's age was <18 years), following data was collected at the time of presentation: Age, Sex, Duration of symptoms (days), Axillary temperature (°C) & Heart rate (beats/min). Five ml of venous blood was collected at the time of presentation and was sent for culture and sensitivity studies and analysis of haematological parameters. 2 ml of aspirate either from joint or bone of suspected septic arthritis or osteomyelitis respectively was sent for culture and sensitivity.
-
b.
Haematological parameters: Estimation of haemoglobin, WBC, differential leukocyte count, platelet count and haematocrit in blood was done by automated cell counter machine (SYSMEX XS-1000i) in haematology department. ESR was estimated by Wintrobe method. C-reactive protein was estimated by nephelometer.
-
c.
Culture and sensitivity: Samples were inoculated on Blood Agar, Mckonkey's Agar, Chocolate Agar and Brain Heart Infusion medium. Agar plates were observed for growth for 24–48 h and sample kept in Brain heart infusion medium for 7 days.
-
d.
Statistical Method: The study parameters were categorised into parametric and non parametric by doing test of normality – Kolmogorov Smirnov test. Parameters found normally distributed were further analysed by univariate analysis using Student T-test. Parameters which showed non normal distribution were further analysed by univariate analysis using Mann Whitney U test. Univariate analysis was done for each parameter to see if there was significant difference between MRSA and non MRSA (including culture negative cases). We repeated comparison between MRSA and culture positive non MRSA cases and between MRSA and culture negative cases so that the influence of the culture negative cases on the larger analysis could be assessed. If more than one parameters were found to be significantly different (P < 0.05), these parameters were further analysed by multivariate logistic regression, to find out significant independent predictor of MRSA with the p value <0.05. Receiver operating characteristic curve analysis was performed for each independent significant predictor to determine the area under the curve. Using Kruskal–Wallis and one way anova test, we compared the outcome of MRSA, non MRSA (culture positive cases) and culture negative cases. Statistical analysis was performed using SPSS software (version 20.0). A two-tailed p value of <0.05 was considered significant.
3. Results
Out of 81 cases, 47 were males (58%) and 34 (42%) were females. Proportion of patient having MRSA bone and joint infection was not significantly different among male and female (p value-0.214). Age of the patients varied from 11days to 45 years among non MRSA group and varied from 25 days to 17 years in MRSA group.
Out of the enrolled 81 cases, culture came positive for 61 patients and was negative for 20 patients. Out of 61 culture positive cases, MRSA had grown in 14 (22%), MSSA in 16 (26%), Klebsiella pneumoniae in 14 (22%), Pseudomonas in six (9%), Streptococcus pyogenes in six (9%), Burkholderia pseudomallei in two, Enterococcus in one, Enterobacter in one, Group D Salmonella in one and Coagulase negative S. aureus in one. In two cases, culture had grown more than one organism (Table 1). In one case of septic arthritis shoulder, Actinomycetes and Pseudomonas had grown in culture while in one case of septic arthritis hip, Klebsiella and Streptococcus had grown. Culture from the site of infection was positive in 51 (63%) cases while Blood culture was found to be positive in 24 (29%) cases.
Table 1.
Frequencies of various causative agents of acute bone and joint infections found in our study.
| Organism | Number |
|---|---|
| MRSA | 14 |
| MSSA | 16 |
| Klebsiella | 14 |
| Pseudomonas | 6 |
| Streptococcus pyogenes | 6 |
| Burkholderia pseudomallei | 2 |
| Enterococcus | 1 |
| Enterobacter | 1 |
| Salmonella | 1 |
| CONSa | 1 |
| Actinomyecetes | 1 |
| Polymicrobial | 2 |
Coagulase negative staphylococcus aureus.
Out of 14 cases of MRSA, 7 patients presented with monoarticular septic arthritis and 1 with polyarticular septic arthritis. 2 had monoosseous osteomyelitis & 1 polyosseous osteomyelitis whereas 3 patients had septic arthritis associated with osteomyelitis.
Out of 81 included cases, two patients died. Both the cases were of MRSA multifocal bone and joint infection with severe systemic illness, one died of pneumonia and the other had a fulminant Liver abscess. No deaths were reported in non MRSA group.
Five patients of MRSA had extra musculoskeletal systemic invasive involvement, while only one patient among non MRSA group had extra musculoskeletal, deeply invasive infection.
