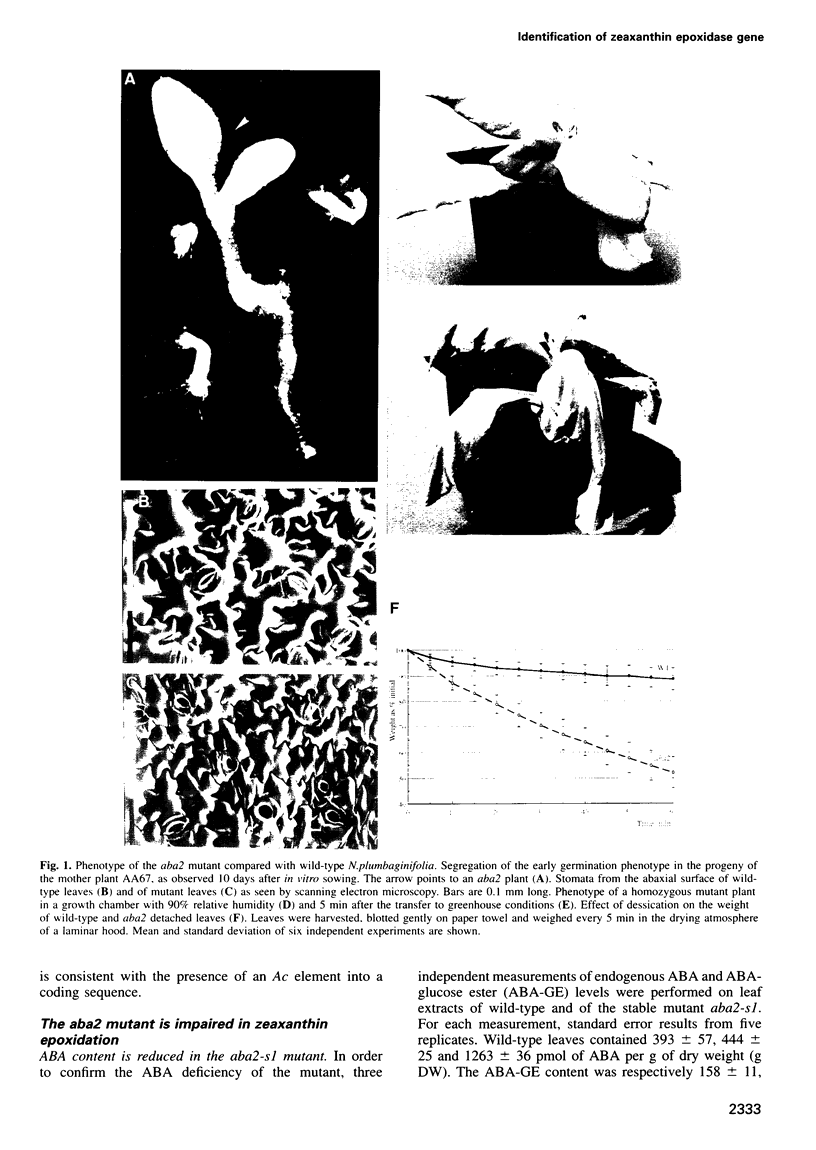
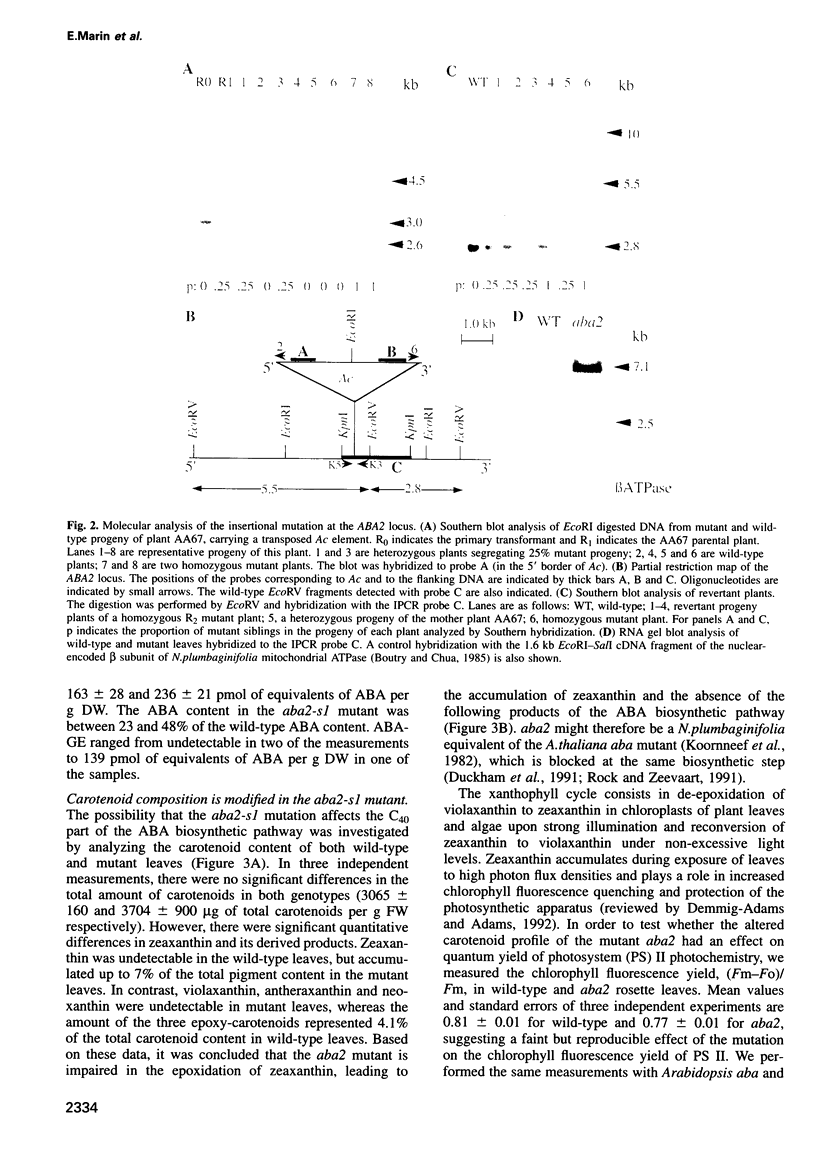
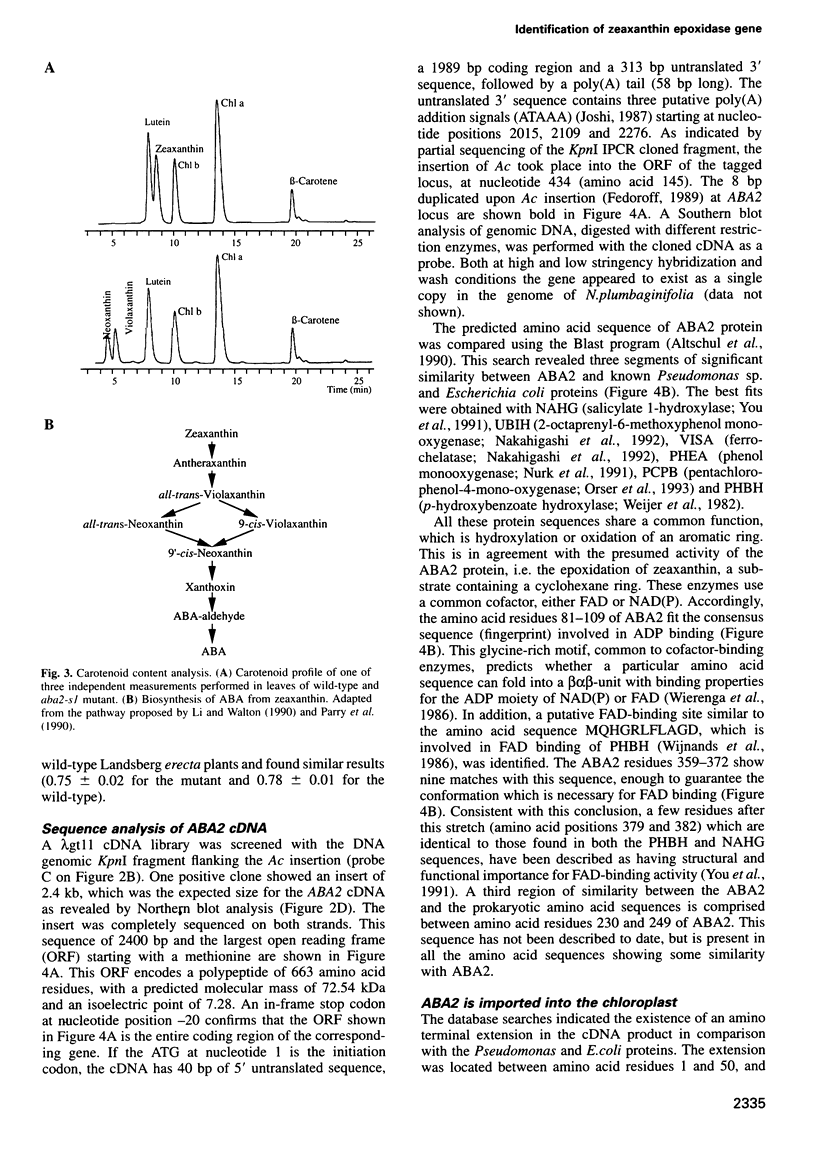
Abstract
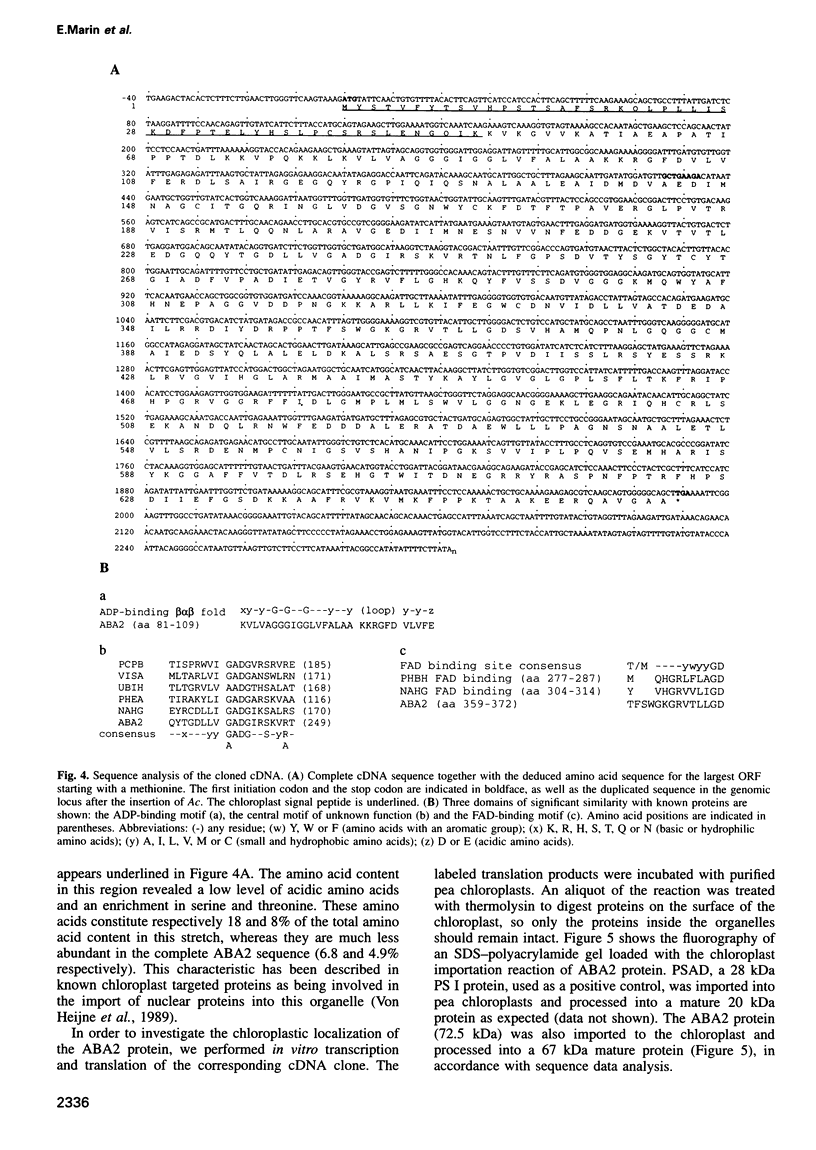
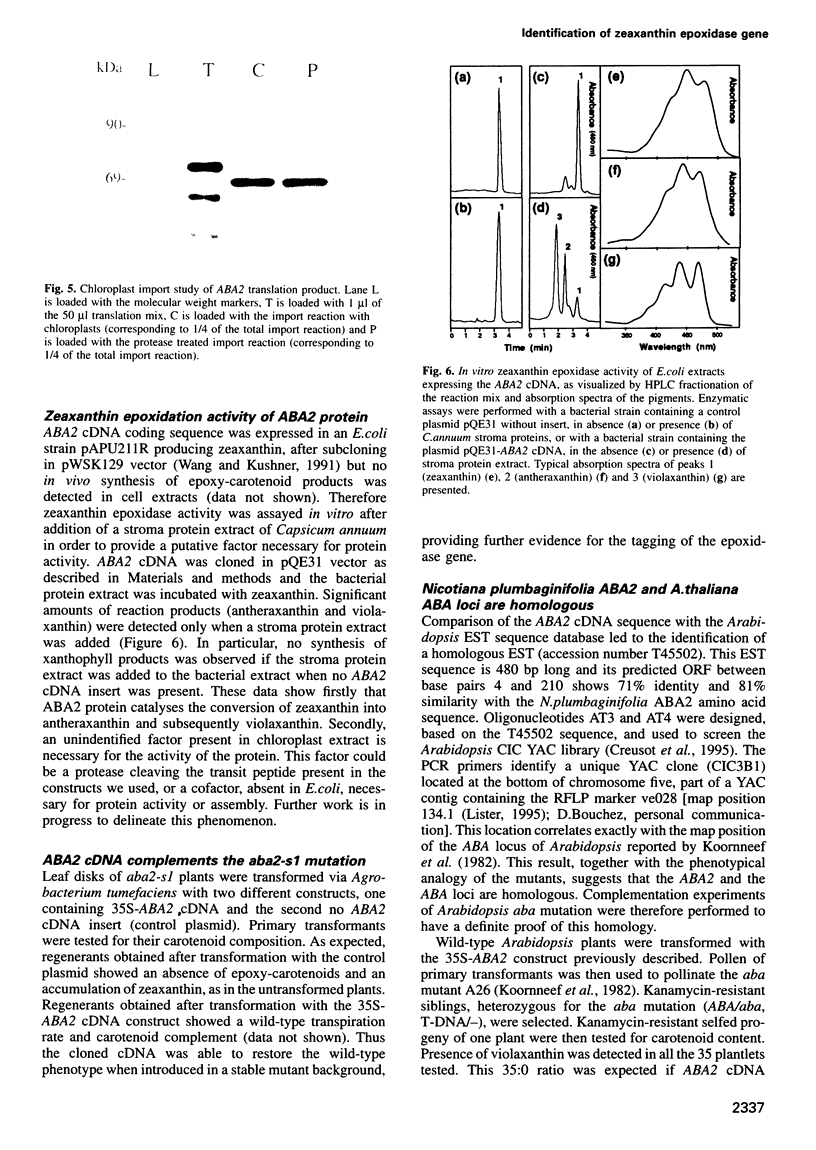
Abscisic acid (ABA) is a plant hormone which plays an important role in seed development and dormancy and in plant response to environmental stresses. An ABA-deficient mutant of Nicotiana plumbaginifolia, aba2, was isolated by transposon tagging using the maize Activator transposon. The aba2 mutant exhibits precocious seed germination and a severe wilty phenotype. The mutant is impaired in the first step of the ABA biosynthesis pathway, the zeaxanthin epoxidation reaction. ABA2 cDNA is able to complement N.plumbaginifolia aba2 and Arabidopsis thaliana aba mutations indicating that these mutants are homologous. ABA2 cDNA encodes a chloroplast-imported protein of 72.5 kDa, sharing similarities with different mono-oxigenases and oxidases of bacterial origin and having an ADP-binding fold and an FAD-binding domain. ABA2 protein, produced in Escherichia coli, exhibits in vitro zeaxanthin epoxidase activity. This is the first report of the isolation of a gene of the ABA biosynthetic pathway. The molecular identification of ABA2 opens the possibility to study the regulation of ABA biosynthesis and its cellular location.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Baker B., Coupland G., Fedoroff N., Starlinger P., Schell J. Phenotypic assay for excision of the maize controlling element Ac in tobacco. EMBO J. 1987 Jun;6(6):1547–1554. doi: 10.1002/j.1460-2075.1987.tb02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutry