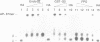Abstract
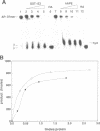
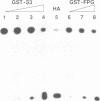
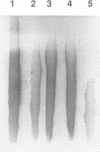
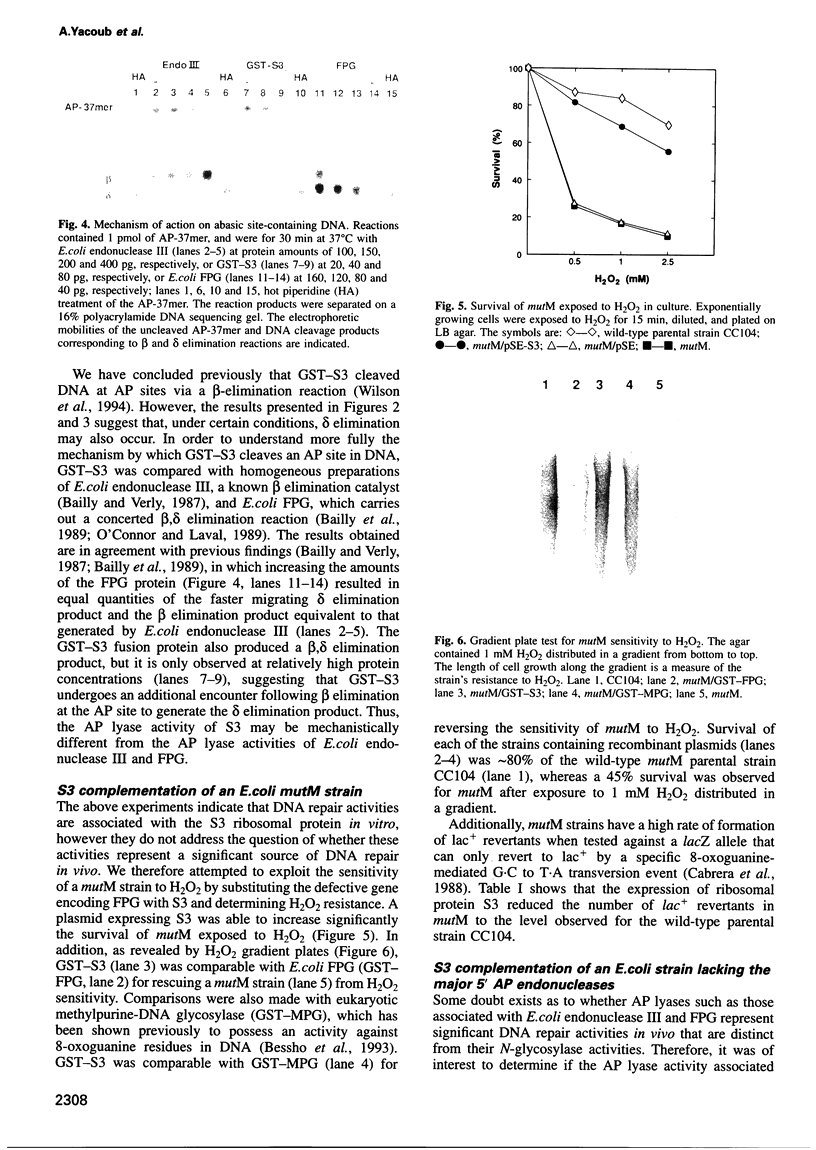
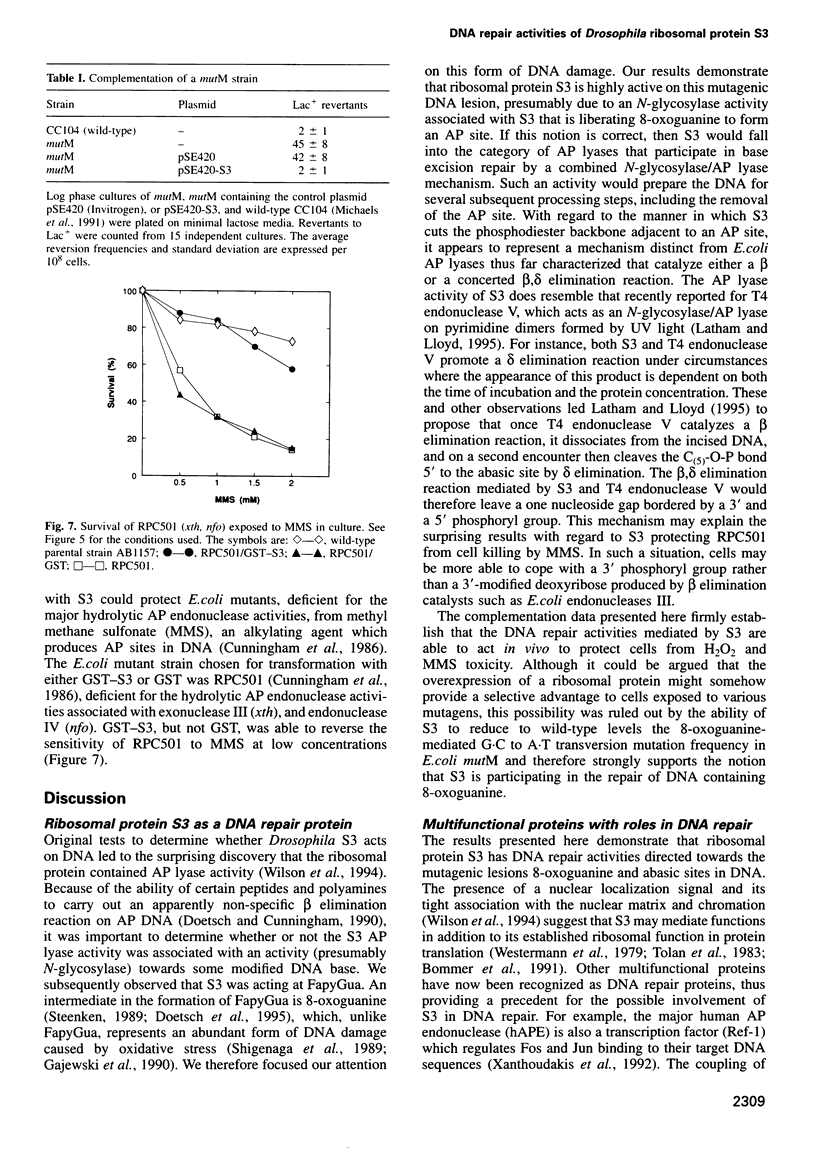
Ionizing radiation and normal cellular respiration form reactive oxygen species that damage DNA and contribute to a variety of human disorders including tumor promotion and carcinogenesis. A major product of free radical DNA damage is the formation of 8-oxoguanine, which is a highly mutagenic base modification produced by oxidative stress. Here, Drosophila ribosomal protein S3 is shown to cleave DNA containing 8-oxoguanine residues efficiently, The ribosomal protein also contains an associated apurinic/apyrimidinic (AP) lyase activity, cleaving phosphodiester bonds via a beta,delta elimination reaction. The significance of this DNA repair activity acting on 8-oxoguanine is shown by the ability of S3 to rescue the H2O2 sensitivity of an Escherichia coli mutM strain (defective for the repair of 8-oxoguanine) and to abolish completely the mutator phenotype of mutM caused by 8-oxoguanine-mediated G-->T transversions. The ribosomal protein is also able to rescue the alkylation sensitivity of an E.coli mutant deficient for the AP endonuclease activities associated with exonuclease III (xth) and endonuclease IV (nfo), indicating for the first time that an AP lyase can represent a significant source of DNA repair activity for the repair of AP sites. These results raise the possibility that DNA repair may be associated with protein translation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailly V., Verly W. G. Escherichia coli endonuclease III is not an endonuclease but a beta-elimination catalyst. Biochem J. 1987 Mar 1;242(2):565–572. doi: 10.1042/bj2420565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly V., Verly W. G., O'Connor T., Laval J. Mechanism of DNA strand nicking at apurinic/apyrimidinic sites by Escherichia coli [formamidopyrimidine]DNA glycosylase. Biochem J. 1989 Sep 1;262(2):581–589. doi: 10.1042/bj2620581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessho T., Roy R., Yamamoto K., Kasai H., Nishimura S., Tano K., Mitra S. Repair of 8-hydroxyguanine in DNA by mammalian N-methylpurine-DNA glycosylase. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8901–8904. doi: 10.1073/pnas.90.19.