Uric acid and ATP from damaged hepatocytes activate IL-1β, a signature inflammasome-dependent cytokine crucial in the pathogenesis of alcoholic liver disease.
Keywords: inflammasome, NLRP3, sterile inflammatory response, DAMPs, liver inflammation
Abstract
Inflammation defines the progression of ALD from reversible to advanced stages. Translocation of bacterial LPS to the liver from the gut is necessary for alcohol-induced liver inflammation. However, it is not known whether endogenous, metabolic danger signals are required for inflammation in ALD. Uric acid and ATP, 2 major proinflammatory danger signals, were evaluated in the serum of human volunteers exposed to a single dose of ethanol or in supernatants of primary human hepatocytes exposed to ethanol. In vitro studies were used to evaluate the role of uric acid and ATP in inflammatory cross-talk between hepatocytes and immune cells. The significance of signaling downstream of uric acid and ATP in the liver was evaluated in NLRP3-deficient mice fed a Lieber-DeCarli ethanol diet. Exposure of healthy human volunteers to a single dose of ethanol resulted in increased serum levels of uric acid and ATP. In vitro, we identified hepatocytes as a significant source of these endogenous inflammatory signals. Uric acid and ATP mediated a paracrine inflammatory cross-talk between damaged hepatocytes and immune cells and significantly increased the expression of LPS-inducible cytokines, IL-1β and TNF-α, by immune cells. Deficiency of NLRP3, a ligand-sensing component of the inflammasome recognizing uric acid and ATP, prevented the development of alcohol-induced liver inflammation in mice and significantly ameliorated liver damage and steatosis. Endogenous metabolic danger signals, uric acid, and ATP are involved in inflammatory cross-talk between hepatocytes and immune cells and play a crucial role in alcohol-induced liver inflammation.
Introduction
Progression of ALD to advanced form is tightly linked to liver inflammation (reviewed in ref. [1]). We have previously demonstrated that the proinflammatory cytokine, IL-1β is required for the development of alcohol-induced liver inflammation, steatosis, and injury and that the pathogenic effect of IL-1β is specific to resident liver macrophages (Kupffer cells) [2]. We also demonstrated that in the mouse model of ALD, the conversion of inactive pro-IL-1β to active IL-1β required inflammasome, an intracellular multicomponent complex that triggers inflammation in response to endogenous danger signals.
Synthesis of active IL-1β is a 2-step process. In the 1st step, microbial-derived signals up-regulate pro-IL-1B, resulting in synthesis of inactive pro-IL-1β. This step is triggered by binding of bacterial LPS, derived from the gut, to the TLR4 on Kupffer cells and provides an initial proinflammatory signal in ALD [3–7]. Deficiency in TLR4 prevents up-regulation of pro-IL-1B in the liver of alcohol-fed mice [8]. In the 2nd step, signals derived from host cells (“danger” signals) activate the inflammasome, resulting in cleavage of pro-IL-1β into the active IL-1β [9]. The nature of endogenous danger signals that activate inflammasome and IL-1β in ALD is not known.
Here, we show that hepatocytes damaged by ethanol release endogenous metabolic danger molecules, uric acid, and ATP, which are recognized by liver immune cells as inflammatory signals. Uric acid and ATP from damaged hepatocytes are required for processing and activation of IL-1β, a cytokine requiring inflammasome activation that is crucial in the pathogenesis of ALD [2]. Our data suggest that the release of these molecules represents signals permissive for the proinflammatory effect of LPS in ALD.
MATERIALS AND METHODS
Animal studies
Six- to 8-week-old female C57Bl/6 WT (The Jackson Laboratory, Bar Harbor, ME, USA), NLRP3-deficient mice (kind gift of Dr. Amy Hise, Case Western Reserve University, Cleveland, OH, USA), all on a C57Bl/6 background, were used. Some animals were fed with the Lieber-DeCarli ad libitum diet (Bioserv, Frenchtown, NJ, USA) with 5% vol/vol ethanol (36%-derived calories); pair-fed control mice matched the alcohol-derived calories with dextran-maltose. Specifically, mice received a fresh Lieber-DeCarli diet in 50 ml feeders daily, between 7:00 PM and 8:00 PM. At the conclusion of the experiment, we collected blood and harvested livers between 8:00 AM and 9:00 AM [2]. Serum ethanol levels did not significantly differ between the genotypes. All animals received proper care in agreement with animal protocols approved by the Institutional Animal Care and Use Committee of the UMASS Medical School (Worcester, MA, USA).
Administration of ethanol to human volunteers
Peripheral blood was obtained by venipuncture with heparin anticoagulation (0.5 μg/ml blood) from 11 healthy volunteers (aged 21–60 years; 9 women, 2 men) from the clerical and professional staff at UMASS Medical Center. Volunteers abstained from alcoholic beverages for at least 48 hours before blood donation. The average alcohol use was <6 drinks/week for women and <9 drinks/week for men. Alcohol-use habits of blood donors were determined by a health assessment questionnaire that incorporates the AUDIT and CAGE tests and Health Screening Survey to identify frequency and quantities of alcohol use. In brief, blood samples (30–120 ml) were obtained on day 1 before alcohol consumption, as well as at indicated time-points after the ethanol uptake. Alcohol was given as vodka, 2 ml/kg body weight (0.85 g ethanol/kg body weight), diluted with orange juice, for a total volume of 300 ml. The alcohol was consumed within 30 minutes. Three male volunteers from the professional staff at UMASS Medical Center that were not exposed to ethanol were used as controls. The study was reviewed and approved by the Institutional Committee for Protection of Human Subjects in Research.
