Abstract
Purpose
Diabetes and certain diabetes medications have been shown to influence breast cancer (BC) risk. Less is known about their relation to BC outcomes. Our objective was to evaluate effects of diabetes and diabetes medications on risk of second breast cancer events (SBCE) and mortality.
Methods
This population-based cohort study was conducted among women diagnosed with early stage (I–II) BC and enrolled in an integrated health plan. Exposures of interest were diabetes and medication classes including insulin, metformin, and sulfonylureas. Outcomes of interest were SBCE defined as recurrence or second primary BC, BC-specific mortality, and all-cause mortality. We used multivariable Cox proportional hazards models to estimate hazard ratios (HR) and 95% confidence intervals (CI) for diabetes and medication use while accounting for potential confounders and competing risks.
Results
Among 4,216 women, 13% developed SBCE during a median follow-up of 6.3 years. 610 women had diabetes of which 76% used oral diabetes medication and/or insulin. Findings suggested that diabetes increased risk of recurrence (HR=1.57;95% CI,1.09–2.25) but not overall SBCE (HR=1.29;95% CI,0.94–1.76) and second primary BC (HR=0.74;95% CI,0.39–1.41). Among women with diabetes, insulin use was associated with increased risks of recurrence (HR=1.94;95% CI,1.08–3.48) and all-cause mortality (HR=2.33;95% CI,1.70–3.20). Metformin use was associated with lower all-cause mortality (HR=0.55;95% CI,0.38–0.79).
Conclusions
Our findings show an association between diabetes and increased recurrence risk, and risk may be greater among insulin users. Metformin may reduce all-cause mortality among BC survivors. Given the growing breast cancer survivor population, further research in larger, more diverse populations is warranted.
Keywords: Breast cancer recurrence, Mortality, Diabetes, Insulin, Metformin, Sulfonylureas
Introduction
There are an estimated 2.8 million breast cancer (BC) survivors in the United States [1]. As this growing population ages, more women are burdened with chronic comorbid conditions such as diabetes and require treatment with multiple medications that could have further implications for health outcomes following BC [2,3].
Diabetes is associated with an increased risk of incident BC [4] possibly through the direct effect of high levels of insulin on breast tissue and/or indirect effects on increases in sex steroids due to inhibition of sex hormone-binding globulin, disruption of adipokines, and increased insulin-like growth factor (IGF)-I production [5]. Diabetes may also be a risk factor for second breast cancer events (SBCE) including recurrence [6] and second primary BC [7]. Also, meta-analyses have described a 49% increased risk of all-cause mortality in BC survivors with diabetes [8]. Some commonly used diabetes medications, such as metformin, may influence the relation between diabetes and BC by decreasing primary BC risk [9], while other diabetes medications, such as insulin glargine, are found to be associated with an increased risk of incident BC in some [10–12] but not all epidemiologic studies [13–15]. Less is known about the relation between diabetes and diabetes medications with risk of SBCE and survival among women with BC.
There is growing evidence to support a possible role for diabetes medications to influence BC outcomes. Metformin, for instance, decreases circulating insulin levels and activates the adenosine monophosphate-activated protein kinase (AMPK) pathway [16]. These effects are relevant to risk of SBCE in that insulin can promote cancer growth through modulators such as insulin-like growth factors, and AMPK activation can suppress several tumorigenic metabolic pathways [16]. As such, metformin is associated with increased rates of pathologic complete response after chemotherapy [17] and reduced tumor proliferation markers in BC patients without diabetes [18]. On the other hand, it is possible that medications such as sulfonylureas that stimulate insulin secretion from beta cells or insulin analogues have increased mitogenicity and carcinogenic potential to influence risk of SBCE [19]. Insulin glargine, in particular, is a long-acting structurally-altered human insulin analogue that has altered binding kinetics for insulin receptors [20] and increased affinity for IGF-I receptors [21]. Recent data suggest that among patients with diabetes, serum of those treated with insulin glargine is more mitogenic to BC cells compared to those treated with regular human insulin or insulin detemir [22].
The objective of this study was to evaluate the relation between diabetes and diabetes medications including insulin, metformin, and sulfonylureas with risks of SBCE, BC-specific mortality, and all-cause mortality.
