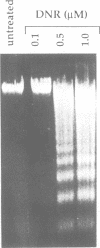
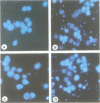
Abstract
The nature of the signaling pathway(s) which initiate drug-triggered apoptosis remains largely unknown and is of fundamental importance in understanding cell death induced by chemotherapeutic agents. Here we show that in the leukemic cell lines U937 and HL-60, daunorubicin, at concentrations which trigger apoptosis, stimulated two distinct cycles of sphingomyelin hydrolysis (approximately 20% decrease at 1 microM) within 4-10 min and 60-75 min with concomitant ceramide generation. We demonstrate that the increase in ceramide levels, which precedes apoptosis, is mediated by a neutral sphingomyelinase and not by ceramide synthase. Indeed, potent ceramide synthase inhibitors such as fumonisin B1 did not affect daunorubicin-triggered sphingomyelin hydrolysis, ceramide generation or apoptosis. In conclusion, we provide evidence that daunorubicin-triggered apoptosis is mediated by a signaling pathway which is initiated by an early sphingomyelin-derived ceramide production.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrieu N., Salvayre R., Jaffrézou J. P., Levade T. Low temperatures and hypertonicity do not block cytokine-induced stimulation of the sphingomyelin pathway but inhibit nuclear factor-kappa B activation. J Biol Chem. 1995 Oct 13;270(41):24518–24524. doi: 10.1074/jbc.270.41.24518. [DOI] [PubMed] [Google Scholar]
- Andrieu N., Salvayre R., Levade T. Comparative study of the metabolic pools of sphingomyelin and phosphatidylcholine sensitive to tumor necrosis factor. Eur J Biochem. 1996 Mar 1;236(2):738–745. doi: 10.1111/j.1432-1033.1996.00738.x. [DOI] [PubMed] [Google Scholar]
- Andrieu N., Salvayre R., Levade T. Evidence against involvement of the acid lysosomal sphingomyelinase in the tumor-necrosis-factor- and interleukin-1-induced sphingomyelin cycle and cell proliferation in human fibroblasts. Biochem J. 1994 Oct 15;303(Pt 2):341–345. doi: 10.1042/bj3030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose R., Verheij M., Haimovitz-Friedman A., Scotto K., Fuks Z., Kolesnick R. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995 Aug 11;82(3):405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- Cocco L., Maraldi N. M., Manzoli F. A., Gilmour R. S., Lang A. Phospholipid interactions in rat liver nuclear matrix. Biochem Biophys Res Commun. 1980 Sep 30;96(2):890–898. doi: 10.1016/0006-291x(80)91439-4. [DOI] [PubMed] [Google Scholar]
- Dive C., Hickman J. A. Drug-target interactions: only the first step in the commitment to a programmed cell death? Br J Cancer. 1991 Jul;64(1):192–196. doi: 10.1038/bjc.1991.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowsky R. T., Kamibayashi C., Mumby M. C., Hannun Y. A. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993 Jul 25;268(21):15523–15530. [PubMed] [Google Scholar]
- Dressler K. A., Mathias S., Kolesnick R. N. Tumor necrosis factor-alpha activates the sphingomyelin signal transduction pathway in a cell-free system. Science. 1992 Mar 27;255(5052):1715–1718. doi: 10.1126/science.1313189. [DOI] [PubMed] [Google Scholar]
- Escargueil-Blanc I., Salvayre R., Nègre-Salvayre A. Necrosis and apoptosis induced by oxidized low density lipoproteins occur through two calcium-dependent pathways in lymphoblastoid cells. FASEB J. 1994 Oct;8(13):1075–1080. doi: 10.1096/fasebj.8.13.7926374. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fisher D. E. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994 Aug 26;78(4):539–542. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- Frankfurt O. S., Byrnes J. J., Seckinger D., Sugarbaker E. V. Apoptosis (programmed cell death) and the evaluation of chemosensitivity in chronic lymphocytic leukemia and lymphoma. Oncol Res. 1993;5(1):37–42. [PubMed] [Google Scholar]