M., Chua N. H. A nuclear gene encoding the beta subunit of the mitochondrial ATP synthase in Nicotiana plumbaginifolia. EMBO J. 1985 Sep;4(9):2159–2165. doi: 10.1002/j.1460-2075.1985.tb03910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuck G., Robbins T., Nijjar C., Ralston E., Courtney-Gutterson N., Dooner H. K. Tagging and Cloning of a Petunia Flower Color Gene with the Maize Transposable Element Activator. Plant Cell. 1993 Apr;5(4):371–378. doi: 10.1105/tpc.5.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Werner-Washburne M., Lubben T. H., Keegstra K. Precursors to two nuclear-encoded chloroplast proteins bind to the outer envelope membrane before being imported into chloroplasts. J Biol Chem. 1985 Mar 25;260(6):3691–3696. [PubMed] [Google Scholar]
- Creusot F., Fouilloux E., Dron M., Lafleuriel J., Picard G., Billault A., Le Paslier D., Cohen D., Chabouté M. E., Durr A. The CIC library: a large insert YAC library for genome mapping in Arabidopsis thaliana. Plant J. 1995 Nov;8(5):763–770. doi: 10.1046/j.1365-313x.1995.08050763.x. [DOI] [PubMed] [Google Scholar]
- Davies W. J., Tardieu F., Trejo C. L. How Do Chemical Signals Work in Plants that Grow in Drying Soil? Plant Physiol. 1994 Feb;104(2):309–314. doi: 10.1104/pp.104.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earp D. J., Lowe B., Baker B. Amplification of genomic sequences flanking transposable elements in host and heterologous plants: a tool for transposon tagging and genome characterization. Nucleic Acids Res. 1990 Jun 11;18(11):3271–3279. doi: 10.1093/nar/18.11.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff N. V. About maize transposable elements and development. Cell. 1989 Jan 27;56(2):181–191. doi: 10.1016/0092-8674(89)90891-x. [DOI] [PubMed] [Google Scholar]
