Abstract
A 47-year-old female with no history of previous illnesses developed cerebral infarction and was diagnosed with lung cancer, specifically EGFR mutation-positive adenocarcinoma, and Trousseau syndrome.
The patient's response to anticoagulant therapy with non-fractionated heparin was very poor; however we were able to control the thrombosis with chemotherapy. She survived for one year and 10 months following treatment with gefitinib, CBDCA + PEM and erlotinib, without recurrence of thrombosis.
Trousseau syndrome carries a poor prognosis and controlling thrombosis is difficult. In this case, the administration of anticancer therapy allowed use to control the patient's thrombosis. Therefore, this case highlights the importance of treating cancer in patients with Trousseau syndrome. In addition, the FDP and D-dimer levels changed in parallel with changes in the CEA level, which suggests that the activity of cancer is related to an internal thrombotic tendency. Hence, changes in the FDP and D-dimer values are associated with the efficacy of treatment with EGFR tyrosine kinase inhibitors and chemotherapy and may function as markers of recurrence.
Keywords: Trousseau syndrome, Lung cancer, EGFR mutation-positive, Chemotherapy, Anticoagulant therapy
1. Introduction
Trousseau syndrome was first described by Armand Trousseau in 1865 as affecting “the patients with internal organ cancer with significant ambiguous phlebothrombosis” [1]. Since then, various reports have assessed the characteristics of whole body thrombosis as a form of paraneoplastic syndrome. Trousseau syndrome has a poor prognosis, and it is very difficult to control the thrombosis; however, it is unknown whether controlling the cancer, which is the original illness, can be used to control the thrombosis [2].
We experienced a case of EGFR mutation-positive pulmonary adenocarcinoma associated with Trousseau syndrome in which we were able to control the thrombosis by treating the cancer with an EGFR tyrosine kinase inhibitor and chemotherapy.
2. Case report
A 47-year-old female with back and muscle pain presented to a local general hospital. A diagnosis of infective endocarditis was suspected, based on the detection of a livedo-like eruption on the finger-tips and in the pericardial fluid on CT. The patient subsequently consulted the department of cardiology at our hospital and experienced left hemiplegia in the ambulatory waiting room. The findings on brain MRI showed acute cerebral infarction of the right frontal lobe. Moreover, deep vein thrombosis and splenic artery thrombosis were detected on contrast-enhanced CT. Based on the presence of a nodule in the right lung on CT and the systemic thrombosis, the patient was thought to have Trousseau syndrome due to lung cancer and was transferred to our department.
The patient had no past medical or family history. Her blood pressure was 156/100 mmHg and her body temperature was 37.3 °C. Her heart and lung sounds were normal and her SpO2 was 95% on room air. The serum FDP level was 105.6 mg/ml, and the D-dimer level was 53.6 mg/ml. The serum CEA level was also increased at 94.5 ng/ml (Table 1). In addition, a chest X-ray film showed a nodule in the hilum of the right lung (Fig. 1), and enhanced CT demonstrated a nodule in the right lower lobe S6, with a second nodule that suggested the presence of metastasis in the right lower lobe. Furthermore, there neoplastic lesions to the second and fourth ribs were detected, and a number of similar lesions were identified in the spine (Fig. 2). Although there were multiple sites of cerebral infarction on brain MRI images, no neoplastic lesions suspicious of metastasis were noted (Fig. 3).
Table 1.
Laboratory findings on admission.