Materials and Methods
Study population and data collection
We conducted a retrospective cohort study within the Commonly Used Medications and Breast Cancer Outcomes (COMBO) study which has been described in detail previously [23,24]. In brief, the source population was enrollees of Group Health (GH), a non-profit integrated delivery system that provides comprehensive health care on a pre-paid basis to approximately 600,000 individuals throughout Washington State and parts of Idaho. GH is located within the geographic reporting region of the western Washington Cancer Surveillance System, a population-based cancer registry and member of the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program [25].
The COMBO cohort included women aged ≥ 18 years, residing within the 13 Washington State counties covered by SEER, diagnosed with an incident, histologically confirmed stage I or II asynchronous (non-bilateral) BC between January 1, 1990 and December 31, 2008, and enrolled in GH’s integrated group practice for 1+ year before and after the incident BC diagnosis unless they died [23,24]. Chart abstraction began in 2009 and continued through August 2011.
Data were collected through health plan administrative databases, SEER, and medical record review from one year before incident BC through the end of study follow-up defined as the earliest of death, disenrollment from GH (>90 day lapse in enrollment), or end of study (chart abstraction date). GH’s administrative data files include demographics, start and end dates of enrollment, inpatient and outpatient diagnoses and procedures, breast screening services and results, pharmacy dispensings, laboratory results, vital signs, and death [26]. The pharmacy database includes all medications dispensed at GH’s outpatient pharmacies as well as claims from contracting pharmacies and is estimated to be 97% complete [26–28]. SEER was the primary source of information for incident BC characteristics including diagnosis year, American Joint Committee on Cancer (AJCC) stage [29], lymph node status, hormone receptor status, and tumor size. Data on BC risk factors and surveillance were obtained from GH’s Breast Cancer Screening Recruitment and Reminder (BSRR) survey taken at the time of each mammography [30]. The source of death data was GH data files that contain date of death for enrollees who die in a hospital while covered by GH or whose survivors inform GH (for instance, to terminate insurance coverage), required reporting of death notice from CMS for Medicare beneficiaries, death information from SEER and an ongoing link to Washington State death tapes [31] which includes records of those who die out of state. Medical record review was done to confirm the incident BC diagnosis and to collect its treatment information, some comorbidities, and details on recurrence and second primary BC that occurred during the follow-up period.
A total of 4,426 potentially eligible subjects underwent medical record review. Women were further excluded on the basis of no medical record (n=72), bilateral disease (n=6), recurrences or second primary BC that were incorrectly identified as incident BC (n=79), and no definitive surgery (n=44). A final cohort of 4,216 women was included in the study after excluding 5 patients who died and 4 patients with metastases that occurred prior to 120 days after completing surgery which was defined as the first date becoming at risk of SBCE.
Exposures
Our exposures of interest included diabetes and use of diabetes medications classes. A woman was defined as having diabetes if she had ≥ 2 outpatient (within a 6-month period) or 1 inpatient encounter that was assigned an ICD-9 code of 250.00–250.93 during the year prior to the incident BC through the end of study follow-up [32–34]. Date of diabetes diagnosis was the first date when either of these criteria were met. Women were defined as users of a particular diabetes medication class including insulin, metformin and sulfonylureas during the follow-up period if they had ≥ 1 dispensing of a medication in the class of interest after the incident BC diagnosis. Presence of diabetes and use of diabetes medication classes were modeled as time-varying covariates that once a woman had the diagnosis or started the medication, she would remain as having diabetes or a user during the remaining of the follow-up. In addition, the three diabetes medication classes were not mutually exclusive. Therefore, medication use among women with diabetes accounted for progression of monotherapy to combination therapy by allowing medications users to have progressive, time-varying exposure to multiple diabetes medication classes [35]. Insulin was further classified as long-acting, short-acting, and rapid-acting.
Outcomes
The primary endpoint of interest was SBCE, defined as the first of recurrence in any regional or distant sites or second primary ductal carcinoma in situ (DCIS) or invasive cancer of the ipsilateral or contralateral breast [36]. Secondary endpoints were BC-specific mortality and all-cause mortality defined based on the cause of death information recorded in the State death tapes.
Statistical analysis
Cox proportional hazards regression models were used to evaluate the association between diabetes and diabetes medication use with risks of SBCE and mortality. We estimated hazard ratios (HR) and their 95% confidence intervals (CI) while adjusting for potential confounders. We modeled time from the incident BC diagnosis with a delayed entry at 120 days post-surgery when a woman was defined to become at risk of a SBCE to the first of SBCE, death, disenrollment from the health plan, or end of study date. Individual events (i.e., recurrence and second primary BC) that made up the composite outcome of SBCE were also modeled to obtain a comprehensive assessment of the medication effects [37]. Specifically, in the analyses of recurrence as an outcome, women were censored at the earliest of disenrollment, end of follow-up, and competing events including death and second primary BC. Analyses for BC-specific mortality and all-cause mortality were modeled in a similar manner but without competing events in the models.