Isolation of primary mouse hepatocytes and LMNCs
Anesthetized animals were perfused by way of portal vein with saline solution, followed by enzymatic digestion, as described in refs. [2, 10]. The hepatocytes were separated by centrifugation, and LMNCs were purified by centrifugation in Percoll gradient. LPS was purchased from Sigma (St. Louis, MO, USA), and IL-1β-specific neutralizing antibody (AF-401) was purchased from R&D Systems (Minneapolis, MN, USA) and used according to the manufacturer’s specifications.
Primary human hepatocytes
Primary human hepatocytes were obtained from the NIH Liver Tissue Cell Distribution System (Minneapolis, MN, USA; Pittsburgh, PA, USA; and Richmond, VA, USA), which was funded by NIH Contract #N01-DK-7-004/HHSN2670070004C. Human hepatocytes were cultured in low-glucose DMEM, supplemented with 10% FBS and 1% insulin, transferrin, and selenium solution (Gibco Life Technologies, Grand Island, NY, USA). Suramin was purchased from Sigma, z-VAD-fmk and ATP were from InvivoGen (San Diego, CA, USA), cytochalasin-D was from EMD-Calbiochem (La Jolla, CA, USA), and Ca-074-Me was from Invitrogen (Grand Island, NY, USA).
Isolation of human PBMCs
Human PBMCs were separated from blood of healthy volunteers by centrifugation in a Ficoll gradient as described in ref. [11].
Biochemical assays
Serum ALT was determined by use of a kinetic method (D-Tek, Bensalem, PA, USA). Colorimetric assays were used to measure serum uric acid (Abcam, Cambridge, MA, USA), liver triglycerides (Wako Chemicals, Richmond, VA, USA), and LDH activity in cell-culture supernatants (Abcam). Chemiluminiscent assay was used to measure ATP in the serum or in cell-culture supernatants (CellTiter-Glo; Promega, Madison, WI, USA).
Cytokine measurement
TNF-α was measured by use of specific anti-mouse ELISA from BioLegend (San Diego, CA, USA). IL-1β was measured by use of specific anti-mouse ELISA (R&D Systems) that recognizes pro-IL-1β and cleaved IL-1β.
Protein quantification
Liver whole-cell lysates were extracted as described previously [2, 12]. Equal amounts of proteins were separated on a polyacrylamide gel and transferred to a nitrocellulose membrane. Target proteins were detected by Western blot and immunostaining with specific primary antibody, followed by HRP-labeled secondary antibody. The specific immunoreactive bands of interest were detected by chemiluminescence (Amersham, Piscataway, NJ, USA). Digital system (ImageQuant LAS 4000; GE Healthcare, Uppsala, Sweden) was used for image acquisition. Blots labeled as “short exposure” and “long exposure” (see Fig. 5) were exposed for 1 or 10 minutes, respectively. Antibodies specific for total caspase-1 and the cleaved p10 fragment of caspase-1 were from Santa Cruz Biotechnology (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Antibody specific for total and cleaved IL-1β was from R&D Systems, and antibody recognizing the total and cleaved forms of caspase-3 was from Cell Signaling Technology (Danvers, MA, USA). β-Tubulin antibodies were from Abcam.
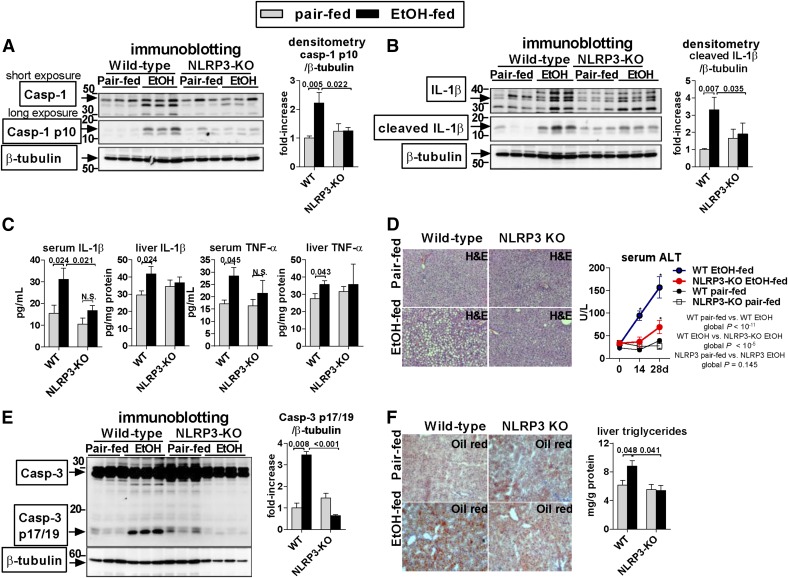
Figure 5. Deficiency of NLRP3 ameliorates alcohol-induced liver inflammation.