- Giraudat J., Parcy F., Bertauche N., Gosti F., Leung J., Morris P. C., Bouvier-Durand M., Vartanian N. Current advances in abscisic acid action and signalling. Plant Mol Biol. 1994 Dec;26(5):1557–1577. doi: 10.1007/BF00016490. [DOI] [PubMed] [Google Scholar]
- Hoff T., Schnorr K. M., Meyer C., Caboche M. Isolation of two Arabidopsis cDNAs involved in early steps of molybdenum cofactor biosynthesis by functional complementation of Escherichia coli mutants. J Biol Chem. 1995 Mar 17;270(11):6100–6107. doi: 10.1074/jbc.270.11.6100. [DOI] [PubMed] [Google Scholar]
- Hugueney P., Badillo A., Chen H. C., Klein A., Hirschberg J., Camara B., Kuntz M. Metabolism of cyclic carotenoids: a model for the alteration of this biosynthetic pathway in Capsicum annuum chromoplasts. Plant J. 1995 Sep;8(3):417–424. doi: 10.1046/j.1365-313x.1995.08030417.x. [DOI] [PubMed] [Google Scholar]
- Hugueney P., Römer S., Kuntz M., Camara B. Characterization and molecular cloning of a flavoprotein catalyzing the synthesis of phytofluene and zeta-carotene in Capsicum chromoplasts. Eur J Biochem. 1992 Oct 1;209(1):399–407. doi: 10.1111/j.1432-1033.1992.tb17302.x. [DOI] [PubMed] [Google Scholar]
- Hundle B., Alberti M., Nievelstein V., Beyer P., Kleinig H., Armstrong G. A., Burke D. H., Hearst J. E. Functional assignment of Erwinia herbicola Eho10 carotenoid genes expressed in Escherichia coli. Mol Gen Genet. 1994 Nov 15;245(4):406–416. doi: 10.1007/BF00302252. [DOI] [PubMed] [Google Scholar]