Our unadjusted model included adjustment for age at diagnosis and AJCC stage for the incident BC. We considered potential confounders a priori and similar to other studies [38–41] further adjusted for the following covariates in the multivariable models: calendar year, hormone receptor status, and primary treatment for the incident BC diagnosis; body mass index (BMI), smoking status, and menopausal status defined at the time of diagnosis; time-varying covariates including endocrine therapy for the incident BC, Charlson comorbidity score [42], statin use, prescription non-steroidal anti-inflammatory (NSAID) medication use, Cox-2 inhibitors, and aspirin, and receipt of a screening mammogram in the prior 12 months.
We included all 4,216 women in the analyses of diabetes on risks of SBCE, BC-specific and all-cause mortality. In separate models, we further evaluated the effects of medication exposures by including the three classes of diabetes medications. Diabetes medications were also evaluated among 610 women with diabetes any time during the year prior to incident BC through end of follow-up. In this subgroup analysis, if the date of diabetes diagnosis occurred after the at risk date for SBCE (i.e. 120 days post-surgery for incident BC), then the date of diabetes became the new at risk date (i.e., the new delayed entry date in the Cox models).
We tested for interaction between the exposures of interest and the logarithm of follow-up time to evaluate proportional hazards assumptions. There was no evidence to suggest violation of the assumptions.
All analyses were performed using SAS statistical software version 9.3 (SAS Institute Inc., Cary, North Carolina). The Institutional Review Board at GH approved this study.
Results
Analyses among all women
Characteristics of the 4,216 women with incident stage I or II BC included in the COMBO study are described in detail elsewhere [23,24] (Table 1). The majority of women in the cohort were postmenopausal and had a Charlson comorbidity score of zero at diagnosis. The majority of incident BC were AJCC stage I, estrogen receptor (ER)-positive/progesterone receptor (PR)-positive, treated with breast-conserving surgery with or without radiation, not treated with chemotherapy, and treated with endocrine therapy.
Table 1.
Descriptive characteristics of 4,216 women included in the COMBO study by SBCE status
| SBCE† | |||
|---|---|---|---|
| All women N=4,216 |
No (n=3,658) | Yes (n=558) | |
| Characteristics at incident breast cancer diagnosis | |||
| Year of diagnosis | |||
| 1990–1994 | 950 (22.5) | 755 (20.6) | 195 (34.9) |
| 1995–1999 | 1191 (28.2) | 1020 (27.9) | 171 (30.6) |
| 2000–2004 | 1201 (28.5) | 1073 (29.3) | 128 (22.9) |
| 2005–2008 | 874 (20.7) | 810 (22.1) | 64 (11.5) |
| Age at diagnosis, years | |||
| Median (interquartile range) | 63 (52–73) | 63 (52–73) | 62 (50–72) |
| 18–39 | 139 (3.3) | 112 (3.1) | 27 (4.8) |
| 40–49 | 646 (15.3) | 544 (14.9) | 102 (18.3) |
| 50–59 | 995 (23.6) | 866 (23.7) | 129 (23.1) |
| 60–69 | 1018 (24.1) | 889 (24.3) | 129 (23.1) |
| 70–79 | 940 (22.3) | 824 (22.5) | 116 (20.8) |
| 80+ | 478 (11.3) | 423 (11.6) | 55 (9.9) |
| Menopausal status at diagnosis | |||
| Peri- or Premenopausal | 1145 (27.2) | 956 (26.1) | 189 (33.9) |
| Postmenopausal | 3071 (72.8) | 2702 (73.9) | 369 (66.1) |
| Body mass index (kg/m2) | |||
| Mean (SD) | 28 (6.2) | 28 (6.2) | 28.3 (6.6) |
| Median | 26.8 | 26.7 | 27.5 |
| <18.5 | 69 (1.6) | 55 (1.5) | 14 (2.5) |
