Abstract
NADPH oxidases (Nox) represent a family of hetero-oligomeric enzymes whose exclusive biological function is the generation of reactive oxygen species (ROS). Nox-derived ROS are essential modulators of signal transduction pathways that control key physiological activities such as cell growth, proliferation, migration, differentiation, and apoptosis, immune responses, and biochemical pathways. Enhanced formation of Nox-derived ROS, which is generally associated with the up-regulation of different Nox subtypes, has been established in various pathologies, namely cardiovascular diseases, diabetes, obesity, cancer, and neurodegeneration. The detrimental effects of Nox-derived ROS are related to alterations in cell signalling and/or direct irreversible oxidative damage of nucleic acids, proteins, carbohydrates, and lipids. Thus, understanding of transcriptional regulation mechanisms of Nox enzymes have been extensively investigated in an attempt to find ways to counteract the excessive formation of Nox-derived ROS in various pathological states. Despite the numerous existing data, the molecular pathways responsible for Nox up-regulation are not completely understood. This review article summarizes some of the recent advances and concepts related to the regulation of Nox expression in the vascular pathophysiology. It highlights the role of transcription factors and epigenetic mechanisms in this process. Identification of the signalling molecules involved in Nox up-regulation, which is associated with the onset and development of cardiovascular dysfunction may contribute to the development of novel strategies for the treatment of cardiovascular diseases.
Keywords: NADPH oxidase, Transcription factors, Epigenetics, Cardiovascular diseases
Graphical abstract
Highlights
-
•
Nox is a unique class of enzymes whose sole function is the generation of ROS.
-
•
Nox-derived ROS play a major role in cell physiology.
-
•
Enhanced expression and activation of Nox has been reported in numerous pathologies.
-
•
Nox expression is regulated via complex transcription factor-epigenetic mechanisms.
-
•
Understanding of Nox regulation is essential to counteract ROS-induced cell damage.
Introduction
Evidence from the last two decades in the field of redox biology have led to a profound change of the dogma that reactive oxygen species (ROS) are detrimental to cells and are predominantly produced as by-products of cellular metabolism and respiration. Since the discovery of vascular NADPH oxidase (Nox) in the late 90s, it has become the focus of continual and extensive research interest due to its exclusive function to produce ROS under normal physiological conditions. Yet, enhanced formation of Nox-derived ROS, which is generally associated with the up-regulation of its expression, has been reported in numerous pathologies such as cardiovascular diseases, cancer, diabetes, obesity, and neurodegenerative disorders. Thus, this activity is currently considered as key pathological trigger of oxidative stress-induced cellular deleterious effects [1–4]. Recently, the first class of Nox1 and Nox4 pharmacological inhibitors, GKT137831, received the approval for phase II clinical study for the treatment of diabetic nephropathy [5,6]. Similarly, beneficial effects of GKT137831 in attenuating oxidative stress-induced vascular injury were reported in experimental models of diabetes-accelerated atherosclerosis [7]. Thus, it has become rapidly evident that understanding of the molecular mechanisms implicated in the regulation of Nox expression and function represents a prerequisite to counteract ROS-induced cell damage and ultimately to prevent organ failure in a large number of pathologies.
Nox has been initially characterized in professional phagocytes, as burst enzyme, having a critical role in the killing the invading pathogens. Structurally, the phagocyte-type Nox contains a membrane-associated protein complex, known as cytochrome b558, comprising the gp91phox/Nox2 and p22phox components, and three cytosolic regulatory subunits (i.e., p40phox, p47phox, and p67phox). In resting cells the Nox complex is dissociated (inactive state) but is rapidly assembled into an active O2•--generating oxidase following the exposure of the phagocytic cells to microbes. Two functionally-related regulatory proteins have been described in non-phagocytes, including Nox organizer 1 (Noxo1) and Nox activator 1 (Noxa1). Later, after its functional characterization in the immune cells, several structurally related but functionally distinct Nox subtypes were identified in numerous non-phagocytic cells including vascular cells. In addition to the archetypical Nox2 phagocyte-type Nox, the oxidase family also comprises Nox1, Nox2, Nox3, Nox4, Nox5, Duox1, and Duox2 isoforms; each of these having a specific function and a distinct pattern of intracellular compartmentalization and tissue distribution [8].
Although it has been extensively demonstrated that the expression of various Nox proteins and ROS production are upregulated by pro-inflammatory cytokines, growth factors, hormones, vasoactive agents, metabolic intermediates, modified lipids and lipoproteins in different cardiovascular cells [9–12], the molecular mechanisms involved in these processes have remained elusive. This review briefly summarizes and discusses some of the latest concepts on the regulation of Nox expression in vascular pathophysiology, emphasizing the role of transcription factors and epigenetic mechanisms.
Multiple ways of Nox activation have been described in various cell types under normal and pathological states. These include the phosphorylation of cytosolic regulatory subunits by protein kinase C (PKC), protein kinase A (PKA), phosphatidylinositol-3-kinase (PI3K), mitogen-activated protein kinases (MAPK), and non-receptor associated protein kinases (e.g., JAK and SRC) [13–18]. Also, protein–protein interactions among Nox and members of the thioredoxin family and transient oscillations in intracellular concentration of various ions may trigger the activation of Nox [19–21]. Hitherto, enhanced level of NADPH oxidase expression has been increasingly implicated as essential mechanisms responsible for excessive and sustained release of ROS in the non-phagocytic cells.
Regulation of Nox enzymes by transcription factors and nuclear receptors
Accumulating evidence suggests that the extent of Nox-driven ROS formation is closely dependent on the level of its expression level [22]. Thus, in addition to direct activation of Nox by phosphorylation-dependent pathways, other mechanisms linked to the regulation of Nox expression have been described. These may include a large spectrum of transcription factors, molecules influencing mRNA stability, and various epigenetic processes such as DNA methylation, post-translational modification of histones, and non-coding RNA [23].
Different Nox enzymes are constitutively expressed in vascular cells (e.g., endothelial cells, smooth muscle cells, fibroblasts, and pericytes), cardiac myocytes, and in circulating and tissue-resident immune cells (e.g., monocytes, macrophages, dendritic cells, and mast cells). Whereas low level of Nox expression and activity was detected under normal physiological conditions, the up-regulation of the various Nox subtypes has been associated with innate and adaptive immune reactions, as well as in vascular wall cells response to injury underlying atherosclerosis, diabetes, obesity, hypertension, and hypoxia [9,24–26] (Fig. 1). Therefore, understanding the molecular transcriptional machinery that controls Nox expression may provide important clues about the role of redox signalling, and in particular of Nox enzymes, in mediating key biological activities in the immune and cardiovascular systems which are often interconnected.
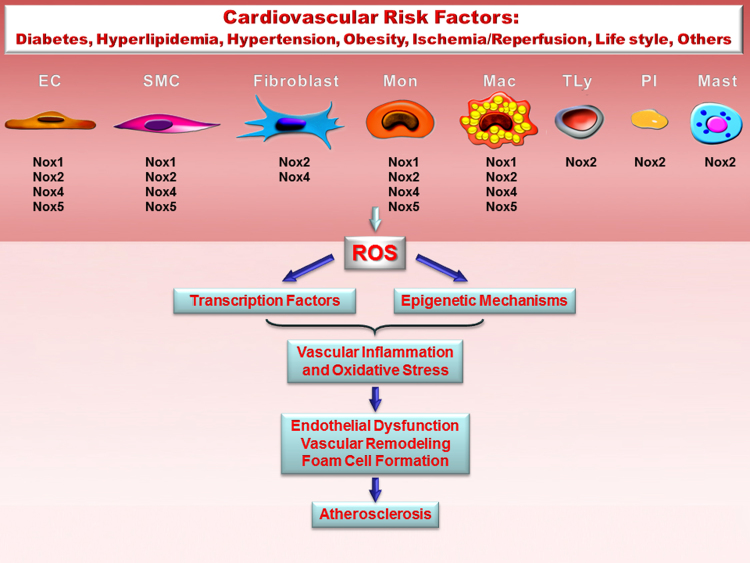
Fig. 1.
Role of Nox-derived ROS signalling in atherosclerosis. Nox are important sources of ROS in the vascular cells and in immune cells interacting with the blood vessels. The diagram shows the distinct expression of Nox subtypes in the cells involved in atheroma formation: endothelial cells (EC), smooth muscle cells (SMC), adventitial fibroblasts, monocytes (Mon), macrophages and foam cells (Mac), T lymphocytes (TLy), platelets (Pl), and mast cells (Mast). Activation of specific signalling pathways by cardiovascular risk factors determines up-regulation of Nox and the ensuing ROS production. Nox-derived ROS play an important role in the regulation of signal transduction and gene expression by activating redox-sensitive transcription factors and epigenetic constituents. Persistent Nox activation induces oxidative stress, a major contributor to atherosclerotic lesions initiation and development.
In previous studies others and we have shown the presence of typical TATA boxes within the core promoter regions of several Nox subunits. TATA cis-acting regulatory elements mediate the recruitment of RNA polymerase II following the formation of a multi-component protein complex formed of various transcription factors such as TFIID, TFIIA, TFIIB, TFIIE, TFIIF, and TFIIH. The formation of the RNA polymerase II – TFII-type transcription factors complex, also known as basal transcriptional complex, is responsible for the constitutive expression of members of the Nox family in different cell types. Besides TATA binding proteins, several other DNA elements, such as CCAC and GAGA boxes that may contribute to the basal promoter activity, were predicted by in silico analysis within the proximal promoters of the human Nox genes [27].
