Abstract
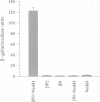
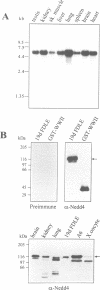
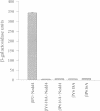
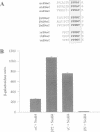
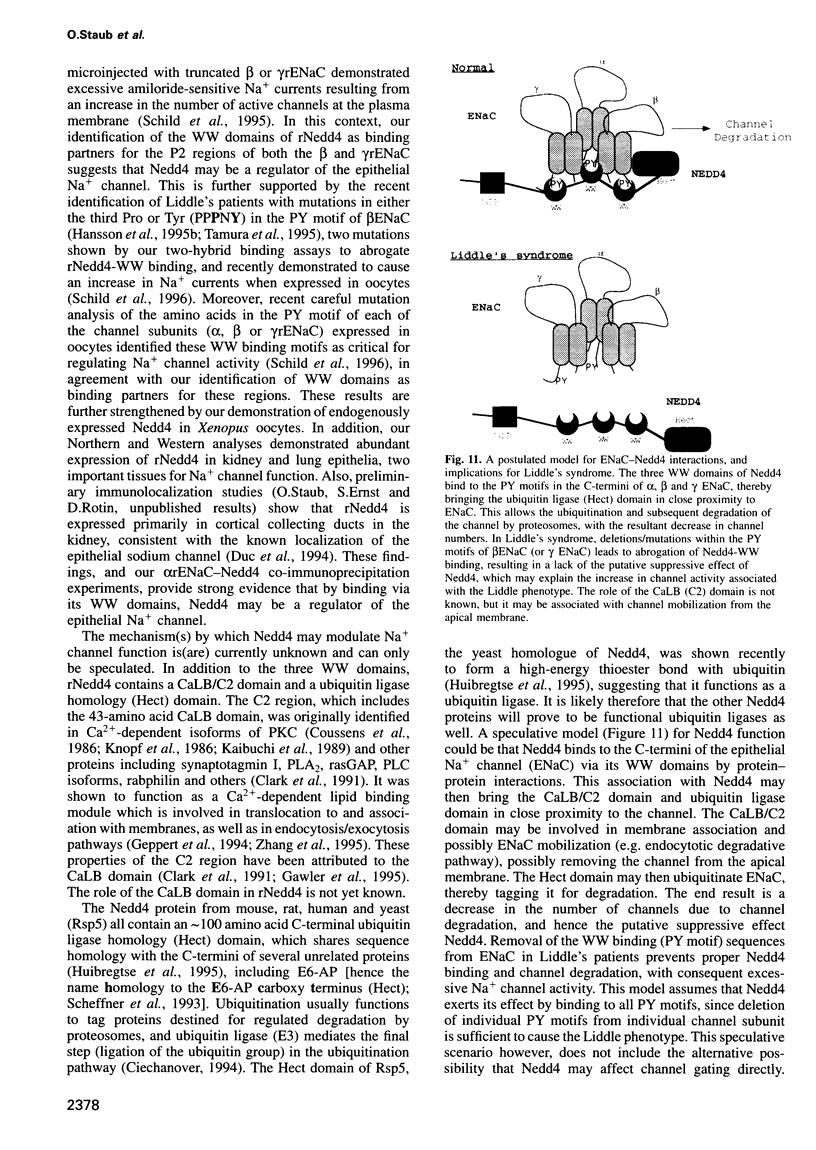
The amiloride-sensitive epithelial sodium channel (ENaC) plays a major role in sodium transport in kidney and other epithelia, and in regulating blood pressure. The channel is composed of three subunits (alphabetagamma) each containing two proline-rich sequences (P1 and P2) at its C-terminus. The P2 regions in human beta and gammaENaC, identical to the rat betagammarENaC, were recently shown to be deleted in patients with Liddle's syndrome (a hereditary form of hypertension), leading to hyperactivation of the channel. Using a yeast two-hybrid screen, we have now identified the rat homologue of Nedd4 (rNedd4) as the binding partner for the P2 regions of beta and gammarENaC. rNedd4 contains a Ca2+ lipid binding (CaLB or C2) domain, three WW domains and a ubiquitin ligase (Hect) domain. Our yeast two-hybrid and in vitro binding studies revealed that the rNedd4-WW domains mediate this association by binding to the P2 regions, which include the PY motifs (XPPXY) of either betarENaC (PPPNY) or gammarENaC (PPPRY). SH3 domains were unable to bind these sequences. Moreover, mutations to Ala of Pro616 or Tyr618 within the betarENaC P2 sequence (to PPANY or PPPNA, respectively), recently described in Liddle's patients, led to abrogation of rNedd4-WW binding. Nedd4-WW domains also bound to the proline-rich C-terminus (containing the sequence PPPAY) of alpharENaC, and endogenous Nedd4 co-immunoprecipitated with alpharENaC expressed in MDCK cells. These results demonstrate that the WW domains of rNedd4 bind to the PY motifs deleted from beta or gammaENaC in Liddle's syndrome patients, and suggest that Nedd4 may be a regulator (suppressor) of the epithelial Na+ channel.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André B., Springael J. Y. WWP, a new amino acid motif present in single or multiple copies in various proteins including dystrophin and the SH3-binding Yes-associated protein YAP65. Biochem Biophys Res Commun. 1994 Dec 15;205(2):1201–1205. doi: 10.1006/bbrc.1994.2793. [DOI] [PubMed] [Google Scholar]
- Benos D. J., Awayda M. S., Ismailov I. I., Johnson J. P. Structure and function of amiloride-sensitive Na+ channels. J Membr Biol. 1995 Jan;143(1):1–18. doi: 10.1007/BF00232519. [DOI] [PubMed] [Google Scholar]
- Bork P., Sudol M. The WW domain: a signalling site in dystrophin? Trends Biochem Sci. 1994 Dec;19(12):531–533. doi: 10.1016/0968-0004(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Breeden L., Nasmyth K. Regulation of the yeast HO gene. Cold Spring Harb Symp Quant Biol. 1985;50:643–650. doi: 10.1101/sqb.1985.050.01.078. [DOI] [PubMed] [Google Scholar]
- Canessa C. M., Horisberger J. D., Rossier B. C. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature. 1993 Feb 4;361(6411):467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- Canessa C. M., Merillat A. M., Rossier B. C. Membrane topology of the epithelial sodium channel in intact cells. Am J Physiol. 1994 Dec;267(6 Pt 1):C1682–C1690. doi: 10.1152/ajpcell.1994.267.6.C1682. [DOI] [PubMed] [Google Scholar]