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiteux S., O'Connor T. R., Laval J. Formamidopyrimidine-DNA glycosylase of Escherichia coli: cloning and sequencing of the fpg structural gene and overproduction of the protein. EMBO J. 1987 Oct;6(10):3177–3183. doi: 10.1002/j.1460-2075.1987.tb02629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiteux S., O'Connor T. R., Lederer F., Gouyette A., Laval J. Homogeneous Escherichia coli FPG protein. A DNA glycosylase which excises imidazole ring-opened purines and nicks DNA at apurinic/apyrimidinic sites. J Biol Chem. 1990 Mar 5;265(7):3916–3922. [PubMed] [Google Scholar]
- Bommer U. A., Lutsch G., Stahl J., Bielka H. Eukaryotic initiation factors eIF-2 and eIF-3: interactions, structure and localization in ribosomal initiation complexes. Biochimie. 1991 Jul-Aug;73(7-8):1007–1019. doi: 10.1016/0300-9084(91)90142-n. [DOI] [PubMed] [Google Scholar]
- Cabrera M., Nghiem Y., Miller J. H. mutM, a second mutator locus in Escherichia coli that generates G.C----T.A transversions. J Bacteriol. 1988 Nov;170(11):5405–5407. doi: 10.1128/jb.170.11.5405-5407.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney J. P., McKnight C., VanEpps S., Kelley M. R. Random rapid amplification of cDNA ends (RRACE) allows for cloning of multiple novel human cDNA fragments containing (CAG)n repeats. Gene. 1995 Apr 3;155(2):289–292. doi: 10.1016/0378-1119(94)00758-k. [DOI] [PubMed] [Google Scholar]
- Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992 Jan 5;267(1):166–172. [PubMed] [Google Scholar]
- Cunningham R. P., Saporito S. M., Spitzer S. G., Weiss B. Endonuclease IV (nfo) mutant of Escherichia coli. J Bacteriol. 1986 Dec;168(3):1120–1127. doi: 10.1128/jb.168.3.1120-1127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Herman T., Chen D. S. Cloning and expression of APE, the cDNA encoding the major human apurinic endonuclease: definition of a family of DNA repair enzymes. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11450–11454. doi: 10.1073/pnas.88.24.11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch P. W., Cunningham R. P. The enzymology of apurinic/apyrimidinic endonucleases. Mutat Res. 1990 Sep-Nov;236(2-3):173–201. doi: 10.1016/0921-8777(90)90004-o. [DOI] [PubMed] [Google Scholar]
