Abstract
Background
As the most common primary bone tumor, osteosarcoma has an improved survival rates with advancement of treatment methods. A higher rate of metastasis, however, leads to the aggravation of the disease. Studies have shown that some genes, namely osteosarcoma metastasis-related genes, participate in the process of tumor metastasis. The peripheral myelin protein 22 (PMP22) gene has recently been found to be abundantly expressed in the oncogenesis of osteosarcoma. Its detailed role and function in the tumor metastasis, however, remains unknown.
Material/Methods
The recombinant retroviral plasmid pcDNA3.1-PMP22 was constructed and used to transfect osteosarcoma cells SOSP-M, whose cell proliferation was measured by MTT method. The formation of tumor cell colony, the cell migration and invasion were also measured. The signal transduction pathway MAPK was further analyzed by Western blotting.
Results
The pcDNA3.1-PMP22 plasmid was confirmed to have a 305bp PMP22 fragment by EcoRI-XhoI dual digestion. Compared to the control group, osteosarcoma cell invasion was significantly facilitated by the transfection of pcDNA3.1-PMP22 plasmid (p<0.05). The recombinant plasmid also significantly potentiated the formation of tumor cell colony and increased the migration and invasion ability of tumor cells (p<0.05 in all cases). Phosphorylated p-ERK and p-P38 were also up-regulated by vector transfection (p<0.05).
Conclusions
Osteosarcoma metastasis-related gene PMP22 participates in the proliferation, invasion, migration and colony formation of osteosarcoma cells possibly via the MAPK signal transduction pathway, providing evidences for further investigation of metastatic mechanism of osteosarcoma.
MeSH Keywords: Cell Migration Assays, Osteosarcoma, Peroxiredoxins
Background
Osteosarcoma usually occurs in children and adolescents, and is the most common primary bone tumor. It is originated from mesenchymal tissues, featured with spindle stromal cells accompanied with bone-like tissues, and commonly occurred during the bone lengthening stage [1,2]. Due to its insignificant clinical symptoms at early stage, osteosarcoma is often misdiagnosed as growing pain or trauma, thereby causing its rapid progression, frequent metastasis, high malignancy and mortality rate, making it one major challenge facing by clinicians and heavy burdens for patients and their families [3,4]. Although the survival rate has been improved with the advancement of surgical, radio-, and chemo-therapy, most osteosarcoma patients still developed multiple metastases, causing unfavorable prognosis [5].
Tumor metastasis-related genes have received lots of research interests as they may help to elucidate the mechanism of tumor metastasis. These genes also have the potency to be as the target for anti-metastatic drug, as well as to provide guidance for evaluating prognosis [6,7]. The expressional alternation of peripheral myelin protein 22 (PMP22) gene expressions can cause motor-sensory neuropathy and is one important pathogenic gene of Carcot-Marie-Tooth disease [8,9]. Recent studies have found high expression of PMP22 in breast cancer cells had, making this gene as an independent prognostic factor evaluating disease-free survival time and overall survival rate of breast cancer patients [10]. PMP22 has also been suggested to be abnormally expressed in various tumors such as prostate cancer, indicating the significant role of this gene in the pathogenesis and development of tumors [11]. DNA microarray analysis has revealed elevated expression of PMP22 in osteosarcoma tissues [12]. The detailed role and function of PMP22 in metastasis of osteosarcoma, however, remains unknown. Therefore this study aimed to construct a recombinant viral plasmid pcDNA3.1-PMP22, which was identified and investigated for its role in osteosarcoma metastasis.
Material and Methods
Culture of osteosarcoma cell line SOSP-M
SOSP-M cell line (ATCC cell bank, US) stored in liquid nitrogen was melt down at 37°C water bath, centrifuged at 1 000 rpm for 3 min, re-suspended in 1 ml fresh DMEM medium (Sigma, US) and transferred into 50-ml culture flask. After incubation at 37°C chamber with 5% CO2 and saturated humidity for 48 hr, cells (1×107 per ml) were inoculated into petri dish with 10% FBS (Bioind, Israel) in DMEM and cultured at 37°C chamber with 5% CO2.