Nox-derived ROS play an important role in the innate immune response, which is the first to react against invading pathogens, but is also crucially involved in regulating the adaptive arm of the immune response. For example, defects in the genes encoding for Nox2/gp91phox (CYBB), p22phox (CYBA), p47phox (NCF1) or p67phox (NCF2) subunits cause a rare inherited immune disorder called chronic granulomatous disease characterized by impaired defense mechanisms against infection. Nox is rapidly activated and its expression is enhanced in professional phagocytes subsequent to microbial infections [28]. Consequently, numerous studies have focused on the role of specific transcription factors that could mediate these effects.
In addition, Nox-derived ROS have been implicated in the regulation of critical signalling pathways associated with the immunologic synapse, controlling the adaptive immune responses in relation to antigen processing and presentation by professional antigen presenting cells. Thus, the transcriptional regulatory mechanisms of the various components comprising the prototypical Nox (i.e., Nox2/gp91phox, p22phox, p47phox, and p67phox) have been extensively investigated in lymphocytes, dendritic cells, and myelomonocytic cells such as monocytes, monocyte-derived macrophages and polymorphonuclear leukocytes (e.g., neutrophils) in an attempt to decipher the link between the expression and function of Nox in immune cells [29–32]. Among the different transcription factors that regulate the expression of Nox2/gp91phox, p22phox, p47phox, and p67phox subunits, PU.1, Elf-1, interferon regulatory factor-1 (IRF-1), and interferon consensus sequence binding (ICSBP) were the most investigated [33,34]. Besides these positive-acting transcriptional regulatory mechanisms, several negative regulators of Nox (i.e., p67phox subunit) expression have been demonstrated in myeloid cells, including HoxA1 that acts in concert with histone deacetylase 2 (HDAC2) [35]. PU.1 transcription factor interacts with a specific purine-rich DNA element in the promoter and enhancer of target genes hence influencing key biological processes in the immune system such as differentiation and activation of macrophages, and maturation of B-cells [36]. Transcriptional regulation of interferon (IFN)-inducible genes mediating the anti-viral and anti-bacterial immune response require the activation of IRF-1 and ICSBP binding proteins that up-regulate the levels of a number of molecules linked to anti-viral (e.g., IFNα/β), antibacterial defense (e.g., inducible NO synthase), as well as anti-proliferative action, and DNA damage/repair responses [37,38].
In addition to Nox, these transcription factors also regulate important genes associated with differentiation, proliferation, and migration of immune cells, and control the expression of a plethora of pro-inflammatory and immune factors such as cytokines, chemokines, growth factors, immunoglobulins and immunoglobulin receptors, and macrophage-specific scavenger receptors [36]. These findings suggest a strong and direct correlation between Nox up-regulation and inflammatory/immune reactions.
Several transcription factors have been identified as critical regulators of pro-inflammatory reactions in the vasculature including nuclear factor kB (NF-kB), activator protein 1 (AP-1), and members of the signal transducer and activator of transcription (STAT) and CCAAT-enhancer binding proteins (C/EBP). Activated NF-kB, AP-1, and STAT correlated with enhanced expression of Nox were detected within atherosclerotic lesions and in the vascular wall of diabetic and hypertensive patients as well as in various experimental animal models [39–42].
NF-kB, AP-1, and STATs are master regulators of numerous genes linked to vascular inflammation and remodelling, the differentiation of circulating immune cells and resident vascular cells. Several redox-sensitive pathways, including Nox-derived ROS, have been demonstrated to directly or indirectly affect the activation of these transcription factors in various cell types following the exposure to cytokines, chemokines, growth factors, vasoactive agents, and modified lipid and lipoproteins [43]. Hence, understanding the link between pro-inflammatory and redox signalling pathways attracts continual interest and is a matter debate in the field of cardiovascular biology and medicine.
The implication of NF-kB in the regulation of Nox transcription was initially demonstrated in murine macrophages [44]. In these cells, interferon γ (IFNγ)/lipopolysaccharide (LPS)-induced gp91phox (Nox2) expression was found to be mediated by activated NF-kB through direct transcription factor-gene promoter interaction mechanisms. In addition, the expression levels of the cytosolic regulatory component p47phox and of the essential subunit p22phox were mediated by NF-kB-related pathways in response to pro-inflammatory conditions. A similar pattern of NF-kB-dependent transcriptional mechanisms of phagocyte-type Nox regulation was further demonstrated in tumor necrosis factor α (TNFα)-treated human monocytes [45]. Previously, we have found several highly conserved NF-kB binding sites within the promoter regions of the human genes coding for Nox1, Nox4, and p22phox subunits, and that NF-kB inhibition reduced the IFNγ/TNFα-upregulated Nox activity and expression in human aortic smooth muscle cells (SMCs) [46,47]. In contrast, despite the presence of several NF-kB sites within the human Nox5 gene promoter, we could not identify direct chromatin interactions. Nonetheless, the fact that Nox5 gene and protein expression levels were significantly reduced in IFNγ-treated SMCs, suggests the existence of an indirect transcriptional mechanism in the regulation of Nox5 expression.
Activation of AP-1 transcription factor has also been linked to vascular response to injury. AP-1 is phosphorylated via multiple mechanisms involving members of the mitogen-activated protein kinase (MAPK) family such as extracellular signal-regulated protein kinase (ERK)1/2, c-Jun amino terminal kinase (JNK), p38 MAPK, and exerts its pro-inflammatory, hypertrophic and hyperplastic effects by activating various targets genes [48,49]. Based on the fact that proinflammatory stimuli activate both AP-1 and Nox, it has been hypothesized that in vascular diseases, overproduction of Nox-derived ROS and AP-1 activation are interrelated. We have demonstrated before that AP-1 physically interacts with the promoter of the human CYBA gene that codes for p22phox essential subunit in angiotensin II (Ang II)/TNFα-stimulated human aortic SMCs. Pharmacological inhibition of various MAPK or direct blockade of AP-1-dependent transcriptional responses by decoy oligodeoxynucleotides (ODN) abolished the Ang II/TNFα-induced Nox1, Nox4, p22phox, p47phox, and p67phox mRNA expression levels and ROS formation in human vascular SMCs [50]. Direct DNA-AP-1 interaction was detected in the promoter of human Nox5 gene in IFNγ-exposed vascular SMCs [51]. A similar AP-1-dependent transcriptional regulatory mechanism has been indicated for the rat Nox1 gene [52]. These data demonstrate that AP-1 is an important regulator of Nox expression and function in various pathological states.
Janus kinase (JAK)/STAT signalling pathway plays a major role in mediating pro-inflammatory and immune responses. In cardiovascular diseases, members of the STAT transcription factor family regulate important genes linked to vascular inflammation. STAT activation is an essential pathogenic mechanism leading to SMC hypertrophy and hyperplasia. We have previously reported that IFNγ-activated JAK regulates Nox expression and function in human aortic SMCs. In addition, STAT1 and STAT3 physically interacted with the promoter of human Nox1, Nox4, and Nox5 genes via highly conserved gamma activated sequences (GAS). Moreover, transient overexpression of STAT1/STAT3 led to a marked up-regulation of the luciferase levels under the control of the promoters of human CYBA (coding for p22phox), NCF1 (coding for p47phox), and NCF2 (coding for p67phox) genes, whereas the decoy ODN-based blockade of STAT1 or STAT3 activities reduced their transcriptional activation [53]. Moreover, pharmacological inhibition of JAK2 by tyrphostin AG490 reduced the Nox activity and expression of Nox1, Nox2, and Nox4 in the aorta of hypercholesterolemic ApoE-deficient mice, a condition that was associated with a significant reduction of atherosclerotic plaque formation [17]. These results clearly support the implication of JAK/STAT signalling in regulation of Nox and the ensuing ROS production in the vascular cells exposed to pro-inflammatory conditions.
Evidence has accumulated suggesting that cytokine-induced activation of non-STAT signalling pathways such as CCAAT/enhancer-binding proteins (C/EBP), regulate important aspects of vascular dysfunction in atherosclerosis [54,55]. C/EBP transcription factors belong to the basic-leucine zipper (bZip) transcription factor family and regulate the expression of a number of genes related to cellular proliferation and differentiation, immune and inflammatory responses, such as cytokines, chemokines, acute phase proteins and immunoglobulins [56,57]. In a previous study we have demonstrated that high glucose-induced nuclear translocation and activation of C/EBPα, C/EBPβ, and C/EBPδ is mediated by activated MAPK in human endothelial cells (ECs) [58]. Dose-dependent increase in C/EBPα, C/EBPβ, and C/EBPδ activation and protein expression levels was detected in human aortic SMCs exposed to IFNγ. These data indicate that the activation of C/EBP transcription factors correlates with the severity of the inflammatory stress in these cells. In addition, we have found that C/EBPα, C/EBPβ, and C/EBPδ regulate Nox1, Nox4, and Nox5 by direct transcriptional mechanisms involving DNA-transcription factor interactions [59]. These data are in good agreement with the former findings about the implication of C/EBPδ in mediating the overactivity of CYBA gene promoter in hypertensive subjects carrying the GG genotype of the CYBA – 930A/G polymorphism [60]. Besides human Nox, the up-regulation of Nox1 by LPS has been shown to be mediated by C/EBPβ and C/EBPδ in mouse macrophages [61].
