Abstract
There are over 5 million Americans with heart failure (HF), the majority of whom are over age 65. Frailty is a systemic syndrome associated with aging that produces subclinical dysfunction across multiple organ systems and leads to an increased risk for morbidity and mortality. The prevalence of frailty is about 10% in community-dwelling elderly and 20% in those with advanced HF, and increases in both cohorts with age. Yet the relationship between the primary frailty of aging and frailty secondary to HF remains poorly defined. Whether the frailty of these two populations share similar etiologies or exist as separate entities is unknown. Teasing apart potential molecular, cellular, and functional differences between the frailty of aging and that of advanced HF has implications for risk stratification, quality of life, and pharmacological and therapeutic interventions for advanced HF patients.
Keywords: frailty, heart failure, sarcopenia, geriatric cardiology
Introduction
The phenotype of heart failure (HF) is changing. Likely due to improved management of chronic diseases such as hypertension and coronary artery disease, as well as interventions that impact survival, the HF population is aging. There are over 5 million Americans with HF, the majority of whom are over age 65. HF incidence doubles with each decade, starting at 5% between ages 65 and 74 years and rises to 20% for those over 80 years of age.1 Common geriatric issues such as multimorbidity, polypharmacy, and frailty are encountered frequently in this population, creating a complex foundation upon which to build HF management plans.
The syndrome of frailty is prevalent both in community-dwelling elders and in individuals with chronic disease. Frailty is a systemic process affecting multiple organ systems, generating a phenotype of weakness, fatigue, lack of physiologic reserve, and decreased tolerance to stressors, and increases the risk of hospitalization, procedural complications, and mortality across all medical and surgical domains. The prevalence of frailty in community-dwelling elders is about 10% and the incidence increases with advancing age.2 As a component of chronic disease, frailty prevalence is about 60%, 40%, and 20% in chronic obstructive pulmonary disease (COPD), end-stage renal disease (ESRD), and systolic HF populations, respectively.3–5 Similar to the aging data, frailty in chronic disease is associated with older age and the presence of additional comorbidities, and predicts increased risk for falls, morbidity, and mortality.3–5 Whether there is an etiological distinction between the primary frailty of aging and frailty secondary to chronic disease is unknown.
At this time, it is not possible to clinically differentiate primary frailty from frailty secondary to chronic disease. With respect to the aging HF population, there is an urgent need to address the potential for this distinction. In addition to guideline-directed medical therapy (GDMT), patients of this demographic segment are increasingly considered for life-prolonging invasive procedures. Between 2008 and 2013, almost 30% of over 9000 left ventricular assist device (LVAD) placements occurred in patients over the age of 65.6 Generally, LVAD placement improves both survival and health-related quality of life (HRQoL), yet both older age and the presence of comorbidities increase the risk of poor outcomes.6 To determine whether the benefit of mechanically assisted hemodynamic support outweighs the risk of the procedure, it is crucial to understand the etiology of frailty in this elderly, advanced HF population. This review will examine the etiology and functional consequences of primary and secondary frailty, and distinguish these changes from those associated with normal aging. Ultimately, understanding the interaction between aging, HF, and frailty, as well as the potential for reversibility, will help guide clinicians and patients in the difficult decisions of advanced HF management.
Primary Frailty of Aging
Complicating the literature on frailty both with respect to primary frailty and frailty associated with chronic disease is the number of working definitions for seemingly similar processes. Sarcopenia is a condition of muscle mass loss and muscle weakness that results in poorer functional status, increased dependence, and increased morbidity and mortality.7 Although muscle mass loss is a normal component of aging,8 in sarcopenic states, not only is there less muscle quantity but the quality of the tissue is also inferior. In 2010, the European Workshop on Sarcopenia in Older People (EWSGOP) defined sarcopenia as meeting two of three criteria: muscle mass loss as determined by imaging; muscle weakness by grip strength; and slowness by gait speed.7 The phenotype of frailty is one that encompasses sarcopenia, and then furthers it with the addition of psychosocial dimensions. Multiple diagnostic tools exist for frailty, but they generally incorporate an assessment of five core elements: shrinking, weakness, slowness, fatigue, and low physical activity.4 As conceptualized by Fried and colleagues in 2001,9 frailty is a constellation of three or more of the following: unintentional weight loss, slow gait speed, weak grip strength, exhaustion, and decreased physical activity. Although the unintentional weight loss of frailty does not specify the tissue type, sarcopenia is a recognized component of this syndrome.9
Pathobiological Mechanisms and Physiological Consequences of Primary Frailty
On a molecular level, the pathobiology of this wasting and weakness process has been correlated with dysregulated immune, endocrine, and neurohormonal systems. Sarcopenic and frail patients have elevated levels of inflammatory markers such as interleukin 6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor alpha (TNFα).10–15 Frailty is also associated with dysregulated glucose management, as frail patients show elevated levels of growth hormone (GH), low levels of insulin-like growth factor-1 (IGF1) and evidence of insulin resistance.16,17 The list continues with a loss of the normal diurnal cortisol pattern,18 lower testosterone levels,19 and increased markers of oxidative stress.12,20 Together, these dysregulated systems create an anabolic–catabolic imbalance, leading to increased muscle catabolism, weight loss, and subclinical organ dysfunction. Upon exposure to additional stressors such as injuries, infections, or surgical procedures, patients with these signaling derangements are more prone to decompensation, hospitalization, and death.