- Canessa C. M., Schild L., Buell G., Thorens B., Gautschi I., Horisberger J. D., Rossier B. C. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994 Feb 3;367(6462):463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Chen H. I., Sudol M. The WW domain of Yes-associated protein binds a proline-rich ligand that differs from the consensus established for Src homology 3-binding modules. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7819–7823. doi: 10.1073/pnas.92.17.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien C. T., Bartel P. L., Sternglanz R., Fields S. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994 Oct 7;79(1):13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Clark J. D., Lin L. L., Kriz R. W., Ramesha C. S., Sultzman L. A., Lin A. Y., Milona N., Knopf J. L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991 Jun 14;65(6):1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- Cohen G. B., Ren R., Baltimore D. Modular binding domains in signal transduction proteins. Cell. 1995 Jan 27;80(2):237–248. doi: 10.1016/0092-8674(95)90406-9. [DOI] [PubMed] [Google Scholar]
- Coussens L., Parker P. J., Rhee L., Yang-Feng T. L., Chen E., Waterfield M. D., Francke U., Ullrich A. Multiple, distinct forms of bovine and human protein kinase C suggest diversity in cellular signaling pathways. Science. 1986 Aug 22;233(4766):859–866. doi: 10.1126/science.3755548. [DOI] [PubMed] [Google Scholar]
- Duc C., Farman N., Canessa C. M., Bonvalet J. P., Rossier B. C. Cell-specific expression of epithelial sodium channel alpha, beta, and gamma subunits in aldosterone-responsive epithelia from the rat: localization by in situ hybridization and immunocytochemistry. J Cell Biol. 1994 Dec;127(6 Pt 2):1907–1921. doi: 10.1083/jcb.127.6.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Chen J. K., Yu H., Simon J. A., Schreiber S. L. Two binding orientations for peptides to the Src SH3 domain: development of a general model for SH3-ligand interactions. Science. 1994 Nov 18;266(5188):1241–1247. doi: 10.1126/science.7526465. [DOI] [PubMed] [Google Scholar]
- Garty H. Molecular properties of epithelial, amiloride-blockable Na+ channels. FASEB J. 1994 May;8(8):522–528. doi: 10.1096/fasebj.8.8.8181670. [DOI] [PubMed] [Google Scholar]
- Gawler D. J., Zhang L. J., Reedijk M., Tung P. S., Moran M. F. CaLB: a 43 amino acid calcium-dependent membrane/phospholipid binding domain in p120 Ras GTPase-activating protein. Oncogene. 1995 Mar 2;10(5):817–825. [PubMed] [Google Scholar]
- Guarente L. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- Hansson J. H., Nelson-Williams C., Suzuki H., Schild L., Shimkets R., Lu Y., Canessa C., Iwasaki T., Rossier B., Lifton R. P. Hypertension caused by a truncated epithelial sodium channel gamma subunit: genetic heterogeneity of Liddle syndrome. Nat Genet. 1995 Sep;11(1):76–82. doi: 10.1038/ng0995-76. [DOI] [PubMed] [Google Scholar]