WT or NLRP3-knockout (KO) mice were fed control (Pair-fed) or alcohol (EtOH) diet. After 4 weeks, we evaluated cleavage of casp-1 (Casp-1; A) by use of an antibody that identifies full-length proform (short exposure) and cleaved form (long exposure) and cleavage of IL-1β in the liver (B). Levels of total IL-1β and TNF-α (C) in the serum and in the liver were evaluated by use of specific ELISA. Liver damage was assessed by liver histology and serum ALT (D) or by the extent of cleavage of caspase-3 (E). Oil Red O staining was performed, and liver triglycerides were measured to evaluate steatosis (F); n = 7–15 (ethanol-fed/genotype); 4–7 (pair-fed/genotype). Numbers in the graphs indicate P values. *P < 0.05 versus baseline. Original magnification, ×200.
Histopathological analysis
Liver sections were stained with H&E or Oil Red O by use of standard protocols analyzed by microscopy.
Statistical analysis
Statistical significance was determined by use of 2-sided t-test; ANOVA and Dunnett’s multiple comparison post-test were used to compare the means of multiple groups. Two-way ANOVA was used (see Fig. 5D) to determine the global effect of genotype or treatment on serum ALT. Unless stated otherwise, data are shown as means ± sem and were considered statistically significant at P < 0.05. We used SPSS 19.0 (IBM SPSS, Chicago, IL, USA) to perform statistical analyses.
RESULTS AND DISCUSSION
Endogenous inflammasome activators, ATP, and uric acid are increased in humans after acute alcohol binge-drinking
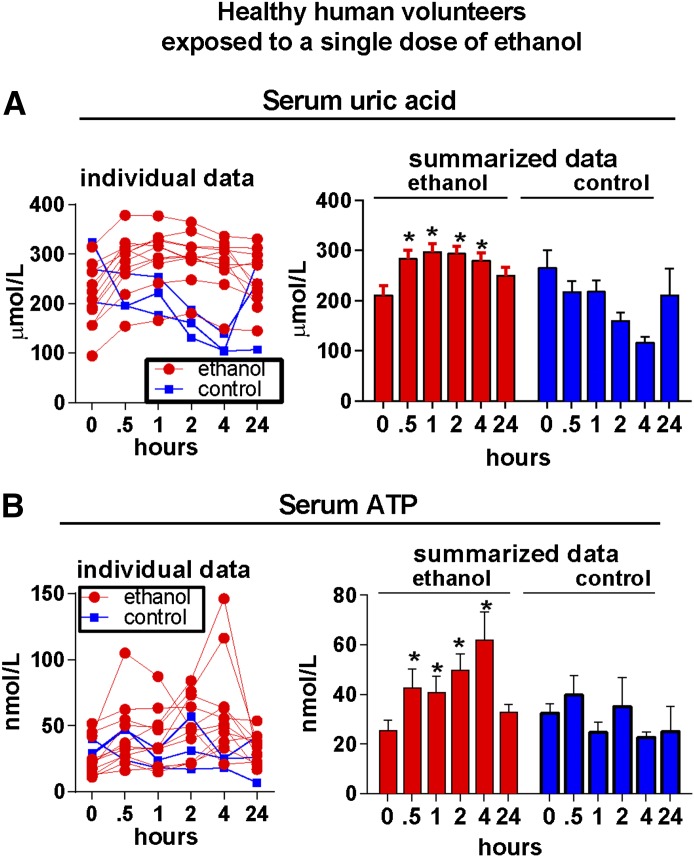
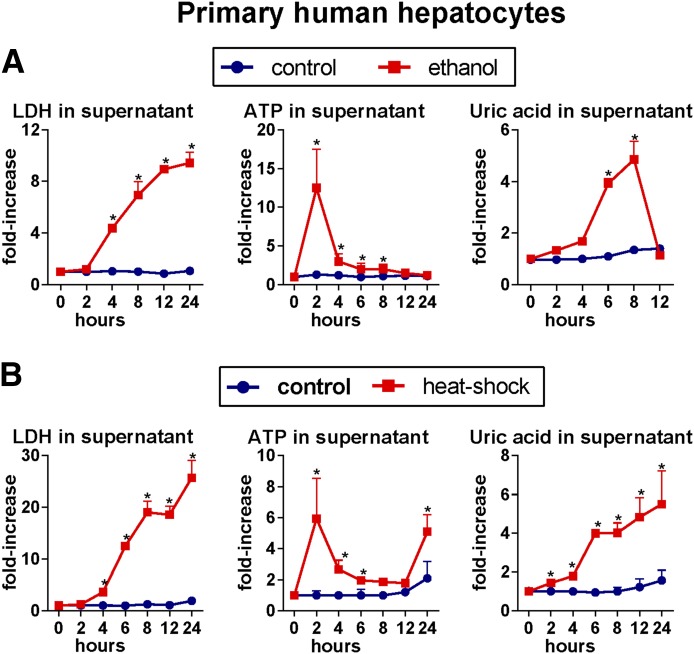
Liver inflammation induced by alcohol is dependent on activation of the inflammasome and IL-1β [2], implying involvement of endogenous danger signals in triggering liver inflammation in ALD. Given the effect of ethanol on mitochondrial function and metabolism of purine nucleotides, we studied the role of ATP and uric acid, 2 well-characterized metabolic danger signals and activators of inflammasome [9]. First, we asked whether alcohol modulates levels of uric acid and ATP. We evaluated uric acid and ATP in the serum of healthy human volunteers after a single binge-drinking of 0.8 mg/kg ethanol. We observed an early and statistically significant increase in serum levels of uric acid (Fig. 1A) and ATP (Fig. 1B) in alcohol-exposed volunteers but not in individuals not exposed to ethanol. In further experiments, we observed that treatment of primary human hepatocytes with ethanol resulted in hepatocyte death and in significantly increased levels of uric acid and ATP in the cell-culture supernatants (Fig. 2A). Similar results were obtained when primary human hepatocytes were exposed to heat shock (HS) (Fig. 2B). These data indicated that alcohol-exposed hepatocytes release significant amounts uric acid and ATP, and this observation was consistent with previous reports that uric acid and ATP are present in high concentrations in hepatocytes [13, 14].