Despite extensive work on altered muscle composition in normal aging, much less is known about this process with respect to frailty. In normal aging, the muscle mass loss has been attributed to the preferential muscle atrophy of fast-twitch glycolytic type II muscle fibers between the third and seventh decade of life,21,22 accounting for decreased muscle cross-sectional area.23 The ultimate result is a fast-to-slow twitch fiber shift in muscle composition with an increased percentage of slow-twitch oxidative type I fibers.24 Data from aging primates demonstrate that these cellular changes are preceded by altered energy metabolism as a result of decreased mitochondrial activity,25 suggesting that the process may represent an adaptation to decreased energy availability. Specific data on frailty-related muscle composition are not yet available. However, other disease states with substantial inflammatory and neurohormonal elements such as sepsis, diabetes, Cushing’s disease, and malignancy-related cachexia show a pattern similar to that seen in aging, with atrophy of type II fibers.24 A large body of evidence suggests a direct role for inflammation in mitochrondrial function, and vice versa. Inflammation causes deficiencies of various mitochrondrial subunits, ultimately resulting in decreased energy production, increased production of reactive oxygen species, and upregulation of nuclear factor kappa B (NFκB). NFκB is a downstream product of TNFα as well as a transcription factor responsible for the production of inflammatory cytokines.26 As such, a dangerous cycle of inflammation and mitochrondrial damage is set in motion, potentially laying the groundwork for the muscle loss, weakness, and exhaustion of the frailty phenotype.
As muscle composition changes, aerobic capacity declines, as well. In healthy individuals over age 60, peak oxygen uptake (VO2) declines by 10%–15% per decade,27 with a correlation between age-related muscle mass loss and aerobic capacity.28 In sarcopenia, a similar relationship is seen between muscle loss and aerobic capacity. In community-dwelling older women without cardiovascular disease, those defined as sarcopenic based on a muscle-mass cutoff29 showed evidence of decreased strength and diminished aerobic capacity as compared to women who did not meet sarcopenic criteria.30 No studies to date have specifically addressed aerobic capacity in patients meeting frailty criteria.
In summary, primary frailty is a syndrome of inflammatory, metabolic, and hormonal derangements that shift homeostasis from an anabolic to a catabolic state. Wasting and weight loss are manifested by loss of muscle fibers, altering muscle composition, which in turn lays the foundation for the phenotypic weakness, slowness, and functional deterioration inherent to frailty syndrome. The potential for treatment and reversibility of the frail state is explored in the following section.
Treatment and Reversal of the Primary Frailty Phenotype
Although significant evidence demonstrates that physical activity reverses or slows the muscle wasting process of aging,31–34 less is known with respect to physical activity and the frailty syndrome. In normal aging, data indicate that directed physical therapy with progressive resistance training increases muscle mass and strength.31–34 However, these mostly small, nonrandomized trials generally do not address outcomes such as mortality, HRQoL, or functional disability.34 Analysis of the literature with respect to sarcopenia and frailty is limited by the lack of consistent definitions and heterogeneity of inclusion criteria. Yet, in patients with specific health problems or existing functional limitations (ie, those who would most likely meet criteria for sarcopenia and frailty), the benefit of resistance training demonstrated a much smaller effect, if any at all.32,35 In larger studies that used validated definitions of frailty, patients identified as “pre-frail” or “transitionally frail” showed a positive response to exercise and balance interventions, resulting in improvements in physical performance and decreased fall risk.36,37 However, those meeting criteria for full frailty actually fared worse, with the intervention group experiencing more injuries requiring medical attention, usually related to increased incidence of falls.37,38 No studies to date have addressed the potential for an exercise-related mortality benefit in sarcopenic or frail patients.
Pharmacological interventions for the treatment and prevention of primary frailty remain in the early stages. Attempts to target the molecular pathways discussed above have been met with mostly disappointing results. Hormone replacement therapy with estrogen, testosterone, or GH have occasionally been shown to increase muscle mass and strength, but the results are mixed.39 There may be a role for the renin–angiotensin–aldosterone system in muscle wasting. Exogenous administration of angiotensin II in animal models precipitates skeletal muscle atrophy with increased proteolysis and elevated levels of reactive oxygen species.40 An observational study found that use of angiotensin-converting enzyme (ACE) inhibitors slowed the progression of muscle-loss-related weakness in elderly women with hypertension,41,42 which may be due to the effects of ACE inhibition on insulin sensitivity and inflammation rather than preservation of cardiac function.43 However, subsequent prospective randomized trials administering angiotensin-receptor or aldosterone antagonists to patients with functional impairments have failed to demonstrate benefit.44,45 Moreover, no studies have addressed pharmacological interventions with respect to mortality benefit in frailty.
In summary, both therapeutic and pharmacologic treatments for frailty have been attempted. Although physical activity improves muscle mass and function in older individuals who are not frail, there appears to be limited benefit in the frail population. Pharmacologic treatments targeting hormonal derangements have been similarly ineffective. Hopefully, future work delineating the etiology of primary frailty will improve upon current capabilities of counteracting and reversing this systemic process.