- Doetsch P. W., Zasatawny T. H., Martin A. M., Dizdaroglu M. Monomeric base damage products from adenine, guanine, and thymine induced by exposure of DNA to ultraviolet radiation. Biochemistry. 1995 Jan 24;34(3):737–742. doi: 10.1021/bi00003a005. [DOI] [PubMed] [Google Scholar]
- Floyd R. A. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis. 1990 Sep;11(9):1447–1450. doi: 10.1093/carcin/11.9.1447. [DOI] [PubMed] [Google Scholar]
- Gajewski E., Rao G., Nackerdien Z., Dizdaroglu M. Modification of DNA bases in mammalian chromatin by radiation-generated free radicals. Biochemistry. 1990 Aug 28;29(34):7876–7882. doi: 10.1021/bi00486a014. [DOI] [PubMed] [Google Scholar]
- Grabowski D. T., Deutsch W. A., Derda D., Kelley M. R. Drosophila AP3, a presumptive DNA repair protein, is homologous to human ribosomal associated protein P0. Nucleic Acids Res. 1991 Aug 11;19(15):4297–4297. doi: 10.1093/nar/19.15.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas K. D., Donahue T. F. SSL2, a suppressor of a stem-loop mutation in the HIS4 leader encodes the yeast homolog of human ERCC-3. Cell. 1992 Jun 12;69(6):1031–1042. doi: 10.1016/0092-8674(92)90621-i. [DOI] [PubMed] [Google Scholar]
- Guzder S. N., Qiu H., Sommers C. H., Sung P., Prakash L., Prakash S. DNA repair gene RAD3 of S. cerevisiae is essential for transcription by RNA polymerase II. Nature. 1994 Jan 6;367(6458):91–94. doi: 10.1038/367091a0. [DOI] [PubMed] [Google Scholar]
- Kane C. M., Linn S. Purification and characterization of an apurinic/apyrimidinic endonuclease from HeLa cells. J Biol Chem. 1981 Apr 10;256(7):3405–3414. [PubMed] [Google Scholar]
- Kelley M. R., Venugopal S., Harless J., Deutsch W. A. Antibody to a human DNA repair protein allows for cloning of a Drosophila cDNA that encodes an apurinic endonuclease. Mol Cell Biol. 1989 Mar;9(3):965–973. doi: 10.1128/mcb.9.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Chubatsu L. S., Admon A., Stahl J., Fellous R., Linn S. Implication of mammalian ribosomal protein S3 in the processing of DNA damage. J Biol Chem. 1995 Jun 9;270(23):13620–13629. doi: 10.1074/jbc.270.23.13620. [DOI] [PubMed] [Google Scholar]
- Latham K. A., Lloyd R. S. Delta-elimination by T4 endonuclease V at a thymine dimer site requires a secondary binding event and amino acid Glu-23. Biochemistry. 1995 Jul 11;34(27):8796–8803. doi: 10.1021/bi00027a031. [DOI] [PubMed] [Google Scholar]