Figure 1. Acute exposure to ethanol increases serum levels of uric acid and ATP in healthy human volunteers.
Peripheral blood was obtained from 11 healthy volunteers, who consumed a single dose of ethanol, or from 3 volunteers not exposed to ethanol, as specified in the Materials and Methods section. Serum levels of uric acid and ATP were measured at indicated time-points. Data are presented as individual data points and as summary statistics (means ± sem). *P < 0.05 versus baseline.
Figure 2. Damaged human hepatocytes release endogenous danger signals, uric acid, and ATP.
Primary murine or human hepatocytes were treated for 3 hours with 800 μM ethanol, and levels of LDH, indicating hepatocyte death; ATP; and uric acid were evaluated in supernatants at indicated time-points (A). Primary murine or human hepatocytes were cultured for 30 minutes at 45°C (HS) or 37°C (control), and levels of LDH, ATP, and uric acid were evaluated in supernatants at indicated time-points (B). Stimulations were performed in triplicates. *P < 0.05 versus baseline.
ATP and soluble uric acid released from damaged primary hepatocytes trigger the release of inflammasome-dependent cytokine IL-1β from immune cells
IL-1β maturation is a 2-step process. LPS induces the pro-IL-1β gene expression, but an additional, inflammasome-mediated signal is required for cleavage of pro-IL-1β protein to mature IL-1β that is released from inflammatory cells [15]. To evaluate whether components released from damaged human hepatocytes serve as a 2nd signal for IL-1β release, we tested primary human hepatocytes and PBMCs. First, we asked whether damaged hepatocytes trigger the release of IL-1β from PBMCs. To increase the sensitivity of our experiments, we used HS as a strong stimulus inducing hepatocyte death. To evaluate whether uric acid and ATP are involved, we used uricase and apyrase to deplete uric acid and ATP, respectively, in the medium.
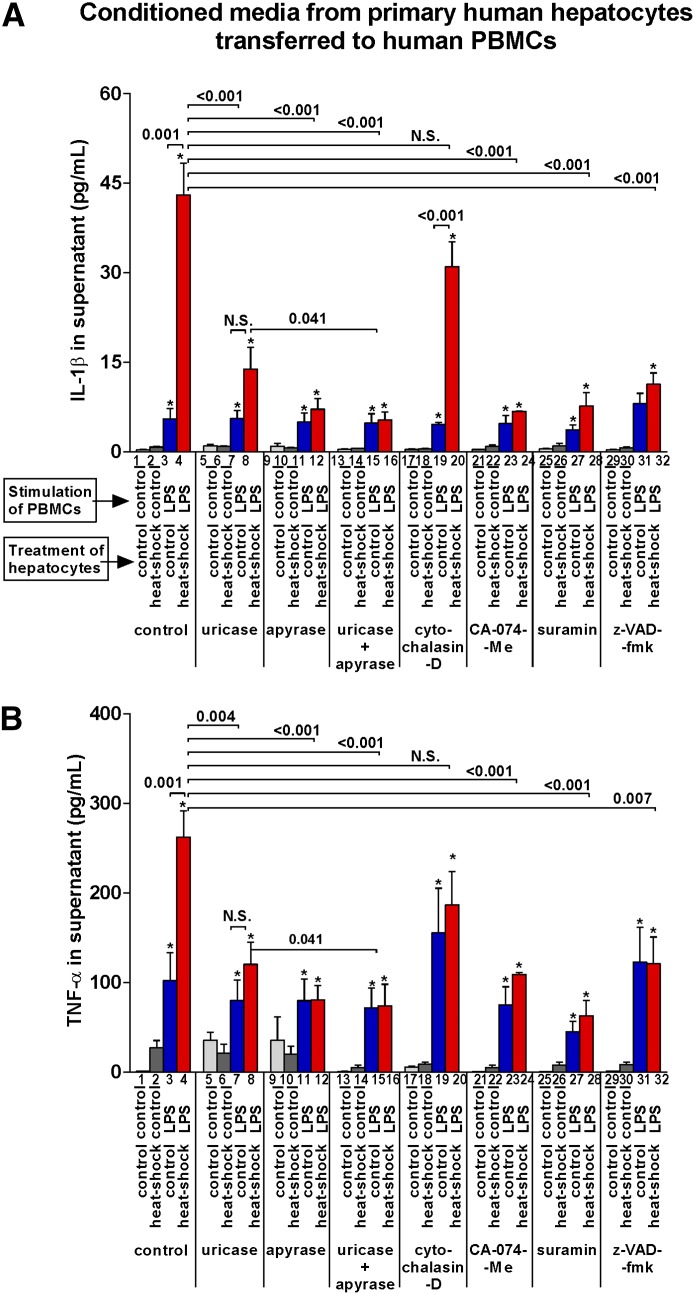
We observed that stimulation of PBMCs with LPS, which provides only the 1st signal that induces pro-IL-1β mRNA, resulted in limited release of IL-1β protein in the supernatant (Fig. 3A, group 3, indicated on x axis). Importantly, the release of IL-1β protein from PBMCs was significantly augmented by addition of supernatants from HS-damaged hepatocytes (Fig. 3A, group 4). This increase was partially abrogated by apyrase or uricase and completely abrogated by a combination of both. These data indicated that uric acid and ATP, released from damaged hepatocytes, act as a 2nd signal for inflammasome activation/IL-1β maturation in PBMCs.
