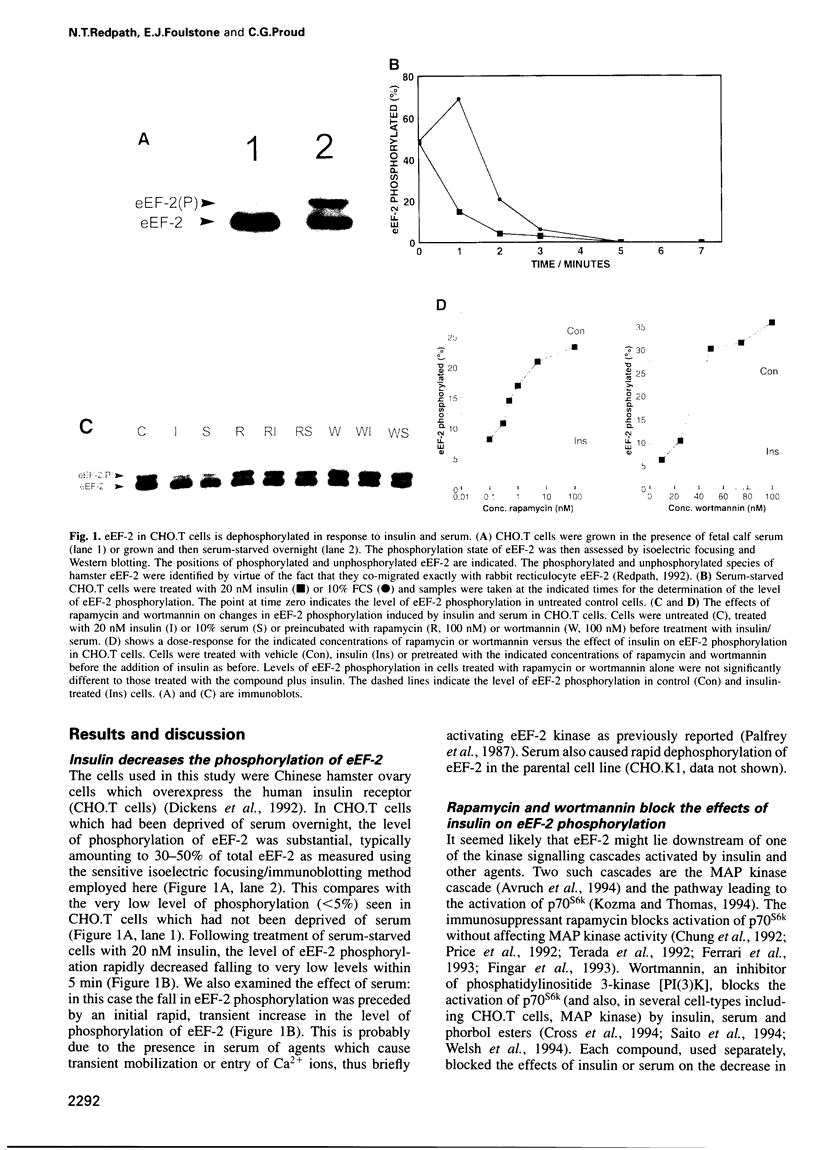
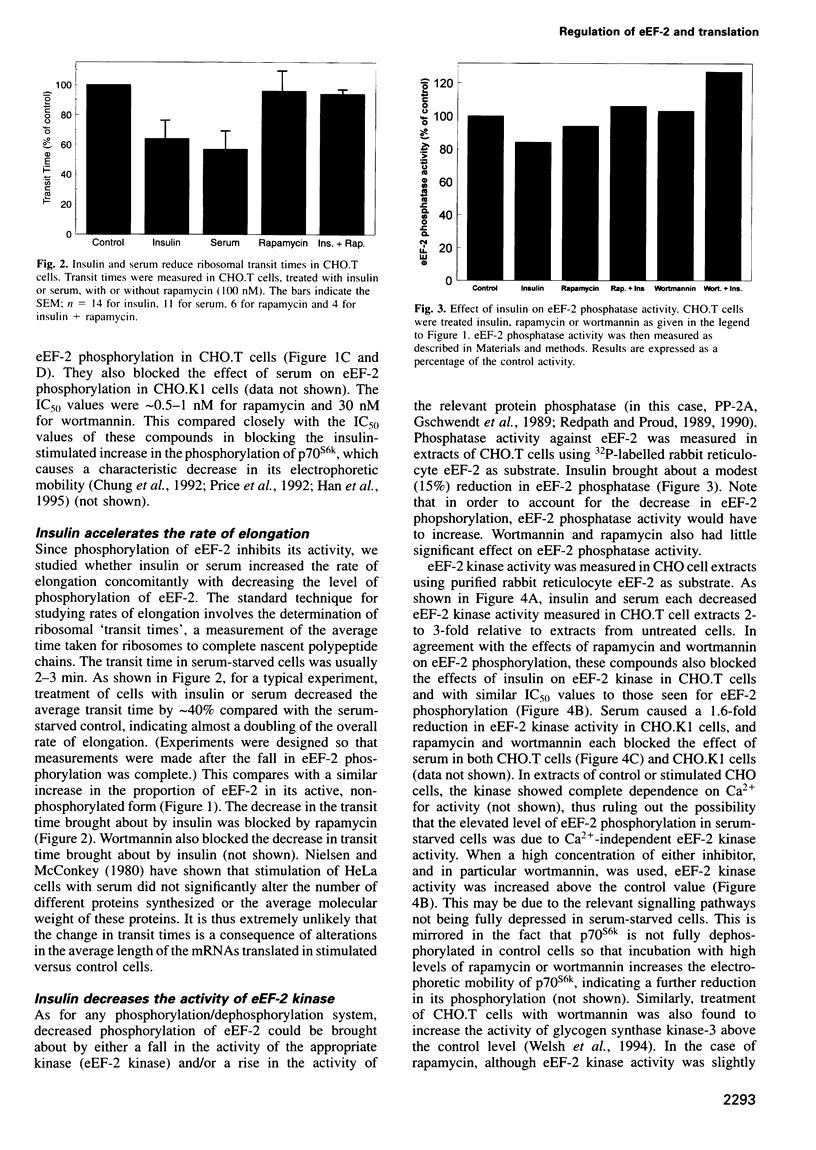
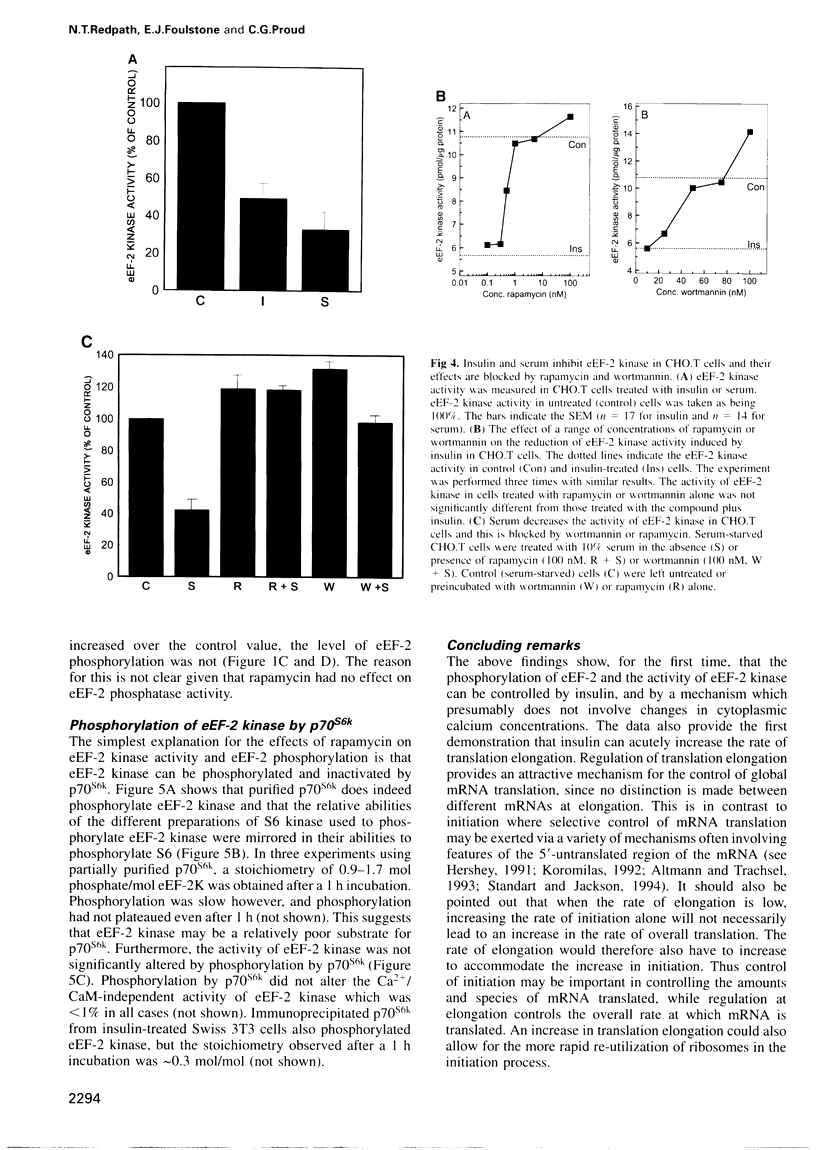
Abstract
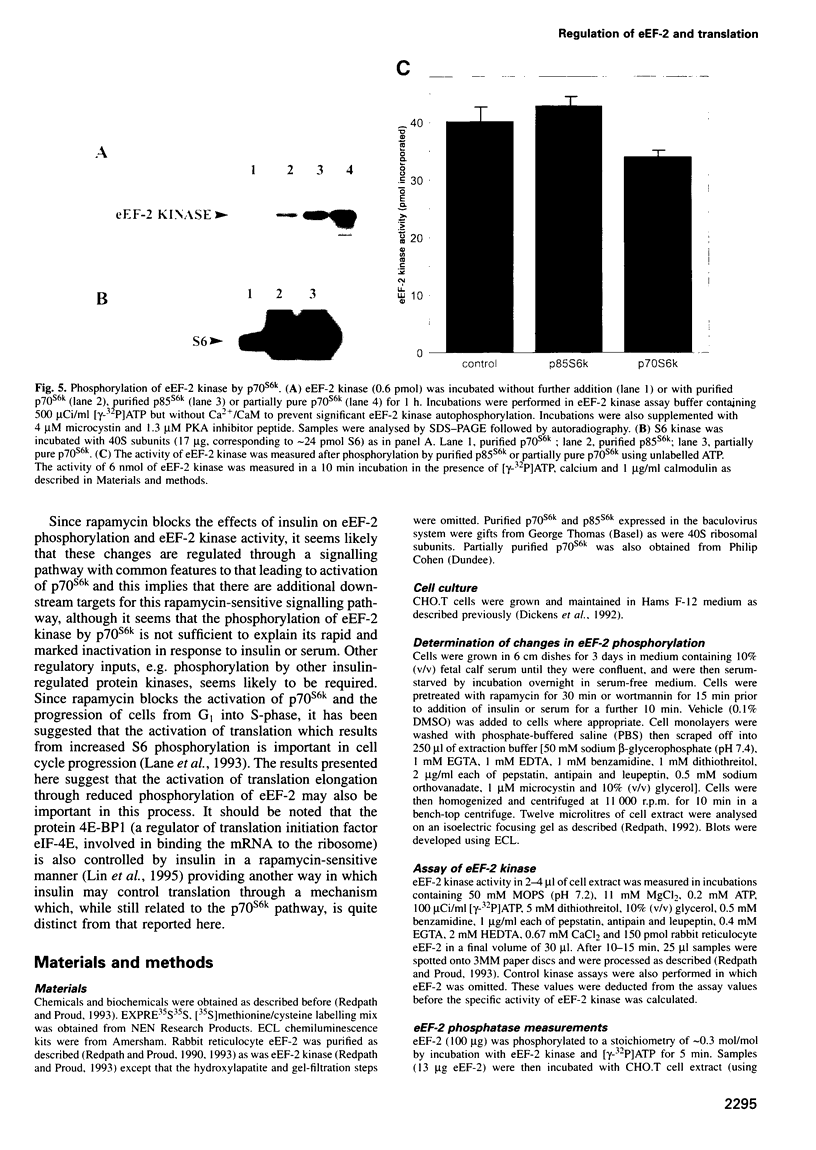
It is well established that insulin and serum stimulate gene expression at the level of mRNA translation in animal cells, and previous studies have mainly focused on the initiation process. Here we show that, in Chinese hamster ovary cells expressing the human insulin receptor, insulin causes decreased phosphorylation of elongation factor eEF-2 and that this is associated with stimulation of the rate of peptide-chain elongation. eEF-2 is phosphorylated by a very specific Ca 2+/calmodulin-dependent protein kinase (eEF-2 kinase) causing its complete inactivation. The decrease in eEF-2 phosphorylation induced by insulin reflects a fall in eEF-2 kinase activity. Rapamycin, a macrolide immunosuppressant which blocks the signalling pathway leading to the stimulation of the 70/85 kDa ribosomal protein S6 kinases, substantially blocks the activation of elongation, the fall in eEF-2 phosphorylation and the decrease in eEF-2 kinase activity, suggesting that p7O S6 kinase (p70s6k) and eEF-2 kinase may tie on a common signalling pathway. Wortmannin, an inhibitor of phosphatidylinositide-3-OH kinase, had similar effects. eEF-2 kinase was phosphorylated in vitro by purified p70s6k but this had no significant effect on the in vitro activity of eEF-2 kinase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann M., Trachsel H. Regulation of translation initiation and modulation of cellular physiology. Trends Biochem Sci. 1993 Nov;18(11):429–432. doi: 10.1016/0968-0004(93)90143-b. [DOI] [PubMed] [Google Scholar]
- Avruch J., Zhang X. F., Kyriakis J. M. Raf meets Ras: completing the framework of a signal transduction pathway. Trends Biochem Sci. 1994 Jul;19(7):279–283. doi: 10.1016/0968-0004(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Brandis J. W., Raff R. A. Elevation of protein synthesis is a complex response to fertilisation. Nature. 1979 Mar 29;278(5703):467–469. doi: 10.1038/278467a0. [DOI] [PubMed] [Google Scholar]