Preparation, identification and transfection of recombinant plasmids
Total RNA was extracted from cultured SOSP-M cells using Trizol reagents (Invitrogen, US) and quantified under UV-spectrometer. In vitro transcription was performed using total RNA as the template to synthesize cDNA using the test kit (Promega, US). Specific primers designed by PrimerPremier 6.0 and synthesized by Shanghai BioTech (China) were used to amplify the target gene using cDNA as the template in a PCR reactor (Model 2400, PE Gene, US). Primer sequences were: PMP22-F, 5′-TGTGC CTTCG CGCCA TGATT-3′; PMP22-R, 5′-GCTTA TCTGA TCTGT CCTTC-3′; Amplification fragment length, 305bp. PCR conditions were: 95°C for 1min, 62°C for 50 sec, 94°C for 30 sec, 60°C for 50 sec and 72°C for 35 sec, repeated for 35 cycles. PCR products were purified from the agarose gel using gel purification kit (Promega, US) and were ligated to empty pcDNA3.1 vector at 3:1 ratio at 4°C for 16 h. Recombinant plasmids were then transferred to JM109 competent cells, which were then cultured in LB-plate for amplification.
Purified pcDNA3.1-PMP22 recombinant plasmids were digested with EcoRI+XhoI restriction enzymes (Promega, US) at 37°C for 4 h. Digested products were separated using 1.5% agarose gel electrophoresis with EB-staining. DNA bands were identified by UV transilluminator.
Purified recombinant plasmids (1 μg/μl, A260/A280=1.8) was used to transfect cultured cells using liposome method following Lipo2000’s manual instruction (Invitrogen, US). In brief, 2 μg plasmids and 5 μl liposome were dissolved in 250 μl DMEM medium respectively. After incubation at room temperature for 20 min, both plasmid and liposome solutions were slowly added into cultured SOSP-M cells. After 24 hour-incubation at 37°C, culture medium was changed. Both control (no plasmid transfection) and empty (transfected with empty plasmids) groups were performed in parallel.
Cell proliferation, clonal formation rate, migration and invasion assays
Following previously reported protocols [13], SOSP-M cells at log phase from each group (transfection, negative and empty control) were seeded into 96-well plate at 5×103 per ml concentration using 10% FBS-DMEM. After 24-hour incubation, supernatants were discarded and 20 μl sterilized MTT (Shanghai BioTech, China) were added into each well at every 24 hours. After 4-hour incubation, 150 μl DMSO (Shanghai BioTech, China) were added into each well whose supernatant solutions have been discarded. The culture plate was then vibrated for 10 min, followed by reading absorption values at 570 nm. The growth inhibition rate was calculated based on light absorption values.
SOSP-M cells at log phase were collected for preparing cell suspensions and were counted. 5 ml of cell suspension solutions were seeded into culture dish containing 50, 100 or 200 cells. After incubation at 37°C with 5% CO2 for 2–3 weeks, culture medium was discarded when the cell colony was visible. After PBS washing, the colony was dried, fixed by methanol for 15 min, stained by Giemsa dye for 10 min, washed and dried. The colony formation rate (in%)=(colony number/seeded cell number)×100%. All experiments were performed in triplicates (N=3).
The streak method was employed to evaluate the migration ability of tumor cells. In brief, cultured cells from all groups were seeded into 6-well plate (5×105 cells per well). Horizontal lines were drawn at the back of the plate by marker pens. After 24 hours when the monolayer has been formed, a horizontal streak was made at the bottom of the dish with reference to the pre-drawn lines. After washing away cells at the streak, remaining cells were further cultured with DMEM containing 5% or 10% FBS for 24 hours. The migration of cells was observed under the microscope. All experiments were performed in triplicates (N=3).