| Hematology | Biochemistry | Serology | |||
|---|---|---|---|---|---|
| RBC | 389 × 104/μl | TP | 6.7 g/dl | CRP | 2.2 mg/dl |
| Hb | 9.8 g/dl | Alb | 3.5 g/dl | ESR | 31 mm/h |
| Hct | 31.8% | α1 | 5.5% | CEA | 94.5 ng/ml |
| WBC | 14000/μl | α2 | 9.3% | KL-6 | 2526U/ml |
| Neu | 87.0% | β | 11.8% | IgA | 232 mg/dl |
| Eos | 1.0% | γ | 15.3% | IgM | 71 mg/dl |
| Bas | 0.0% | T-Bil | 0.7 mg/dl | IgG | 1074 mg/dl |
| Mo | 2.0% | D-Bil | 0.1 mg/dl | C3 | 132.0 mg/dl |
| Lym | 5.0% | AST | 41IU/l | C4 | 33.9 m |
| Plt | 15.2 × 104/μl | ALT | 19IU/l | ||
| LDH | 872IU/l | ||||
| Coagulation-Fibrinolysis | ALP | 3617IU/l | |||
| Fib | 165 mg/dl | γ-GTP | 159IU/l | ||
| PT | 78% | Amy | 306IU/l | ||
| PT-INR | 1.11 | CPK | 781IU/l | ||
| APTT | 35.9sec | UA | 4.1 mg/dl | ||
| AT-Ⅲ | 84.7% | BUN | 10 mg/dl | ||
| FDP | 105.6 μg/ml | Cr | 0.51 mg/dl | ||
| D-dimer | 53.6 μg/ml | Na | 136mEq/l | ||
| TAT | 27.2 μg/ml | K | 3.9mEq/l | ||
| PIC | 3.0 ng/ml | Cl | 101mEq/l | ||
| Ca | 9.1 mg/dl | ||||
| FBS | 85 mg/dl | ||||
| HbA1c | 5.4% | ||||
| HDL-cho | 38 mg/dl | ||||
| LDL-cho | 141 mg/dl | ||||
| TG | 159 mg/dl | ||||
Fig. 1.
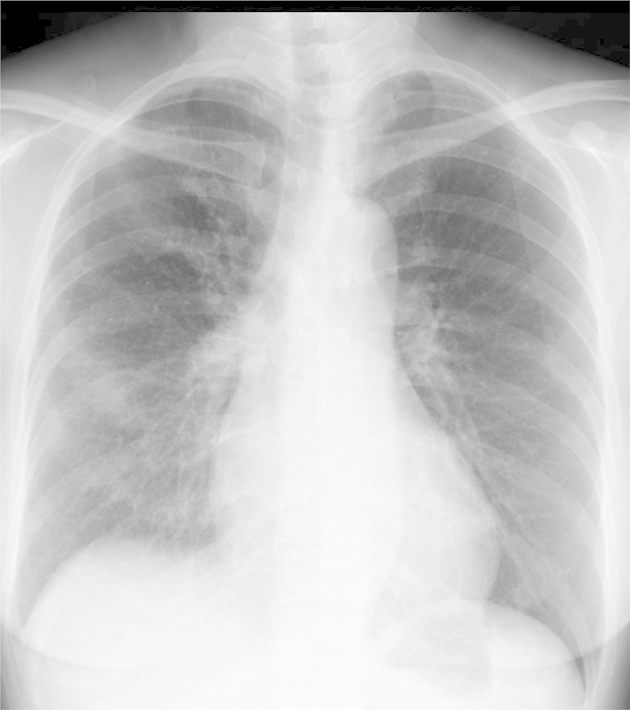
Chest X-ray film. A nodulare shadow was seen near the hilum of the right lung. Neoplastic changes were also noted in the right fourth rib.
Fig. 2.
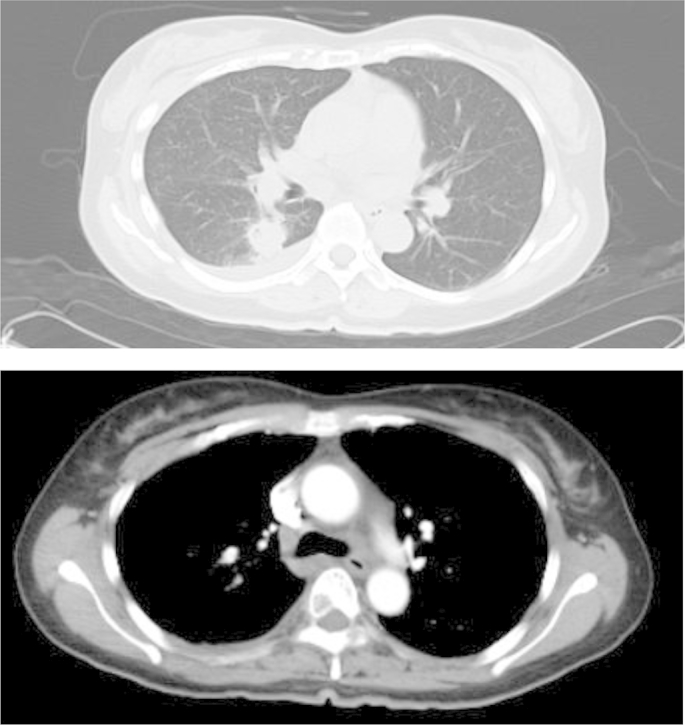
Chest high-resolution CT film. A nodule with marginal irregularity, internal heterogeneity and spiculation was found in the right lung lobs S6. In addition, neoplastic lesions were detected in the second and fourth ribs, with several similar lesions in the spine.