We found that among MRSA cases, median duration of symptoms was 7 days, the median temperature was 37.5 °C, the median total WBC count was 10,250/mm3, the median ESR was 57 mm/h, the median CRP was 19.1 mg/L and the median platelet count was 320,000/mm3 while mean heart rate was 134/m, ANC was 7444/mm3, Hb was 9.9 g% and haematocrit was 30.28%.
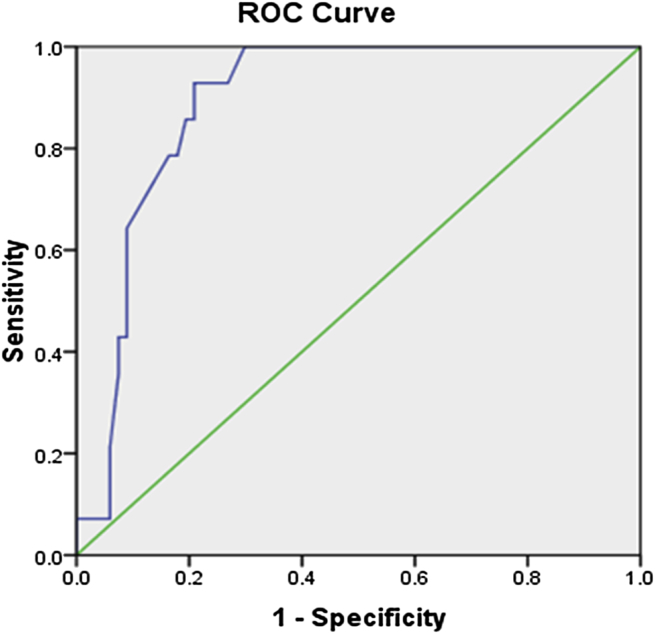
On statistical analysis, heart rate, absolute neutrophil count (ANC), PCV and haemoglobin (Hb) were found to be normally distributed while parameters like duration of symptoms, temperature, total WBC count, ESR, CRP and Platelet count showed non normal distribution. By univariate analysis statistically significant difference was seen only in CRP (P < 0.001) among MRSA and other cases (Table 2). Through ROC curve, the cut off value of CRP was calculated (Fig. 1). At CRP >13.9 mg/L, MRSA bone and joint infection could be predicted with 92.9% sensitivity and 79.1% specificity. Area under the curve came out to be 89.1%. Using 13.9 mg/L as a cut off value, forward multivariate logistic regression model showed CRP to be statistically significant predictor of MRSA septic arthritis and osteomyelitis (P < 0.001). By multivariate logistic regression, we found that the positive predictive value of CRP >13.9 mg/L was 48.1%, when compared with all other culture positive or negative cases of acute haematogenous osteomyelitis and septic arthritis. By Kruskal–Wallis and one way Anova test, we compared MRSA with non MRSA culture positive cases and cases with culture proven no growth so that influence of culture negative cases on larger analysis can be assessed (Table 3).
Table 2.
Comparison of central tendencies of parameters among MRSA and others and p value after univariate analysis of parameters.
| Parameters | MRSA | Others | p value |
|---|---|---|---|
| Heart ratea (beats/min) | 134 ± 31 | 127 ± 24 | 0.353 |
| ANCa (/mm3) | 7444 ± 3457 | 7725 ± 2880 | 0.749 |
| PCVa (%) | 30.28 ± 4.54 | 33.49 ± 5.74 | 0.053 |
| Hba (g%) | 9.90 ± 1.68 | 10.53 ± 1.95 | 0.265 |
| Duration of symptomsb (days) | 7 (4−10) | 7 (3−10) | 0.945 |
| Temperatureb (°C) | 37.5 (37−38.07) | 37.8 (37.2−38.3) | 0.486 |
| WBCb (/mm3) | 10,250 (8650−17,000) | 11,800 (9800−14,700) | 0.438 |
| ESRb (mm/h) | 57 (50−63.5) | 50 (42−60) | 0.172 |
| CRPb(mg/L) | 19.1 (17.5−24.12) | 9.4 (6.08−13.2) | <0.001 |
| Platelet countb (/mm3) | 320,000 (186,750−470,500) | 240,000 (210,000−320,000) | 0.375 |
P value is significant for the parameters in bold.
Mean (±Standard deviation).
Median (25th – 75th percentile).
Fig. 1.