In evaluating cell invasive ability, SOSP-M cells from all groups 48 hours after transfection were further cultured in non-serum medium for 24 hours to cover the bottom and upper face of the membrane of Transwell chamber (Hyclone, US). The chamber was further covered with dilution buffer containing 50 mg/L Matrigel and dried at 4°C. After draining all solutions, cells were further cultured for 30 min at 37°C with 50 μl non-serum medium containing 10 g/L BSA. Then the Transwell chambers were placed into 24-well plate, which was cultured using DMEM containing 10% FBS. Tumor cell suspensions cultured using non-serum medium were added inside the chamber. In the control group, Transwell chambers without Matrigel were used to incubate tumor cells under the same condition. Cells from all groups were cultured for 48 hours, followed by PBS washing of the chamber to remove cells at the upper surface of the membrane. Those cells were fixed in cold ethanol and stained for 30 min. Cells at the lower surface of the membrane from 10 fields were counted and averaged. All experiments were performed in triplicates (N=3).
Western blot assays for the effect on MAPK signal transduction pathway
The potential effect of PMP22 plasmid transfection on MAPK signal transduction pathway was analyzed by Western blot method. After reaching a confluent rate more than 80%, cells were lysed on ice for 30 min, followed by ultrasound vibration and centrifugation by 10,000 g at 4°C for 15 min. Supernatants after lysis were saved and quantified for protein contents. Cell lyse were mixed with elution buffer and heated at 95°C for 5 min. After separation in 10% SDS-PAGE electrophoresis, proteins were transferred to PVDF membrane, on which non-specific binding sites were blocked by 5% defatted milk powder incubation at room temperature for 2 hours. Primary antibody (1:1 000 dilution, Cell signaling, US) was incubated at 4°C overnight. Mouse anti-rabbit IgG secondary antibody (1:2 000 dilution, Santa Cruz, US) was then added for 30-min incubation at room temperature in dark. After rinsing in PSBT, enhanced ECL chromogenic substrates (Amersham Biosci, US) were used to develop the membrane for 1 min, followed by X-ray exposure. Images were captured by Bio-Rad imaging software and optical density of bands was analyzed by Quantity One software. Anti-actin antibody worked as the internal reference. All experiments were repeated for four times (N=4).
Statistical analysis
All measurement data were presented as mean ± standard deviation. A comparison among multiple groups was performed by analysis of variance (ANOVA) following previous established methods [14,15]. Further between-group-comparison was done by Dunnett’s test. All enumeration data were tested by chi-square test. A statistical significance was identified when p<0.05. All statistical analyses were done using the SPSS11.5 software package.
Results
Identification of recombinant retroviral plasmids by dual enzyme digestion
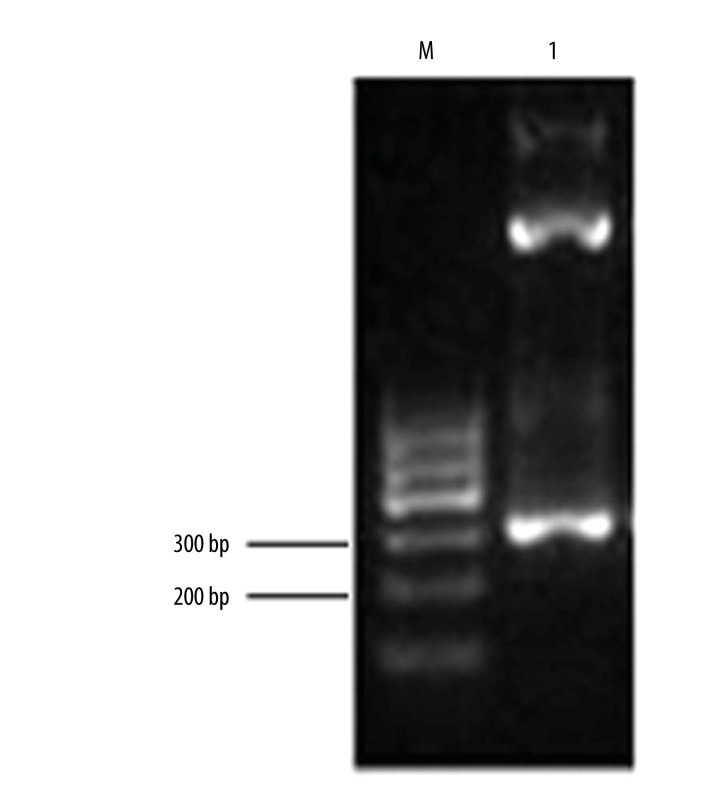
We applied both EcoRI and XhoI restriction enzymes and PCR amplification to identify the recombinant pcDNA3.1-PMP22 plasmids. The insertion fragment was found to have a length about 300 bp (Figure 1), suggesting the successful construction of retroviral plasmid pcDNA3.1-PMP22 plasmid.