Fig. 3.
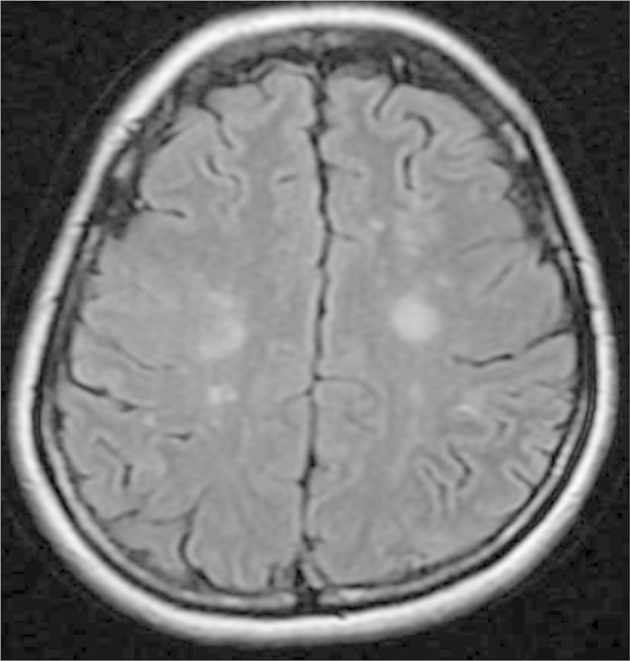
Brain MRI film. Multiple sites of cerebral infarction were identified. However, no brain metastasis was observed.
We subsequently diagnosed the patient with stage Ⅳ lung cancer, specifically EGFR mutation-positive mutation positive adenocarcinoma based on the findings of a percutaneous CT-guided lung biopsy and started her on treatment with gefitinib and anticoagulants.
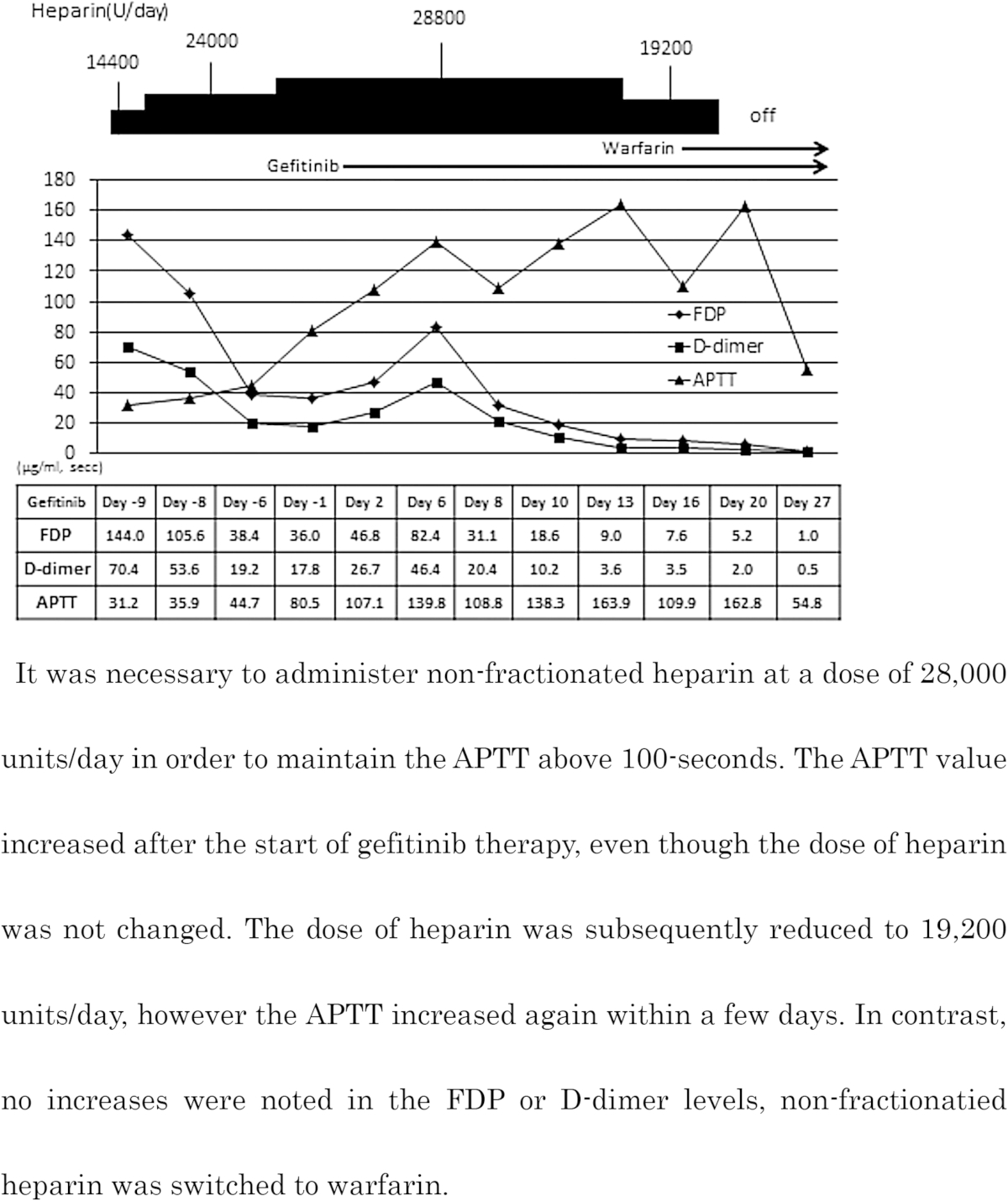
The changes in the APTT, FDP and D-dimer values were monitored during treatment (Table 2). The APTT was poorly controlled at 40 s under anticoagulant therapy with non-fractionated heparin at a dose of 14,400 units/day. We gradually increased the dose to 28,800 units/day, which effectively maintained the APTT at 100 s. In addition, the levels of FDP and D-dimer were initially decreased by the anticoagulant therapy, then gradually increased, even when the APTT was maintained at over 100 s. However, a trend toward reduction was observed following treatment with gefitinib on day 6, and the values ultimately reached the normal ranges. Therefore, the dose of non-fractionated heparin was reduced and the ragimen was switched to oral warfarin, with no increases in the FDP or D-dimer levels.
Table 2.
Transition of the anticoagulant therapy over the first month.
After two months of gefitinib therapy, the serum FDP and D-dimer levels increased, and, after three months of treatment, the CEA level exhibited an upward trend. Meanwhile, CT images showed stable disease (SD); however, we were concerned about the potential for recurrence of thrombosis. Therefore, the patient received a second-line chemotherapy using CBDCA + PEM. Thereafter, the CEA, FDP and D-dimer levels displayed a tendencyto decrease. The second-line chemotherapy was administered four times, followed by maintenance