Frailty Secondary to Heart Failure
The constellation of muscle loss, weakness, and exhaustion has been a long recognized component of HF. In addition to sarcopenia and frailty, cardiac cachexia, defined as loss of >5% of one’s body weight over 6 months,46 is a third term referring to the wasting process of this population. Depending on the definition used, studies in HF find about a 20% prevalence of sarcopenia, a 20%–50% prevalence of frailty, and 15% prevalence of cardiac cachexia.47–49 All predict increased risk of hospitalization and mortality, along with decreased HRQoL.49 Notably, the risk for HF-related frailty rises dramatically with age, as a 30% prevalence has been identified in patients younger than age 70, versus a 52% prevalence in those 70 years or older.50
Pathobiological Mechanisms of HF-Related Frailty and Physiological Consequences
Similar to that seen in community-dwelling elders, the wasting process of HF is likely related to an anabolic–catabolic imbalance in which initially adaptive neurohormonal mechanisms and autonomic nervous system activation yield detrimental systemic effects over time. Consistent evidence exists for TNFα, IL-1, and IL-6 upregulation,51–54 along with abnormalities in the GH/IGF-1 axis, cortisol regulation,51 and uric acid production55 in HF-related frailty. In addition to HF precipitating frailty, the reverse is also true. Frail community-dwelling elders are more likely to develop de novo HF then their non-frail counterparts.56 In animal models, exogenous administration of TNFα as well as transgenic overexpression of inflammatory cytokines results in left ventricular remodeling and increased mortality,57,58 implicating a significant contribution of systemic inflammation to HF development. Therefore, similar inflammatory and neurohormonal components contribute to the etiology of both primary frailty and frailty secondary to HF.
Cellular and molecular alterations to muscle cell composition are frequently noted in patients with symptomatic HF, but are slightly different than those of normal aging and strict inflammatory processes. Similar to aging, fast-twitch glycolytic type II fibers are atrophic.59 Unlike normal aging, however, the total percentage of fast-twitch type II fibers actually increases as compared to slow-twitch oxidative type I fibers, resulting in an overall slow-to-fast twitch fiber shift.59 Interestingly, this slow-to-fast twitch fiber shift is also seen in deconditioning due to extended bed rest60 and microgravity,61 and is hypothesized to be related to the easy muscle fatigability seen in HF patients.62 In contrast to peripheral skeletal muscles, diaphragmatic muscle biopsies taken during LVAD placement or heart transplant show a fast-to-slow twitch fiber shift with an increase in type I fibers at the expense of type II.63 It is unknown whether this fast-to-slow twitch fiber shift is related to the higher diaphragmatic workload of HF patients, the underlying chronic inflammatory state, or a combination of the two. Skeletal muscle changes in HF are therefore complex and site-specific, and most likely represent a mixed picture of chronic deconditioning and inflammation. However, the specific relationship between these muscle changes and HF-related frailty has yet to be fully explored.
Although decreased functional capacity is a component of frailty, the correlation between functional capacity and HF-related frailty is infrequently addressed. Reduced exercise tolerance due to fatigue and dyspnea is an independent predictor of mortality and rehospitalization.64 In HF patients, both diminished strength and decreased aerobic capacity correlate with decreased muscle mass.47,65 In one study of 200 HF patients, New York Heart Association (NYHA) class II–III, 19% had evidence of muscle wasting by dual energy X-ray absorptiometry scan. Handgrip strength, gait speed, and absolute peak VO2 were all significantly reduced in the group with muscle wasting as compared to those without.47 In addition, the amount of skeletal muscle apoptosis seen on peripheral skeletal muscle biopsy corresponded with decreased aerobic capacity, while the percentage of fast-twitch type II muscle fibers was inversely related.66 Interestingly, the relationship between aerobic capacity and muscle composition remains similar whether or not HF patients meet criteria for cardiac cachexia,67 implying that the functional consequences of altered muscle composition manifest before full cachexia is evident.51 This phenomenon may be explained by the hypothesis that, similar to frailty, exercise intolerance in this population relates to impaired oxygen utilization by skeletal muscles due to inflammation, oxidative stress, and mitochondrial dysfunction.68–70 Therefore, although an important component in HF prognosis, much about the cellular, molecular, and functional consequences specific to frailty in HF have yet to be elucidated. Information extrapolated from HF-related muscle wasting suggests that there are striking similarities between the molecular fingerprints of primary frailty and HF-related frailty, although notable differences exist with respect to muscle cell composition. Further work to understand the molecular and cellular etiology of HF-related frailty as distinct from primary frailty may have implications for prevention, treatment, and reversibility, which will be further discussed below.
Treatment and Reversal of HF-Related Frailty
Exercise may impart an overall benefit to HF patients with respect to mortality,71 improvements in HRQoL, and decreased risk of HF-related hospitalizations,72,73 but the relationship between exercise and HF-related frailty is incompletely understood. Data from Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) showed that after adjustment for known predictors of mortality, exercise training was associated with a reduced risk for mortality and hospitalization.74 HF-ACTION further demonstrated safety of exercise in a population of patients NYHA class II—IV, improvements in six-minute walk tests, exercise time, peak oxygen consumption, HRQoL, and depressive symptoms.74–76 Exercise also affects intrinsic HF-related muscle changes. In a prospective, randomized, age-matched control trial, Gielen and colleagues (2012) showed increased force endurance and peak VO2 of HF patients via a 4-week endurance training program.77 These improvements correlated with a post-training increase in IGF-1 as well as decreased markers of muscle apoptosis. Others have shown that physical training increases peak VO2 and decreases circulating levels of TNFα and IL-6,78 although these findings are not consistently replicated.79 Therefore, there are signals that physical activity may positively alter the hormonal, inflammatory, and metabolic parameters of HF-related frailty. However, no studies to date have specifically addressed the impact of these molecular changes with specific respect to frailty reversibility or mortality.
As discussed above, in addition to predicting incident HF, inflammation is associated with both primary frailty and frailty secondary to HF. However, attempts to alter the inflammatory milieu of HF have met with disappointing results. Small studies of patients with moderate to severe HF found that TNFα-inhibition with infliximab or etanercept improved both functional status and ejection fraction. However, larger randomized trials found no such benefit; in fact, there was a trend toward harm, as TNFα-inhibition seemed to increase HF-related hospitalization and death.80,81 The reason for this lack of benefit remains unclear. One theory suggests that the redundancy of the inflammatory pathway allows TNFα-specific elements to be bypassed with few downstream consequences.81 Another is that inhibition may ultimately result in a “rebound effect” or a potentiation of TNFα activity over the long term.80,81 However, these medications are safe and effective in other inflammatory diseases such as rheumatoid arthritis, ankylosing spondylitis, and psoriasis. They have never been applied to treatment or prevention of primary frailty or the de novo development of HF in this population, nor is there an understanding of their impact on HF-related frailty.