Figure 1.
Identification of pcDNA3.1-PMP22 plasmids. M, DNA ladder; Lane 1, PCR fragments after enzymatic digestion.
PMP22 facilitates the proliferation of SOSP-M cells
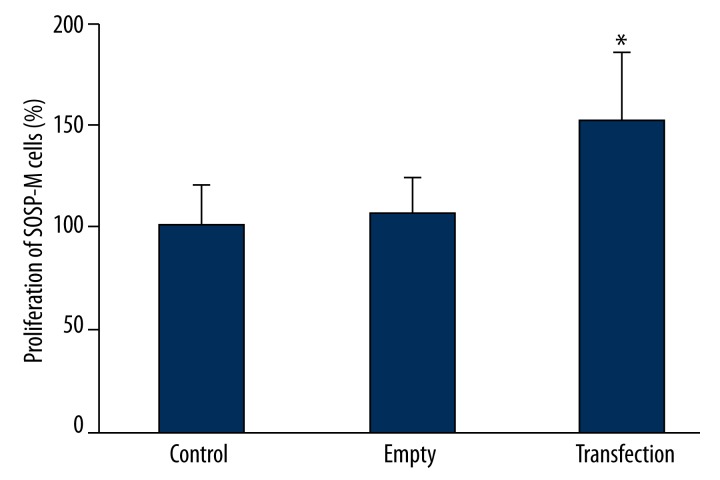
Using MTT assay to detect the effect of PMP22 transfection on tumor proliferation, results (Figure 2) showed that after transfecting pcDNA3.1-PMP22 plasmid, osteosarcoma cells had significantly elevated proliferation when compared to either of the control or empty group (p<0.05).
Figure 2.
The effect of PMP22 on SOSP-M cell proliferation. * p<0.05 compared to control group.
Colony formation of SOSP-M cell was elevated by PMP22
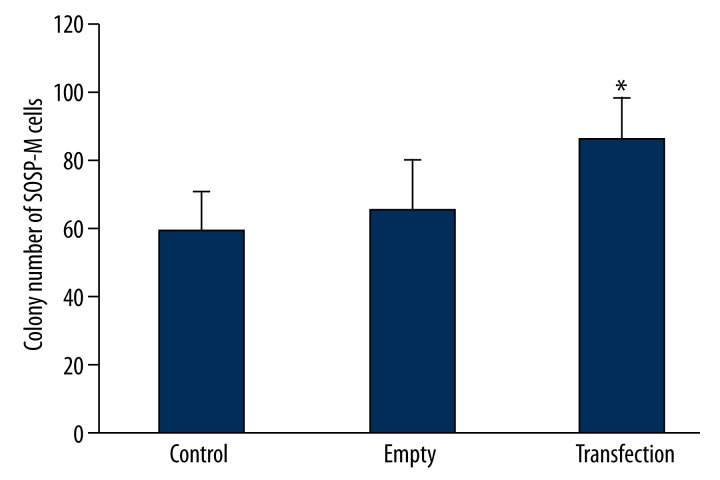
We further utilized plate colony formation assay to detect the effect of PMP22 on the SOSP-M cell’s colony formation rate. Results (Figure 3) showed that SOSP-M cells transfected with pcDNA3.1-PMP22 plasmids had significantly higher colony formation rates when compared to those in control group (p<0.05).
Figure 3.
The effect of PMP22 on the colony formation of PMP22. * p<0.05 compared to control group.