Receiver operating characteristic curve of CRP on comparison of MRSA with all other cases.
Table 3.
Comparison of parameters among MRSA, non MRSA culture positive cases and culture negative cases.
| Parameters | MRSA (14) | Non-MRSA (47) | No growth (20) | p value |
|---|---|---|---|---|
| Heart ratea (beats/min) | 134 ± 31 | 129 ± 24 | 124 ± 23 | 0.502 |
| ANCa (/mm3) | 7444 ± 3457 | 8165 ± 2772 | 6689 ± 2930 | 0.168 |
| PCVa (%) | 30.28 ± 4.54 | 33.08 ± 6.23 | 34.45 ± 4.39 | 0.103 |
| Hba (g%) | 9.90 ± 1.68 | 10.24 ± 1.91 | 11.24 ± 1.91 | 0.078 |
| Duration of symptomsb (days) | 7 (4−10) | 7 (4−10) | 5.5 (3−10) | 0.674 |
| Temperatureb (°C) | 37.5 (37−38.07) | 37.8 (37.2−38.3) | 37.5 (37.05−38) | 0.594 |
| WBCb (/mm3) | 10,250 (8650−17,000) | 12,200 (10,000−14,200) | 11,500 (9125−15,900) | 0.738 |
| ESRb (mm/h) | 57 (50−63.5) | 52 (45−60) | 49 (36.25−58.75) | 0.204 |
| CRPb(mg/L) | 19.1 (17.5−24.12) | 11 (8−14.3) | 8 (3.575−10.7) | <0.001 |
| Platelet countb (/mm3) | 320,000 (186,750−470,500) | 240,000 (210,000−320,000) | 234,000 (184,750−305,000) | 0.58 |
P value is significant for the parameters in bold.
Mean (±Standard deviation).
Median (25th – 75th percentile).
4. Discussion
Staphylococcus aureus has been the most common cause of acute haematogenous osteoarticular infections. In the last two decades the incidence of MRSA has shown an upward trend around the world.1–3 We also found S. aureus to be the most common cause of acute haematogenous osteomyelitis and septic arthritis in our population with the prevalence of MSSA being 26% while that of MRSA being 22%.
As already observed, extremes of ages are known to be affected more by MRSA.13 In our study, 6 cases of MRSA were children less than one year of age.
MRSA is also known to cause more virulent and deeply invasive infections. In our study, out of 14 cases of MRSA, 3 developed DVT, Two of these were further complicated by pulmonary embolism. Two patients had pneumonia and one of them also developed a pericardial effusion while one developed a fulminant liver abscess. Other studies have also reported a significantly higher complication rates particularly DVT and pulmonary complications in patients with MRSA osteoerticular infections.7,8,13,14
Early diagnosis and aggressive antibiotic and surgical treatment can prevent mortality and morbidity associated with MRSA infections. However the diagnosis is mainly reliant on isolation of causative organism by culture which may take minimum 48–72 h and has a positivity rate of only 50–70%. Even in our study we could isolate causative organism only in 75% (61 out of 81) cases. Local sample had grown organism in 63% (51) while blood culture was positive in 29% (24) cases.
It has been reported that patients with MRSA infection show significant increase in serum levels of inflammatory markers, grade of temperature and extent of osteoarticular involvement compared to patients with non MRSA infection.9,15,16
Hawkshed et al performed a retrospective analysis of 71 cases of age four months to 19 years and found that in MRSA acute haematogenous osteomyelitis the median duration of elevated temperature was 3.06 days, the temperature was 102.03 °F, WBC count was 13,740/mm3, ESR was 73.5 mm/h and CRP was 9.5 mg/L. These parameters were significantly different (p < 0.01) in MRSA group of acute haematogenous osteomyelitis comparing with MSSA, other culture positive cases and culture negative groups.15 Similarly Ju et al, in the retrospective study of 129 patients from 1 to 18 years, found that the MRSA osteomyelitis differs significantly from MSSA osteomyelitis in terms of the number of patients with inability to bear weight by affected extremity, temperature (mean = 38.8 °C), heart rate (mean = 114/m), total WBC (mean = 16,920/mm3), ANC (mean = 13,100/mm3), haematocrit (mean = 32.5%), platelet count (mean = 514,000/mm3), ESR (mean = 93.2 mm/h) and CRP (mean = 19.5 mg/L).17 Kini et al performed a retrospective observational study from India, comparing community acquired MRSA acute bone and joint infection in patients <18 years and found that in MRSA cases, the mean days of fever (6.2), temperature (101.8 °F), Hb (9.1 g%), haematocrit (32.1%), total WBC count (14,200/mm3), ESR (39.3 mm/h), CRP (37.3 mg/dl), differed significantly compared to MSSA infected patients.3
One of the reasons of not having significant difference among the most of the clinical and haematological parameters of MRSA and non MRSA bone and joint infections analysed in our study may be having 57% population of our study <1 year age out of which 78% were <1 month. These patients have immature immune system and thereby may have normal temperature, WBC and other inflammatory markers.18 Other reason might be the less number of MRSA cases in our study.