- Goldkorn T., Dressler K. A., Muindi J., Radin N. S., Mendelsohn J., Menaldino D., Liotta D., Kolesnick R. N. Ceramide stimulates epidermal growth factor receptor phosphorylation in A431 human epidermoid carcinoma cells. Evidence that ceramide may mediate sphingosine action. J Biol Chem. 1991 Aug 25;266(24):16092–16097. [PubMed] [Google Scholar]
- Gong J., Traganos F., Darzynkiewicz Z. A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Anal Biochem. 1994 May 1;218(2):314–319. doi: 10.1006/abio.1994.1184. [DOI] [PubMed] [Google Scholar]
- Haimovitz-Friedman A., Kan C. C., Ehleiter D., Persaud R. S., McLoughlin M., Fuks Z., Kolesnick R. N. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994 Aug 1;180(2):525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman J. A. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992 Sep;11(2):121–139. doi: 10.1007/BF00048059. [DOI] [PubMed] [Google Scholar]
- Jarvis W. D., Kolesnick R. N., Fornari F. A., Traylor R. S., Gewirtz D. A., Grant S. Induction of apoptotic DNA damage and cell death by activation of the sphingomyelin pathway. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):73–77. doi: 10.1073/pnas.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns L. D., Sarr T., Ranges G. E. Inhibition of ceramide pathway does not affect ability of TNF-alpha to activate nuclear factor-kappa B. J Immunol. 1994 Jun 15;152(12):5877–5882. [PubMed] [Google Scholar]
- Kapuściński J., Skoczylas B. Simple and rapid fluorimetric method for DNA microassay. Anal Biochem. 1977 Nov;83(1):252–257. doi: 10.1016/0003-2697(77)90533-4. [DOI] [PubMed] [Google Scholar]
- Kim M. Y., Linardic C., Obeid L., Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J Biol Chem. 1991 Jan 5;266(1):484–489. [PubMed] [Google Scholar]
- Kolesnick R. N. 1,2-Diacylglycerols but not phorbol esters stimulate sphingomyelin hydrolysis in GH3 pituitary cells. J Biol Chem. 1987 Dec 15;262(35):16759–16762. [PubMed] [Google Scholar]
- Kolesnick R. Ceramide: a novel second messenger. Trends Cell Biol. 1992 Aug;2(8):232–236. doi: 10.1016/0962-8924(92)90310-j. [DOI] [PubMed] [Google Scholar]
- Kolesnick R., Golde D. W. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994 May 6;77(3):325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Levade T., Tempesta M. C., Salvayre R. The in situ degradation of ceramide, a potential lipid mediator, is not completely impaired in Farber disease. FEBS Lett. 1993 Aug 30;329(3):306–312. doi: 10.1016/0014-5793(93)80243-n. [DOI] [PubMed] [Google Scholar]
- Linardic C. M., Hannun Y. A. Identification of a distinct pool of sphingomyelin involved in the sphingomyelin cycle. J Biol Chem. 1994 Sep 23;269(38):23530–23537. [PubMed] [Google Scholar]
- Ling Y. H., Priebe W., Perez-Soler R. Apoptosis induced by anthracycline antibiotics in P388 parent and multidrug-resistant cells. Cancer Res. 1993 Apr 15;53(8):1845–1852. [PubMed] [Google Scholar]
- Mathias S., Dressler K. A., Kolesnick R. N. Characterization of a ceramide-activated protein kinase: stimulation by tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10009–10013. doi: 10.1073/pnas.88.22.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey D. J., Aguilar-Santelises M., Hartzell P., Eriksson I., Mellstedt H., Orrenius S., Jondal M. Induction of DNA fragmentation in chronic B-lymphocytic leukemia cells. J Immunol. 1991 Feb 1;146(3):1072–1076. [PubMed] [Google Scholar]
- Obeid L. M., Linardic C. M., Karolak L. A., Hannun Y. A. Programmed cell death induced by ceramide. Science. 1993 Mar 19;259(5102):1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Okazaki T., Bell R. M., Hannun Y. A. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. Role in cell differentiation. J Biol Chem. 1989 Nov 15;264(32):19076–19080. [PubMed] [Google Scholar]