PMP22 transfection potentiated tumor cell migration
The streak assay was further used to reveal the effect of PMP22 on the migration ability of SOSP-M cells. Results (Figure 4) showed that the transfection of empty plasmids did not affect the migration of tumor cells. Those with pcDNA3.1-PMP22 plasmid transfection, however, had significantly elevated migration levels.
Figure 4.
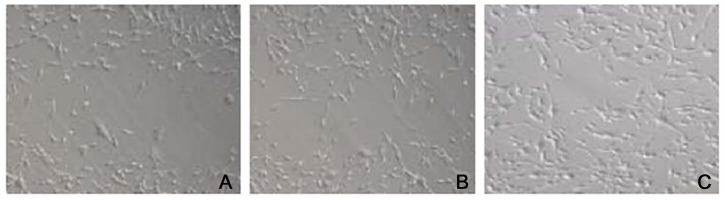
The effect of PMP22 on migration of SOSP-M cells.(A) control group; (B) empty group; (C) pcDNA3.1-PMP22 transfection group.
PMP22 can facilitate invasive ability of SOSP-M cells
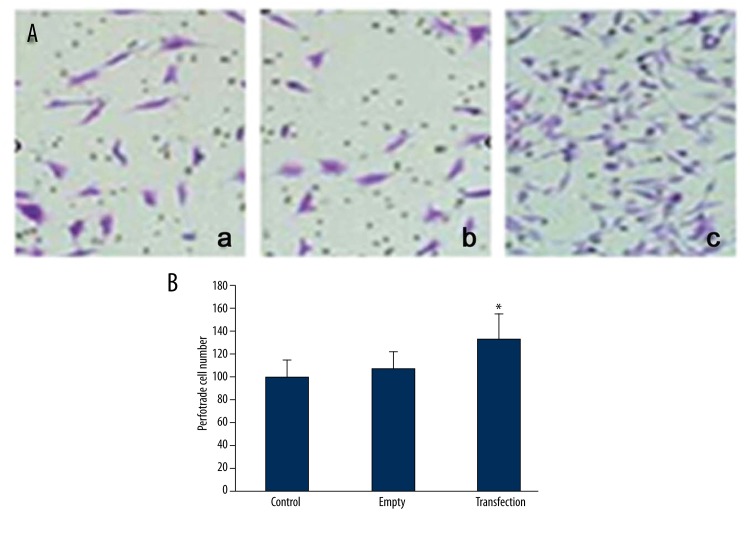
We further used Tranwell chamber assay to quantify the influence of PMP22 on the invasive ability of SOSP-M cells. Results showed that the transfection of empty plasmids did not affect the cell invasive ability as no significant changes of perforated cell numbers existed between empty and control group (Figure 5, p>0.05). Cells transfected with pcDNA3.1-PMP22 plasmids, however, showed significantly higher number of perforated cells when compared to the control group (Figure 5, p<0.05), suggesting that PMP22 can potentiate the invasive ability of osteosarcoma cells.
Figure 5.
The effect of PMP22 on the invasive ability of SOSP-M cells. (A) showed representative staining images from all groups (a, control; b, empty; c, transfection); (B) showed quantitative results of perforated cell numbers in all groups. * p<0.05 compared to those in control group.