- Hansson J. H., Schild L., Lu Y., Wilson T. A., Gautschi I., Shimkets R., Nelson-Williams C., Rossier B. C., Lifton R. P. A de novo missense mutation of the beta subunit of the epithelial sodium channel causes hypertension and Liddle syndrome, identifying a proline-rich segment critical for regulation of channel activity. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11495–11499. doi: 10.1073/pnas.92.25.11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt R. P., Poulain-Godefroy O., Billotte J., Kraehenbuhl J. P., Fasel N. Highly inducible synthesis of heterologous proteins in epithelial cells carrying a glucocorticoid-responsive vector. Gene. 1992 Feb 15;111(2):199–206. doi: 10.1016/0378-1119(92)90687-k. [DOI] [PubMed] [Google Scholar]
- Hofmann K., Bucher P. The rsp5-domain is shared by proteins of diverse functions. FEBS Lett. 1995 Jan 23;358(2):153–157. doi: 10.1016/0014-5793(94)01415-w. [DOI] [PubMed] [Google Scholar]
- Huibregtse J. M., Scheffner M., Beaudenon S., Howley P. M. A family of proteins structurally and functionally related to the E6-AP ubiquitin-protein ligase. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2563–2567. doi: 10.1073/pnas.92.7.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi K., Fukumoto Y., Oku N., Takai Y., Arai K., Muramatsu M. Molecular genetic analysis of the regulatory and catalytic domains of protein kinase C. J Biol Chem. 1989 Aug 15;264(23):13489–13496. [PubMed] [Google Scholar]
- Knopf J. L., Lee M. H., Sultzman L. A., Kriz R. W., Loomis C. R., Hewick R. M., Bell R. M. Cloning and expression of multiple protein kinase C cDNAs. Cell. 1986 Aug 15;46(4):491–502. doi: 10.1016/0092-8674(86)90874-3. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Tomooka Y., Noda M. Identification of a set of genes with developmentally down-regulated expression in the mouse brain. Biochem Biophys Res Commun. 1992 Jun 30;185(3):1155–1161. doi: 10.1016/0006-291x(92)91747-e. [DOI] [PubMed] [Google Scholar]
- Lingueglia E., Renard S., Waldmann R., Voilley N., Champigny G., Plass H., Lazdunski M., Barbry P. Different homologous subunits of the amiloride-sensitive Na+ channel are differently regulated by aldosterone. J Biol Chem. 1994 May 13;269(19):13736–13739. [PubMed] [Google Scholar]
- Lingueglia E., Voilley N., Waldmann R., Lazdunski M., Barbry P. Expression cloning of an epithelial amiloride-sensitive Na+ channel. A new channel type with homologies to Caenorhabditis elegans degenerins. FEBS Lett. 1993 Feb 22;318(1):95–99. doi: 10.1016/0014-5793(93)81336-x. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Baltimore D. Signalling through SH2 and SH3 domains. Trends Cell Biol. 1993 Jan;3(1):8–13. doi: 10.1016/0962-8924(93)90194-6. [DOI] [PubMed] [Google Scholar]
- McDonald F. J., Snyder P. M., McCray P. B., Jr, Welsh M. J. Cloning, expression, and tissue distribution of a human amiloride-sensitive Na+ channel. Am J Physiol. 1994 Jun;266(6 Pt 1):L728–L734. doi: 10.1152/ajplung.1994.266.6.L728. [DOI] [PubMed] [Google Scholar]
- Nelson R. M., Long G. L. A general method of site-specific mutagenesis using a modification of the Thermus aquaticus polymerase chain reaction. Anal Biochem. 1989 Jul;180(1):147–151. doi: 10.1016/0003-2697(89)90103-6. [DOI] [PubMed] [Google Scholar]
- O'Brodovich H. M. The role of active Na+ transport by lung epithelium in the clearance of airspace fluid. New Horiz. 1995 May;3(2):240–247. [PubMed] [Google Scholar]
- Renard S., Lingueglia E., Voilley N., Lazdunski M., Barbry P. Biochemical analysis of the membrane topology of the amiloride-sensitive Na+ channel. J Biol Chem. 1994 Apr 29;269(17):12981–12986. [PubMed] [Google Scholar]