Besides NF-kB, AP-1, STAT, and C/EBP, other pro-inflammatory and vascular remodelling-promoting transcription factors, namely activating transcription factor-1 (ATF-1), Ets-1, E2F, have been implicated in the regulation of vascular Nox [62–64]. Based on the fact that NF-kB, AP-1, and STAT transcription factors are redox-sensitive, the existence of a positive feed-back loop whereby Nox-derived ROS contribute to self-upregulation should be considered. Taken together, one can conclude that multiple alternative pro-inflammatory transcription factors converge to Nox regulation in cardiovascular cells. Yet, understanding the precise biological significance of the apparent redundant signalling pathways requires further investigation.
Other than pro-inflammatory transcription factors, several negative regulators of Nox expression have been demonstrated, such as members of the peroxisome proliferator-activated receptor (PPAR) family, which comprises 3 major isoforms, namely, PPARα, PPARβ/δ, and PPARγ. These receptors function as ligand-activated transcription factors following their dimerization with the retinoid X receptor (RXR). PPARs control the expression of a large number of genes that are involved in energy homeostasis and fatty acid and glucose metabolism [65]. Compelling evidence exist that selective activation of the various PPAR isotypes by synthetic agonists negatively regulate Nox expression and activity, and reduce inflammation in a number of clinical and experimental models of cardiovascular diseases [66]. Nevertheless, the precise molecular mechanisms supporting the antioxidant and anti-inflammatory effects of PPARα, PPARβ/δ, and PPARγ agonists are not entirely clear [67,68]. Conversely, it was shown that genetic ablation of PPARα could abolish hypertension and attenuate atherosclerotic plaque development in mice [69]. An interesting up-regulatory mechanism of Nox expression and activity by PPARα agonists has been identified in human and mouse macrophages, and a significant reduction in Nox activity and expression was detected in macrophages of PPARα-deficient mice. These reports indicate that Nox-derived ROS may elicit anti-inflammatory activities by inducing the formation of endogenous PPARα ligands, such as lipid peroxidation products that are generated following degradation of oxidized low-density lipoprotein [70]. Activation of Nox by PRARα agonists was demonstrated in mouse embryonic stem cells and was implicated in the process of cardiomyogenesis [71]. These data show the need to delineate the relationship among PPARs and Nox and the actual function of PPARs in the vasculature [72]. In addition, it is not known whether Nox subtypes are direct targets of PPARs or whether their expression is indirectly affected. Several lines of evidence support the concept that activation of PPARs down-regulate the expression levels and also negatively interfere by direct protein–protein interactions in the activation of several pro-inflammatory transcription factors such as NF-kB, AP-1, and STAT [73]. Based on the fact that NF-kB, AP-1, and STAT1/3 are important regulators of Nox it can be hypothesized that the effects of PPAR agonists on Nox expression and activity in vascular pathologies are partially mediated by such negative regulatory interactions.
Although numerous data related to the role of synthetic PPAR agonizts exist, less is known about the nature of endogenous ligands that mediate these processes. It has been suggested that oxidative derivatives of the fatty acids might serve as such ligands. Emerging evidence indicate that at low and physiological levels lipid peroxidation products of polyunsaturated fatty acids (PUFAs), namely 4-hydroxy-2E-hexenal (4-HHE), 4-hydroxy-2E-nonenal (4-HNE), and 4-hydroxy-2E,6Z-dodecadienal (4-HDDE), function as natural endogenous activators for various PPAR isotypes [74–76]. We have recently found that high glucose induced the synthesis of lipid peroxidation-based endogenous ligands for PPARα and PRARβ/δ in human aortic SMCs [77]. Moreover, we have demonstrated that high glucose-increased expression and function of Nox is mediated by 4-HNE-activated PPARα and PPARβ/δ. Interestingly, in silico analysis of the human Nox1, Nox4, and Nox5 gene proximal promoters indicated the absence of typical PPAR elements (PPRE) suggesting that PPARα and PPARβ/δ regulate Nox expression via indirect transcriptional mechanisms [77]. Of particular importance is that all PPARs could regulate the expression of the target genes by interacting with an intermediate transcription factor such as Sp1 [78,79]. In good agreement with these observations are our previous studies on the ability of Sp1 to form complexes with the promoter of Nox5 gene in human vascular SMCs exposed to pro-inflammatory conditions, whereas several highly conserved Sp1 elements were identified by in silico analysis in the promoters of human Nox1 and Nox4 genes [51,77]. Our data demonstrate the existence of a novel “lipid peroxidation products–PPARs–Nox axis” as an alternative mechanism of Nox regulation in diabetes. Also, this study highlights a novel redox sensing function of the PPAR family in vascular cells in diabetes. A schematic conceptual depiction of the crosstalk among pro-inflammatory transcription factors and PPARs converging to Nox regulation is presented in Fig. 2.
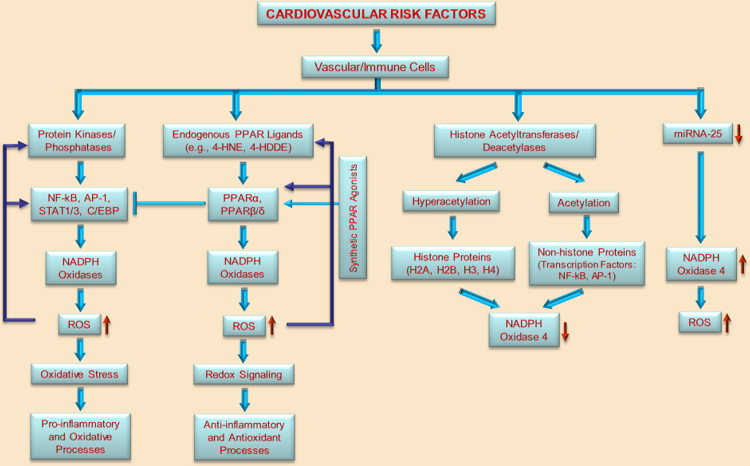
Fig. 2.
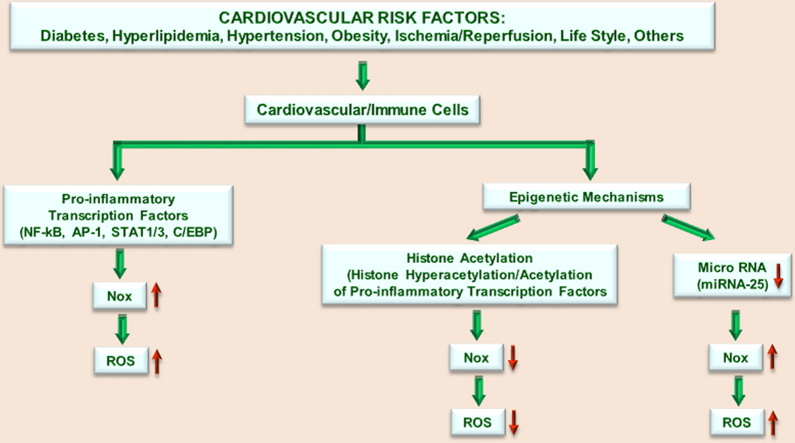
Concept diagram depicting the potential transcriptional regulation of Nox by transcription factors and epigenetic mechanisms in vascular/immune cells in response to cardiovascular risk factors. Activation of specific protein kinases/phosphatases leads to the activation of specific pro-inflammatory transcription factors (e.g., NF-kB, AP-1, STAT1/3, and C/EBP) that regulate the expression of various Nox subtypes by direct and indirect mechanisms. Enhanced Nox-derived ROS formation induces oxidative stress that triggers pro-inflammatory and oxidative processes in the vasculature. Alternative to the classical pro-inflammatory pathway of Nox activation, various lipid peroxidation products (e.g., 4-HNE, 4-HDDE) generated via enzymatic or non-enzymatic mechanisms may function as natural endogenous ligands for PPARs. Activated PPARα or PPARβ/δ act as negative regulators of pro-inflammatory pathways by interfering with NF-kB, AP-1 or STAT1/3 signalling. PPAR-mediated Nox up-regulation and ROS formation contribute to the generation of lipid peroxidation products with PPARα or PPARβ/δ-activating functions. Activation of PPARs by synthetic agonists may compensate the exacerbated pro-inflammatory and oxidative reactions in response to vascular insults. The diagram illustrates that several epigenetic mechanisms namely, histone acetylation (hyperacetylation of nucleosomal histones or acetylation of pro-inflammatory transcription factors) and micro RNA (i.e., down-regulation of miRNA-25) differentially regulate Nox4 expression and activity.