In our study we found only CRP being significantly different predictor in MRSA and other cases including culture positive non MRSA and culture negative cases. Through ROC curve we found out the cut off value of CRP >13.9 mg/L to maximise the sensitivity (92.9%) and specificity (79.1%). The predictivity of MRSA acute haematogenous septic arthritis and osteomyelitis was 48.1% from other non MRSA and culture negative cases, if CRP >13.9 mg/L.
In the study of Ju et al, predictivity of MRSA osteomyelitis was 92%, if all four parameters were present, which includes body temperature >38 °C, haematocrit <34%, WBC >12,000/mm3 and CRP >13 mg/L.17 When any three parameters were found, MRSA predictivity was 45% and for any two parameters, it was 10%. Kini et al, found predicitivity of MRSA osteoarticular infection of 94% when all seven parameters, hb < 9.5 g%, PCV <34%, CRP >32 mg/dl and ANC >65%, body temperature >100.41 °F, ESR >35 mm/h and WBC >14,000/mm3, were found at presentation. Predictivity of MRSA was only 65%, if six parameters were present.3
To form a predictive algorithm for MRSA acute haematogenous osteomyelitis and septic arthritis, it is required to perform multicentric, prospective study categorising different age groups, involving larger number of MRSA and non MRSA cases, to find out predictivity of different parameters in Indian population.
The limitations of the present study are less sample sizes and inclusion of all age group in the study. Also it seems prudent to study the role of particular genetic virulence factors in the MRSA strains.
5. Conclusion
-
•
We found in our study that at a cut off value of 13.9 mg/L, CRP has a positive predictive value of 48.1% for the diagnosis of acute haematogenous MRSA osteoarticular infections at presentation.
-
•
Estimation of the serum CRP levels at presentation for a patient presenting with the acute haematogenous osteoarticular infection can aid in distinguishing an MRSA infection from a non MRSA one. This can be a useful guide to early institution of appropriate antibiotic.
Authors’ contribution
RA did data collection & statistical analysis DS performed interim analysis, proof reading and editing the manuscript; PD performed formulation and editing of manuscript and DKP did proof reading and editing of the manuscript.
Conflicts of interest
All authors have none to declare.
Acknowledgements
The above work was funded by the Institute in which it was carried out (JIPMER, Puducherry, India).
References
- 1.Arnold S.R., Elias D., Buckingham S.C., Thomas E.D., Novais E., Arkader A. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop. 2006;26:703–708. doi: 10.1097/01.bpo.0000242431.91489.b4. [DOI] [PubMed] [Google Scholar]
- 2.Young T.P., Maas L., Thorp A.W., Brown L. Etiology of septic arthritis in children: an update for the new millennium. Am J Emerg Med. 2011;29:899–902. doi: 10.1016/j.ajem.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Kini A.R., Shetty V., Kumar A.M., Shetty S.M., Shetty A. Community-associated, methicillin-susceptible, and methicillin-resistant Staphylococcus aureus bone and joint infections in children: experience from India. J Pediatr Orthop Part B. 2013;22:158–166. doi: 10.1097/BPB.0b013e32835c530a. [DOI] [PubMed] [Google Scholar]
- 4.National Nosocomial Infections Surveillance System National Nosocomial Infections Surveillance (NNIS) System Report(2002), data summary from January 1992 to June 2002, issued August 2002. Am J Infect Control. 2002;30:458–475. doi: 10.1067/mic.2002.130032. [DOI] [PubMed] [Google Scholar]