With respect to HF-related frailty, GDMT targeting the neurohormonal and autonomic nervous system dysfunction of systolic HF may pose complex advantages and disadvantages. Treatment recommendations for systolic HF include β-blockers (BB), ACE inhibitors or angiotensin receptor blockers, aldosterone antagonists, and diuretics to improve morbidity, hospitalizations, and mortality.82 The β-adrenergic signaling pathway stimulates an anabolic response in skeletal muscle via increased protein synthesis and decreased degradation83; and administration of low-dose β-agonists in animal models increases muscle mass and force (for review, see Lynch & Ryall 2008),83 a seemingly beneficial effect with respect to the muscle wasting process of frailty. Therefore, the BB recommendation of GDMT would be assumed to have deleterious consequences. Somewhat counterintuitively, but perhaps due to indirect effects on the renin–angiotensin system, Scherer and colleagues (2013) found that appropriate uptitration of BBs improves HRQoL in elderly HF patients with reported benefits in general health, vitality, and physical function, although physical capacity was not objectively quantified.84 Regarding the renin–angiotensin–aldosterone system, as discussed above, administration of ACE inhibitors in normal aging may have a modest effect in slowing the progression of muscle-related weakness, although results are variable.41–43 In HF, early studies showed that 12 weeks of ACE inhibitor administration increased exercise tolerance and improved NYHA functional class by one grade,85 and therefore indirectly suggest that ACE inhibition may improve frailty status. However, these results preceded current recommendations for GDMT. There has been no direct assessment of GDMT on HF-related frailty.
Theoretically, the recipients of heart transplants or artificial circulatory support are the best population to assess for potential improvement of HF-related frailty. On a molecular level, data from Lund and colleagues (2012) show reversibility of some hormonal derangements common in HF-related frailty after transplant, including a normalization of the GH/IGF-1 axis,86 although the relationship of this molecular change to reversal of the frailty phenotype was not addressed in this study. Functionally, heart transplant patients undergo some improvement in peak VO2, but do not improve to levels equal to control populations. Interestingly, reported factors affecting exercise capacity changed from dyspnea to leg fatigue in a post-transplant population,87 implying an element of permanence to some musculoskeletal consequences of HF. With respect to components of frailty, reversal of weight loss has been consistently documented in this setting. After undergoing heart transplant, patients gain an average of 10 kg in the first year.88,89 This does not appear to be a consequence of glucocorticoid use, as increased steroid dose is actually inversely related to weight gain and likely a marker of rejection episodes. It is important to note, however, that age is inversely correlated with weight gain after transplant, as younger patients (<48 years of age) gain significantly more weight than older ones,88,89 suggesting a spectrum of frailty reversibility based on patient age.
If there is an age-related spectrum of frailty reversibility, understanding the impact of circulatory support in an elderly HF population is crucial. Toward this end, Khawaja et al (2014) addressed some molecular, cellular, and functional elements of frailty reversibility in a slightly older population of patients receiving an LVAD as a bridge to transplant.90 Study participants were on average 62 years old, as compared to an average age of 50 years in the heart transplant population. By LVAD explant, GH levels decreased significantly, accompanied by a normalization of the GH/IGF-1 ratio. Rectus abdominis muscle biopsies at explant showed a decreased proportion of type II muscle fibers with relative increase of type I muscle fibers as compared to implant, and grip strength improved over time. The results of this small study suggest that circulatory support with an LVAD has the ability to reverse some of the metabolic, structural, and functional derangements associated with HF and frailty in a slightly older population than previously analyzed.
Given that frailty is a risk factor for morbidity and mortality in the elderly advanced HF population, the potential for frailty reversibility is an important element of management decisions. Although evidence supports that some frailty components may improve in response to physical therapy, GDMT, and more advanced circulatory support, how this relates to the entire frailty phenotype, as well as future risk for morbidity and mortality, is unknown. Also unknown is whether these results hold true for elderly patients (ie, over age 65) with advanced HF. Additional work to address the relationship between age, HF management, and frailty reversibility may help to improve outcomes as well as enhance conversations between clinicians and patients with respect to treatment options and goals of care (Fig. 1).
Figure 1.
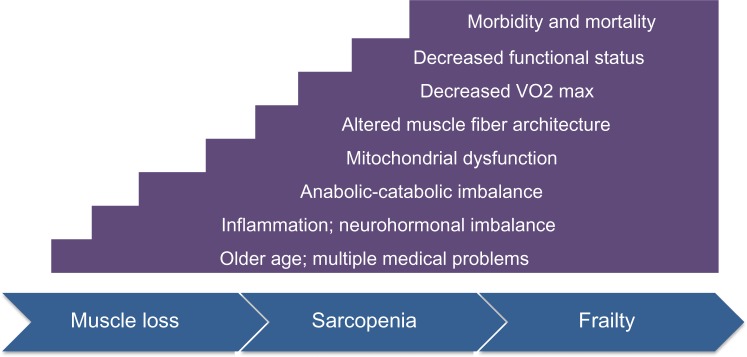
Progression to frailty in aging and heart failure. The primary frailty of aging and HF-related frailty have significant similarities. Older age and multiple medical problems are risk factors for the development of both types of frailty. The pathophysiological mechanisms of both include elevated inflammatory markers (ie, TNFα and IL-6), as well as dysregulated neurohormonal systems (ie, cortisol and insulin signaling). With respect to skeletal muscle, both groups have evidence of mitochondrial dysfunction and a shift in the cellular architecture of the muscle fibers. These molecular and structural changes result in decreased aerobic capacity, impaired functional status, and ultimately increased morbidity and mortality.