- Chung J., Kuo C. J., Crabtree G. R., Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992 Jun 26;69(7):1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- Cross D. A., Alessi D. R., Vandenheede J. R., McDowell H. E., Hundal H. S., Cohen P. The inhibition of glycogen synthase kinase-3 by insulin or insulin-like growth factor 1 in the rat skeletal muscle cell line L6 is blocked by wortmannin, but not by rapamycin: evidence that wortmannin blocks activation of the mitogen-activated protein kinase pathway in L6 cells between Ras and Raf. Biochem J. 1994 Oct 1;303(Pt 1):21–26. doi: 10.1042/bj3030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demolle D., Lecomte M., Boutherin-Falson O., Cragoe E. J., Jr, Nairn A. C., Boeynaems J. M. Amiloride analogs induce the phosphorylation of elongation factor-2 in vascular endothelial cells. Mol Pharmacol. 1990 Jun;37(6):827–832. [PubMed] [Google Scholar]
- Dickens M., Chin J. E., Roth R. A., Ellis L., Denton R. M., Tavaré J. M. Characterization of insulin-stimulated protein serine/threonine kinases in CHO cells expressing human insulin receptors with point and deletion mutations. Biochem J. 1992 Oct 1;287(Pt 1):201–209. doi: 10.1042/bj2870201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Pearson R. B., Siegmann M., Kozma S. C., Thomas G. The immunosuppressant rapamycin induces inactivation of p70s6k through dephosphorylation of a novel set of sites. J Biol Chem. 1993 Aug 5;268(22):16091–16094. [PubMed] [Google Scholar]
- Fingar D. C., Hausdorff S. F., Blenis J., Birnbaum M. J. Dissociation of pp70 ribosomal protein S6 kinase from insulin-stimulated glucose transport in 3T3-L1 adipocytes. J Biol Chem. 1993 Feb 5;268(4):3005–3008. [PubMed] [Google Scholar]
- Fischer I., Arfin S. M., Moldave K. Regulation of translation. Analysis of intermediary reactions in protein synthesis in exponentially growing and stationary phase Chinese hamster ovary cells in culture. Biochemistry. 1980 Apr 1;19(7):1417–1425. doi: 10.1021/bi00548a024. [DOI] [PubMed] [Google Scholar]