- 5.Indian Network for Surveillance of Antimicrobial Resistance (INSAR) Group, India Methicillin resistant Staphylococcus aureus (MRSA) in India: prevalence & susceptibility pattern. Indian J Med Res. 2013;137:363–369. [PMC free article] [PubMed] [Google Scholar]
- 6.Song J.-H., Hsueh P.-R., Chung D.R. Spread of methicillin-resistant Staphylococcus aureus between the community and the hospitals in Asian countries: an ANSORP study. J Antimicrob Chemother. 2011 May 1;66:1061–1069. doi: 10.1093/jac/dkr024. [DOI] [PubMed] [Google Scholar]
- 7.Vander Have K.L., Karmazyn B., Verma M. Community-associated methicillin-resistant Staphylococcus aureus in acute musculoskeletal infection in children: a game changer. J Pediatr Orthop. 2009 Dec;29:927–931. doi: 10.1097/BPO.0b013e3181bd1e0c. [DOI] [PubMed] [Google Scholar]
- 8.Bouchoucha S., Benghachame F., Trifa M. Deep venous thrombosis associated with acute hematogenous osteomyelitis in children. Orthop Traumatol Surg Res. 2010 Dec;96:890–893. doi: 10.1016/j.otsr.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Bouchoucha S., Drissi G., Trifa M. Epidemiology of acute hematogenous osteomyelitis in children: a prospective study over a 32 months period. Tunis Médicale. 2012 Jun;90:473–478. [PubMed] [Google Scholar]
- 10.Deshpande S.S., Taral N., Modi N., Singrakhia M. Changing epidemiology of neonatal septic arthritis. J Orthop Surg [Internet] 2004 doi: 10.1177/230949900401200103. [cited 2013 Jul 21];12(1) [DOI] [PubMed] [Google Scholar]
- 11.Saavedra-Lozano J., Mejías A., Ahmad N. Changing trends in acute osteomyelitis in children: impact of methicillin-resistant Staphylococcus aureus infections. J Pediatr Orthop. 2008 Aug;28:569–575. doi: 10.1097/BPO.0b013e31817bb816. [DOI] [PubMed] [Google Scholar]
- 12.Dartnell J., Ramachandran M., Katchburian M. Haematogenous acute and subacute paediatric osteomyelitis a systematic review of the literature. J Bone Jt Surg Br. 2012;94:584–595. doi: 10.1302/0301-620X.94B5.28523. [DOI] [PubMed] [Google Scholar]
- 13.Elixhauser A., Steiner C. 2007. Infections with Methicillin-resistant Staphylococcus aureus (MRSA) in US Hospitals, 1993–2005.http://www.ncbi.nlm.nih [cited 2013 Aug 31]; Available from: [PubMed] [Google Scholar]
- 14.Gonzalez B.E., Teruya J., Mahoney D.H. Venous thrombosis associated with Staphylococcal osteomyelitis in children. Pediatrics. 2006 May 1;117:1673–1679. doi: 10.1542/peds.2005-2009. [DOI] [PubMed] [Google Scholar]
- 15.Hawkshead J.J., Patel N.B., Steele R.W., Heinrich S.D. Comparative severity of pediatric osteomyelitis attributable to methicillin-resistant versus methicillin-sensitive Staphylococcus aureus. J Pediatr Orthop. 2009 Feb;29:85–90. doi: 10.1097/BPO.0b013e3181901c3a. [DOI] [PubMed] [Google Scholar]
- 16.Gillet Y., Dohin B., Dumitrescu O. Osteoarticular infections with staphylococcus aureus secreting Panton-Valentine leucocidin. Arch Pédiatrie Organe Off Sociéte Française Pédiatrie. 2007 Oct;14(suppl 2):S102–S107. doi: 10.1016/s0929-693x(07)80043-1. [DOI] [PubMed] [Google Scholar]
- 17.Ju K.L., Zurakowski D., Kocher M.S. Differentiating between methicillin-resistant and methicillin-sensitive Staphylococcus aureus osteomyelitis in children: an evidence-based clinical prediction algorithm. J Bone Jt Surg Am. 2011 Sep 21;93:1693–1701. doi: 10.2106/JBJS.J.01154. [DOI] [PubMed] [Google Scholar]
- 18.Frederiksen B., Christiansen P., Knudsen F.U. Acute osteomyelitis and septic arthritis in the neonate, risk factors and outcome. Eur J Pediatr. 1993 Jul;152:577–580. doi: 10.1007/BF01954084. [DOI] [PubMed] [Google Scholar]