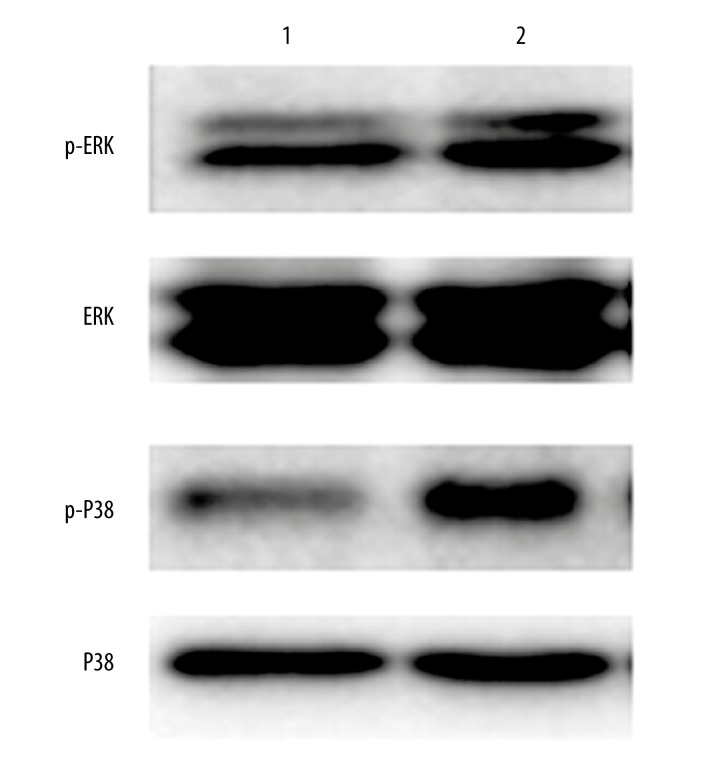
PMP22 activates MAPK pathway
Using Western blotting assay, we found significantly elevated expressional levels of p-ERK and p-P38 in transfected cells when compared to control ones (Figure 6). This result suggests that PMP22 may exert its facilitating role of osteosarcoma cell invasion, migration and colony formation via the activation of MAPK pathway.
Figure 6.
The effect of PMP22 on MAPK pathway. Lane 1, control cells; Lane 2, pcDNA3.1-PMP22 transfected cells.
Discussion
As a primary malignant bone cancer, osteosarcoma commonly occurred in young people and had a higher mortality rate due to its rapid progression and higher malignancy. Although treatment has been improved in recent years, more than half of patients still suffered from failure treatment due to its tendency to metastasis, thereby making the metastatic mechanism receive more research interests [12]. With the advanced study about tumor genomics and proteomics, the identification of osteosarcoma metastasis-related genes has the potency to reveal the invasion mechanism of the tumor and to be the molecular target for clinical treatment [16,17].
Differential expressions of genes have been discovered in tumor tissues as a result of advanced gene chip technique. Certain genes have dramatic expressional alternations during the early stage of oncogenesis while other genes had differential expressional profiles in late-stage cancer cells. In addition, some genes had significant alternated expressional patterns during the whole stage of tumor’s occurrence and progression. Current opinions agree that the metastasis of osteosarcoma is a complex process involving multiple factors in a cascade, accompanying with the higher metastatic activity of tumor cells. Previous studies have indicated the participation of certain genes in the metastasis of osteosarcoma and named them osteosarcoma metastasis-related genes [18–20]. Therefore it is of critical values for the treatment against osteosarcoma to identify and confirm such genes, in addition to the functional characterization of their roles in the occurrence, progression and prognosis prediction [21,22].
Some groups used DNA microarray analysis to identify a cohort of 13 genes with elevated expression levels in the metastatic osteosarcoma samples when compared to primary tumors [23,24]. Among all those candidate genes, CD73 and IRF4 are closely related to the anti-oxidation of osteosarcoma cells and activation of lymphocytes. It is known that Ezrin protein is included in the osteosarcoma metastasis-related gene family. A study in the animal model using DNA microarray analysis found 7 genes with significant expressional variations between higher metastatic and lower metastatic sub-groups of osteosarcoma cells. Some of those genes are correlated with the proliferation and invasion ability of tumors [23,24]. Past studies reported that PMP22 mainly exerted its functions in the motor-sensory neuropathy [25]. Recent studies revealed its elevated expression in various malignant tumors, including breast cancer and prostate cancer, and its correlation with patients’ prognosis, thereby suggesting its potential relationship with the invasion ability of the tumor [10,11]. DNA-microarray analysis found elevated expression of PMP22 in osteosarcoma tissues especially in those with high prevalence of metastasis [26]. These results suggest the potential oncogene role of PMP22 in the occurrence of osteosarcoma, but leaving its detailed mechanisms unresolved. This study successfully constructed a pcDNA3.1-PMP22 recombinant plasmid, which was confirmed to facilitate cell proliferation and colony formation of cultured osteosarcoma cells, suggesting the trophic function of PMP22 in osteosarcoma pathogenesis. Further assays revealed that PMP22 could potentiate the migration and invasion of tumor cells, indicating the direct relationship between osteosarcoma metastasis and PMP22, which can work as an osteosarcoma metastasis-related gene. Some studies have confirmed the important role of MAPK in oncogenesis of osteosarcoma via its participation in tumor cell proliferation, differentiation, migration, and invasion by DNA microarray analysis [27]. This study demonstrated a higher phosphorylation level of ERK and p38 in the MAPK signal pathway, suggesting the possible involvement of PMP22 in osteosarcoma via its effect on MAPK signal pathway.