- Grove J. R., Banerjee P., Balasubramanyam A., Coffer P. J., Price D. J., Avruch J., Woodgett J. R. Cloning and expression of two human p70 S6 kinase polypeptides differing only at their amino termini. Mol Cell Biol. 1991 Nov;11(11):5541–5550. doi: 10.1128/mcb.11.11.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwendt M., Kittstein W., Mieskes G., Marks F. A type 2A protein phosphatase dephosphorylates the elongation factor 2 and is stimulated by the phorbol ester TPA in mouse epidermis in vivo. FEBS Lett. 1989 Nov 6;257(2):357–360. doi: 10.1016/0014-5793(89)81571-6. [DOI] [PubMed] [Google Scholar]
- Han J. W., Pearson R. B., Dennis P. B., Thomas G. Rapamycin, wortmannin, and the methylxanthine SQ20006 inactivate p70s6k by inducing dephosphorylation of the same subset of sites. J Biol Chem. 1995 Sep 8;270(36):21396–21403. doi: 10.1074/jbc.270.36.21396. [DOI] [PubMed] [Google Scholar]
- Hassell J. A., Engelhardt D. L. The regulation of protein synthesis in animal cells by serum factors. Biochemistry. 1976 Apr 6;15(7):1375–1381. doi: 10.1021/bi00652a004. [DOI] [PubMed] [Google Scholar]
- Hershey J. W. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- Hille M. B., Albers A. A. Efficiency of protein synthesis after fertilisation of sea urchin eggs. Nature. 1979 Mar 29;278(5703):469–471. doi: 10.1038/278469a0. [DOI] [PubMed] [Google Scholar]
- Hincke M. T., Nairn A. C. Phosphorylation of elongation factor 2 during Ca(2+)-mediated secretion from rat parotid acini. Biochem J. 1992 Mar 15;282(Pt 3):877–882. doi: 10.1042/bj2820877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball S. R., Vary T. C., Jefferson L. S. Regulation of protein synthesis by insulin. Annu Rev Physiol. 1994;56:321–348. doi: 10.1146/annurev.ph.56.030194.001541. [DOI] [PubMed] [Google Scholar]
- Koromilas A. E., Lazaris-Karatzas A., Sonenberg N. mRNAs containing extensive secondary structure in their 5' non-coding region translate efficiently in cells overexpressing initiation factor eIF-4E. EMBO J. 1992 Nov;11(11):4153–4158. doi: 10.1002/j.1460-2075.1992.tb05508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma S. C., Ferrari S., Thomas G. Unmasking a growth factor/oncogene-activated S6 phosphorylation cascade. Cell Signal. 1989;1(3):219–225. doi: 10.1016/0898-6568(89)90039-9. [DOI] [PubMed] [Google Scholar]
