Abstract
Background
Prolonged intensive care unit (ICU) stay is a predictor of mortality. The length of ICU stay has never been considered as a variable in an additive scoring system. How could this variable be integrated into a scoring system? Does this integration improve mortality prediction?
Material/Methods
The ‘modified CArdiac SUrgery Score’ (CASUS) was generated by implementing the length of stay as a new variable to the ‘additive CASUS’. The ‘logistic CASUS’ already considers this variable. We defined outcome as ICU mortality and statistically compared the three CASUS models. Discrimination, comparison of receiver operating characteristic curves (DeLong’s method), and calibration (observed/expected ratio) were analyzed on days 1–13.
Results
Between 2007 and 2010, we included 5207 cardiac surgery patients in this prospective study. The mean age was 67.2±10.9 years. The mean length of ICU stay was 4.6±7.0 days and ICU mortality was 5.9%. All scores had good discrimination, with a mean area under the curve of 0.883 for the additive and modified, and 0.895 for the ‘logistic CASUS’. DeLong analysis showed superiority in favor of the logistic model as from day 5. The calibration of the logistic model was good. We identified overestimation (days 1–5) and accurate (days 6–9) calibration for the additive and ‘modified CASUS’. The ‘modified CASUS’ remained accurate but the ‘additive CASUS’ tended to underestimate the risk of mortality (days 10–13).
Conclusions
The integration of length of ICU stay as a variable improves mortality prediction significantly. An ‘ICU-day’ variable should be included into a logistic but not an additive model.
MeSH Keywords: Biostatistics, Cardiac Surgical Procedures, Intensive Care
Background
No improvement of disease severity after cardiac surgery extends the duration of intensive care unit (ICU) stay. However, the definition of a prolonged postoperative ICU stay in cardiac surgery is controversial. It varies from greater than 24 hours to more than 14 days after ICU admission [1–6]. Nevertheless, a prolonged ICU stay is related to operative and postoperative problems, stands as a surrogate for morbidity [7], and is actually a strong predictor of mortality, as experienced in the praxis [4,5,7–9]. The association of prolonged ICU stay and mortality was merely investigated but never considered in intensive care risk stratification models. To the best of our knowledge, the ICU length of stay (ICULOS) was never included as a variable in an additive scoring system, in spite of its predictive power.
When our group published the ‘additive CArdiac SUrgery Score’ (additive CASUS) (Table 1) in 2005, by combining descriptors of mortality and multiorgan dysfunction, the importance of ICULOS was disregarded [10]. The ‘additive CASUS’ was evaluated in 3230 patients, recently validated in a population of 6007 patients [11], and showed high accuracy in other independent patient subsets [12–14].
Table 1.
Additive and ‘modified CASUS’.
| Organ system | Descriptor | Score points | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||
| Additive CASUS | Modified CASUS | Respiratory | PaO2 /FiO2 (mmHg/%) | Extubated | >250 | 151–250 | 75–150 | <75 |
| Renal | Creatinine (mg/dl) | <1.2 | 1.2–2.2 | 2.3–4.0 | 4.1–5.5 | >5.5 | ||
| CVVH/dialysis | No | Yes | ||||||
| Liver | Bilirubin (mg/dl) | <1.2 | 1.2–3.5 | 3.6–7.0 | 7.1–14.0 | >14.0 | ||
| Cardiovascular | PAR=HR×CVP/MAP | <10.1 | 10.1–15.0 | 15.1–20.0 | 20.1–30.0 | >30.0 | ||
| Lactic acid (mmol/l) | <2.1 | 2.1–4.0 | 4.1–8.0 | 8.1–12.0 | >12.0 | |||
| IABP | No | Yes | ||||||
| VAD | No | Yes | ||||||
| Coagulation | Platelets ×10≥/μL | > 120 | 81–120 | 51–80 | 21–50 | <21 | ||
| Central nervous | Neurologic state | Normal | Confused | Sedated | Diffuse neuropathy | |||
| ICU-day/ICULOS | 1 or 2 | 3–5 | 6–8 | 9–12 | ≥13 | |||
CVVH – continuous venovenous hemofiltration; FiO2 – fraction of inspired oxygen; IABP – intraaortic balloon pump; ICU – intensive care unit; ICULOS – ICU length of stay; PAR – pressure-adjusted heart rate (=HR: heart rate × CVP: central venous pressure/MAP: mean arterial blood pressure); PaO2 – partial oxygen pressure; VAD – ventricular assist device.