Figure 3. ATP and soluble uric acid derived from damaged primary human hepatocytes trigger release of IL-1β and TNF-a from immune cells.
Primary human hepatocytes seeded on 6-well plates were incubated at 37°C or 45°C (HS). Supernatant was collected at 8 hours. In the meantime, primary human PBMCs were isolated from 3 healthy donors, seeded on 96-well plates and incubated with or without LPS (100 ng/ml) for 2 hours. Meanwhile, supernatant harvested from human hepatocytes was preincubated with control media, uricase (10 U/ml), and/or apyrase (1 U/ml) for 1 hour at 37°C. PBMCs were incubated with cytochalasin-D (10 μg/ml), CA-074-Me (10 μM), suramin (0.1 mM), or z-VAD-fmk (10 μM) for 1 hour. Three-fourths of PBMC media was then replaced with appropriate hepatocyte supernatant. After 8 hours, PBMC supernatant was collected, and IL-1β (A) and TNF-α (B) were measured. Stimulations were performed in triplicates. *P < 0.05 versus baseline; N.S., not significant.
Consistent with the mechanisms by which ATP or uric acid activate inflammasome, the release of IL-1β triggered by supernatants from HS hepatocytes required active inflammasome (inhibited by z-VAD-fmk, groups 29–32), purinergic signaling (inhibited by suramin in Fig. 3A, groups 25–28), or cathepsin B-dependent signaling downstream of endosomes (inhibited by CA-074-Me in Fig. 3A, groups 21–24), respectively. However, induction of IL-1β seemed to be independent of endocytosis because of cytochalasin-D, an inhibitor of endocytosis (Fig. 3A, groups 17–20).
We also observed that the increased levels of IL-1β resulted in significantly enhanced levels of TNF-α and that inhibition of IL-1β release by uricase, apyrase, z-VAD-fmk, suramin, or CA-074-Me was closely associated with decreased secretion of TNF-α (Fig. 3B). This was consistent with the role of IL-1β in triggering the release of TNF-α via IL-1R signaling (refs. [16–18], and see below).
ATP and soluble uric acid released from alcohol-exposed primary hepatocytes trigger the release of inflammasome-dependent cytokine IL-1β from liver immune cells
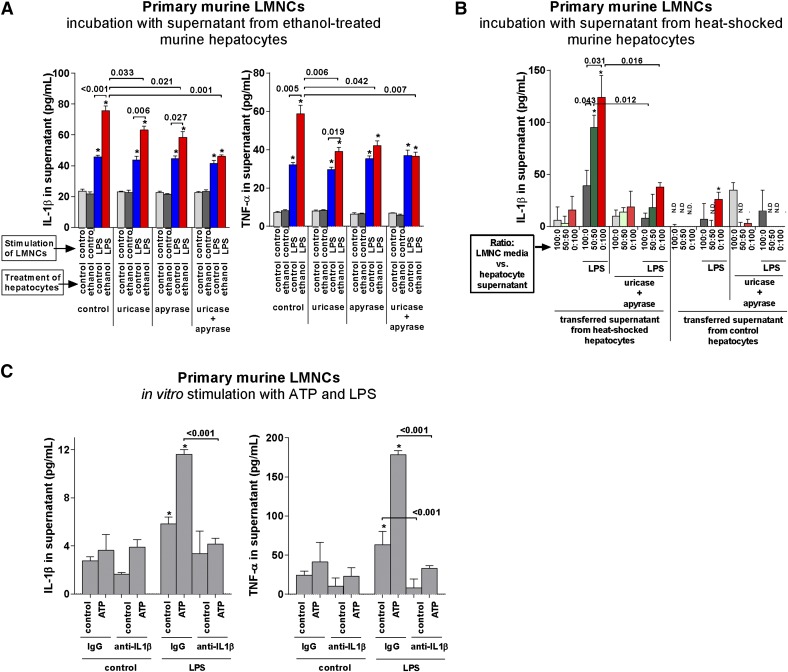
In the next set of experiments, we asked whether uric acid and ATP released from damaged hepatocytes would activate liver immune cells (LMNCs). To answer that question, we used primary hepatocytes and LMNCs isolated from WT mice. In this scenario, we used ethanol to induce hepatocyte damage. Similar to experiment with human hepatocytes and PBMCs, we used uricase and apyrase to deplete uric acid or ATP, respectively. We observed that stimulation of LMNCs with LPS resulted in limited release of IL-1β, which was significantly augmented by addition of supernatants from alcohol-damaged hepatocytes (Fig. 4A). This increase was partially abrogated by apyrase or uricase and completely abrogated by a combination of both, supporting our finding of involvement of uric acid and ATP as the 2nd signal for inflammasome activation/IL-1β maturation. The increased levels of IL-1β were associated with significantly enhanced levels of TNF-α (Fig. 4A, right).