PEM therapy. Consequently, the serum levels of CEA, FDP and D-dimer gradually increased after the administration of the fourth cycle of maintenance therapy. We then switched the regimen to the third-line chemotherapy with erlotinib. After approximately one month, the patient developed marked pleural effusion and appetite loss in association with further increases in the CEA, FDP and D-dimer levels. She ultimately survived for one year and 10 months after the first treatment, with no episodes of thrombosis recurrence.
3. Discussion
Trousseau syndrome is a paraneoplastic syndrome that produces neurological symptoms associated with latent malignant tumors. This condition is recognized to cause systemic thrombosis as well as brain infarction due to enhancement of the coagulant system induced by the malignant tumor [2] and has been reported in connection with gastric, lung, pancreatic and ovarian cancers. Mucin-producing adenocarcinoma is a frequent histologic type in such cases [3,4], and 1–25% of cancer patients have been shown to have phlebothrombosis depending on the primary site (histologic type) [5], stage of disease and cycle of treatment. The survival rate is 12% over one year, compared to 36% in patients without thrombi [6]. Therefore, it is necessary consider the potential for malignant tumors in patients with thrombosis.
As a coagulation mechanism activated by malignant tumors, the tumor cells produce tissue factor, tumor pro-coagulants and cellularity coagulants, such as factor V receptors and fibrinolysis proteins, as well as fibrinolysis inhibitor, which activate the coagulation cascade. The activation of coagulation is promoted by interactions between blood platelets, monocytes and endothelial cells via the actions of various cytokines and tumor antigens, with consequent immune complex thrombogenesis [2].