In 2012, we published the ‘logistic CASUS’ (Table 2) based on a logistic formula that calculates mortality prediction in percentage [15]. Different predictors of organ dysfunction gained a specific weight by their β-coefficients. This score is not only the first logistic model for cardiac surgical ICU patients, but is also the first system that integrates the ‘ICU-day’ as a variable [15].
Table 2.
‘Logistic CASUS’.
| Descriptor | β-coefficient | |
|---|---|---|
| PaO2/FiO2 (mmHg/%) | −0.0013498 | |
| Serum creatinine (mg/dl) | 0.3063634 | |
| CVVH/dialysis | No | 0 |
| Yes | 0.4109223 | |
| Serum bilirubin (mg/dl) | 0.2214784 | |
| PAR=HR×CVP/MAP | 0.0642751 | |
| Lactic acid (mmol/l) | 0.2115760 | |
| IABP | No | 0 |
| Yes | 0.6770239 | |
| VAD | No | 0 |
| Yes | 2.2976240 | |
| Platelets ×10≥/μL | −0.0042251 | |
| Neurologic state | Normal | 0 |
| Confused | 0.4736275 | |
| Sedated | 0.7020852 | |
| Dif. neuropath. | 1.4715528 | |
| ICU-day | 1 | 0 |
| 2 | 0.0097085 | |
| 3 | 0.8372058 | |
| 4 | 1.0426010 | |
| 5 | 1.2561380 | |
| 6 | 1.4975238 | |
| 7 | 1.6260023 | |
| 8 | 1.9023001 | |
| 9 | 2.1064412 | |
| 10 | 2.2268852 | |
| 11 | 2.2597632 | |
| 12 | 2.3782868 | |
| 13–∞ | 2.5289064 | |
| Constant | −5.6412079 | |
CVVH – continuous venovenous hemofiltration; Dif. neuropath. – diffuse neuropathy; FiO2 – fraction of inspired oxygen; IABP – intraaortic balloon pump; ICU – intensive care unit; PAR – pressure-adjusted heart rate (=HR: heart rate × CVP: central venous pressure/MAP: mean arterial blood pressure); PaO2 – partial oxygen pressure; VAD – ventricular assist device.
In this study, 2 questions were addressed: What is the best way to integrate the ICULOS into a scoring system? Does the integration of ICULOS improve mortality prediction? To answer these questions, we devised the ‘modified CASUS’ (Table 1) by integrating ‘ICU-day’ as a new variable into our additive model. Then, we statistically compared the ‘additive CASUS’, the ‘modified CASUS’, and the ‘logistic CASUS’ in a large cardiac surgery subset of patients.
Material and Methods
General information
The study is based on an evaluation of prospectively collected data of all consecutive adult patients admitted to our ICU after cardiac surgery between January 1st 2007 and December 31st 2010. It was approved by the Institutional Review Board of our university (approval number: 2809-05/10). We considered only the first admission for patients who were readmitted to the ICU during the study period. Outcome was defined as ICU mortality. Please find further information on the data collection in our previous study [15].
Score calculation of the ‘modified CASUS’
We calculated the ‘modified CASUS’ by adding ICULOS as an additional variable to the ‘additive CASUS’ to assess prolonged ICU stay in an individual patient. The new score (‘modified CASUS’) contains, accordingly, 11 variables. The total number of points ranges from 0 to 44.
The new variable (ICULOS) was divided into 5 categories. Each category was affiliated with points. During the first 48 hours, the points remain 0 for each patient, but the maximum number of points is 4 in case an ICU stay exceeds 12 days. Table 1 demonstrates the additive and the ‘modified CASUS’.
Statistical analyses
Discrimination was evaluated with receiver operating characteristic curves (ROC). The area under the curve (AUC) was used to indicate the discriminative ability of the parameters. For the comparison of the new ‘modified CASUS’ with the additive and the ‘logistic CASUS’, we used the method of DeLong [16]. Calibration was analyzed according to the observed versus expected ratio (O/E ratio). An O/E ratio of 1.0 indicates perfect calibration, while an O/E ratio <1 or >1 implies overestimation and underestimation, respectively. Discrimination statistics focus on an individual patient’s outcome, whereas calibration assesses the general population level. A value of p<0.05 was defined as significant. Statistical analyses were performed from ICU day 1 (operative day) (n=5207) to day 13 (n=353). Please find detailed information on the applied statistical methods in our previous study [15].