| 18.5–24.9 | 1453 (34.6) | 1269 (34.8) | 184 (33.3) |
| 25.0–29.9 | 1362 (32.5) | 1186 (32.6) | 176 (31.8) |
| 30.0–34.9 | 766 (18.3) | 666 (18.3) | 100 (18.1) |
| 35+ | 546 (13.0) | 467 (12.8) | 79 (14.3) |
| Unknown | 20 | 15 | 5 |
| Smoking status at diagnosis | |||
| Current | 253 (6.0) | 230 (6.3) | 23 (4.1) |
| Past | 352 (8.3) | 318 (8.7) | 34 (6.1) |
| Never/Unknown | 3611 (85.6) | 3110 (85.0) | 501 (89.8) |
| AJCC stage | |||
| I | 2648 (62.8) | 2384 (65.2) | 264 (47.3) |
| IIA | 1078 (25.6) | 906 (24.8) | 172 (30.8) |
| IIB | 490 (11.6) | 368 (10.1) | 122 (21.9) |
| ER/PR status | |||
| ER(−)/PR(−) | 667 (15.8) | 531 (14.5) | 136 (24.4) |
| ER(+)/PR(−) | 383 (9.1) | 319 (8.7) | 64 (11.5) |
| ER(−)/PR(+) | 61 (1.4) | 47 (1.3) | 14 (2.5) |
| ER(+)/PR(+) | 2888 (68.5) | 2572 (70.3) | 316 (56.6) |
| ER and/or PR unknown | 217 (5.1) | 189 (5.2) | 28 (5.0) |
| HER2 status | |||
| Test performed | 2074 (79.7) | 1874 (80.5) | 200 (73.0) |
| Positive/borderline | 353 (17.0) | 311 (16.6) | 42 (21.0) |
| Negative | 1714 (82.6) | 1556 (83.0) | 158 (79.0) |
| No result | 7 (0.3) | 7 (0.4) | 0 (0.0) |
| Surgical procedure | |||
| Mastectomy including radical ± radiation | 1521 (36.1) | 1289 (35.2) | 232 (41.6) |
| Breast conserving, + radiation | 2172 (51.5) | 1927 (52.7) | 245 (43.9) |
| Breast conserving, no radiation | 523 (12.4) | 442 (12.1) | 81 (14.5) |
| Other treatment | |||
| Any chemotherapy | 1376 (32.6) | 1142 (31.2) | 234 (41.9) |
| Completed course | 1212 (88.1) | 1003 (87.8) | 209 (89.3) |
| Any endocrine therapy | 2363 (56.0) | 2101 (57.4) | 262 (47.0) |
| Charlson score at diagnosis | |||
| 0 | 3229 (76.6) | 2784 (76.1) | 445 (79.7) |
| 1 | 704 (16.7) | 625 (17.1) | 79 (14.2) |
| 2+ | 283 (6.7) | 249 (6.8) | 34 (6.1) |
| Diabetes | |||
| Diabetes (ever) throughout follow-up | 610 (14.5) | 539 (14.7) | 71 (12.7) |
| At BC diagnosis (year prior to BC) | 329 (53.9) | 294 (54.5) | 35 (49.3) |
| After BC diagnosis through follow-up | 281 (46.1) | 245 (45.5) | 36 (50.7) |
| Diabetes medication use in year prior to breast cancer diagnosis* | |||
| Any oral or injectable medications | 277 (6.6) | 241 (6.6) | 36 (6.5) |
| Any oral medications | 225 (5.3) | 200 (5.5) | 25 (4.5) |
| Metformin | 106 (47.1) | 97 (48.5) | 9 (36.0) |
| Sulfonylurea | 175 (77.8) | 152 (76.0) | 23 (92.0) |
| Insulin | 103 (2.4) | 88 (2.4) | 15 (2.7) |
| Long-acting | 12 (11.7) | 11 (12.5) | 1 (6.7) |
| Short-acting | 88 (85.4) | 74 (84.1) | 14 (93.3) |
| Rapid-acting | 16 (15.5) | 13 (14.8) | 3 (20.0) |
| Characteristics throughout follow-up | |||
| Diabetes medication use throughout follow-up* | |||
| Any oral or injectable medications | 464 (11.0) | 408 (11.2) | 56 (10.0) |
| Any oral medications | 403 (9.6) | 358 (9.8) | 45 (8.1) |
| Metformin | 275 (68.2) | 248 (69.3) | 27 (60.0) |
| Sulfonylurea | 307 (76.2) | 270 (75.4) | 37 (82.2) |
| Insulin | 246 (5.8) | 218 (6.0) | 28 (5.0) |
| Long-acting | 47 (19.1) | 43 (19.7) | 4 (14.3) |
| Short-acting | 198 (80.5) | 173 (79.4) | 25 (89.3) |
| Rapid-acting | 62 (25.2) | 56 (25.7) | 6 (21.4) |
| % Follow-up years with yearly screening mammogram | |||
| <50% | 939 (22.3) | 793 (21.7) | 146 (26.2) |
| 50%–80% | 1439 (34.1) | 1284 (35.1) | 155 (27.8) |
| >80% | 1838 (43.6) | 1581 (43.2) | 257 (46.1) |
| Years of follow-up | |||
| Mean (SD) | 7.1 (4.3) | 7.5 (4.3) | 4.4 (3.5) |
| Median | 6.3 | 6.7 | 3.3 |
SBCE = second breast cancer event includes recurrence or second primary breast cancer, in situ and invasive.