Hypoxia represents a key major pathological event leading to structural and functional changes in the cardiovascular system. Up-regulation of Nox expression and activity under hypoxic condition has been observed in various experimental settings, both in vitro and in vivo [80]. Hypoxia-induced transcriptional responses are mediated by a specific family of transcription factors named hypoxia-inducible factors (HIF-1α, HIF-2α, and HIF-3α) whose expression and function is tightly regulated by the level of molecular oxygen. Moreover, the expression of various HIF isoforms, such as HIF-1, has been reported to be up-regulated by ROS, possible generated by activated Nox, and oxidative stress-activated pro-inflammatory transcription factors (e.g., NF-kB) [81,82]. Among the various Nox subtypes, the transcription of Nox4 has been shown to be directly regulated by HIF-1α in pulmonary artery SMCs under hypoxic conditions [83]. Similarly, HIF-1α-induced Nox2 transcription has been indicated as an important mechanism of angiogenesis triggered by Nox-derived ROS [84]. Thus, the induction of Nox expression by HIF-1α represents an important compensatory feed-back mechanism that maintains the physiologic level of ROS in cells after prolonged and intermittent hypoxia.
Epigenetic regulation of Nox enzymes
Recent evidence indicates that in addition to transcription factors and their up-stream regulators (i.e., receptors, protein kinases/phosphatases); dysregulation of epigenetic mechanisms plays a major role in the pathoetiology of cardiovascular diseases [85–89]. In particular, it has been demonstrated that epigenetic pathways are implicated in the regulation of Nox expression and function in various cell types [90]. Three major epigenetic systems exist, namely DNA methylation, posttranslational modification of nucleosomal histones, and non-coding RNA [23].
DNA methylation of cytosine residues at the C5 position by DNA methyltransferases represents the major mechanism of gene silencing in the genome. Thus, aberrant methylation of the CpG islands/shores and even CpG sites in the promoters/enhancers of target genes may repress gene expression. The expression of protein-encoding transcripts for Nox1, Nox2, Nox4, and Nox5 subtypes has been demonstrated in all vascular cells by means of different mRNA expression assays. Hitherto, the direct implication of DNA methylation in the regulation of Nox subtypes expression in the vascular cells has not been demonstrated yet. Presumably, the induction of several Nox subtypes under certain pathological states is mediated by transcription-dependent mechanisms. Still, the silencing of Duox1 and Duox2 gene expression by hypermethylation of the CpG islands present within the promoter regions of both genes was demonstrated in human lung cancer cells [91].
Post-translational modifications of nucleosomal core histones (H2A, H2B, H3, and H4) at conserved lysine residues located on the NH2-terminus regions are catalyzed by specialized enzymes and include acetylation, methylation, phosphorylation, and SUMOylation. Changes in chromatin conformation due to post-translational modification of histones modulate the accessibility of transcription factors to their cognate DNA elements thus affecting gene expression. As a general principle, specific histone modifications induce the transition from a transcriptional silent chromatin (heterochromatin) to a transcriptional active chromatin (euchromatin). Yet, multiple histone markers have been related to gene expression and repression. Consequently, histone modifications occurring within the enhancer/promoter region can induce or repress the expression of the target gene [92,93].
Histone acetylation represents one of the major epigenetic mechanisms regulating gene expression, and it generally triggers transcriptional activation. An overall increase in cellular histone acetylation was demonstrated in several models of cardiovascular disorders, including atherosclerosis, hypertension, coronary heart disease, cardiomyopathy, and heart failure [94–99]. The histone acetylation state is regulated by two groups of specialized epigenetic enzymes, namely histone acetyltransferases (HAT) and histone deacetylases (HDAC). Thus far two types of HAT have been described, namely type A (i.e., p300/CBP, GNAT, MYST, Basal TF, and NRCF) and type B (HAT1, HAT2, HAT4, HatB3.1, and Rtt109). The HDAC family is divided into four major classes, specifically zinc-dependent class I (HDAC-1, -2, -3, and -8), zinc-dependent class II (HDAC-4, -5, -6, -7, -9, and -10), NAD(+)-dependent class III (SIRT-1 to -7), and zinc-dependent class IV (HDAC11). Members of the HAT and HDAC family display a specific pattern of cellular expression and compartmentalization and are responsible for precise biological activities. Most isotypes are located within the nucleus whereas others elicit their function in the cytoplasm. Evidence exist that selective members of either HAT and HDAC can be imported into the nucleus from the cytoplasm and vice versa. Of particular importance is that some HAT/HDAC isoforms can act on non-histone proteins such as transcription factors and transcriptional co-activators/repressors thereby affecting their function [99].
Pharmacological inhibitors of HDAC class I and II have emerged as important alternative anti-cancer agents (clinical trials phase 1–3) [100–102]. Interestingly, it has been demonstrated that HDAC inhibitors efficiently reduce neointima hyperplasia and prevent ischemia/reperfusion injury in the failing hearts [103–105]. Thus, member of the HDAC family may be important therapeutic targets in various cardiovascular pathologies. Interestingly, it has been shown that pharmacological inhibition of HDAC reduces the transcriptional activity and expression of Nox4 in human ECs [106]. Based on the fact that histone acetylation promotes transcriptional activation of the genes, these data put into a new light the role of histone acetylation in the regulation of gene expression. The authors elegantly showed that increased acetylation of histones following the exposure of the cells to diverse HDAC inhibitors (e.g., scriptaid, suberoylanilide hydroxamic acid, and trichostatin A) prevented the binding of AP-1 transcription factor and RNA polymerase 2A to the promoter of the human Nox4 gene. Similar findings were reported in human pulmonary ECs, in which the gene and protein expression levels of Nox4 were significantly reduced by HDAC class I inhibitors [107]. Besides histone hyperacetylation, it has been demonstrated that acetylation of non-histone proteins such as transcription factors (e.g., NF-kB, AP-1) and transcriptional co-activators/repressors [108,109] may determine either transcriptional activation or repression of the target genes. Reportedly, activation of SIRT1, a class IV HDAC, displayed anti-aging and anti-oxidant cardiovascular effects partially by down-regulation of Nox-derived ROS production [109,110]. Interestingly, it has been indicated that valproic acid, a potential HADC inhibitor, elicits pro-oxidant effects in the cancer cells [111]. It is yet to be investigated to what extent the dysregulation of histone acetylation influences the expression and function of Nox subtypes in other vascular cells or immune cells interacting with the blood vessels. Nevertheless, evidence is accumulating that Nox-derived ROS play a major role in histone modification and chromatin conformational changes, thus influencing key biological activities and pathological manifestations [112–115].
Non-coding RNAs are important regulators of gene expression. MicroRNAs (miRNAs) are a family of endogenous short (22–25 nt), non-coding RNAs that negatively control gene expression at the post-transcriptional level by binding to specific sequences located within the 3′UTR of target mRNAs. Several miRNAs (e.g., miRNA-21, miRNA-210, miRNA-34a, and miRNA-146a/b) have been shown to be expressed differentially in the stable plaque versus unstable plaque. In addition, it was shown that specific miRNA expression patterns may predict long-termed cardiovascular events and several miRNA-dependent mechanisms are directly linked to the pathophysiology of cardiovascular diseases [116]. Yet, the precise role and the associated molecular mechanisms of Nox regulation by miRNAs are scantly elucidated. Based on the fact that miRNA negatively regulate the gene expression by post-transcriptional mechanisms, it has been suggested that Nox expression is tightly regulated by several miRNAs under physiological conditions. In contrast, down-regulation or repression of particular Nox-specific miRNAs may lead to the up-regulation of oxidase complex in various pathological states. In the line with hypothesis, it has been demonstrated that miRNA-25 directly targets the 3′UTR of the human Nox4 gene. Down-regulation of miRNA-25 expression as observed under various pathological conditions (diabetes, hypercholesterolemia) has been implicated as important post-transcriptional regulatory mechanism leading to Nox4 up-regulation and consequent ROS production [117,118]. Besides direct post-transcriptional regulation of Nox by miRNAs, emerging evidence indicate that ROS, possibly generated by activated Nox regulate the expression of several miRNAs thus contributing to the maintenance of vascular homeostasis or controlling key mechanisms in various pathologies [119].
Conclusions
Nox expression and function is regulated via multiple mechanisms. The data summarized in this review attest to a complex interplay among transcription factors, co-activators/-repressors, nuclear receptors, and epigenetic mechanisms converge to Nox up-regulation in several cardiovascular disorders. Thus, understanding mechanisms and revealing the signalling molecules responsible for the increased expression and activation of Nox that is associated with the onset and development of cardiovascular dysfunction may contribute to the prevention and treatment of cardiovascular diseases.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Acknowledgments
We acknowledge the skilful assistance of Ms. Marilena Daju in figure preparation. This work was supported by the European Foundation for the Study of New Horizons Grant to A. Manea in collaboration with S. Sasson, Romanian National Authority for Scientific Research (CNCS – UEFISCDI, project numbers PN-II-ID-PCE-2011-3-0548, PN-II-RU-TE-2011-3-0142, PNII-TE 65/2010). G. Manda, S. Sasson, and A. Manea were supported by the European Cooperation in Science and Technology (COST Action BM1203/EU-ROS). S.-A. Manea and A. Constantin acknowledge the support of the strategic grant POSDRU/159/1.5/S/133391-financed by the European Social Found within the Sectorial Operational Program Human Resources Development 2007–2013. S. Sasson is the Adolf D. and Horty Storch Chair in Pharmaceutical Sciences, at the Faculty of Medicine, The Hebrew University of Jerusalem, Israel. He is affiliated with the David R. Bloom Center for Pharmacy and the Dr. Adolf and Klara Brettler Center for Research of Molecular Pharmacology and Therapeutics in the Hebrew University.