Results
Population characteristics
This study included 5207 ICU patients admitted over a period of 4 years; 37.6% were female and the mean age was 67.2±10.9 years. The types of surgical procedures are shown in Table 3. The mean length of ICU stay was 4.6±7.0 days. ICU mortality was 5.9% (n=305). The preoperative mean additive EuroSCORE was 6.3±3.7 and mean logistic EuroSCORE was 9.9±12.9. No sex-based differences were present. There were no missing data in this study.
Table 3.
Operations during the study period.
| Operations | Number | % |
|---|---|---|
| CABG | 2755 | 52.9 |
| Isolated valve surgery | 1187 | 22.8 |
| Combined CABG and valve surgery | 692 | 13.3 |
| Aortic surgery | 323 | 6.2 |
| Cardiac transplantation | 52 | 1.0 |
| Others (including congenital heart defects, tumors, pulmonary embolectomy, assist device implantation) | 198 | 3.8 |
| Total | 5207 | 100.0 |
CABG – coronary artery bypass grafting.
Results of the statistical analyses
Table 4 summarizes the discriminatory power, the comparison of the 3 models’ AUC curves by DeLong’s method, and the calibration of the models. The 3 scores had good discrimination on all days, with AUCs of ≥0.838 for the additive and ‘modified CASUS’, and ≥0.855 for the ‘logistic CASUS’. The best results of all scores were on day 2 (0.957). The additive and ‘modified CASUS’ demonstrated the smallest AUCs on day 12, whereas the ‘logistic CASUS’ showed the smallest AUC on day 10. In general, the ‘logistic CASUS’ showed better AUC curves compared to the other 2 models, throughout the statistical analysis.
Table 4.
Statistical results.
| ICU-day (patients) | Scoring model | ROC-analysis | De Long | O/E ratio | |
|---|---|---|---|---|---|
| AUC | 95%-CI | p-value | |||
| 1 (5207) | Add_CASUS | 0.905 | 0.887–0.924 | 1.0000 | 0.6207 |
| Log_CASUS | 0.908 | 0.888–0.928 | 0.5963 | 1.0011 | |
| Mod_CASUS | 0.905 | 0.887–0.924 | 0.6211 | ||
| 2 (5159) | Add_CASUS | 0.957 | 0.938–0.976 | 1.0000 | 0.6118 |
| Log_CASUS | 0.957 | 0.938–0.977 | 0.9934 | 0.9924 | |
| Mod_CASUS | 0.957 | 0.938–0.976 | 0.6109 | ||
| 3 (2372) | Add_CASUS | 0.935 | 0.920–0.950 | 1.0000 | 0.7888 |
| Log_CASUS | 0.939 | 0.928–0.949 | 0.6460 | 0.9976 | |
| Mod_CASUS | 0.935 | 0.925–0.946 | 0.8264 | ||
| 4 (1612) | Add_CASUS | 0.912 | 0.904–0.920 | 1.0000 | 0.8289 |
| Log_CASUS | 0.921 | 0.907–0.936 | 0.2691 | 0.9984 | |
| Mod_CASUS | 0.912 | 0.902–0.921 | 0.8419 | ||
| 5 (1165) | Add_CASUS | 0.893 | 0.886–0.901 | 1.0000 | 0.8691 |
| Log_CASUS | 0.911 | 0.905–0.918 | 0.0005 | 0.9986 | |
| Mod_CASUS | 0.893 | 0.886–0.901 | 0.8967 | ||
| 6 (888) | Add_CASUS | 0.888 | 0.883–0.894 | 1.0000 | 0.9277 |
| Log_CASUS | 0.902 | 0.896–0.908 | 0.0039 | 1.0015 | |
| Mod_CASUS | 0.888 | 0.881–0.896 | 0.9416 | ||
| 7 (713) | Add_CASUS | 0.878 | 0.874–0.882 | 1.0000 | 0.9688 |
| Log_CASUS | 0.892 | 0.886–0.898 | 0.0018 | 0.9989 | |