- Joshi C. P. Putative polyadenylation signals in nuclear genes of higher plants: a compilation and analysis. Nucleic Acids Res. 1987 Dec 10;15(23):9627–9640. doi: 10.1093/nar/15.23.9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjarulff S., Okkels J. S. Cloning and sequencing of a full-length cDNA clone encoding the PSI-D subunit of photosystem I from barley. Plant Physiol. 1993 Jan;101(1):335–336. doi: 10.1104/pp.101.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Walton D. C. Violaxanthin is an abscisic Acid precursor in water-stressed dark-grown bean leaves. Plant Physiol. 1990 Mar;92(3):551–559. doi: 10.1104/pp.92.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti I. B., Murphy J. F., Shaw J. G., Hunt A. G. Plants that express a potyvirus proteinase gene are resistant to virus infection. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6110–6114. doi: 10.1073/pnas.90.13.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion-Poll A., Marin E., Bonnefoy N., Pautot V. Transposition of the maize autonomous element Activator in transgenic Nicotiana plumbaginifolia plants. Mol Gen Genet. 1993 Apr;238(1-2):209–217. doi: 10.1007/BF00279549. [DOI] [PubMed] [Google Scholar]
- Moore R., Smith J. D. Graviresponsiveness and abscisic-acid content of roots of carotenoid-deficient mutants of Zea mays L. Planta. 1985;164:126–128. [PubMed] [Google Scholar]