- Lindahl T. Uracil-DNA glycosylase from Escherichia coli. Methods Enzymol. 1980;65(1):284–290. doi: 10.1016/s0076-6879(80)65038-1. [DOI] [PubMed] [Google Scholar]
- Michaels M. L., Pham L., Cruz C., Miller J. H. MutM, a protein that prevents G.C----T.A transversions, is formamidopyrimidine-DNA glycosylase. Nucleic Acids Res. 1991 Jul 11;19(13):3629–3632. doi: 10.1093/nar/19.13.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya M., Ou C., Bodepudi V., Johnson F., Takeshita M., Grollman A. P. Site-specific mutagenesis using a gapped duplex vector: a study of translesion synthesis past 8-oxodeoxyguanosine in E. coli. Mutat Res. 1991 May;254(3):281–288. doi: 10.1016/0921-8777(91)90067-y. [DOI] [PubMed] [Google Scholar]
- O'Connor T. R., Laval F. Isolation and structure of a cDNA expressing a mammalian 3-methyladenine-DNA glycosylase. EMBO J. 1990 Oct;9(10):3337–3342. doi: 10.1002/j.1460-2075.1990.tb07534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor T. R., Laval J. Physical association of the 2,6-diamino-4-hydroxy-5N-formamidopyrimidine-DNA glycosylase of Escherichia coli and an activity nicking DNA at apurinic/apyrimidinic sites. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5222–5226. doi: 10.1073/pnas.86.14.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue-Geile K., Geiser J. R., Shu M., Miller C., Wool I. G., Meisler A. I., Pipas J. M. Ribosomal protein genes are overexpressed in colorectal cancer: isolation of a cDNA clone encoding the human S3 ribosomal protein. Mol Cell Biol. 1991 Aug;11(8):3842–3849. doi: 10.1128/mcb.11.8.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibutani S., Takeshita M., Grollman A. P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991 Jan 31;349(6308):431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- Shigenaga M. K., Gimeno C. J., Ames B. N. Urinary 8-hydroxy-2'-deoxyguanosine as a biological marker of in vivo oxidative DNA damage. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9697–9701. doi: 10.1073/pnas.86.24.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T., Morimoto K. Increased formation of 8-hydroxydeoxyguanosine, an oxidative DNA damage, in lymphoblasts from Fanconi's anemia patients due to possible catalase deficiency. Carcinogenesis. 1993 Jun;14(6):1115–1120. doi: 10.1093/carcin/14.6.1115. [DOI] [PubMed] [Google Scholar]