Conclusion
The frailty phenotype is associated with both advancing age and progressive HF and increases the risk for morbidity and mortality in both populations. At this time, clinical differentiation of the primary frailty of aging and frailty secondary to HF is not possible. In both, a propensity for an anabolic–catabolic imbalance appears to be driven by similar molecular mechanisms of systemic inflammation, oxidative stress, mitochondrial dysfunction, and neurohormonal dysregulation. These molecular derangements result in first structural and then functional musculoskeletal consequences. This process lays the foundation for the weight loss, weakness, fatigue, and functional deterioration that define the frailty phenotype.
Using the available data, it appears that there are subtle differences between the pathophysiology and recovery potential of primary frailty and HF-related frailty, which could have implications for management decisions. The prevalence of HF-related frailty is higher than that of age-matched community-dwelling elderly, demonstrating an earlier age of onset of frailty in this population. Perhaps, circulatory derangements trigger the neurohormonal and inflammatory milieu, which in turn initiates the underlying catabolic process of frailty. Interestingly, as compared to muscle loss associated with aging, fiber changes in HF favor a slow-to-fast twitch fiber shift due to higher overall percentage of type II fibers. The reason for this difference is unknown. Additionally, this population appears to have a more robust response to physical therapy as compared to those with primary frailty. Finally, there is a potential role for GDMT beyond preservation of left ventricular function, with some suggestion that BBs and ACE inhibitors improve frailty status. The caveat, however, is that the majority of evidence related to frailty in the HF population is extrapolated from studies of weight loss and cachexia, not frailty per se, in this population. Continuing to tease apart true differences between primary frailty and HF-related frailty may ultimately improve success with respect to therapeutic and pharmacological interventions.
However, primary frailty and HF-related frailty are most likely not mutually exclusive. Whether the elderly HF population manifests components of both primary and secondary frailty is unknown. About 30% of patients receiving LVADs are over age 65, the majority of whom receive the device as destination therapy. Although preliminary data suggest that circulatory support positively affects some elements of HF-related frailty, there is no information with respect to this very elderly cohort. If primary and secondary frailty coexist, potentially the balance between the two will contribute to reversibility potential. To assist with clinical differentiation, perhaps assessments may be aided by additional tests, such as muscle biopsies, to define frailty etiology and predict the potential for reversibility. Ongoing investigations are addressing the prevalence of frailty in this population as well as the potential for LVAD-mediated frailty reversibility. These results will further the discussion with respect to the risks and benefits of advanced circulatory support in an elderly HF population. Until that time, continued use of a standardized frailty definition and systematic operationalized approach to pre- and post-procedural frailty assessments will add to our knowledge. Ultimately, understanding the interaction between aging, HF, and frailty and clarifying the distinction between primary and secondary frailty will help guide difficult life-altering decisions for elderly HF patients.
List of Abbreviations
- ACE
angiotensin-converting enzyme
- BB
β-blockers
- COPD
chronic obstructive pulmonary disease
- CRP
C-reactive protein
- ESRD
end stage renal disease
- GDMT
guideline-directed medical therapy
- GH
growth hormone
- HF
heart failure
- HRQoL
health-related quality of life
- IGF1
insulin-like growth factor-1
- IL
interleukin
- LVAD
left ventricular assist device
- NFκB
nuclear factor kappa B
- NYHA
New York Heart Association
- TNFα
tumor necrosis factor alpha
- VO2
peak oxygen uptake
Footnotes
ACADEMIC EDITOR: Thomas E. Vanhecke, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: SP discloses personal fees and non-financial support from Thoratec Inc, outside the work presented here. DSG discloses no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Analyzed the data: DG, SP. Wrote the first draft of the manuscript: DG, SP. Contributed to the writing of the manuscript: DG, SP. Agree with manuscript results and conclusions: DG, SP. Jointly developed the structure and arguments for the paper: DG, SP. Made critical revisions and approved final version: DG, SP. Both authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8(1):30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–92. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 3.Park SK, Richardson CR, Holleman RG, Larson JL. Frailty in people with COPD, using the National Health and Nutrition Evaluation Survey dataset (2003–2006) Heart Lung. 2013;42(3):163–70. doi: 10.1016/j.hrtlng.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63(8):747–62. doi: 10.1016/j.jacc.2013.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61(6):896–901. doi: 10.1111/jgs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant. 2014;33(6):555–64. doi: 10.1016/j.healun.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in older people. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004;286(1):E92–101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- 9.Fried LP, Tangen CM, Walston J, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 10.Yao X, Li H, Leng SX. Inflammation and immune system alterations in frailty. Clin Geriatr Med. 2011;27(1):79–87. doi: 10.1016/j.cger.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubbard RE, O’Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13(9B):3103–9. doi: 10.1111/j.1582-4934.2009.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serviddio G, Romano AD, Greco A, et al. Frailty syndrome is associated with altered circulating redox balance and increased markers of oxidative stress. Int J Immunopathol Pharmacol. 2009;22(3):819–27. doi: 10.1177/039463200902200328. [DOI] [PubMed] [Google Scholar]