- Nakahigashi K., Miyamoto K., Nishimura K., Inokuchi H. Isolation and characterization of a light-sensitive mutant of Escherichia coli K-12 with a mutation in a gene that is required for the biosynthesis of ubiquinone. J Bacteriol. 1992 Nov;174(22):7352–7359. doi: 10.1128/jb.174.22.7352-7359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurk A., Kasak L., Kivisaar M. Sequence of the gene (pheA) encoding phenol monooxygenase from Pseudomonas sp. EST1001: expression in Escherichia coli and Pseudomonas putida. Gene. 1991 Jun 15;102(1):13–18. doi: 10.1016/0378-1119(91)90531-f. [DOI] [PubMed] [Google Scholar]
- Orser C. S., Lange C. C., Xun L., Zahrt T. C., Schneider B. J. Cloning, sequence analysis, and expression of the Flavobacterium pentachlorophenol-4-monooxygenase gene in Escherichia coli. J Bacteriol. 1993 Jan;175(2):411–416. doi: 10.1128/jb.175.2.411-416.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock C. D., Bowlby N. R., Hoffmann-Benning S., Zeevaart J. A. The aba Mutant of Arabidopsis thaliana (L.) Heynh. Has Reduced Chlorophyll Fluorescence Yields and Reduced Thylakoid Stacking. Plant Physiol. 1992 Dec;100(4):1796–1801. doi: 10.1104/pp.100.4.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock C. D., Heath T. G., Gage D. A., Zeevaart J. A. Abscisic alcohol is an intermediate in abscisic Acid biosynthesis in a shunt pathway from abscisic aldehyde. Plant Physiol. 1991 Oct;97(2):670–676. doi: 10.1104/pp.97.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock C. D., Zeevaart J. A. The aba mutant of Arabidopsis thaliana is impaired in epoxy-carotenoid biosynthesis. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7496–7499. doi: 10.1073/pnas.88.17.