Not mutually exclusive
There were 432 (10%) women who experienced a recurrence and 153 (4%) second primary BC during study follow-up, yielding a total of 558 (13%) women having a SBCE (with 415 recurrences and 143 second primary BC as the first SBCE events). Median time from incident BC diagnosis to the SBCE was 3.3 years. Among recurrences, 67% were distant, 32% local or regional, and 1% DCIS. Among second primary cancers, 21% were DCIS, 49% stage I, 21% stage II, 4% stage III/IV, and 5% unknown stage. At the end of study follow-up, 22% of women died, and 6% were BC-specific.
Women that experienced a SBCE were more likely to be peri- or premenopausal, diagnosed with AJCC stage II, lymph node positive, ER− and/or PR-negative, tumor size >2 cm, HER2-positive, treated by mastectomy, treated with chemotherapy, not treated with endocrine therapy, and detected by a diagnostic versus screening mammography compared to women without a SBCE during the follow-up period (Table 1).
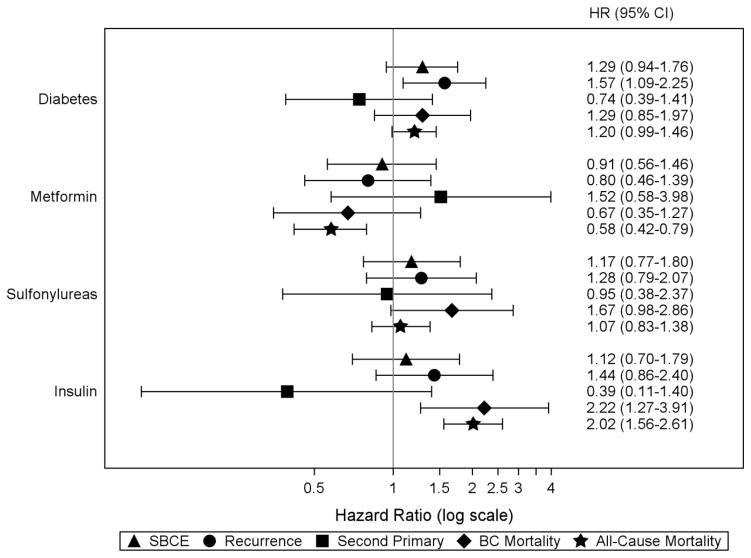
Unadjusted and adjusted models did not differ substantially, so we present only results from adjusted models. We observed a statistically significant association between diabetes diagnosis and an increased risk of recurrence (HR=1.57;95% CI,1.09–2.25), but not of SBCE (HR=1.29;95% CI,0.94–1.76) or second primary BC (HR=0.74;95% CI,0.39–1.41) (Figure 1). Results suggested a slightly elevated risk of both BC-specific and all-cause mortality among women with diabetes compared to those without, though the confidence intervals included 1.0.
Figure 1.