Heparin is considered to be the first-choice agent for controlling thrombosis associated with Trousseau syndrome, and it has been reported that the administration of low-molecular-weight heparin improves the mortality rate compared to that achieved with non-fractionated haparin [7]. In addition, it has been demonstrated that dalteparin sodium, a form of low-molecular-weight heparin, is more effective than warfarin in preventing the recurrence of thromboembolism as well as the risk of hemorrhage [8]. As to why the effects of warfarin are inferior to those of heparin, Wahrenbrock et al. reported that heparin, but not warfarin, inhibits the function of cancer-derived mucin, which activates blood platelets, causing microangiopathy [9].
In addition, a new series of anticoagulant medicines have recently been released. One of themedicines is dabigatran forming IIa factor (thrombin) and thrombin-TM complex, which acts as a direct thrombin reversal agent. The others are rivaroxaban, apixaban and edxaban, which directly connect to the S1 pocket of the active center of the Xa factor, thus inhibiting Xa activity. These new medicines are termed novel oral anticoaglants (NOACs). NOACs allow for better or equivalent control of thrombosis than warfarin in cases of stroke and systemic thrombosis [10–13]. At present, no studies have compared the efficacy of heparin with the NOACs in the treatment of systemic thrombosis or Trousseau syndrome. However, the main action of heparin is the inhibitory effect toward thrombin and Xa factor, which is closer to the anticoagulant mechanism of NOACs than warfarin. Therefore, the NOACs may be more effective for treatment of the Trousseau syndrome than warfarin. The NOACs have an additional advantage in that they can administered orally. In consideration of the difficulties in controlling thrombosis without anticancer therapy, we are hopeful that NOACs achieve their expected effect.
We used non-fractionated heparin in this case. The ability to control the patient's thrombosis was poor because we were unable to promptly maintain the APTT level and the FDP and D-dimer values exhibited an upward trend even when the APTT was 100-s under treatment with a large dose of non-fractionated heparin. Furthermore, the serum levels of FDP and D-dimer increased continually until gefitinib therapy was initiated. However, these values gradually decreased following the administration of gefitinib and did not increase again, even when the non-fractionated heparin was switched to warfarin.