Conclusions
Osteosarcoma metastasis-related gene PMP22 participates in the oncogenesis, progression, and metastasis of osteosarcoma, possibly via its effects on the MAPK signal transduction pathway. This study provides evidence for the metastatic mechanism of osteosarcoma, although its detailed pathways require further in-depth investigations.
Footnotes
Source of support: Departmental sources
References
- 1.Trosman SJ, Krakovitz PR. Pediatric Maxillary and Mandibular Tumors. Otolaryngol Clin North Am. 2014;48(1):101–19. doi: 10.1016/j.otc.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Vijayakumar V, Lowery R, Zhang X, et al. Pediatric Osteosarcoma: A Single Institution’s Experience. South Med J. 2014;107(11):671–75. doi: 10.14423/SMJ.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 3.Kim SS, Park YK. Significance of MTA1 in the molecular characterization of osteosarcoma. Cancer Metastasis Rev. 2014;33(4):981–91. doi: 10.1007/s10555-014-9523-3. [DOI] [PubMed] [Google Scholar]
- 4.Sun L, Li Y, Zhang J, et al. Prognostic value of pathologic fracture in patients with high grade localized osteosarcoma: A systemic review and meta-analysis of cohort studies. J Orthop Res. 2015;33(1):131–39. doi: 10.1002/jor.22734. [DOI] [PubMed] [Google Scholar]
- 5.Ebrahimzadeh MH, Vahedi E, Ganji R, Bozorgnia S. Skeletal sarcoma on the site of retained war bullet fragments and a literature review on long-term complications of retained war shells. Arch Bone Jt Surg. 2013;1(2):107–11. [PMC free article] [PubMed] [Google Scholar]
- 6.Salah S, Toubasi S. Factors predicting survival following complete surgical remission of pulmonary metastasis inosteosarcoma. Mol Clin Oncol. 2015;3(1):157–62. doi: 10.3892/mco.2014.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagao-Kitamoto H, Setoguchi T, Kitamoto S, et al. Ribosomal protein S3 regulates GLI2-mediated osteosarcoma invasion. Cancer Lett. 2015;356(2 Pt B):855–61. doi: 10.1016/j.canlet.2014.10.042. [DOI] [PubMed] [Google Scholar]
- 8.Chumakov I, Milet A, Cholet N, et al. Polytherapy with a combination of three repurposed drugs (PXT3003) down-regulates Pmp22 over-expression and improves myelination, axonal and functional parameters in models of CMT1A neuropathy. Orphanet J Rare Dis. 2014;9(1):201. doi: 10.1186/s13023-014-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosso G, Liashkovich I, Gess B, et al. Unravelling crucial biomechanical resilience of myelinated peripheral nerve fibres provided by the Schwann cell basal lamina and PMP22. Sci Rep. 2014;4:7286. doi: 10.1038/srep07286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winslow S, Leandersson K, Larsson C. Regulation of PMP22 mRNA by G3BP1 affects cell proliferation in breast cancer cells. Mol Cancer. 2013;12(1):156. doi: 10.1186/1476-4598-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong D, Heinze G, Pils D, et al. Gene expression of PMP22 is an independent prognostic factor for disease-free and overall survival in breast cancer patients. BMC Cancer. 2010;10:682. doi: 10.1186/1471-2407-10-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Zhu X, Li H, et al. Piperine inhibits proliferation of human osteosarcoma cells via G2/M phase arrest and metastasisby suppressing MMP-2/-9 expression. Int Immunopharmacol. 2014;24(1):50–58. doi: 10.1016/j.intimp.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Jing W, Chen Y, Lu L, et al. Human umbilical cord blood-derived mesenchymal stem cells producing IL15 eradicate established pancreatic tumor in syngeneic mice. Mol Cancer Ther. 2014;13(8):2127–37. doi: 10.1158/1535-7163.MCT-14-0175. [DOI] [PubMed] [Google Scholar]