Risk of SBCE, breast cancer-specific mortality, and all-cause mortality in relation to diabetes and diabetes medication classes* among all women in the COMBO cohort
Abbreviations: SBCE second breast cancer event (recurrence and second primary BC), BC breast cancer, HR hazard ratio, CI confidence interval
Note: all hazard ratios are adjusted for age at diagnosis (18–49, 50–59, 60–69, 70–79, 80+ years); diagnosis year (1990–1994, 1995–1999, 2000–2004, 2005–2008); AJCC stage (I, IIA, IIB); hormone receptor status (estrogen receptor [ER]−/progesterone receptor [PR] −, ER +/PR −, ER −/PR +, ER +/PR +, and ER and/or PR unknown); primary treatment for initial BC (mastectomy, breast conserving surgery with radiation, breast conserving surgery without radiation); endocrine therapy for the incident BC (yes/no, time-varying); body mass index (BMI) at diagnosis (<18.5, 18.5–24.9, 25.0–29.9, 30.0–34.9, 35+ kg/m2); smoking status at diagnosis (current, past, never/unknown); menopausal status at diagnosis (peri- or premenopausal, postmenopausal); Charlson co-morbidity score (0, 1, 2+, time-varying); statin use (yes/no, time-varying); prescription non-steroidal anti-inflammatory medication use, Cox-2 inhibitors, and aspirin (yes/no, time-varying); and receipt of screening mammogram in the 12 months prior to events (yes/no, time-varying).
* Hazard ratio for diabetes is from a model that did not adjust for diabetes medication use; hazard ratios for individual diabetes medication classes are from a separate model adjusting for all diabetes medication classes of interest (ever/never use, time-varying) and diabetes diagnosis (yes/no, time-varying)
Associations between oral diabetes medication classes and SBCE were not statistically significant but point estimates suggested an increased risk of BC-specific mortality with use of sulfonylureas (HR=1.67;95% CI,0.98–2.86). Metformin users had a non-significant decrease in risk of BC-specific mortality (HR=0.67;95% CI,0.35–1.27) and a significant decrease in risk of all-cause mortality (HR=0.58;95% CI,0.42–0.79) (Figure 1). Compared to non-users, insulin users had an increased risk of BC-specific mortality (HR=2.22;95% CI,1.27–3.91) and all-cause mortality (HR=2.02;95% CI,1.56–2.61).
Analyses among women with diabetes
Among the 610 women with diabetes, most women were postmenopausal, had BMI ≥ 30 kg/m2, and a Charlson comorbidity score of one or greater at diagnosis (Table 2). The majority of incident BC among women with diabetes were ER-positive/PR-positive, treated with breast-conserving surgery with or without radiation, not treated with chemotherapy, and treated with endocrine therapy. Compared to non-users, insulin users were more likely to be diagnosed with AJCC stage I BC, not treated with chemotherapy, nor endocrine therapy, and have higher BMI at diagnosis.
Table 2.
Descriptive characteristics of 610 women with diabetes in the COMBO study by insulin use
| Insulin use | |||
|---|---|---|---|
| All women with diabetes N=610 |
Non-user (n=364) | User Yes (n =246) | |
| Characteristics at incident breast cancer diagnosis | |||
| Year of diagnosis | |||
| 1990–1994 | 97 (15.9) | 54 (14.8) | 43 (17.5) |
| 1995–1999 | 200 (32.8) | 119 (32.7) | 81 (32.9) |
| 2000–2004 | 181 (29.7) | 109 (29.9) | 72 (29.3) |
| 2005–2008 | 132 (21.6) | 82 (22.5) | 50 (20.3) |
| Age at diagnosis, years | |||
| Median | 67 (58–74) | 67 (56–74) | 67 (59–74) |
| 18–39 | 5 (0.8) | 3 (0.8) | 2 (0.8) |
| 40–49 | 35 (5.7) | 16 (4.4) | 19 (7.7) |
| 50–59 | 122 (20.0) | 83 (22.8) | 39 (15.9) |
| 60–69 | 197 (32.3) | 114 (31.3) | 83 (33.7) |
| 70–79 | 179 (29.3) | 103 (28.3) | 76 (30.9) |
| 80+ | 72 (11.8) | 45 (12.4) | 27 (11.0) |
| Menopausal status at diagnosis | |||
| Peri- or Premenopausal | 81 (13.3) | 45 (12.4) | 36 (14.6) |
| Postmenopausal | 529 (86.7) | 319 (87.6) | 210 (85.4) |
| Body mass index (kg/m2) | |||
| Mean (SD) | 32.1 (6.9) | 31.5 (6.1) | 33.1 (7.8) |