- Rotin D., Bar-Sagi D., O'Brodovich H., Merilainen J., Lehto V. P., Canessa C. M., Rossier B. C., Downey G. P. An SH3 binding region in the epithelial Na+ channel (alpha rENaC) mediates its localization at the apical membrane. EMBO J. 1994 Oct 3;13(19):4440–4450. doi: 10.1002/j.1460-2075.1994.tb06766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saumon G., Basset G. Electrolyte and fluid transport across the mature alveolar epithelium. J Appl Physiol (1985) 1993 Jan;74(1):1–15. doi: 10.1152/jappl.1993.74.1.1. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Huibregtse J. M., Vierstra R. D., Howley P. M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993 Nov 5;75(3):495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- Schiestl R. H., Gietz R. D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989 Dec;16(5-6):339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Schild L., Canessa C. M., Shimkets R. A., Gautschi I., Lifton R. P., Rossier B. C. A mutation in the epithelial sodium channel causing Liddle disease increases channel activity in the Xenopus laevis oocyte expression system. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5699–5703. doi: 10.1073/pnas.92.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets R. A., Warnock D. G., Bositis C. M., Nelson-Williams C., Hansson J. H., Schambelan M., Gill J. R., Jr, Ulick S., Milora R. V., Findling J. W. Liddle's syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell. 1994 Nov 4;79(3):407–414. doi: 10.1016/0092-8674(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Snyder P. M., McDonald F. J., Stokes J. B., Welsh M. J. Membrane topology of the amiloride-sensitive epithelial sodium channel. J Biol Chem. 1994 Sep 30;269(39):24379–24383. [PubMed] [Google Scholar]
- Sudol M., Chen H. I., Bougeret C., Einbond A., Bork P. Characterization of a novel protein-binding module--the WW domain. FEBS Lett. 1995 Aug 1;369(1):67–71. doi: 10.1016/0014-5793(95)00550-s. [DOI] [PubMed] [Google Scholar]
- Voilley N., Lingueglia E., Champigny G., Mattéi M. G., Waldmann R., Lazdunski M., Barbry P. The lung amiloride-sensitive Na+ channel: biophysical properties, pharmacology, ontogenesis, and molecular cloning. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):247–251. doi: 10.1073/pnas.91.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtek A. B., Hollenberg S. M., Cooper J. A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993 Jul 16;74(1):205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- Yu H., Chen J. K., Feng S., Dalgarno D. C., Brauer A. W., Schreiber S. L. Structural basis for the binding of proline-rich peptides to SH3 domains. Cell. 1994 Mar 11;76(5):933–945. doi: 10.1016/0092-8674(94)90367-0. [DOI] [PubMed] [Google Scholar]
- Zhang J. Z., Davletov B. A., Südhof T. C., Anderson R. G. Synaptotagmin I is a high affinity receptor for clathrin AP-2: implications for membrane recycling. Cell. 1994 Sep 9;78(5):751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Zhang J. Z., Davletov B. A., Südhof T. C., Anderson R. G. Synaptotagmin I is a high affinity receptor for clathrin AP-2: implications for membrane recycling. Cell. 1994 Sep 9;78(5):751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]