Figure 4. ATP and soluble uric acid derived from damaged primary murine hepatocytes trigger release of IL-1β and TNF-α from liver immune cells.
(A) Primary murine hepatocytes were in vitro exposed to 900 mM ethanol for 2 hours, followed by washing and by postexposure incubation in alcohol- and serum-free media for 12 hours. Afterward, hepatocyte supernatant was collected, LPS (100 ng/ml), uricase (0.1 unit/ml), or apyrase (1 unit/ml) was added, and the hepatocyte supernatant was used to replace 75% of media of nonstimulated LMNCs that had already been seeded in a 96-well plate for 1 hour. Five hours after addition of hepatocyte media to LMNCs, IL-1β and TNF-α were measured in the supernatant. (B) LMNCs were seeded on 96-well plates. After 1 hour, media were replaced with a new media, admixed with cell-free supernatant from HS or control hepatocytes at indicated ratios, and LPS (100 ng/ml), uricase (0.1 unit/ml), or apyrase (1 unit/ml) was added. Five hours afterward, supernatants were collected, and IL-1β was measured. (C) LMNCs were seeded on 96-well plates. Cells were primed with LPS (10 ng/ml). After 30 minutes, IL-1β-neutralizing antibody or anti-goat IgG antibody (0.25 µg/ml each) was added. After 30 minutes, cells were stimulated with ATP (5 mM). Six hours afterward, supernatants were collected and analyzed for IL-1β and TNF-α by ELISA. Numbers in the graphs indicate P values. *P < 0.05 versus baseline.
Next, we asked whether the effect of hepatocyte-derived danger signals on LMNCs is dose dependent. To increase the sensitivity of the in vitro system, we used HS to induce the sufficient extent of hepatocyte death. With the use of increasing concentrations of conditioned hepatocyte media, we observed increasing secretion of IL-1β by LPS-primed LMNCs. This effect was abrogated completely by adding uricase or apyrase (Fig. 4B). Taken together, these data indicated that the effect of hepatocyte-derived uric acid and ATP on inflammasome activation/IL-1β release from LMNCs was dose dependent (Fig. 4B).
Finally, we used murine LMNCs to investigate the role of IL-1β signaling in induction of TNF-α. Stimulation of LMNCs with LPS resulted in a significant, albeit minor, increase in IL-1β in the cell-culture supernatant (Fig. 4C, left). A further significant increase was achieved with addition of ATP as a 2nd signal to LMNCs. This increase was abrogated by neutralization of IL-1β by use of a specific antibody, reflecting the dependency of IL-1β induction on an autocrine loop mediated by an IL-1R [17–19]. Along with increased levels of IL-1β, addition of ATP to LPS-stimulated LMNCs significantly increased levels of TNF-α in the supernatant (2.8-fold; Fig. 4C, right). This increase was attributable to increased levels of IL-1β, as stimulation of LMNCs in the presence of neutralizing antibodies against IL-1β did not result in any increase of TNF-α compared with baseline conditions (Fig. 4C, right). These findings were consistent with the previously reported role of IL-1β in triggering the release of TNF-α via IL-1R-dependent signaling [16–18], and extended the role of IL-1β in amplification of TNF-α expression to LMNCs. Furthermore, these findings support our previous findings showing that low concentrations of IL-1β may have a major impact on inflammatory signaling in ALD [2].
Overall, these data suggested the presence of cross-talk between alcohol-damaged hepatocytes and liver immune cells and demonstrated that inflammatory activation of LMNCs is augmented by hepatocyte-derived uric acid and ATP and is dependent on a feed-forward induction via inflammasome activation and release of IL-1β.
Liver inflammation in ALD requires NLRP3, a sensor of metabolic danger
Our data indicated that uric acid and ATP, released from damaged hepatocytes, are required for activation of liver immune cells. The mechanism by which uric acid and ATP activate inflammation requires the NLRP3 inflammasome [20]. With the use of the NLRP3, immune cells recognize endogenous danger signals, including ATP, uric acid, and crystalline substances, and trigger activation of caspase-1 and maturation of IL-1β [9]. Therefore, we evaluated mice lacking NLRP3 and asked whether NLRP3 provides a mechanistic link among hepatocyte-derived danger signals, inflammasome activation, and liver inflammation in ALD.
Deficiency of NLRP3 prevented alcohol-induced activation of the inflammasome in the liver, as indicated by the diminished levels of the cleaved p10 fragment of caspase-1 (Fig. 5A) and cleaved IL-1β in the liver and in the serum (Fig. 5B and C) compared with WT mice. Active, secreted IL-1β up-regulates inflammatory cytokines, including TNF-α, promotes steatosis, and sensitizes hepatocyte to cytotoxicity induced by TNF-α [2, 12, 19, 21]. Accordingly, lack of activation of inflammasome and IL-1β in NLRP3-deficient mice was accompanied by protection from liver inflammation, steatosis, and damage, as indicated by the absence of alcohol-induced up-regulation of TNF-α and IL-1β in the liver and in the serum (Fig. 5C), improved findings on H&E and amelioration of an ALT increase in the serum (Fig. 5D), absent cleavage of proapoptotic caspase-3 in the liver (Fig. 5E), and decreased lipid accumulation (Fig. 5F). The extent of protection from liver inflammation, injury, and steatosis in NLRP3-knockout mice was comparable with that observed previously in mice deficient in caspase-1, the effector molecule of inflammasomes [2], indicating that NLRP3 is a major activator of the inflammasome in ALD.