References
- 1.Ushio-Fukai M., Zafari A.M., Fukui T., Ishizaka N., Griendling K.K. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J. Biol. Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 2.Rajagopalan S., Kurz S., Münzel T., Tarpey M., Freeman B.A., Griendling K.K., Harrison D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warnholtz A., Nickenig G., Schulz E., Macharzina R., Bräsen J.H., Skatchkov M., Heitzer T., Stasch J.P., Griendling K.K., Harrison D.G., Böhm M., Meinertz T., Münzel T. Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin-angiotensin system. Circulation. 1999;99:2027–2033. doi: 10.1161/01.cir.99.15.2027. [DOI] [PubMed] [Google Scholar]
- 4.San Martin A., Griendling K.K. NADPH oxidases: progress and opportunities. Antioxid. Redox Signal. 2014;20:2692–2694. doi: 10.1089/ars.2014.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altenhöfer S., Radermacher K.A., Kleikers P.W., Wingler K., Schmidt H.H. Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxid. Redox Signal. 2014 doi: 10.1089/ars.2013.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorin Y., Cavaglieri R.C., Khazim K., Lee D.Y., Bruno F., Thakur S., Fanti P., Szyndralewiez C., Barnes J.L., Block K., Abboud H.E. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am. J. Physiol. Renal. Physiol. 2015 doi: 10.1152/ajprenal.00396.2014. ajprenal.00396.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray S.P., Di Marco E., Okabe J., Szyndralewiez C., Heitz F., Montezano A.C., de Haan J.B., Koulis C., El-Osta A., Andrews K.L., Chin-Dusting J.P., Touyz R.M., Wingler K., Cooper M.E., Schmidt H.H., Jandeleit-Dahm K.A. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation. 2013;127:1888–1902. doi: 10.1161/CIRCULATIONAHA.112.132159. [DOI] [PubMed] [Google Scholar]
- 8.Selemidis S., Sobey C.G., Wingler K., Schmidt H.H., Drummond G.R. NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacol. Ther. 2008;120:254–291. doi: 10.1016/j.pharmthera.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Touyz R.M., Chen X., Tabet F., Yao G., He G., Quinn M.T., Pagano P.J., Schiffrin E.L. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ. Res. 2002;90:1205–1213. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- 10.Takeya R., Ueno N., Kami K., Taura M., Kohjima M., Izaki T., Nunoi H., Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J. Biol. Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- 11.Viedt C., Fei J., Krieger-Brauer H.I., Brandes R.P., Teupser D., Kamimura M., Katus H.A., Kreuzer J. Role of p22phox in angiotensin II and plateletderived growth factor AA induced activator protein 1 activation in vascular smooth muscle cells. J. Mol. Med. 2004;82:31–38. doi: 10.1007/s00109-003-0500-5. [DOI] [PubMed] [Google Scholar]
- 12.Kim J.S., Diebold B.A., Babior B.M., Knaus U.G., Bokoch G.M. Regulation of Nox1 activity via protein kinase A-mediated phosphorylation of NoxA1 and 14-3-3 binding. J. Biol. Chem. 2007;282:34787–34800. doi: 10.1074/jbc.M704754200. [DOI] [PubMed] [Google Scholar]
- 13.Yamamori T., Inanami O., Nagahata H., Kuwabara M. Phosphoinositide 3-kinase regulates the phosphorylation of NADPH oxidase component p47(phox) by controlling cPKC/PKCdelta but not Akt. Biochem. Biophys. Res. Commun. 2004;316:720–730. doi: 10.1016/j.bbrc.2004.02.108. [DOI] [PubMed] [Google Scholar]
- 14.Kilpatrick L.E., Sun S., Li H., Vary T.C., Korchak H.M. Regulation of TNF-induced oxygen radical production in human neutrophils: role of delta-PKC. J. Leukoc. Biol. 2010;87:153–164. doi: 10.1189/jlb.0408230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y.S., Morgan M.J., Choksi S., Liu Z.G. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol. Cell. 2007;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Gianni D., Bohl B., Courtneidge S.A., Bokoch G.M. The involvement of the tyrosine kinase c-Src in the regulation of reactive oxygen species generation mediated by NADPH oxidase-1. Mol. Biol. Cell. 2008;19:2984–2994. doi: 10.1091/mbc.E08-02-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenyo I.M., Florea I.C., Raicu M., Manea A. Tyrphostin AG490 reduces NAPDH oxidase activity and expression in the aorta of hypercholesterolemic apolipoprotein E-deficient mice. Vascul. Pharmacol. 2011;54:100–106. doi: 10.1016/j.vph.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Chen F., Yu Y., Haigh S., Johnson J., Lucas R., Stepp D.W., Fulton D.J. Regulation of NADPH oxidase 5 by protein kinase C isoforms. PLoS One. 2014;9:e88405. doi: 10.1371/journal.pone.0088405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janiszewski M., Lopes L.R., Carmo A.O., Pedro M.A., Brandes R.P., Santos C.X., Laurindo F.R. Regulation of NAD(P)H oxidase by associated protein disulfide isomerase in vascular smooth muscle cells. J. Biol. Chem. 2005;280(49):40813–40819. doi: 10.1074/jbc.M509255200. [DOI] [PubMed] [Google Scholar]
- 20.Miller F.J., Jr, Filali M., Huss G.J., Stanic B., Chamseddine A., Barna T.J., Lamb F.S. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ. Res. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 21.Pandey D., Gratton J.P., Rafikov R., Black S.M., Fulton D.J. Calcium/calmodulin-dependent kinase II mediates the phosphorylation and activation of NADPH oxidase 5. Mol. Pharmacol. 2011;80:407–415. doi: 10.1124/mol.110.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorescu D., Weiss D., Lassègue B., Clempus R.E., Szöcs K., Sorescu G.P., Valppu L., Quinn M.T., J.D. Lambeth, J.D. Vega, W.R. Taylor, K.K. Griendling. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 23.Hassler M.R., Egger G. Epigenomics of cancer-emerging new concepts. Biochimie. 2012;94:2219–2230. doi: 10.1016/j.biochi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.BelAiba R.S., Djordjevic T., Petry A., Diemer K., Bonello S., Banfi B., Hess J., Pogrebniak A., Bickel C., Görlach A. NOX5 variants are functionally active in endothelial cells. Free Radic. Biol. Med. 2007;42:446–459. doi: 10.1016/j.freeradbiomed.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 25.Manea A., Simionescu M. Nox enzymes and oxidative stress in atherosclerosis. Front. Biosci. (Schol Ed.) 2012;4:651–670. doi: 10.2741/s291. [DOI] [PubMed] [Google Scholar]
- 26.Manea A., Manea S.A., Gan A.M., Constantin A., Fenyo I.M., Raicu M., Muresian H., Simionescu M. Human monocytes and macrophages express NADPH oxidase 5; a potential source of reactive oxygen species in atherosclerosis. Biochem. Biophys. Res. Commun. 2015;461:172–179. doi: 10.1016/j.bbrc.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Moreno M.U., San José G., Orbe J., Páramo J.A., Beloqui O., Díez J., Zalba G. Preliminary characterisation of the promoter of the human p22(phox) gene: identification of a new polymorphism associated with hypertension. FEBS Lett. 2003;542:27–31. doi: 10.1016/s0014-5793(03)00331-4. [DOI] [PubMed] [Google Scholar]
- 28.Rada B., Leto T.L. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib. Microbiol. 2008;15:164–187. doi: 10.1159/000136357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savina A., Jancic C., Hugues S., Guermonprez P., Vargas P., Moura I.C., Lennon-Duménil A.M., Seabra M.C., Raposo G., Amigorena S. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 30.Jackson S.H., Devadas S., Kwon J., Pinto L.A., Williams M.S. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat. Immunol. 2004;5:818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 31.Ma J., Becker C., Lowell C.A., Underhill D.M. Dectin-1-triggered recruitment of light chain 3 protein to phagosomes facilitates major histocompatibility complex class II presentation of fungal-derived antigens. J. Biol. Chem. 2012;287:34149–34156. doi: 10.1074/jbc.M112.382812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crotzer V.L., Matute J.D., Arias A.A., Zhao H., Quilliam L.A., Dinauer M.C., Blum J.S. Cutting edge: NADPH oxidase modulates MHC class II antigen presentation by B cells. J. Immunol. 2012;189:3800–3804. doi: 10.4049/jimmunol.1103080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eklund E.A., Kakar R. Recruitment of CREB-binding protein by PU.1, IFN-regulatory factor-1, and the IFN consensus sequence-binding protein is necessary for IFN-gamma-induced p67phox and gp91phox expression. J. Immunol. 1999;163:6095–6105. [PubMed] [Google Scholar]