| Mod_CASUS | 0.878 | 0.871–0.885 | 0.9883 | ||
| 8 (601) | Add_CASUS | 0.857 | 0.853–0.862 | 1.0000 | 1.0232 |
| Log_CASUS | 0.881 | 0.875–0.886 | <.0001 | 1.0016 | |
| Mod_CASUS | 0.857 | 0.851–0.864 | 0.9967 | ||
| 9 (506) | Add_CASUS | 0.856 | 0.851–0.860 | 1.0000 | 1.0897 |
| Log_CASUS | 0.871 | 0.868–0.875 | <.0001 | 0.9989 | |
| Mod_CASUS | 0.856 | 0.850–0.862 | 1.0008 | ||
| 10 (448) | Add_CASUS | 0.846 | 0.841–0.859 | 1.0000 | 1.1113 |
| Log_CASUS | 0.855 | 0.852–0.858 | 0.0072 | 1.0007 | |
| Mod_CASUS | 0.846 | 0.840–0.852 | 1.0089 | ||
| 11 (415) | Add_CASUS | 0.852 | 0.847–0.856 | 1.0000 | 1.1146 |
| Log_CASUS | 0.866 | 0.863–0.869 | <.0001 | 1.0018 | |
| Mod_CASUS | 0.852 | 0.846–0.858 | 1.0123 | ||
| 12 (379) | Add_CASUS | 0.838 | 0.834–0.842 | 1.0000 | 1.1621 |
| Log_CASUS | 0.857 | 0.854–0.860 | <.0001 | 1.0010 | |
| Mod_CASUS | 0.838 | 0.832–0.844 | 1.0212 | ||
| 13 (353) | Add_CASUS | 0.856 | 0.851–0.861 | 1.0000 | 1.2015 |
| Log_CASUS | 0.878 | 0.875–0.881 | <.0001 | 1.0022 | |
| Mod_CASUS | 0.856 | 0.851–0.861 | 1.0322 | ||
Add.: additive; AUC – area under curve; CI – confidential interval; ICU – intensive care unit; Log. – logistic; Mod. – modified; O/E – observed/expected; p-value – comparison of the new modified CASUS with the additive and the logistic CASUS according to DeLong’s method; ROC – receiver operating characteristic.
For the ICU days 1 up to 4, the differences between AUCs were not significant. However, the DeLong analysis showed a significant p-value in favor of the logistic model from day 5 up to day 13. The AUCs of the additive and ‘modified CASUS’ were identical on all days and therefore had p-value=1.000 according to DeLong’s method.
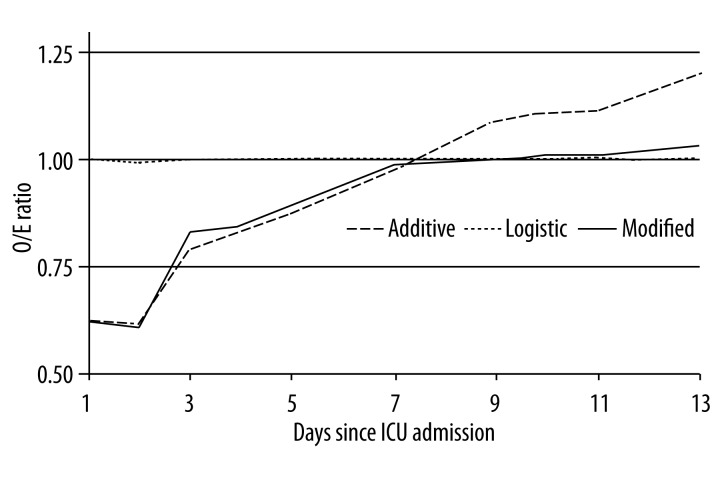
The calibration of the logistic model was good on all days and better than the additive and ‘modified CASUS’, especially during the early postoperative period (Figure 1). Both scores overestimated the mortality on day 1 up to day 5 (O/E <0.9). From day 6 up to day 9, the additive and ‘modified CASUS’ calibrated well (O/E 0.9–1.1). We detected a positive effect by integrating ‘ICU-day’ as a new variable in the ‘modified CASUS’ because its calibration remained appropriate (O/E <1.1) from day 10 up to day 13. The ‘additive CASUS’ tended to underestimate the risk of mortality from day 10 to day 13 (O/E >1.1) (Figure 1).
Figure 1.
Calibration of the 3 CASUS models. ICU – intensive care unit; O/E – observed/expected.
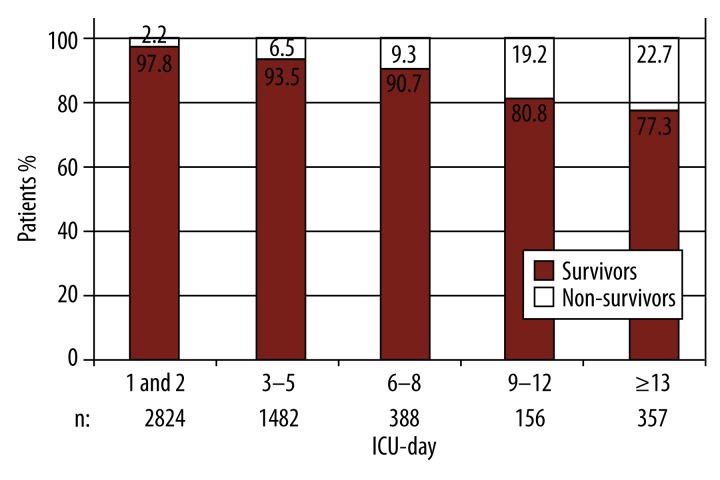
Figure 2 shows the patients’ distribution to survivors and non-survivors. The 5 groups were arranged in a manner similar to the ‘modified CASUS’ categories according to the length of ICU stay. It can be clearly seen that the portion of non-survivors increased the longer an ICU stay persisted: 2.2% for patients discharged within 48 hours versus 22.7% for those admitted for longer than 12 days.