- 13.Schaap LA, Pluijm SMF, Deeg DJH, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119(6):e9–17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 14.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57(5):M326–32. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 15.Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59(3):242–8. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 16.Kalyani RR, Varadhan R, Weiss CO, Fried LP, Cappola AR. Frailty status and altered glucose-insulin dynamics. J Gerontol A Biol Sci Med Sci. 2012;67(12):1300–6. doi: 10.1093/gerona/glr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2(10):819–29. doi: 10.1016/S2213-8587(14)70034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johar H, Emeny RT, Bidlingmaier M, et al. Blunted diurnal cortisol pattern is associated with frailty: a cross-sectional study of 745 participants aged 65 to 90 years. J Clin Endocrinol Metab. 2014;99(3):E464–8. doi: 10.1210/jc.2013-3079. [DOI] [PubMed] [Google Scholar]
- 19.O’Connell MDL, Tajar A, Roberts SA, Wu FCW. Do androgens play any role in the physical frailty of ageing men? Int J Androl. 2011;34(3):195–211. doi: 10.1111/j.1365-2605.2010.01093.x. [DOI] [PubMed] [Google Scholar]
- 20.Semba RD, Ferrucci L, Sun K, et al. Oxidative stress and severe walking disability among older women. Am J Med. 2007;120(12):1084–9. doi: 10.1016/j.amjmed.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lexell J. Human aging, muscle mass, and fiber type composition. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1995;50:11–6. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 22.Larsson L. Histochemical characteristics of human skeletal muscle during aging. Acta Physiol Scand. 1983;117(3):469–71. doi: 10.1111/j.1748-1716.1983.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 23.Nilwik R, Snijders T, Leenders M, et al. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol. 2013;48(5):492–8. doi: 10.1016/j.exger.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol. 2013;45(10):2191–9. doi: 10.1016/j.biocel.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Pugh TD, Conklin MW, Evans TD, et al. A shift in energy metabolism anticipates the onset of sarcopenia in rhesus monkeys. Aging Cell. 2013;12(4):672–81. doi: 10.1111/acel.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.López-Armada MJ, Riveiro-Naveira RR, Vaamonde-García C, Valcárcel-Ares MN. Mitochondrial dysfunction and the inflammatory response. Mitochondrion. 2013;13(2):106–18. doi: 10.1016/j.mito.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Rogers MA, Hagberg JM, Martin WH, Ehsani AA, Holloszy JO. Decline in VO2 max with aging in master athletes and sedentary men. J Appl Physiol. 1990;68(5):2195–9. doi: 10.1152/jappl.1990.68.5.2195. [DOI] [PubMed] [Google Scholar]
- 28.Toth MJ, Gardner AW, Ades PA, Poehlman ET. Contribution of body composition and physical activity to age-related decline in peak VO2 in men and women. J Appl Physiol. 1994;77(2):647–52. doi: 10.1152/jappl.1994.77.2.647. [DOI] [PubMed] [Google Scholar]
- 29.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 30.de Oliveira RJ, Bottaro M, Motta AM, et al. Association between sarcopenia-related phenotypes and aerobic capacity indexes of older women. J Sports Sci Med. 2009;8(3):337–43. [PMC free article] [PubMed] [Google Scholar]
- 31.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990;263(22):3029–34. [PubMed] [Google Scholar]
- 32.Mangione KK, Miller AH, Naughton IV. Cochrane review: improving physical function and performance with progressive resistance strength training in older adults. Phys Ther. 2010;90(12):1711–5. doi: 10.2522/ptj.20100270. [DOI] [PubMed] [Google Scholar]
- 33.Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database of Systematic Reviews 2009. (3) doi: 10.1002/14651858.CD002759.pub2. Art. No.: CD002759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keysor JJ, Jette AM. Have we oversold the benefit of late-life exercise? J Gerontol A Biol Sci Med Sci. 2001;56(7):M412–23. doi: 10.1093/gerona/56.7.m412. [DOI] [PubMed] [Google Scholar]
- 35.Wolf SL, Sattin RW, Kutner M, O’Grady M, Greenspan AI, Gregor RJ. Intense tai chi exercise training and fall occurrences in older, transitionally frail adults: a randomized, controlled trial. J Am Geriatr Soc. 2003;51(12):1693–701. doi: 10.1046/j.1532-5415.2003.51552.x. [DOI] [PubMed] [Google Scholar]
- 36.Wolf SL, O’Grady M, Easley KA, Guo Y, Kressig RW, Kutner M. The influence of intense Tai Chi training on physical performance and hemodynamic outcomes in transitionally frail, older adults. J Gerontol A Biol Sci Med Sci. 2006;61(2):184–9. doi: 10.1093/gerona/61.2.184. [DOI] [PubMed] [Google Scholar]
- 37.Faber MJ, Bosscher RJ, Chin A, Paw MJ, van Wieringen PC. Effects of exercise programs on falls and mobility in frail and pre-frail older adults: a multicenter randomized controlled trial. Arch Phys Med Rehabil. 2006;87(7):885–96. doi: 10.1016/j.apmr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Binder EF, Schechtman KB, Ehsani AA, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002;50(12):1921–8. doi: 10.1046/j.1532-5415.2002.50601.x. [DOI] [PubMed] [Google Scholar]