- 34.Kakar R., Kautz B., Eklund E.A. JAK2 is necessary and sufficient for interferon-gamma-induced transcription of the gene encoding gp91PHOX. J. Leukoc. Biol. 2005;77:120–127. doi: 10.1189/jlb.0704429. [DOI] [PubMed] [Google Scholar]
- 35.Lindsey S., Zhu C., Lu Y.F., Eklund E.A. HoxA10 represses transcription of the gene encoding p67phox in phagocytic cells. J. Immunol. 2005;175:5269–5279. doi: 10.4049/jimmunol.175.8.5269. [DOI] [PubMed] [Google Scholar]
- 36.Celada A., Borràs F.E., Soler C., Lloberas J., Klemsz M., van Beveren C., McKercher S., Maki R.A. The transcription factor PU.1 is involved in macrophage proliferation. J. Exp. Med. 1996;184:61–69. doi: 10.1084/jem.184.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maruyama S., Kanoh M., Matsumoto A., Kuwahara M., Yamashita M., Asano Y. A novel function of interferon regulatory factor-1: inhibition of Th2 cells by down-regulating the Il4 gene during Listeria infection. Int. Immunol. 2015;27:143–152. doi: 10.1093/intimm/dxu092. [DOI] [PubMed] [Google Scholar]
- 38.Shi L., Perin J.C., Leipzig J., Zhang Z., Sullivan K.E. Genome-wide analysis of interferon regulatory factor I binding in primary human monocytes. Gene. 2011;487:21–28. doi: 10.1016/j.gene.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gareus R., Kotsaki E., Xanthoulea S., van der Made I., Gijbels M.J., Kardakaris R., Polykratis A., Kollias G., de Winther M.P., Pasparakis M. Endothelial cell-specific NF-kappaB inhibition protects mice from atherosclerosis. Cell Metab. 2008;8:372–383. doi: 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Manea S.A., Manea A., Heltianu C. Inhibition of JAK/STAT signaling pathway prevents high-glucose-induced increase in endothelin-1 synthesis in human endothelial cells. Cell Tissue Res. 2010;340:71–79. doi: 10.1007/s00441-010-0936-1. [DOI] [PubMed] [Google Scholar]
- 41.Recio C., Oguiza A., Lazaro I., Mallavia B., Egido J., Gomez-Guerrero C. Suppressor of cytokine signaling 1-derived peptide inhibits Janus kinase/signal transducers and activators of transcription pathway and improves inflammation and atherosclerosis in diabetic mice. Arterioscler. Thromb. Vasc. Biol. 2014;34:1953–1960. doi: 10.1161/ATVBAHA.114.304144. [DOI] [PubMed] [Google Scholar]
- 42.Sozen E., Karademir B., Yazgan B., Bozaykut P., Ozer N.K. Potential role of proteasome on c-jun related signaling in hypercholesterolemia induced atherosclerosis. Redox Biol. 2014;2:732–738. doi: 10.1016/j.redox.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Winther M.P., Kanters E., Kraal G., Hofker M.H. Nuclear factor kappaB signaling in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2005;25:904–914. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- 44.Anrather J., Racchumi G., Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J. Biol. Chem. 2006;281:5657–5667. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- 45.Gauss K.A., Nelson-Overton L.K., Siemsen D.W., Gao Y., DeLeo F.R., Quinn M.T. Role of NF-kappaB in transcriptional regulation of the phagocyte NADPH oxidase by tumor necrosis factor-alpha. J. Leukoc. Biol. 2007;82:729–741. doi: 10.1189/jlb.1206735. [DOI] [PubMed] [Google Scholar]
- 46.Manea A., Manea S.A., Gafencu A.V., Raicu M. Regulation of NADPH oxidase subunit p22(phox) by NF-kB in human aortic smooth muscle cells. Arch. Physiol. Biochem. 2007;113:163–172. doi: 10.1080/13813450701531235. [DOI] [PubMed] [Google Scholar]
- 47.Manea A., Tanase L.I., Raicu M., Simionescu M. Transcriptional regulation of NADPH oxidase isoforms, Nox1 and Nox4, by nuclear factor-kappaB in human aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 2010;396:901–907. doi: 10.1016/j.bbrc.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Izumi Y., Kim S., Zhan Y., Namba M., Yasumoto H., Iwao H. Important role of angiotensin II-mediated c-Jun NH(2)-terminal kinase activation in cardiac hypertrophy in hypertensive rats. Hypertension. 2000;36:511–516. doi: 10.1161/01.hyp.36.4.511. [DOI] [PubMed] [Google Scholar]
- 49.Ahn J.D., Morishita R., Kaneda Y., Lee S.J., Kwon K.Y., Choi S.Y., Lee K.U., Park J.Y., Moon I.J., Park J.G., Yoshizumi M., Ouchi Y., Lee I.K. Inhibitory effects of novel AP-1 decoy oligodeoxynucleotides on vascular smooth muscle cell proliferation in vitro and neointimal formation in vivo. Circ. Res. 2002;90:1325–1332. doi: 10.1161/01.res.0000023200.19316.d5. [DOI] [PubMed] [Google Scholar]
- 50.Manea A., Manea S.A., Gafencu A.V., Raicu M., Simionescu M. AP-1-dependent transcriptional regulation of NADPH oxidase in human aortic smooth muscle cells: role of p22phox subunit. Arterioscler. Thromb. Vasc. Biol. 2008;28:878–885. doi: 10.1161/ATVBAHA.108.163592. [DOI] [PubMed] [Google Scholar]
- 51.Manea A., Manea S.A., Florea I.C., Luca C.M., Raicu M. Positive regulation of NADPH oxidase 5 by proinflammatory-related mechanisms in human aortic smooth muscle cells. Free Radic. Biol. Med. 2012;52:1497–1507. doi: 10.1016/j.freeradbiomed.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 52.Cevik M.O., Katsuyama M., Kanda S., Kaneko T., Iwata K., Ibi M., Matsuno K., Kakehi T., Cui W., Sasaki M., Yabe-Nishimura C. The AP-1 site is essential for the promoter activity of NOX1/NADPH oxidase, a vascular superoxide-producing enzyme: possible involvement of the ERK1/2-JunB pathway. Biochem. Biophys. Res. Commun. 2008;374:351–355. doi: 10.1016/j.bbrc.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 53.Manea A., Tanase L.I., Raicu M., Simionescu M. Jak/STAT signaling pathway regulates nox1 and nox4-based NADPH oxidase in human aortic smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2010;30:105–112. doi: 10.1161/ATVBAHA.109.193896. [DOI] [PubMed] [Google Scholar]
- 54.Kelkenberg U., Wagner A.H., Sarhaddar J., Hecker M., von der Leyen H.E. CCAAT/enhancer-binding protein decoy oligodeoxynucleotide inhibition of macrophage-rich vascular lesion formation in hypercholesterolemic rabbits. Arterioscler. Thromb. Vasc. Biol. 2002;22:949–954. doi: 10.1161/01.atv.0000017198.16727.27. [DOI] [PubMed] [Google Scholar]
- 55.Zhou A.X., Wang X., Lin C.S., Han J., Yong J., Nadolski M.J., Boren J., Kaufman R.J., Tabas I. C/EBP-homologous protein (CHOP) in vascular smooth muscle cells regulates their proliferation in aortic explants and atherosclerotic lesions. Circ. Res. 2015;114:305602. doi: 10.1161/CIRCRESAHA.116.305602. CIRCRESAHA.114.305602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cardinaux J.R., Allaman I., Magistretti P.J. Pro-inflammatory cytokines induce the transcription factors C/EBPbeta and C/EBPdelta in astrocytes. Glia. 2000;29:91–97. [PubMed] [Google Scholar]
- 57.Ramji D.P., Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem. J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manea S.A., Todirita A., Manea A. High glucose-induced increased expression of endothelin-1 in human endothelial cells is mediated by activated CCAAT/enhancer-binding proteins. PLoS One. 2013;8:e84170. doi: 10.1371/journal.pone.0084170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manea S.A., Todirita A., Raicu M., Manea A. C/EBP transcription factors regulate NADPH oxidase in human aortic smooth muscle cells. J. Cell. Mol. Med. 2014;18:1467–1477. doi: 10.1111/jcmm.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.San José G., Moreno M.U., Oliván S., Beloqui O., Fortuño A., Díez J., Zalba G. Functional effect of the p22phox-930A/G polymorphism on p22phox expression and NADPH oxidase activity in hypertension. Hypertension. 2004;44:163–169. doi: 10.1161/01.HYP.0000134790.02026.e4. [DOI] [PubMed] [Google Scholar]