Taken together, these data further indicated that endogenous danger signals, uric acid, and ATP are involved in inflammatory cross-talk between hepatocytes and immune cells in ALD and that their effect is mediated via the NLRP3 inflammasome.
In this study, we found that alcohol-induced hepatocyte damage and hepatocyte-derived danger molecules, uric acid and ATP, triggered secretion of IL-1β from liver immune cells. In light of our previous results showing that inflammasome-dependent IL-1β is crucially involved in the pathogenesis of ALD [22], our data strongly support the hypothesis that hepatocyte-derived uric acid and ATP represent an endogenous metabolic danger signal for activation of inflammasome/IL-1β in ALD. Our results suggest that uric acid and ATP represent the 2nd signal in inflammasome activation, thus enabling the 1st signal, represented by gut-derived LPS and dependent on Kupffer cell-specific TLR4 signaling [3–8], to initiate liver inflammation. To the best of our knowledge, this concept has not been reported previously in the context of ALD.
Our results strongly support several novel aspects of inflammation in ALD. First, alcohol-induced metabolic stress results in release of the endogenous danger signals, uric acid and extracellular ATP, from hepatocytes, leading to inflammasome activation in liver immune cells. Second, activation of innate immune cells is triggered by alcohol-induced metabolic danger signals, supporting the hypothesis that outcomes of metabolic signaling in the liver may trigger innate-immune signaling in ALD. Third, development of liver inflammation in ALD seems to be dependent, not only on gut-derived LPS but also on endogenous ligands released from damaged hepatocytes and cellular cross-talk between hepatocytes and immune cells.
Our findings of protection from liver inflammation in alcohol-fed mice deficient in NLRP3 are consistent with a report by Shulga and Pastorino [23], demonstrating that activation of murine Kupffer cells by ethanol requires NLRP3, a ligand-sensing component of inflammasomes that detects ATP and uric acid [9]. The requirement for NLRP3 in the development of ALD is supported further by the study of Cui et al. [24], who demonstrated protection from liver inflammation, damage, and steatosis in NLRP3-deficient mice subjected to acute-on-chronic exposure to ethanol. A report by DeSantis et al. [25] demonstrated that mice deficient in NLRP3 were not protected from alcohol-induced liver inflammation. Our previous data demonstrated the requirement for ASC, an adaptor molecule immediately downstream of NLRP3, for activation of inflammasome in ALD. The indispensability of ASC in ALD narrows the choice of inflammasome activators to 2 molecules: NLRP3, supported by these data and reports by Shulga and Pastorino [23] and Cui et al. [24], or an alternative intracellular ligand receptor, AIM2 [9], implied by the negative findings of DeSantis et al. [25]. AIM2 recognizes cytoplasmic dsDNA [9], and its role in ALD has yet to be elucidated.
In summary, our data indicate that hepatocytes, damaged by ethanol, release endogenous danger molecules, uric acid, and ATP, which are recognized by liver immune cells as inflammatory signals. The final common pathway of this signaling is most likely represented by the activation of inflammasome, which is required for processing and activation of IL-1β, a key inflammatory cytokine involved in the pathogenesis of alcoholic steatohepatitis [2]. These data may provide the basis for future mechanistic studies, as well as for clinical interventions in liver diseases.
Acknowledgments
This work was supported by Grants AA017729 and AA021907 from the U.S. National Institutes of Health National Institute on Alcohol Abuse and Alcoholism (to G.S.). Core resources, supported by the U.S. Diabetes Endocrinology Research Center (Grant DK32520), were also used. The authors thank Anna Cerny for excellent technical assistance.
Glossary
- AIM2
absent in melanoma 2
- ALD
alcoholic liver disease
- ASC
apoptosis-associated speck-like protein containing a caspase-recruitment domain
- HS
heat shock
- LDH
lactate dehydrogenase
- LMNC
liver mononuclear cell
- NLRP3
nucleotide-binding oligomerization domain-like receptor family, pyrin domain containing 3
- UMASS
University of Massachusetts
- WT
wild-type
- z-VAD-fmk,
z-Val-Ala-Asp-fluoromethylketone
AUTHORSHIP
J.P., A.I.-V., and G.S. designed the research. J.P., A.I.-V., B.S., A.S., and G.S. performed the research. E.A.K.-J. and K.A.F. contributed new reagents/analytic tools. J.P., A.I.-V., K.K., and G.S. analyzed data. J.P., A.I.-V., A.S., E.A.K.-J., K.A.F., and G.S. wrote the paper.
DISCLOSURES
There are no conflicts of interest to declare.