- Kozma S. C., Thomas G. p70s6k/p85s6k: mechanism of activation and role in mitogenesis. Semin Cancer Biol. 1994 Aug;5(4):255–260. [PubMed] [Google Scholar]
- Lane H. A., Fernandez A., Lamb N. J., Thomas G. p70s6k function is essential for G1 progression. Nature. 1993 May 13;363(6425):170–172. doi: 10.1038/363170a0. [DOI] [PubMed] [Google Scholar]
- Lin T. A., Kong X., Saltiel A. R., Blackshear P. J., Lawrence J. C., Jr Control of PHAS-I by insulin in 3T3-L1 adipocytes. Synthesis, degradation, and phosphorylation by a rapamycin-sensitive and mitogen-activated protein kinase-independent pathway. J Biol Chem. 1995 Aug 4;270(31):18531–18538. doi: 10.1074/jbc.270.31.18531. [DOI] [PubMed] [Google Scholar]
- Mitsui K., Brady M., Palfrey H. C., Nairn A. C. Purification and characterization of calmodulin-dependent protein kinase III from rabbit reticulocytes and rat pancreas. J Biol Chem. 1993 Jun 25;268(18):13422–13433. [PubMed] [Google Scholar]
- Nairn A. C., Palfrey H. C. Identification of the major Mr 100,000 substrate for calmodulin-dependent protein kinase III in mammalian cells as elongation factor-2. J Biol Chem. 1987 Dec 25;262(36):17299–17303. [PubMed] [Google Scholar]
- Nielsen P. J., McConkey E. H. Evidence for control of protein synthesis in HeLa cells via the elongation rate. J Cell Physiol. 1980 Sep;104(3):269–281. doi: 10.1002/jcp.1041040302. [DOI] [PubMed] [Google Scholar]
- Palfrey H. C., Nairn A. C., Muldoon L. L., Villereal M. L. Rapid activation of calmodulin-dependent protein kinase III in mitogen-stimulated human fibroblasts. Correlation with intracellular Ca2+ transients. J Biol Chem. 1987 Jul 15;262(20):9785–9792. [PubMed] [Google Scholar]
- Palfrey H. C. Presence in many mammalian tissues of an identical major cytosolic substrate (Mr 100 000) for calmodulin-dependent protein kinase. FEBS Lett. 1983 Jun 27;157(1):183–190. doi: 10.1016/0014-5793(83)81142-9. [DOI] [PubMed] [Google Scholar]