Figure 2.
Survivors and non-survivors according to length of ICU stay. ICU – intensive care unit; n – number of patients.
Discussion
Definition and incidence of prolonged ICU stay
The definition of a prolonged ICU stay in cardiac surgery is controversial. Uncomplicated surgery and uneventful ICU stay allow an ICU discharge on the second day, when the pathophysiological sequels of the cardiac operation fade away [4–7]. This is in conformity to our discharge policy (Table 4), which points to a prolonged ICU stay in 45.6% of our patients. Nevertheless, some studies define prolonged ICU stay as from 7 up to 14 days after cardiac surgery [1–3].
Lagercrantz et al. defined prolonged ICU stay as more than 10 days, and consequently reported it for 3.5% of their study population [17]. In contrast to this, Silberman et al. set the cutoff at 48 hours and included 73% of their patients in the prolonged ICU stay group [7]. Accordingly, the incidence of prolonged ICU stay after cardiac surgery diverges widely depending on its definition [1–5,7–9,17–27].
Risk factors for a prolonged ICU stay
During recent years, a large number of pre-, intra-, and postoperative risk factors for prolonged ICU stay were identified. In a study of 10759 patients, Bucerius et al. postulated that preoperative predictors of prolonged ICU stay were: cardiac, pulmonary, and renal failure; advanced age; diabetes; hypertension; peripheral and cerebrovascular disease; and atrial fibrillation [4,5,7,8,17–19,26,28].
The urgency, duration, and complexity of cardiac surgery and a prolonged cardio-pulmonary-bypass time were identified as intraoperative risk factors for an extended ICU admission [4,5,7,8,18,20].
According to Hein et al., postoperative cardiac failure is an independent risk factor for a prolonged ICU stay [19]. Moreover, low ejection fraction was postulated as a risk factor by several authors [7,18,19,26]. This status is often accompanied by the need for high-dose catecholamine therapy and intra-aortic balloon pump, which are both risk factors themselves [4,8,18–20]. In addition to respiratory failure [4,7,19,20,28], renal insufficiency was also identified as a risk factor [4,5,7,8,17,19,20]. Other strong postoperative predictors are blood transfusion, re-exploration [4,18], stroke, and sepsis [7]. Benetis et al. postulated the readmission to ICU as a cofactor of significantly higher mortality after cardiac surgery [29]. Furthermore, the rare complications of deep sternal wound infection and post-sternotomy mediastinitis might lead to a prolonged ICU stay and end in increased mortality [30]. Finally, a postoperative peripheral cytopenia in patients after orthotropic heart transplantation might extend the duration of intensive care unit stay [31].
Influence of prolonged ICU stay on short-term survival
Patients that require a prolonged ICU stay have a higher short-term mortality [3–5,7–9,18], due to cardiac reasons [7,17] or multiorgan dysfunction [2,17,21–24]. Heimrath et al. reported a short-term mortality after prolonged ICU stay of 10%, which was significantly higher than the 1.2% for patients after non-prolonged ICU stay [5]. Hein et al. observed a significantly increased ICU mortality in patients with an ICU stay longer than 3 days in comparison to patients with ICULOS <3 days [19]. Hassan et al. postulated prolonged ICU stay as a predictor of short-term mortality, with an odds ratio of 20.9 [8]. Mahesh et al. reported that patients who required a prolonged ICU stay had a significantly higher ICU mortality compared to the control group (10% versus 0.6%, respectively; p-value <0.001) [4]. Soppa et al. subdivided patients with prolonged ICU stay into 2 groups (group A: 5–10 days; group B: >10 days). ICU mortality was 13% for group A compared to 20% for group B [9]. In 2013, Silberman et al. compared mortality rates according to ICULOS after cardiac surgery [7]. They distributed 6385 patients into 3 groups (group 1: <2 days; group 2: 3–14 days; group 3: >14 days). The early postoperative mortality rates were significantly different (2%, 8%, and 40%, respectively).