REFERENCES
- 1.Gao B., Seki E., Brenner D. A., Friedman S., Cohen J. I., Nagy L., Szabo G., Zakhari S. (2011) Innate immunity in alcoholic liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G516–G525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrasek J., Bala S., Csak T., Lippai D., Kodys K., Menashy V., Barrieau M., Min S. Y., Kurt-Jones E. A., Szabo G. (2012) IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Invest. 122, 3476–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adachi Y., Moore L. E., Bradford B. U., Gao W., Thurman R. G. (1995) Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology 108, 218–224. [DOI] [PubMed] [Google Scholar]
- 4.Adachi Y., Bradford B. U., Gao W., Bojes H. K., Thurman R. G. (1994) Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology 20, 453–460. [PubMed] [Google Scholar]
- 5.Bode C., Bode J. C. (2005) Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol. Clin. Exp. Res. 29 (11 Suppl) 166S–171S. [DOI] [PubMed] [Google Scholar]
- 6.Hritz I., Mandrekar P., Velayudham A., Catalano D., Dolganiuc A., Kodys K., Kurt-Jones E., Szabo G. (2008) The critical role of Toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology 48, 1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bala S., Marcos M., Gattu A., Catalano D., Szabo G. (2014) Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS ONE 9, e96864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inokuchi S., Tsukamoto H., Park E., Liu Z. X., Brenner D. A., Seki E. (2011) Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol. Clin. Exp. Res. 35, 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroder K., Tschopp J. (2010) The inflammasomes. Cell 140, 821–832. [DOI] [PubMed] [Google Scholar]
- 10.Petrasek J., Dolganiuc A., Csak T., Nath B., Hritz I., Kodys K., Catalano D., Kurt-Jones E., Mandrekar P., Szabo G. (2011) Interferon regulatory factor 3 and type I interferons are protective in alcoholic liver injury in mice by way of crosstalk of parenchymal and myeloid cells. Hepatology 53, 649–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolganiuc A., Oak S., Kodys K., Golenbock D. T., Finberg R. W., Kurt-Jones E., Szabo G. (2004) Hepatitis C core and nonstructural 3 proteins trigger Toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology 127, 1513–1524. [DOI] [PubMed] [Google Scholar]
- 12.Petrasek J., Dolganiuc A., Csak T., Kurt-Jones E. A., Szabo G. (2011) Type I interferons protect from Toll-like receptor 9-associated liver injury and regulate IL-1 receptor antagonist in mice. Gastroenterology 140, 697–708 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi Y., Evans J. E., Rock K. L. (2003) Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425, 516–521. [DOI] [PubMed] [Google Scholar]
- 14.Feranchak A. P., Lewis M. A., Kresge C., Sathe M., Bugde A., Luby-Phelps K., Antich P. P., Fitz J. G. (2010) Initiation of purinergic signaling by exocytosis of ATP-containing vesicles in liver epithelium. J. Biol. Chem. 285, 8138–8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroder K., Zhou R., Tschopp J. (2010) The NLRP3 inflammasome: a sensor for metabolic danger? Science 327, 296–300. [DOI] [PubMed] [Google Scholar]
- 16.Dinarello C. A. (2009) Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550. [DOI] [PubMed] [Google Scholar]
- 17.Granowitz E. V., Clark B. D., Vannier E., Callahan M. V., Dinarello C. A. (1992) Effect of interleukin-1 (IL-1) blockade on cytokine synthesis: I. IL-1 receptor antagonist inhibits IL-1-induced cytokine synthesis and blocks the binding of IL-1 to its type II receptor on human monocytes. Blood 79, 2356–2363. [PubMed] [Google Scholar]
- 18.Granowitz E. V., Vannier E., Poutsiaka D. D., Dinarello C. A. (1992) Effect of interleukin-1 (IL-1) blockade on cytokine synthesis: II. IL-1 receptor antagonist inhibits lipopolysaccharide-induced cytokine synthesis by human monocytes. Blood 79, 2364–2369. [PubMed] [Google Scholar]
- 19.Cosgrove B. D., Cheng C., Pritchard J. R., Stolz D. B., Lauffenburger D. A., Griffith L. G. (2008) An inducible autocrine cascade regulates rat hepatocyte proliferation and apoptosis responses to tumor necrosis factor-alpha. Hepatology 48, 276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tschopp J., Schroder K. (2010) NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 10, 210–215. [DOI] [PubMed] [Google Scholar]
- 21.Miura K., Kodama Y., Inokuchi S., Schnabl B., Aoyama T., Ohnishi H., Olefsky J. M., Brenner D. A., Seki E. (2010) Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology 139, 323–334 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lippai D., Bala S., Petrasek J., Csak T., Levin I., Kurt-Jones E. A., Szabo G. (2013) Alcohol-induced IL-1β in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J. Leukoc. Biol. 94, 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shulga N., Pastorino J. G. (2014) Hexokinase II binding to mitochondria is necessary for Kupffer cell activation and is potentiated by ethanol exposure. J. Biol. Chem. 289, 26213–26225. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Cui K., Yan G., Xu C., Chen Y., Wang J., Zhou R., Bai L., Lian Z., Wei H., Sun R., Tian Z. (2015) Invariant NKT cells promote alcohol-induced steatohepatitis through interleukin-1β in mice. J. Hepatol. pii: S0168-8278, 00956–00958. [DOI] [PubMed] [Google Scholar]
- 25.DeSantis D. A., Ko C. W., Liu Y., Liu X., Hise A. G., Nunez G., Croniger C. M. (2013) Alcohol-induced liver injury is modulated by Nlrp3 and Nlrc4 inflammasomes in mice. Mediators Inflamm. 2013, 751374. [DOI] [PMC free article] [PubMed] [Google Scholar]