The changes in the CEA, FDP and D-dimer levels are shown in Table 3. The CEA level exhibited a similar trend under the second-line and third-line treatments. With respect to the incidence of tumor recurrence under treatment, no significant changes were noted in the size of the primary tumor in this case, and zoledronic acid and denosumab, bone-specific agents, were used to treat the bone metastases. Therefore, monitoring the patient's condition based on imaging findings was difficult, and thus measuring the levels of CEA, FDP and D-dimer was helpful for assessing the effects of treatment and detecting recurrence. In addition, we determined the appropriate timing for changing the treatment regimen primarily by monitoring increases in the CEA, FDP and D-dimer levels.
Table 3.
Changes in serum levels of CEA, FDP and D-dimer after the start of gefitinib.
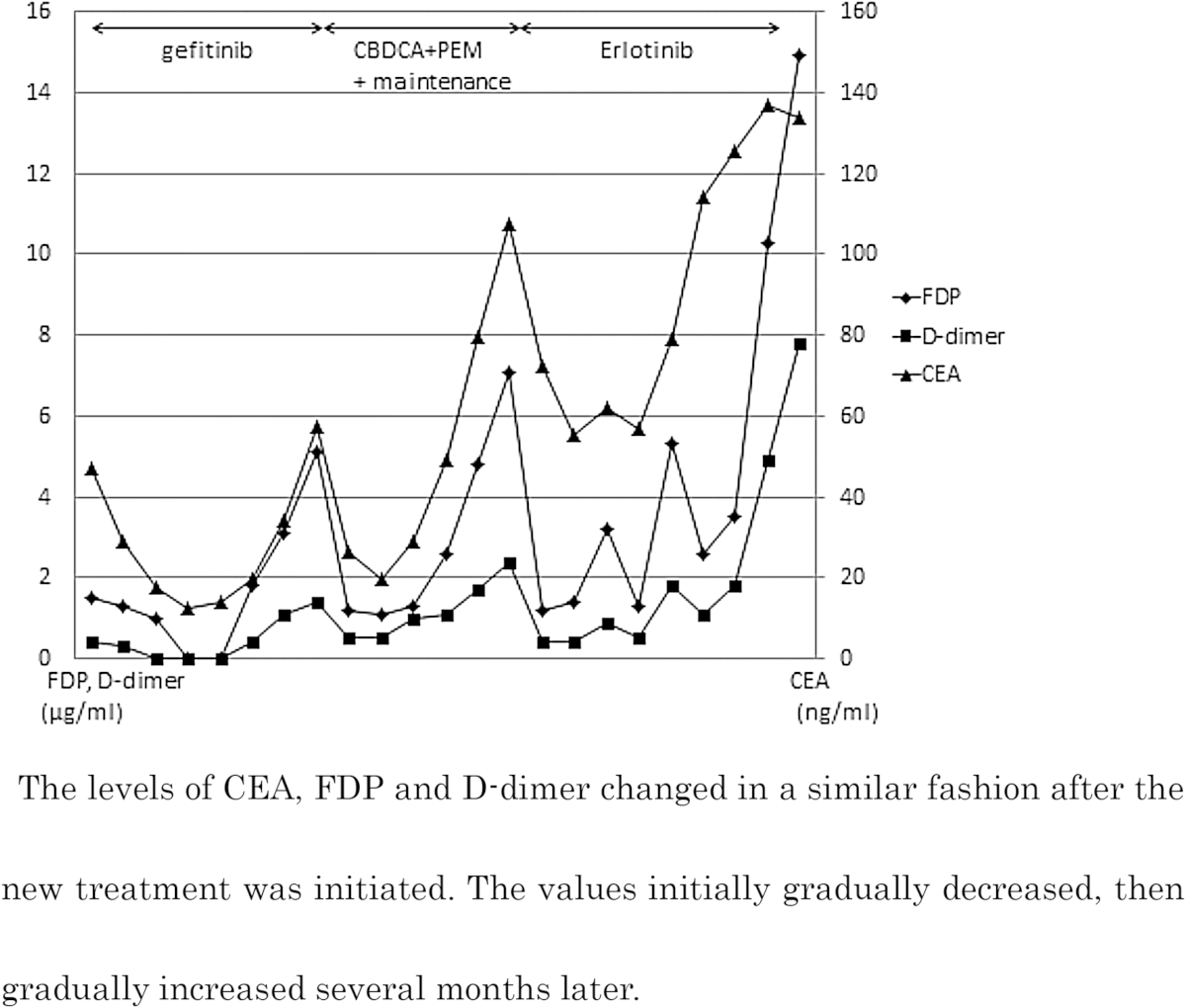
The parallel changes in the CEA level and FDP and D-dimer values suggest that the activity of the patient's cancer was related to an internal thrombotic tendency and that the changes of in the FDP and D-dimer values were associated with the effects of the chemotherapy. In other words, the patient's response to normal anticoagulant therapy was very poor, whereas providing treatment for the original illness successfully controlled the thrombosis. In addition, our findings indicate that measuring the FDP and D-dimer levels is helpful for evaluating the status of the tumor.
In patients with Trousseau syndrome, the ability to control the cancer is poor when the level of control of the thrombosis is poor, in association with a poor prognosis [14,15,16]. In contrast, there are many cases reports in which achieving control of the cancer helped to control the thrombosis [17,18]. However, although there are no previous-reports in which the authors were able to control the thrombosis by successfully treating the underlying cancer, there is a report in which the physicians successfully controlled the thrombosis to some extent without providing anticancer therapy [19].
The use of oral warfarin was continued in this case, although it is unclear whether this medicine had an effect. Therefore, a similar level of control may have been obtained even if we had not used warfarin. Hence, there is room for discussion regarding whether it is necessary to continue anticoagulant therapy in cases in which anticancer therapy is successful and level of control of thrombosis is good.
Contributor Information
Hiroaki Masubuchi, Email: hmasubuc@gunma-u.ac.jp.
Toshitaka Maeno, Email: mutoyu03@gunma-u.ac.jp.
Megumi Uchida, Email: m22_omegumi@yahoo.co.jp.
Shunichi Kono, Email: contra.since2005@gmail.com.
Masafumi Suzuki, Email: masafumi.suzuki1229@gmail.com.
Masao Takemura, Email: takehope1111@yahoo.co.jp.
Aya Yamaguchi, Email: koity6_23dayo@yahoo.co.jp.