- 39.Burton LA, Sumukadas D. Optimal management of sarcopenia. Clin Interv Aging. 2010;2010(5):217–28. doi: 10.2147/cia.s11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sukhanov S, Semprun-Prieto L, Yoshida T, et al. Angiotensin II, oxidative stress and skeletal muscle wasting. Am J Med Sci. 2011;342(2):143–7. doi: 10.1097/MAJ.0b013e318222e620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Witham MD, Sumukadas D, McMurdo MET. ACE inhibitors for sarcopenia – as good as exercise training? Age Ageing. 2008;37(4):363–5. doi: 10.1093/ageing/afn124. [DOI] [PubMed] [Google Scholar]
- 42.Onder G, Penninx BW, Balkrishnan R, et al. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. 2002;359(9310):926–30. doi: 10.1016/s0140-6736(02)08024-8. [DOI] [PubMed] [Google Scholar]
- 43.Haehling von S, Steinbeck L, Doehner W, Springer J, Anker SD. Muscle wasting in heart failure: an overview. Int J Biochem Cell Biol. 2013;45(10):2257–65. doi: 10.1016/j.biocel.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 44.Burton LA, Sumukadas D, Witham MD, Struthers AD, McMurdo MET. Effect of spironolactone on physical performance in older people with self-reported physical disability. Am J Med. 2013;126(7):590–7. doi: 10.1016/j.amjmed.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sumukadas D, Band M, Miller S, et al. Do ACE inhibitors improve the response to exercise training in functionally impaired older adults? A randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2014;69(6):736–43. doi: 10.1093/gerona/glt142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans WJ, Morley JE, Argilés J, et al. Cachexia: a new definition. Clin Nutr. 2008;27(6):793–9. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 47.Fülster S, Tacke M, Sandek A, et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF) Eur Heart J. 2013;34(7):512–9. doi: 10.1093/eurheartj/ehs381. [DOI] [PubMed] [Google Scholar]
- 48.Haykowsky MJ, Timmons MP, Kruger C, McNeely M, Taylor DA, Clark AM. Meta-analysis of aerobic interval training on exercise capacity and systolic function in patients with heart failure and reduced ejection fractions. Am J Cardiol. 2013;111(10):1466–9. doi: 10.1016/j.amjcard.2013.01.303. [DOI] [PubMed] [Google Scholar]
- 49.Anker SD, Ponikowski P, Varney S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349(9058):1050–3. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 50.Lupón J, González B, Santaeugenia S, et al. Prognostic implication of frailty and depressive symptoms in an outpatient population with heart failure. Rev Esp Cardiol. 2008;61(8):835–42. [PubMed] [Google Scholar]
- 51.Anker SD, Ponikowski PP, Clark AL, et al. Cytokines and neurohormones relating to body composition alterations in the wasting syndrome of chronic heart failure. Eur Heart J. 1999;20(9):683–93. doi: 10.1053/euhj.1998.1446. [DOI] [PubMed] [Google Scholar]
- 52.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323(4):236–41. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 53.McMurray J, Abdullah I, Dargie HJ, Shapiro D. Increased concentrations of tumour necrosis factor in “cachectic” patients with severe chronic heart failure. Br Heart J. 1991;66(5):356–8. doi: 10.1136/hrt.66.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anker SD, Clark AL, Kemp M, et al. Tumor necrosis factor and steroid metabolism in chronic heart failure: possible relation to muscle wasting. J Am Coll Cardiol. 1997;30(4):997–1001. doi: 10.1016/s0735-1097(97)00262-3. [DOI] [PubMed] [Google Scholar]
- 55.Haehling von S, Doehner W, Anker SD. Nutrition, metabolism, and the complex pathophysiology of cachexia in chronic heart failure. Cardiovasc Res. 2007;73(2):298–309. doi: 10.1016/j.cardiores.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 56.Khan H, Kalogeropoulos AP, Georgiopoulou VV, et al. Frailty and risk for heart failure in older adults: the health, aging, and body composition study. Am Heart J. 2013;166(5):887–94. doi: 10.1016/j.ahj.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bozkurt B, Kribbs SB, Clubb FJ, Jr, et al. Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation. 1998;97(14):1382–91. doi: 10.1161/01.cir.97.14.1382. [DOI] [PubMed] [Google Scholar]
- 58.Sivasubramanian N, Coker ML, Kurrelmeyer KM, et al. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2001;104(7):826–31. doi: 10.1161/hc3401.093154. [DOI] [PubMed] [Google Scholar]
- 59.Mancini DM, Coyle E, Coggan A, et al. Contribution of intrinsic skeletal muscle changes to 31P NMR skeletal muscle metabolic abnormalities in patients with chronic heart failure. Circulation. 1989;80(5):1338–46. doi: 10.1161/01.cir.80.5.1338. [DOI] [PubMed] [Google Scholar]
- 60.Borina E, Pellegrino MA, D’Antona G, Bottinelli R. Myosin and actin content of human skeletal muscle fibers following 35 days bed rest. Scand J Med Sci Sports. 2010;20(1):65–73. doi: 10.1111/j.1600-0838.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- 61.Caiozzo VJ, Baker MJ, Herrick RE, Tao M, Baldwin KM. Effect of spaceflight on skeletal muscle: mechanical properties and myosin isoform content of a slow muscle. J Appl Physiol. 1994;76(4):1764–73. doi: 10.1152/jappl.1994.76.4.1764. [DOI] [PubMed] [Google Scholar]
- 62.Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990;81(2):518–27. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- 63.Tikunov B, Levine S, Mancini D. Chronic congestive heart failure elicits adaptations of endurance exercise in diaphragmatic muscle. Circulation. 1997;95(4):910–6. doi: 10.1161/01.cir.95.4.910. [DOI] [PubMed] [Google Scholar]
- 64.Francis DP, Shamim W, Davies LC, et al. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2) Eur Heart J. 2000;21(2):154–61. doi: 10.1053/euhj.1999.1863. [DOI] [PubMed] [Google Scholar]