- 61.Maitra U., Singh N., Gan L., Ringwood L., Li L. IRAK-1 contributes to lipopolysaccharide-induced reactive oxygen species generation in macrophages by inducing NOX-1 transcription and Rac1 activation and suppressing the expression of antioxidative enzymes. J. Biol. Chem. 2009;284:35403–35411. doi: 10.1074/jbc.M109.059501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Katsuyama M., Fan C., Arakawa N., Nishinaka T., Miyagishi M., Taira K., Yabe-Nishimura C. Essential role of ATF-1 in induction of NOX1, a catalytic subunit of NADPH oxidase: involvement of mitochondrial respiratory chain. Biochem. J. 2005;386:255–261. doi: 10.1042/BJ20041180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ni W., Zhan Y., He H., Maynard E., Balschi J.A., Oettgen P. Ets-1 is a critical transcriptional regulator of reactive oxygen species and p47(phox) gene expression in response to angiotensin II. Circ. Res. 2007;101:985–994. doi: 10.1161/CIRCRESAHA.107.152439. [DOI] [PubMed] [Google Scholar]
- 64.Zhang L., Sheppard O.R., Shah A.M., Brewer A.C. Positive regulation of the NADPH oxidase NOX4 promoter in vascular smooth muscle cells by E2F. Free Radic. Biol. Med. 2008;45:679–685. doi: 10.1016/j.freeradbiomed.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 65.Plutzky J. The PPAR-RXR transcriptional complex in the vasculature: energy in the balance. Circ. Res. 2011;108:1002–1016. doi: 10.1161/CIRCRESAHA.110.226860. [DOI] [PubMed] [Google Scholar]
- 66.Balakumar P., Rose M., Singh M. PPAR ligands: are they potential agents for cardiovascular disorders? Pharmacology. 2007;80:1–10. doi: 10.1159/000102594. [DOI] [PubMed] [Google Scholar]
- 67.Calkin A.C., Cooper M.E., Jandeleit-Dahm K.A., Allen T.J. Gemfibrozil decreases atherosclerosis in experimental diabetes in association with a reduction in oxidative stress and inflammation. Diabetologia. 2006;49:766–774. doi: 10.1007/s00125-005-0102-6. [DOI] [PubMed] [Google Scholar]
- 68.Quintela A.M., Jiménez R., Gómez-Guzmán M., Zarzuelo M.J., Galindo P., Sánchez M., Vargas F., Cogolludo A., Tamargo J., Pérez-Vizcaíno F., Duarte J. Activation of peroxisome proliferator-activated receptor-β/-δ (PPARβ/δ) prevents endothelial dysfunction in type 1 diabetic rats. Free Radic. Biol. Med. 2012;53:730–741. doi: 10.1016/j.freeradbiomed.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 69.Tordjman K.M., Semenkovich C.F., Coleman T., Yudovich R., Bak S., Osher E., Vechoropoulos M., Stern N. Absence of peroxisome proliferator-activated receptor-alpha abolishes hypertension and attenuates atherosclerosis in the Tsukuba hypertensive mouse. Hypertension. 2007;50:945–951. doi: 10.1161/HYPERTENSIONAHA.107.094268. [DOI] [PubMed] [Google Scholar]
- 70.Teissier E., Nohara A., Chinetti G., Paumelle R., Cariou B., Fruchart J.C., Brandes R.P., Shah A., Staels B. Peroxisome proliferator-activated receptor alpha induces NADPH oxidase activity in macrophages, leading to the generation of LDL with PPAR-alpha activation properties. Circ. Res. 2004;95:1174–1182. doi: 10.1161/01.RES.0000150594.95988.45. [DOI] [PubMed] [Google Scholar]
- 71.Sharifpanah F., Wartenberg M., Hannig M., Piper H.M., Sauer H. Peroxisome proliferator-activated receptor alpha agonists enhance cardiomyogenesis of mouse ES cells by utilization of a reactive oxygen species-dependent mechanism. Stem Cells. 2008;26:64–71. doi: 10.1634/stemcells.2007-0532. [DOI] [PubMed] [Google Scholar]
- 72.Yagil C., Yagil Y. Peroxisome proliferator-activated receptor alpha:friend or foe? Hypertension. 2007;50:847–850. doi: 10.1161/HYPERTENSIONAHA.107.100461. [DOI] [PubMed] [Google Scholar]
- 73.Chinetti G., Fruchart J.C., Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors with functions in the vascular wall. Z. Kardiol. 2001;90:125–132. doi: 10.1007/s003920170034. [DOI] [PubMed] [Google Scholar]
- 74.Riahi Y., Cohen G., Shamni O., Sasson S. Signaling and cytotoxic functions of 4-hydroxyalkenals. Am. J. Physiol. Endocrinol. Metab. 2010;299:E879–E886. doi: 10.1152/ajpendo.00508.2010. [DOI] [PubMed] [Google Scholar]
- 75.Riahi Y., Sin-Malia Y., Cohen G., Alpert E., Gruzman A., Eckel J., Staels B., Guichardant M., Sasson S. The natural protective mechanism against hyperglycemia in vascular endothelial cells: roles of the lipid peroxidation product 4-hydroxydodecadienal and peroxisome proliferator-activated receptor delta. Diabetes. 2010;59:808–818. doi: 10.2337/db09-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cohen G., Riahi Y., Shamni O., Guichardant M., Chatgilialoglu C., Ferreri C., Kaiser N., Sasson S. Role of lipid peroxidation and PPAR-δ in amplifying glucose-stimulated insulin secretion. Diabetes. 2011;60:2830–2842. doi: 10.2337/db11-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manea A., Manea S.A., Todirita A., Albulescu I.C., Raicu M., Sasson S., Simionescu M. High-glucose-increased expression and activation of NADPH oxidase in human vascular smooth muscle cells is mediated by 4-hydroxynonenal-activated PPARα and PPARβ/δ. Cell Tissue Res. 2015 doi: 10.1007/s00441-015-2120-0. Feb 27. [DOI] [PubMed] [Google Scholar]
- 78.Gizard F., Amant C., Barbier O., Bellosta S., Robillard R., Percevault F., Sevestre H., Krimpenfort P., Corsini A., Rochette J., Glineur C., Fruchart J.C., Torpier G., Staels B. PPAR alpha inhibits vascular smooth muscle cell proliferation underlying intimal hyperplasia by inducing the tumor suppressor p16INK4a. J. Clin. Invest. 2005;115:3228–3238. doi: 10.1172/JCI22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okazaki M., Iwasaki Y., Nishiyama M., Taguchi T., Tsugita M., Nakayama S., Sevestre H., Krimpenfort P., Corsini A., Rochette J., Glineur C., Fruchart J.C., Torpier G., Staels B. PPARbeta/delta regulates the human SIRT1 gene transcription via Sp1. Endocrine J. 2010;57:403–413. doi: 10.1507/endocrj.k10e-004. [DOI] [PubMed] [Google Scholar]
- 80.Wolin M.S., Ahmad M., Gupte S.A. Oxidant and redox signaling in vascular oxygen sensing mechanisms: basic concepts, current controversies, and potential importance of cytosolic NADPH. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L159–L173. doi: 10.1152/ajplung.00060.2005. [DOI] [PubMed] [Google Scholar]
- 81.Goyal P., Weissmann N., Grimminger F., Hegel C., Bader L., Rose F., Fink L., Ghofrani H.A., Schermuly R.T., Schmidt H.H., Seeger W., Hänze J. Upregulation of NAD(P)H oxidase 1 in hypoxia activates hypoxia-inducible factor 1 via increase in reactive oxygen species. Free Radic. Biol. Med. 2004;36:1279–1288. doi: 10.1016/j.freeradbiomed.2004.02.071. [DOI] [PubMed] [Google Scholar]
- 82.Bonello S., Zähringer C., BelAiba R.S., Djordjevic T., Hess J., Michiels C., Kietzmann T., Görlach A. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler. Thromb. Vasc. Biol. 2007;27:755–761. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- 83.Diebold I., Petry A., Hess J., Görlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol. Biol. Cell. 2010;21:2087–2096. doi: 10.1091/mbc.E09-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Diebold I., Petry A., Sabrane K., Djordjevic T., Hess J., Görlach A. The HIF1 target gene NOX2 promotes angiogenesis through urotensin-II. J. Cell Sci. 2012;125:956–964. doi: 10.1242/jcs.094060. [DOI] [PubMed] [Google Scholar]
- 85.Cooper M.E., El-Osta A. Epigenetics: mechanisms and implications for diabetic complications. Circ. Res. 2010;107:1403–1413. doi: 10.1161/CIRCRESAHA.110.223552. [DOI] [PubMed] [Google Scholar]
- 86.Chen S., Feng B., George B., Chakrabarti R., Chen M., Chakrabarti S. Transcriptional coactivator p300 regulates glucose-induced gene expression in endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2010;298:E127–E137. doi: 10.1152/ajpendo.00432.2009. [DOI] [PubMed] [Google Scholar]
- 87.Okabe J., Orlowski C., Balcerczyk A., Tikellis C., Thomas M.C., Cooper M.E., El-Osta A. Distinguishing hyperglycemic changes by Set7 in vascular endothelial cells. Circ. Res. 2012;110:1067–1076. doi: 10.1161/CIRCRESAHA.112.266171. [DOI] [PubMed] [Google Scholar]
- 88.Paneni F., Costantino S., Battista R., Castello L., Capretti G., Chiandotto S., Scavone G., Villano A., Pitocco D., Lanza G., Volpe M., Lüscher T.F., Cosentino F. Adverse epigenetic signatures by histone methyltransferase set7 contribute to vascular dysfunction in patients with type 2 diabetes. Circ. Cardiovasc. Genet. 2014;114:000671. doi: 10.1161/CIRCGENETICS.114.000671. pii: CIRCGENETICS.114.000671. [DOI] [PubMed] [Google Scholar]