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle R. P., Morch M. D. Stop making sense: or Regulation at the level of termination in eukaryotic protein synthesis. FEBS Lett. 1988 Aug 1;235(1-2):1–15. doi: 10.1016/0014-5793(88)81225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd T. C., Dekker B. M., Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989 Mar 25;17(6):2362–2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijer W. J., Hofsteenge J., Vereijken J. M., Jekel P. A., Beintema J. J. Primary structure of p-hydroxybenzoate hydroxylase from Pseudomonas fluorescens. Biochim Biophys Acta. 1982 Jun 4;704(2):385–388. doi: 10.1016/0167-4838(82)90170-4. [DOI] [PubMed] [Google Scholar]
- Wierenga R. K., Terpstra P., Hol W. G. Prediction of the occurrence of the ADP-binding beta alpha beta-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986 Jan 5;187(1):101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]
- Wijnands R. A., Weijer W. J., Müller F., Jekel P. A., van Berkel W. J., Beintema J. J. Chemical modification of tyrosine residues in p-hydroxybenzoate hydroxylase from Pseudomonas fluorescens: assignment in sequence and catalytic involvement. Biochemistry. 1986 Jul 29;25(15):4211–4218. doi: 10.1021/bi00363a007. [DOI] [PubMed] [Google Scholar]
- You I. S., Ghosal D., Gunsalus I. C. Nucleotide sequence analysis of the Pseudomonas putida PpG7 salicylate hydroxylase gene (nahG) and its 3'-flanking region. Biochemistry. 1991 Feb 12;30(6):1635–1641. doi: 10.1021/bi00220a028. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Steppuhn J., Herrmann R. G. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989 Apr 1;180(3):535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]