- 14.Hu YC, Wu L, Yan LF, et al. Predicting subtypes of thymic epithelial tumors using CT: new perspective based on a comprehensive analysis of 216 patients. Sci Rep. 2014;4:6984. doi: 10.1038/srep06984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu YC, Yan LF, Wu L, et al. Intravoxel incoherent motion diffusion-weighted MR imaging of gliomas: efficacy in preoperative grading. 2014;4:7208. doi: 10.1038/srep07208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vormoor B, Knizia HK, Batey MA, et al. Development of a preclinical orthotopic xenograft model of ewing sarcoma and other human malignant bone disease using advanced in vivo imaging. PLoS One. 2014;9(1):e85128. doi: 10.1371/journal.pone.0085128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang LY, Gatenby EL, Kamida A, et al. Global gene expression analysis of canine osteosarcoma stem cells reveals a novel role for COX-2 in tumour initiation. PLoS One. 2014;9(1):e83144. doi: 10.1371/journal.pone.0083144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Yang Z, Meng Z, et al. Knockdown of TRPM8 suppresses cancer malignancy and enhances epirubicin-induced apoptosis in human osteosarcoma cells. Int J Biol Sci. 2013;10(1):90–102. doi: 10.7150/ijbs.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jentzsch T, Robl B, Husmann M, et al. Expression of MSH2 and MSH6 on a Tissue Microarray in Patients with Osteosarcoma. Anticancer Res. 2014;34(12):6961–72. [PubMed] [Google Scholar]
- 20.Ahmed H, Salama A, Salem SE, Bahnassy AA. A case of synchronous double primary breast carcinoma and osteosarcoma: Mismatch repair genes mutations as a possible cause for multiple early onset malignant tumors. Am J Case Rep. 2012;13:218–23. doi: 10.12659/AJCR.883382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai X, Ma W, He X, Jha RK. Review of therapeutic strategies for osteosarcoma, chondrosarcoma, and Ewing’s sarcoma. Med Sci Monit. 2011;17(8):RA177–90. doi: 10.12659/MSM.881893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghule P, Kadam PA, Jambhekar N, et al. p53 gene gets altered by various mechanisms: studies in childhood sarcomas and retinoblastoma. Med Sci Monit. 2006;12(12):BR385–96. [PubMed] [Google Scholar]
- 23.Kimura Y, Sumiyoshi M. Antitumor and antimetastatic actions of dihydroxycoumarins (esculetin or fraxetin) through the inhibition of M2 macrophage differentiation in tumor-associated macrophages and/or G1 arrest in tumor cells. Eur J Pharmacol. 2014;746C:115–25. doi: 10.1016/j.ejphar.2014.10.048. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Tang YJ, Li ZH, et al. KiSS1 inhibits growth and invasion of osteosarcoma cells through inhibition of the MAPK pathway. Eur J Histochem. 2013;57(4):e30. doi: 10.4081/ejh.2013.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan T, Wunder JS, Gokgoz N, et al. COPS3 amplification and clinical outcome in osteosarcoma. Cancer. 2007;109(9):1870–76. doi: 10.1002/cncr.22595. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez RK, Maegbaek ML, Liede A, et al. Bone metastases, skeletal-related events, and survival among children with cancer in Denmark. J Pediatr Hematol Oncol. 2014;36(7):528–33. doi: 10.1097/MPH.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 27.Li GD, Cai ZD, Zhang YQ, et al. Gene profiling of MAPK pathway in human osteosarcoma. Zhonghua Zhong Liu Za Zhi. 2009;31(5):340–45. [PubMed] [Google Scholar]