- 89.Paneni F., Costantino S., Volpe M., Lüscher T.F., Cosentino F. Epigenetic signatures and vascular risk in type 2 diabetes: a clinical perspective. Atherosclerosis. 2013;230:191–197. doi: 10.1016/j.atherosclerosis.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 90.Hayes P., Knaus U.G. Balancing reactive oxygen species in the epigenome: NADPH oxidases as target and perpetrator. Antioxid. Redox Signal. 2013;18:1937–1945. doi: 10.1089/ars.2012.4895. [DOI] [PubMed] [Google Scholar]
- 91.Luxen S., Belinsky S.A., Knaus U.G. Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res. 2008;68:1037–1045. doi: 10.1158/0008-5472.CAN-07-5782. [DOI] [PubMed] [Google Scholar]
- 92.Biterge B., Schneider R. Histone variants: key players of chromatin. Cell Tissue Res. 2014;356:457–466. doi: 10.1007/s00441-014-1862-4. [DOI] [PubMed] [Google Scholar]
- 93.Vogel T., Lassmann S. Epigenetics: development, dynamics and disease. Cell Tissue Res. 2014;356:451–455. doi: 10.1007/s00441-014-1916-7. [DOI] [PubMed] [Google Scholar]
- 94.Wierda R.J., Geutskens S.B., Jukema J.W., Quax P.H., van den Elsen P.J. Epigenetics in atherosclerosis and inflammation. J. Cell. Mol. Med. 2010;14:1225–1240. doi: 10.1111/j.1582-4934.2010.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Usui T., Okada M., Mizuno W., Oda M., Ide N., Morita T., Hara Y., Yamawaki H. HDAC4 mediates development of hypertension via vascular inflammation in spontaneous hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H1894–H1904. doi: 10.1152/ajpheart.01039.2011. [DOI] [PubMed] [Google Scholar]
- 96.Nührenberg T., Gilsbach R., Preissl S., Schnick T., Hein L. Epigenetics in cardiac development, function, and disease. Cell Tissue Res. 2014;356:585–600. doi: 10.1007/s00441-014-1887-8. [DOI] [PubMed] [Google Scholar]
- 97.Eom G.H., Nam Y.S., Oh J.G., Choe N., Min H.K., Yoo E.K., Kang G., Nguyen V.H., Min J.J., Kim J.K., Lee I.K., Bassel-Duby R., Olson E.N., Park W.J., Kook H. Regulation of acetylation of histone deacetylase 2 by p300/CBP-associated factor/histone deacetylase 5 in the development of cardiac hypertrophy. Circ Res. 2014;114:1133–1143. doi: 10.1161/CIRCRESAHA.114.303429. [DOI] [PubMed] [Google Scholar]
- 98.Turgeon P.J., Sukumar A.N., Marsden P.A. Epigenetics of cardiovascular disease – a New “Beat” in coronary artery disease. Med. Epigenet. 2014;2:37–52. doi: 10.1159/000360766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Y., Miao X., Liu Y., Li F., Liu Q., Sun J., Cai L. Dysregulation of histone acetyltransferases and deacetylases in cardiovascular diseases. Oxid. Med. Cell. Longev. 641979. 2014;2014;2014 doi: 10.1155/2014/641979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Verma M., Banerjee H.N. Epigenetic inhibitors. Methods Mol. Biol. 2015;1238:469–485. doi: 10.1007/978-1-4939-1804-1_24. [DOI] [PubMed] [Google Scholar]
- 101.Li X., Xu W. HDAC1/3 dual selective inhibitors – new therapeutic agents for the potential treatment of cancer. Drug. Discov. Ther. 2014;8:225–228. doi: 10.5582/ddt.2014.01034. [DOI] [PubMed] [Google Scholar]
- 102.Lakshmaiah K.C., Jacob L.A., Aparna S., Lokanatha D., Saldanha S.C. Epigenetic therapy of cancer with histone deacetylase inhibitors. J. Cancer Res. Ther. 2014;10:469–478. doi: 10.4103/0973-1482.137937. [DOI] [PubMed] [Google Scholar]
- 103.Findeisen H.M., Gizard F., Zhao Y., Qing H., Heywood E.B., Jones K.L., Cohn D., Bruemmer D. Epigenetic regulation of vascular smooth muscle cell proliferation and neointima formation by histone deacetylase inhibition. Arterioscler. Thromb. Vasc. Biol. 2011;31:851–860. doi: 10.1161/ATVBAHA.110.221952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Usui T., Morita T., Okada M., Yamawaki H. Histone deacetylase 4 controls neointimal hyperplasia via stimulating proliferation and migration of vascular smooth muscle cells. Hypertension. 2014;63:397–403. doi: 10.1161/HYPERTENSIONAHA.113.01843. [DOI] [PubMed] [Google Scholar]
- 105.Xie M., Kong Y., Tan W., May H., Battiprolu P.K., Pedrozo Z., Wang Z.V., Morales C., Luo X., Cho G., Jiang N., Jessen M.E., Warner J.J., Lavandero S., Gillette T.G., Turer A.T., Hill J.A. Histone deacetylase inhibition blunts ischemia/reperfusion injury by inducing cardiomyocyte autophagy. Circulation. 2014;129:1139–1151. doi: 10.1161/CIRCULATIONAHA.113.002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Siuda D., Zechner U., El Hajj N., Prawitt D., Langer D., Xia N., Horke S., Pautz A., Kleinert H., Förstermann U., Li H. Transcriptional regulation of Nox4 by histone deacetylases in human endothelial cells. Basic Res. Cardiol. 2012;107:283. doi: 10.1007/s00395-012-0283-3. [DOI] [PubMed] [Google Scholar]
- 107.Zelko I.N., Folz R.J. Regulation of oxidative stress in pulmonary artery endothelium: modulation of EC-SOD and NOX4 expression using HDAC class I inhibitors. Am. J. Respir. Cell Mol. Biol. 2015 doi: 10.1165/rcmb.2014-0260OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Quivy V., Van Lint C. Regulation at multiple levels of NF-kappaB-mediated transactivation by protein acetylation. Biochem. Pharmacol. 2004;68:1221–1229. doi: 10.1016/j.bcp.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 109.Zarzuelo M.J., López-Sepúlveda R., Sánchez M., Romero M., Gómez-Guzmán M., Ungvary Z., Pérez-Vizcaíno F., Jiménez R., Duarte J. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem. Pharmacol. 2013;85:1288–1296. doi: 10.1016/j.bcp.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 110.Gano L.B., Donato A.J., Pasha H.M., Hearon C.M., Jr, Sindler A.L., Seals D.R. The SIRT1 activator SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am. J. Physiol. Heart Circ. Physiol. 2014;307:H1754–H1763. doi: 10.1152/ajpheart.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kawai Y., Arinze I.J. Valproic acid-induced gene expression through production of reactive oxygen species. Cancer Res. 2006;66:6563–6569. doi: 10.1158/0008-5472.CAN-06-0814. [DOI] [PubMed] [Google Scholar]
- 112.Zhao T.C., Zhang L.X., Cheng G., Liu J.T. gp-91 mediates histone deacetylase inhibition-induced cardioprotection. Biochim. Biophys. Acta. 2010;1803:872–880. doi: 10.1016/j.bbamcr.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sanders Y.Y., Liu H., Liu G., Thannickal V.J. Epigenetic mechanisms regulate NADPH oxidase-4 expression in cellular senescence. Free Radic. Biol. Med. 2014;79C:197–205. doi: 10.1016/j.freeradbiomed.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 114.He M., Zhang B., Wei X., Wang Z., Fan B., Du P., Zhang Y., Jian W., Chen L., Wang L., Fang H., Li X., Wang P.A., Yi F. HDAC4/5-HMGB1 signalling mediated by NADPH oxidase activity contributes to cerebral ischaemia/reperfusion injury. J. Cell. Mol. Med. 2013;17:531–542. doi: 10.1111/jcmm.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Matsushima S., Kuroda J., Ago T., Zhai P., Park J.Y., Xie L.H., Tian B., Sadoshima J. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circ. Res. 2013;112:651–663. doi: 10.1161/CIRCRESAHA.112.279760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Najafi-Shoushtari S.H. MicroRNAs in cardiometabolic disease. Curr. Atheroscler. Rep. 2011;13:202–207. doi: 10.1007/s11883-011-0179-y. [DOI] [PubMed] [Google Scholar]
- 117.Fu Y., Zhang Y., Wang Z., Wang L., Wei X., Zhang B., Wen Z., Fang H., Pang Q., Yi F. Regulation of NADPH oxidase activity is associated with miRNA-25-mediated NOX4 expression in experimental diabetic nephropathy. Am. J. Nephrol. 2010;32:581–589. doi: 10.1159/000322105. [DOI] [PubMed] [Google Scholar]
- 118.Varga Z.V., Kupai K., Szűcs G., Gáspár R., Pálóczi J., Faragó N., Zvara A., Puskás L.G., Rázga Z., Tiszlavicz L., Bencsik P., Görbe A., Csonka C., Ferdinandy P., Csont T. MicroRNA-25-dependent up-regulation of NADPH oxidase 4 (NOX4) mediates hypercholesterolemia-induced oxidative/nitrative stress and subsequent dysfunction in the heart. J. Mol. Cell Cardiol. 2013;62:111–121. doi: 10.1016/j.yjmcc.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 119.Ranjan R., Lee Y.G., Karpurapu M., Syed M.A., Chung S., Deng J., Jeong J.J., Zhao G., Xiao L., Sadikot R.T., Weiss M.J., Christman J.W., Park G.Y. p47phox and reactive oxygen species production modulate expression of microRNA-451 in macrophages. Free Radic. Res. 2015;49:25–34. doi: 10.3109/10715762.2014.974037. [DOI] [PMC free article] [PubMed] [Google Scholar]