- 65.Harrington D, Anker SD, Chua TP, et al. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol. 1997;30(7):1758–64. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- 66.Adams V, Jiang H, Yu J, et al. Apoptosis in skeletal myocytes of patients with chronic heart failure is associated with exercise intolerance. J Am Coll Cardiol. 1999;33(4):959–65. doi: 10.1016/s0735-1097(98)00626-3. [DOI] [PubMed] [Google Scholar]
- 67.Filippatos GS, Kanatselos C, Manolatos DD, et al. Studies on apoptosis and fibrosis in skeletal musculature: a comparison of heart failure patients with and without cardiac cachexia. Int J Cardiol. 2003;90(1):107–13. doi: 10.1016/s0167-5273(02)00535-1. [DOI] [PubMed] [Google Scholar]
- 68.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301(6):H2181–90. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 69.Middlekauff HR. Making the case for skeletal myopathy as the major limitation of exercise capacity in heart failure. Circ Heart Fail. 2010;3(4):537–46. doi: 10.1161/CIRCHEARTFAILURE.109.903773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosca MG, Hoppel CL. Mitochondrial dysfunction in heart failure. Heart Fail Rev. 2013;18(5):607–22. doi: 10.1007/s10741-012-9340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Piepoli MF, Davos C, Francis DP, Coats AJS. ExTraMATCH collaborative. exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) BMJ. 2004;328(7433):189. doi: 10.1136/bmj.37938.645220.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davies EJ, Moxham T, Rees K, et al. Exercise training for systolic heart failure: cochrane systematic review and meta-analysis. Eur J Heart Fail. 2010;12(7):706–15. doi: 10.1093/eurjhf/hfq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taylor RS, Sagar VA, Davies EJ, et al. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev. 2014;4:CD003331. doi: 10.1002/14651858.CD003331.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Connor CM, Whellan DJ, Lee KL, et al. HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Flynn KE, Piña IL, Whellan DJ, et al. HF-ACTION Investigators Effects of exercise training on health status in patients with chronic heart failure: HF- ACTION randomized controlled trial. JAMA. 2009;301(14):1451–9. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blumenthal JA, Babyak MA, O’Connor C, et al. Effects of exercise training on depressive symptoms in patients with chronic heart failure: the HF-ACTION randomized trial. JAMA. 2012;308(5):465–74. doi: 10.1001/jama.2012.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gielen S, Sandri M, Kozarez I, et al. Exercise training attenuates MuRF-1 expression in the skeletal muscle of patients with chronic heart failure independent of age: the randomized Leipzig Exercise Intervention in Chronic Heart Failure and Aging Catabolism Study. Circulation. 2012;125(22):2716–27. doi: 10.1161/CIRCULATIONAHA.111.047381. [DOI] [PubMed] [Google Scholar]
- 78.Adamopoulos S, Parissis J, Karatzas D, et al. Physical training modulates proinflammatory cytokines and the soluble Fas/soluble Fas ligand system in patients with chronic heart failure. J Am Coll Cardiol. 2002;39(4):653–63. doi: 10.1016/s0735-1097(01)01795-8. [DOI] [PubMed] [Google Scholar]
- 79.Conraads VM, Beckers P, Bosmans J, et al. Combined endurance/resistance training reduces plasma TNF-alpha receptor levels in patients with chronic heart failure and coronary artery disease. Eur Heart J. 2002;23(23):1854–60. doi: 10.1053/euhj.2002.3239. [DOI] [PubMed] [Google Scholar]
- 80.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107(25):3133–40. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 81.Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109(13):1594–602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 82.Yancy CW, Jessup M, Bozkurt B, et al. American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 83.Lynch GS, Ryall JG. Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev. 2008;88(2):729–67. doi: 10.1152/physrev.00028.2007. [DOI] [PubMed] [Google Scholar]
- 84.Scherer M, Düngen HD, Inkrot S, et al. Determinants of change in quality of life in the Cardiac Insufficiency Bisoprolol Study in Elderly (CIBIS-ELD) Eur J Intern Med. 2013;24(4):333–8. doi: 10.1016/j.ejim.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 85.Dössegger L, Aldor E, Baird MG, et al. Influence of angiotensin converting enzyme inhibition on exercise performance and clinical symptoms in chronic heart failure: a multicentre, double-blind, placebo-controlled trial. Eur Heart J. 1993;14(suppl C):18–23. doi: 10.1093/eurheartj/14.suppl_c.18. [DOI] [PubMed] [Google Scholar]
- 86.Lund LH, Freda P, Williams JJ, LaManca JJ, LeJemtel TH, Mancini DM. Growth hormone resistance in severe heart failure resolves after cardiac transplantation. Eur J Heart Fail. 2009;11(5):525–8. doi: 10.1093/eurjhf/hfp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Habedank D, Ewert R, Hummel M, Wensel R, Hetzer R, Anker SD. Changes in exercise capacity, ventilation, and body weight following heart transplantation. Eur J Heart Fail. 2007;9(3):310–6. doi: 10.1016/j.ejheart.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 88.Baker AM, Levine TB, Goldberg AD, Levine AB. Natural history and predictors of obesity after orthotopic heart transplantation. J Heart Lung Transplant. 1992;11(6):1156–9. [PubMed] [Google Scholar]
- 89.Williams JJ, Lund LH, LaManca J, et al. Excessive weight gain in cardiac transplant recipients. J Heart Lung Transplant. 2006;25(1):36–41. doi: 10.1016/j.healun.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 90.Khawaja T, Chokshi A, Ji R, et al. Ventricular assist device implantation improves skeletal muscle function, oxidative capacity, and growth hormone/insulin-like growth factor-1 axis signaling in patients with advanced heart failure. J Cachexia Sarcopenia Muscle. 2014;5(4):297–305. doi: 10.1007/s13539-014-0155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