The incidence of short-term mortality diverges widely depending on the definition of prolonged ICU stay [3,18,28]. In Figure 2 we display the correlation of increased mortality and long ICU stay in our patient population.
‘ICU-day’ as a variable in an additive scoring system
According to several authors, the ICULOS is a strong predictor of mortality [4,5,7–9]. However, the ICULOS has never been included as a variable in an additive scoring system.
The general approach to assess the influence of a long ICU stay on mortality is by dividing a population into groups. Although there is no consistent definition of prolonged ICU stay, this division is based on 1 or several cut-off points [1–5,7–9,17–27]. This has the potential risk of wrongly converting the continuous nature of a prolonged ICU stay into a dichotomous or categorical unit and might therefore lead to inaccurate mortality prediction.
By integrating ‘ICU-day’ as a categorical variable into an additive model, we created the ‘modified CASUS’. Due to the new variable, we identified a more accurate mortality prediction in calibration statistics on patients with a long ICU stay. We failed to demonstrate an improved outcome prediction on the level of an individual patient, since the discrimination of additive and ‘modified CASUS’ was identical.
Despite its high influence on mortality, the lack of improved individual outcome prediction might be why ICULOS was never considered as a variable in an additive scoring system.
‘ICU-day’ as a variable of a logistic model
In accordance with our results, Silberman et al. postulated a proportional increase of negative outcome as a consequence of prolonged ICU stay [7]. Especially among elective surgical patients, this effect arises not immediately after cardiac surgery, but after some days, when organ dysfunctions evolve [32].
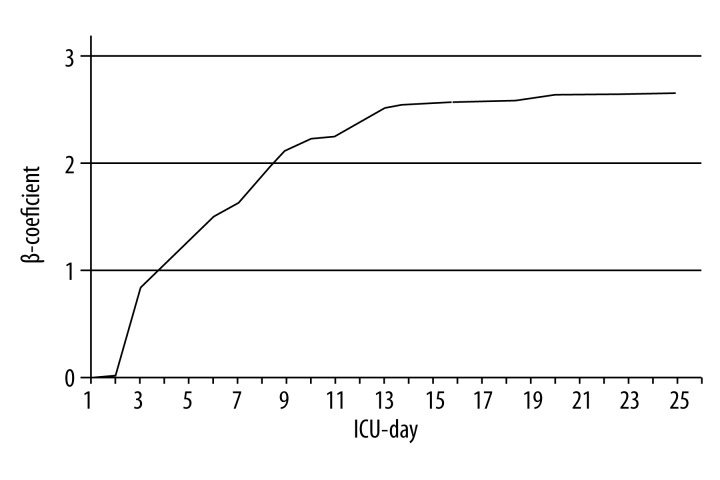
‘ICU-day’ is an established variable of the ‘logistic CASUS’ [15]. The score’s logistic character maintains the continuous nature of a prolonged ICU stay and its progressive influence on mortality. Table 2 captures the increasing weight of the ‘ICU-day’ variable by demonstrating the parameter’s β-coefficients, which remain consistent after the 13th postoperative day (Figure 3) [15].
Figure 3.
Variable ‘ICU-day’ in ‘logistic CASUS’: course of β-coefficients from day 1 to day 25. ICU – intensive care unit.
During the study’s conception phase, we calculated the ‘logistic CASUS’ with and without the ‘ICU-day’ variable to detect its particular effect on mortality prediction. This approach was later dismissed because it necessitated the total recalculation of the score’s β-coefficients, which affects not only the ‘ICU-day’ variable, but also the other 10 parameters and the recalculated β-coefficients would have generated misleading results.
The ‘logistic CASUS’ showed a significantly improved accuracy in mortality prediction as from day 5, which demonstrates the importance of the ‘ICU-day’ variable during a long ICU stay. The improved accuracy applies to the levels of the individual patient and the general population. Thus, the ‘logistic CASUS’ is superior to the other 2 models.
Conclusions
We detected a significant influence of prolonged ICU stay on adverse outcome. This effect arises on the fifth day. To significantly improve mortality prediction, the length of ICU stay should be considered and ICULOS should be included as a variable in postoperative scoring systems. The significant improvement of mortality prediction for an individual patient was observed in a logistic model, but not in an additive ICU scoring system. We therefore recommend the integration of ‘ICU-day’ into a logistic score. In this regard, additive systems might be replaced by logistic models, which could gain the user’s attention.