| Median | 31.2 | 30.7 | 32.3 |
| <18.5 | 5 (0.8) | 2 (0.5) | 3 (1.2) |
| 18.5–24.9 | 78 (12.8) | 46 (12.6) | 32 (13.1) |
| 25.0–29.9 | 173 (28.4) | 119 (32.7) | 54 (22.0) |
| 30.0–34.9 | 175 (28.7) | 103 (28.3) | 72 (29.4) |
| 35+ | 178 (29.2) | 94 (25.8) | 84 (34.3) |
| Unknown | 1 | 0 | 1 |
| Smoking status at diagnosis | |||
| Current | 37 (6.1) | 16 (4.4) | 21 (8.5) |
| Past | 59 (9.7) | 42 (11.5) | 17 (6.9) |
| Never/Unknown | 514 (84.3) | 306 (84.1) | 208 (84.6) |
| AJCC stage | |||
| I | 383 (62.8) | 212 (58.2) | 171 (69.5) |
| IIA | 150 (24.6) | 103 (28.3) | 47 (19.1) |
| IIB | 77 (12.6) | 49 (13.5) | 28 (11.4) |
| ER/PR status | |||
| ER(−)/PR(−) | 93 (15.2) | 53 (14.6) | 40 (16.3) |
| ER(+)/PR(−) | 45 (7.4) | 31 (8.5) | 14 (5.7) |
| ER(−)/PR(+) | 8 (1.3) | 3 (0.8) | 5 (2.0) |
| ER(+)/PR(+) | 441 (72.3) | 264 (72.5) | 177 (72.0) |
| ER and/or PR unknown | 23 (3.8) | 13 (3.6) | 10 (4.1) |
| HER2 status | |||
| Test performed | 312 (77.6) | 189 (78.1) | 123 (76.9) |
| Positive/borderline | 56 (17.9) | 37 (19.6) | 19 (15.4) |
| Negative | 256 (82.1) | 152 (80.4) | 104 (84.6) |
| Surgical procedure | |||
| Mastectomy including radical ± radiation | 228 (37.4) | 132 (36.3) | 96 (39.0) |
| Breast conserving, + radiation | 311 (51.0) | 183 (50.3) | 128 (52.0) |
| Breast conserving, no radiation | 71 (11.6) | 49 (13.5) | 22 (8.9) |
| Other treatment | |||
| Any chemotherapy | 157 (25.7) | 104 (28.6) | 53 (21.5) |
| Completed course | 133 (84.7) | 92 (88.5) | 41 (77.4) |
| Any endocrine therapy | 358 (58.7) | 224 (61.5) | 134 (54.5) |
| Charlson score at diagnosis | |||
| 0 | 209 (34.3) | 163 (44.8) | 46 (18.7) |
| 1 | 223 (36.6) | 116 (31.9) | 107 (43.5) |
| 2+ | 178 (29.2) | 85 (23.4) | 93 (37.8) |
| Diabetes | |||
| Diabetes (ever) throughout follow-up | 610 (14.5) | 364 (100) | 246 (100) |
| At BC diagnosis (year prior to BC) | 329 (53.9) | 159 (43.7) | 170 (69.1) |
| After BC diagnosis through follow-up | 281 (46.1) | 205 (56.3) | 76 (30.9) |
| Diabetes medication use in year prior to breast cancer diagnosis* | |||
| Any oral or injectable medications | 277 (45.4) | 96 (26.4) | 181 (73.6) |
| Any oral medications | 225 (36.9) | 94 (25.8) | 131 (53.3) |
| Metformin | 106 (47.1) | 38 (40.4) | 68 (51.9) |
| Sulfonylurea | 175 (77.8) | 71 (75.5) | 104 (79.4) |
| Insulin | 103 (16.9) | 103 (41.9) | |
| Long-acting | 12 (11.7) | 12 (11.7) | |
| Short-acting | 88 (85.4) | 88 (85.4) | |
| Rapid-acting | 16 (15.5) | 16 (15.5) | |
| Characteristics throughout follow-up | |||
| Oral diabetes medication use during follow-up* | |||
| Any oral or injectable medications | 464 (76.1) | 218 (59.9) | 246 (100) |
| Any oral medications | 403 (66.1) | 218 (59.9) | 185 (75.2) |
| Metformin | 275 (45.1) | 141 (38.7) | 134 (54.5) |
| Sulfonylureas | 307 (50.3) | 155 (42.6) | 152 (61.8) |
| Insulin | 246 (40.3) | 246 (100) | |
| Long-acting | 47 (7.7) | 47 (19.1) | |
| Short-acting | 198 (32.5) | 198 (80.5) | |
| Rapid-acting | 62 (10.2) | 62 (25.2) | |
| % Follow-up years with yearly screening mammogram | |||
| <50% | 161 (26.4) | 93 (25.5) | 68 (27.6) |
| 50%–80% | 207 (33.9) | 117 (32.1) | 90 (36.6) |
| >80% | 242 (39.7) | 154 (42.3) | 88 (35.8) |
| Years of follow-up | |||
| Mean (SD) | 6.8 (3.8) | 6.8 (3.8) | 6.8 (3.7) |
| Median | 6.5 | 6.5 | 6.4 |
Not mutually exclusive
During follow-up, 76% had ever used oral or injectable diabetes medications during the study follow-up, and 30% used both insulin and oral medications. Individually or in combination, 40% used insulin, 45% used metformin, and 50% used sulfonylureas (Table 2). Among the 246 insulin users, 19% used long-acting insulin, 80% used short-acting insulin, and 25% used rapid-acting insulin analogues alone or in combination. Compared to these women, those with diabetes that did not use oral or injectable diabetes medications during follow-up had higher median age, lower proportions of obese women and lower Charlson scores (Online Resource 1).