Koichi Yamaguchi, Email: ckpnt341@yahoo.co.jp.
Masahiko Kanbe, Email: laihg_rd@yahoo.co.jp.
Shinsuke Kitahara, Email: shinkita.s50@gmail.com.
Kenichiro Hara, Email: pourta5311@yahoo.co.jp.
Shiro Hara, Email: hara4600@gmail.com.
Nozomi Aoki, Email: veuveclicquot@jcom.home.ne.jp.
Tatsuo Suga, Email: sugat@gunma-u.ac.jp.
Masahiko Kurabayashi, Email: mkuraba@gunma-u.ac.jp.
References
- 1.Trousseau A. 2nd. The Sydenham Society; Paris, France: 1865. Phlegmasia alba dolens.Clinique Medicale de 1’Hotel-Dieu De Paris; pp. 654–712. [Google Scholar]
- 2.Uchiyama S., Terashi H., Shimizu Y. Antiphospholipid syndrome and Trousseau's syndrome. Jap J. Stroke. 2005;27:547–552. [Google Scholar]
- 3.Sutherland D.E., Weitz I.C., Liebman H.A. Thromboembolic complications of cancer: Epidemiology, pathogenesis, diagnosis, andtreatment. Am. J. Hematol. 2003;72:43–52. doi: 10.1002/ajh.10263. [DOI] [PubMed] [Google Scholar]
- 4.Umehara F., Nomoto M., Yanazume S. A case of cerebral infarction due to nonbacterial thrombotic endocarditis associated with ovarian cancer. Jap J. Stroke. 2006;28(2):306–312. [Google Scholar]
- 5.Brose K.M., Lee A.Y. Cancer-associated thrombosis:prevention and treatment. Curr. Oncol. 2008;15(Suupl 1):58–67. doi: 10.3747/co.2008.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szrensen H.T., Mellemkjaer L., Olsen J.H. Prognosis of cancers associated with venous thromboembolism. N. Engl. J. Med. 2000;343:1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 7.Kakkar A.K., LemQine N.R., Scully M.F. Tissue factor expression correlates with histological grade in human pancreatic cancer. Br. J. Surg. 1995;82:1101–1104. doi: 10.1002/bjs.1800820831. [DOI] [PubMed] [Google Scholar]
- 8.Lee A.Y., Levine M.N., Baker R.I. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N. Engl. J. Med. 2003;349(2):146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 9.Wahrenbrock M., Borsig L., Le D. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J. Clin. lnvest. 2003;112:853–862. doi: 10.1172/JCI18882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connolly S.J., Ezekowitz M.D., Yusuf S. RE-LY steering committee and investigator. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009;363:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 11.Patel M.R., Mahaffey K.W., Garg J. The ROCKET AF investigatoers. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 12.Granger C.B., Alexander J.H., McMurray J.J. ARISTOTLE committees and investigators. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 13.Giugliano R.P., Ruff C.T., Braunwald E. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 14.Kawakami N., Matuo S., Moriyama T. A case of Trousseau's syndrome with Recurrent Cerebral Infarction. Pract. Curr. 2007;17(6):741–746. [Google Scholar]
- 15.Igarashi N., Takahashi H., Yoshida S. Two cases of Trousseau Syndrome that occurred anatomically and involved repeated multifocal cerebral infarction. Jap J. Cancer Clin. 2009;55(7):531–535. [Google Scholar]
- 16.Kawaguchi S., Ishiguro A., Suzuki K. A case of unresectable advanced gastric cancer with Trousseau Syndrome. Jap J. Cancer Chemother. 2009;36(2):317–320. [PubMed] [Google Scholar]
- 17.Kato N., Tanaka T., Yamamoto Y. “Cancerous” cerenral infarction associated with overian cancer: A case of Trousseau Syndrome. Tokyo Jikeikai Med. J. 2009;24(4):153–158. [Google Scholar]
- 18.Kikuchi S., Sawa N., Nishiwaki T. Development of Trousseau syndrome in a patient awaiting surgery for a pancreatic body tumor: A case report. Jap. J. Stroke. 2011;33(1):119–122. [Google Scholar]
- 19.Isono T., Wada H., Kobayashi T. A case of Trousseau Syndrome caused by pancreatic cancer with liver metastasis during chemotherapy. Jap. J. Cancer Clincs. 2008;54(8):701–705. [Google Scholar]