- Tchou J., Kasai H., Shibutani S., Chung M. H., Laval J., Grollman A. P., Nishimura S. 8-oxoguanine (8-hydroxyguanine) DNA glycosylase and its substrate specificity. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4690–4694. doi: 10.1073/pnas.88.11.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolan D. R., Hershey J. W., Traut R. T. Crosslinking of eukaryotic initiation factor eIF3 to the 40S ribosomal subunit from rabbit reticulocytes. Biochimie. 1983 Jul;65(7):427–436. doi: 10.1016/s0300-9084(83)80062-5. [DOI] [PubMed] [Google Scholar]
- Wang Z., Svejstrup J. Q., Feaver W. J., Wu X., Kornberg R. D., Friedberg E. C. Transcription factor b (TFIIH) is required during nucleotide-excision repair in yeast. Nature. 1994 Mar 3;368(6466):74–76. doi: 10.1038/368074a0. [DOI] [PubMed] [Google Scholar]
- Westermann P., Heumann W., Bommer U. A., Bielka H., Nygard O., Hultin T. Crosslinking of initiation factor eIF-2 to proteins of the small subunit of rat liver ribosomes. FEBS Lett. 1979 Jan 1;97(1):101–104. doi: 10.1016/0014-5793(79)80061-7. [DOI] [PubMed] [Google Scholar]
- Wilson D. M., 3rd, Deutsch W. A., Kelley M. R. Cloning of the Drosophila ribosomal protein S3: another multifunctional ribosomal protein with AP endonuclease DNA repair activity. Nucleic Acids Res. 1993 May 25;21(10):2516–2516. doi: 10.1093/nar/21.10.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. M., 3rd, Deutsch W. A., Kelley M. R. Drosophila ribosomal protein S3 contains an activity that cleaves DNA at apurinic/apyrimidinic sites. J Biol Chem. 1994 Oct 14;269(41):25359–25364. [PubMed] [Google Scholar]
- Wilson D. M., 3rd, Emanuele N. V., Jurgens J. K., Kelley M. R. Prolactin message in brain and pituitary of adult male rats is identical: PCR cloning and sequencing of hypothalamic prolactin cDNA from intact and hypophysectomized adult male rats. Endocrinology. 1992 Nov;131(5):2488–2490. doi: 10.1210/endo.131.5.1339346. [DOI] [PubMed] [Google Scholar]
- Wood M. L., Dizdaroglu M., Gajewski E., Essigmann J. M. Mechanistic studies of ionizing radiation and oxidative mutagenesis: genetic effects of a single 8-hydroxyguanine (7-hydro-8-oxoguanine) residue inserted at a unique site in a viral genome. Biochemistry. 1990 Jul 31;29(30):7024–7032. doi: 10.1021/bi00482a011. [DOI] [PubMed] [Google Scholar]
- Xanthoudakis S., Miao G., Wang F., Pan Y. C., Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992 Sep;11(9):3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]