- Okazaki T., Bielawska A., Domae N., Bell R. M., Hannun Y. A. Characteristics and partial purification of a novel cytosolic, magnesium-independent, neutral sphingomyelinase activated in the early signal transduction of 1 alpha,25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J Biol Chem. 1994 Feb 11;269(6):4070–4077. [PubMed] [Google Scholar]
- Potmesil M., Kirschenbaum S., Israel M., Levin M., Khetarpal V. K., Silber R. Relationship of adriamycin concentrations to the DNA lesions induced in hypoxic and euoxic L1210 cells. Cancer Res. 1983 Aug;43(8):3528–3533. [PubMed] [Google Scholar]
- Quillet-Mary A., Mansat V., Duchayne E., Come M. G., Allouche M., Bailly J. D., Bordier C., Laurent G. Daunorubicin-induced internucleosomal DNA fragmentation in acute myeloid cell lines. Leukemia. 1996 Mar;10(3):417–425. [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Rivas C. I., Golde D. W., Vera J. C., Kolesnick R. N. Involvement of the sphingomyelin pathway in autocrine tumor necrosis factor signaling for human immunodeficiency virus production in chronically infected HL-60 cells. Blood. 1994 Apr 15;83(8):2191–2197. [PubMed] [Google Scholar]
- Ross W. E., Glaubiger D., Kohn K. W. Qualitative and quantitative aspects of intercalator-induced DNA strand breaks. Biochim Biophys Acta. 1979 Mar 28;562(1):41–50. doi: 10.1016/0005-2787(79)90124-2. [DOI] [PubMed] [Google Scholar]
- Schütze S., Potthoff K., Machleidt T., Berkovic D., Wiegmann K., Krönke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced "acidic" sphingomyelin breakdown. Cell. 1992 Nov 27;71(5):765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- Skladanowski A., Konopa J. Adriamycin and daunomycin induce programmed cell death (apoptosis) in tumour cells. Biochem Pharmacol. 1993 Aug 3;46(3):375–382. doi: 10.1016/0006-2952(93)90512-u. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Solary E., Bertrand R., Kohn K. W., Pommier Y. Differential induction of apoptosis in undifferentiated and differentiated HL-60 cells by DNA topoisomerase I and II inhibitors. Blood. 1993 Mar 1;81(5):1359–1368. [PubMed] [Google Scholar]
- Spangler M., Coetzee M. L., Katyal S. L., Morris H. P., Ove P. Some biochemical characteristics of rat liver and Morris hepatoma nuclei and nuclear membranes. Cancer Res. 1975 Nov;35(11 Pt 1):3131–3135. [PubMed] [Google Scholar]
- Speth P. A., Linssen P. C., Boezeman J. B., Wessels H. M., Haanen C. Leukemic cell and plasma daunomycin concentrations after bolus injection and 72 h infusion. Cancer Chemother Pharmacol. 1987;20(4):311–315. doi: 10.1007/BF00262582. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Melzner I. Studies in vitro on the biosynthesis of ceramide and sphingomyelin. A reevaluation of proposed pathways. Hoppe Seylers Z Physiol Chem. 1980 May;361(5):755–771. doi: 10.1515/bchm2.1980.361.1.755. [DOI] [PubMed] [Google Scholar]
- Tamiya-Koizumi K., Umekawa H., Yoshida S., Kojima K. Existence of Mg2+-dependent, neutral sphingomyelinase in nuclei of rat ascites hepatoma cells. J Biochem. 1989 Oct;106(4):593–598. doi: 10.1093/oxfordjournals.jbchem.a122901. [DOI] [PubMed] [Google Scholar]
- Tritton T. R. Cell surface actions of adriamycin. Pharmacol Ther. 1991;49(3):293–309. doi: 10.1016/0163-7258(91)90060-y. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Matthews T. J., Bolognesi D. P., Bell R. M. Changes in bioactive lipids, alkylacylglycerol and ceramide, occur in HIV-infected cells. Biochem Biophys Res Commun. 1992 Aug 31;187(1):209–216. doi: 10.1016/s0006-291x(05)81480-9. [DOI] [PubMed] [Google Scholar]
- Wiegmann K., Schütze S., Machleidt T., Witte D., Krönke M. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell. 1994 Sep 23;78(6):1005–1015. doi: 10.1016/0092-8674(94)90275-5. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A., Kerrigan D., Michaels S. Cytotoxicity and DNA strand breaks by 5-iminodaunorubicin in mouse leukemia L1210 cells: comparison with adriamycin and 4'-(9-acridinylamino)methanesulfon-m-anisidide. Cancer Res. 1982 Jul;42(7):2687–2691. [PubMed] [Google Scholar]