- Pause A., Belsham G. J., Gingras A. C., Donzé O., Lin T. A., Lawrence J. C., Jr, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5'-cap function. Nature. 1994 Oct 27;371(6500):762–767. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- Price D. J., Grove J. R., Calvo V., Avruch J., Bierer B. E. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science. 1992 Aug 14;257(5072):973–977. doi: 10.1126/science.1380182. [DOI] [PubMed] [Google Scholar]
- Proud C. G. Peptide-chain elongation in eukaryotes. Mol Biol Rep. 1994 May;19(3):161–170. doi: 10.1007/BF00986958. [DOI] [PubMed] [Google Scholar]
- Redpath N. T. High-resolution one-dimensional polyacrylamide gel isoelectric focusing of various forms of elongation factor-2. Anal Biochem. 1992 May 1;202(2):340–343. doi: 10.1016/0003-2697(92)90115-n. [DOI] [PubMed] [Google Scholar]
- Redpath N. T., Proud C. G. Activity of protein phosphatases against initiation factor-2 and elongation factor-2. Biochem J. 1990 Nov 15;272(1):175–180. doi: 10.1042/bj2720175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redpath N. T., Proud C. G. Molecular mechanisms in the control of translation by hormones and growth factors. Biochim Biophys Acta. 1994 Jan 13;1220(2):147–162. doi: 10.1016/0167-4889(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Redpath N. T., Proud C. G. Purification and phosphorylation of elongation factor-2 kinase from rabbit reticulocytes. Eur J Biochem. 1993 Mar 1;212(2):511–520. doi: 10.1111/j.1432-1033.1993.tb17688.x. [DOI] [PubMed] [Google Scholar]
- Redpath N. T., Proud C. G. The tumour promoter okadaic acid inhibits reticulocyte-lysate protein synthesis by increasing the net phosphorylation of elongation factor 2. Biochem J. 1989 Aug 15;262(1):69–75. doi: 10.1042/bj2620069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryazanov A. G., Natapov P. G., Shestakova E. A., Severin F. F., Spirin A. S. Phosphorylation of the elongation factor 2: the fifth Ca2+/calmodulin-dependent system of protein phosphorylation. Biochimie. 1988 May;70(5):619–626. doi: 10.1016/0300-9084(88)90245-3. [DOI] [PubMed] [Google Scholar]