Acknowledgements
We thank Dr. rer. pol. Thomas Lehmann (Institute of Medical Statistics, Computer Sciences and Documentation of University of Jena/Germany) for his statistical consulting.
Appendix
The additive and ‘logistic CASUS’, as well as the 2013 published third generation score RACE (RApide Clinical Evaluation), can be calculated online (English version: http://www.cardiac-icu.org; German version: http://www.cardiac-icu.de) or downloaded on a personal digital assistant or smartphone. The download is available for free in the iTunes App store: http://itunes.apple.com/us/app/cardiac-icu/id389965786?mt=8.
Footnotes
Conflicts of interest
The authors declare that they have neither financial nor non-financial competing interests.
Source of support: Departmental sources
References
- 1.Bashour CA, Yared JP, Ryan TA, et al. Long-term survival and functional capacity in cardiac surgery patients after prolonged intensive care. Crit Care Med. 2000;28:3847–53. doi: 10.1097/00003246-200012000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Engoren M, Buderer NF, Zacharias A. Long-term survival and health status after prolonged mechanical ventilation after cardiac surgery. Crit Care Med. 2000;28:2742–49. doi: 10.1097/00003246-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Williams MR, Wellner RB, Hartnett EA, et al. Long-term survival and quality of life in cardiac surgical patients with prolonged intensive care unit length of stay. Ann Thorac Surg. 2002;73:1472–78. doi: 10.1016/s0003-4975(02)03464-1. [DOI] [PubMed] [Google Scholar]
- 4.Mahesh B, Choong CK, Goldsmith K, et al. Prolonged stay in intensive care unit is a powerful predictor of adverse outcomes after cardiac operations. Ann Thorac Surg. 2012;94:109–16. doi: 10.1016/j.athoracsur.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Heimrath OP, Buth KJ, Légaré JF. Long-term outcomes in patients requiring stay of more than 48 hours in the intensive care unit following coronary bypass surgery. J Crit Care. 2007;22:153–58. doi: 10.1016/j.jcrc.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Doering LV, Esmailian F, Imperial-Perez F, Monsein S. Determinants of intensive care unit length of stay after coronary artery bypass graft surgery. Heart Lung. 2001;30:9–17. doi: 10.1067/mhl.2001.112502. [DOI] [PubMed] [Google Scholar]
- 7.Silberman S, Bitran D, Fink D, et al. Very prolonged stay in the intensive care unit after cardiac operations: early results and late survival. Ann Thorac Surg. 2013;96:15–22. doi: 10.1016/j.athoracsur.2013.01.103. [DOI] [PubMed] [Google Scholar]
- 8.Hassan A, Anderson C, Kypson A, et al. Clinical outcomes in patients with prolonged intensive care unit length of stay after cardiac surgical procedures. Ann Thorac Surg. 2012;93:565–69. doi: 10.1016/j.athoracsur.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Soppa G, Woodford C, Yates M, et al. Functional status and survival after prolonged intensive care unit stay following cardiac surgery. Interact Cardiovasc Thorac Surg. 2013;16:750–54. doi: 10.1093/icvts/ivt046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hekmat K, Kroener A, Stuetzer H, et al. Daily assessment of organ dysfunction and survival in intensive care unit cardiac surgical patients. Ann Thorac Surg. 2005;79:1555–62. doi: 10.1016/j.athoracsur.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Hekmat K, Doerr F, Kroener A, et al. Prediction of mortality in intensive care unit cardiac surgical patients. Eur J Cardiothorac Surg. 2010;38:104–9. doi: 10.1016/j.ejcts.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 12.Doerr F, Badreldin AM, Heldwein MB, et al. A comparative study of four intensive care outcome prediction models in cardiac surgery patients. J Cardiothorac Surg. 2011;6:21. doi: 10.1186/1749-8090-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badreldin AM, Kroener A, Heldwein MB, et al. Prognostic value of daily cardiac surgery score (CASUS) and its derivatives in cardiac surgery patients. Thorac Cardiovasc Surg. 2010;58:392–97. doi: 10.1055/s-0030-1250080. [DOI] [PubMed] [Google Scholar]
- 14.Badreldin AM, Doerr F, Ismail MM, et al. Comparison between Sequential Organ Failure Assessment Score (SOFA) and Cardiac Surgery Score (CASUS) for mortality prediction after cardiac surgery. Thorac Cardiovasc Surg. 2012;60:35–42. doi: 10.1055/s-0030-1270943. [DOI] [PubMed] [Google Scholar]