- Ryazanov A. G., Rudkin B. B., Spirin A. S. Regulation of protein synthesis at the elongation stage. New insights into the control of gene expression in eukaryotes. FEBS Lett. 1991 Jul 22;285(2):170–175. doi: 10.1016/0014-5793(91)80798-8. [DOI] [PubMed] [Google Scholar]
- Ryazanov A. G., Shestakova E. A., Natapov P. G. Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature. 1988 Jul 14;334(6178):170–173. doi: 10.1038/334170a0. [DOI] [PubMed] [Google Scholar]
- Saito Y., Vandenheede J. R., Cohen P. The mechanism by which epidermal growth factor inhibits glycogen synthase kinase 3 in A431 cells. Biochem J. 1994 Oct 1;303(Pt 1):27–31. doi: 10.1042/bj3030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standart N., Jackson R. Translational regulation. Y the message is masked? Curr Biol. 1994 Oct 1;4(10):939–941. doi: 10.1016/s0960-9822(00)00212-8. [DOI] [PubMed] [Google Scholar]
- Terada N., Lucas J. J., Szepesi A., Franklin R. A., Takase K., Gelfand E. W. Rapamycin inhibits the phosphorylation of p70 S6 kinase in IL-2 and mitogen-activated human T cells. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1315–1321. doi: 10.1016/s0006-291x(05)81549-9. [DOI] [PubMed] [Google Scholar]
- Welsh G. I., Foulstone E. J., Young S. W., Tavaré J. M., Proud C. G. Wortmannin inhibits the effects of insulin and serum on the activities of glycogen synthase kinase-3 and mitogen-activated protein kinase. Biochem J. 1994 Oct 1;303(Pt 1):15–20. doi: 10.1042/bj3030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh G. I., Proud C. G. Glycogen synthase kinase-3 is rapidly inactivated in response to insulin and phosphorylates eukaryotic initiation factor eIF-2B. Biochem J. 1993 Sep 15;294(Pt 3):625–629. doi: 10.1042/bj2940625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh G. I., Proud C. G. Regulation of protein synthesis in Swiss 3T3 fibroblasts. Rapid activation of the guanine-nucleotide-exchange factor by insulin and growth factors. Biochem J. 1992 May 15;284(Pt 1):19–23. doi: 10.1042/bj2840019. [DOI] [PMC free article] [PubMed] [Google Scholar]