- 15.Doerr F, Badreldin AM, Bender EM, et al. Outcome prediction in cardiac surgery: the first logistic scoring model for cardiac surgical intensive care patients. Minerva Anestesiol. 2012;78:879–86. [PubMed] [Google Scholar]
- 16.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 17.Lagercrantz E, Lindblom D, Sartipy U. Survival and quality of life in cardiac surgery patients with prolonged intensive care. Ann Thorac Surg. 2010;89:490–95. doi: 10.1016/j.athoracsur.2009.09.073. [DOI] [PubMed] [Google Scholar]
- 18.Bucerius J, Gummert JF, Walther T, et al. Predictors of prolonged ICU stay after on-pump versus off-pump coronary artery bypass grafting. Intensive Care Med. 2004;30:88–95. doi: 10.1007/s00134-003-1950-5. [DOI] [PubMed] [Google Scholar]
- 19.Hein OV, Birnbaum J, Wernecke K, et al. Prolonged intensive care unit stay in cardiac surgery: risk factors and long-term-survival. Ann Thorac Surg. 2006;81:880–85. doi: 10.1016/j.athoracsur.2005.09.077. [DOI] [PubMed] [Google Scholar]
- 20.Gersbach P, Tevaearai H, Revelly JP, et al. Are there accurate predictors of long-term vital and functional outcomes in cardiac surgical patients requiring prolonged intensive care? Eur J Cardiothorac Surg. 2006;29:466–72. doi: 10.1016/j.ejcts.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 21.Chertow GM, Levy EM, Hammermeister KE, et al. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–48. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 22.Ostermann ME, Taube D, Morgan CJ, Evans TW. Acute renal failure following cardiopulmonary bypass: a changing picture. Intensive Care Med. 2000;26:565–71. doi: 10.1007/s001340051205. [DOI] [PubMed] [Google Scholar]
- 23.Pappalardo F, Franco A, Landoni G, et al. Long-term outcome and quality of life of patients requiring prolonged mechanical ventilation after cardiac surgery. Eur J Cardiothorac Surg. 2004;25:548–52. doi: 10.1016/j.ejcts.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 24.Kern H, Redlich U, Hotz H, et al. Risk factors for prolonged ventilation after cardiac surgery using APACHE II, SAPS II, and TISS: comparison of three different models. Intensive Care Med. 2001;27:407–15. doi: 10.1007/s001340000802. [DOI] [PubMed] [Google Scholar]
- 25.Bapat V, Allen D, Young C, et al. Survival and quality of life after cardiac surgery complicated by prolonged intensive care. J Card Surg. 2005;20:212–17. doi: 10.1111/j.1540-8191.2005.200413.x. [DOI] [PubMed] [Google Scholar]
- 26.Gaudino M, Girola F, Piscitelli M, et al. Long-term survival and quality of life of patients with prolonged postoperative intensive care unit stay: unmasking an apparent success. J Thorac Cardiovasc Surg. 2007;134:465–69. doi: 10.1016/j.jtcvs.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 27.Mazzoni M, De Maria R, Bortone F, et al. Long-term outcome of survivors of prolonged intensive care treatment after cardiac surgery. Ann Thorac Surg. 2006;82:2080–87. doi: 10.1016/j.athoracsur.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 28.Hortal J, Giannella M, Pérez MJ, et al. Incidence and risk factors for ventilator-associated pneumonia after major heart surgery. Intensive Care Med. 2009;35:1518–25. doi: 10.1007/s00134-009-1523-3. [DOI] [PubMed] [Google Scholar]
- 29.Benetis R, Sirvinskas E, Kumpaitiene B, et al. A case-control study of readmission to the intensive care unit after cardiac surgery. Med Sci Monit. 2013;19:148–52. doi: 10.12659/MSM.883814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dohmen PM. Post-sternotomy mediastinitis after cardiac surgery. Med Sci Monit. 2014;20:59–60. doi: 10.12659/MSM.890279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urbanowicz T, Straburzyńska-Migaj E, Klotzka A, et al. Induction therapy, tacrolimus plasma concentration, and duration if intensive care unit stay are risk factors for peripheral leucopenia following heart transplantation. Ann Transplant. 2014;19:494–98. doi: 10.12659/AOT.890816. [DOI] [PubMed] [Google Scholar]
- 32.Le Gall JR, Klar J, Lemeshow S, et al. The Logistic Organ Dysfunction system. A new way to assess organ dysfunction in the intensive care unit. ICU Scoring Group. JAMA. 1996;276:802–10. doi: 10.1001/jama.276.10.802. [DOI] [PubMed] [Google Scholar]