At the end of study follow-up, 58 (9%) of the 610 women with diabetes had a recurrence and 16 (3%) had a second primary BC, giving a total of 71 (12%) women experienced a SBCE (with 57 recurrences and 14 second primary BC as the first SBCE). There were 187 (31%) deaths of all causes of which 37 were due to BC. Since there were few second primary BC and BC-specific deaths observed in this subgroup, our analyses were limited to evaluating the composite SBCE outcome, recurrences, and all-cause mortality.
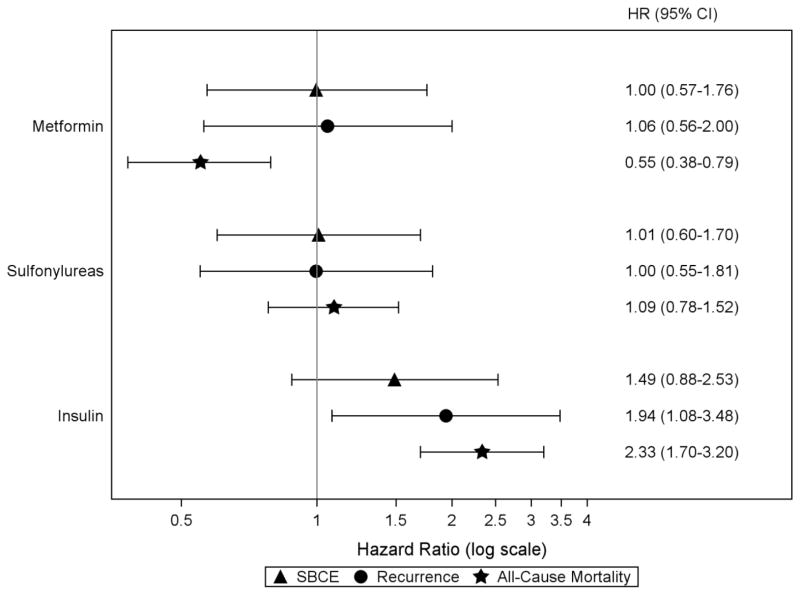
Results from the multivariable analyses of women with diabetes are shown in Figure 2. There were no significant associations between insulin use and risk of SBCE (HR=1.49;95% CI,0.88–2.53). However, insulin use was associated with an increased risk of BC recurrence (HR=1.94;95% CI,1.08–3.48) and all-cause mortality (HR=2.33;95% CI,1.70–3.20). These associations with recurrence and all-cause mortality were seen in all types of insulin use: use of long-acting insulin only, rapid-/short-acting insulin only, or both (Online Resource 1).
Figure 2.
Risk of SBCE, recurrence, and all-cause mortality in relation to diabetes medication classes among women with diabetes in the COMBO cohort
Abbreviations: SBCE second breast cancer event (recurrence and second primary BC), BC breast cancer, HR hazard ratio, CI confidence interval
Note: all hazard ratios for individual diabetes medication classes are adjusted for other medication classes of interest (ever/never use, time-varying); age at diagnosis (18–49, 50–59, 60–69, 70–79, 80+ years); diagnosis year (1990–1994, 1995–1999, 2000–2004, 2005–2008); AJCC stage (I, IIA, IIB); hormone receptor status (estrogen receptor [ER] −/progesterone receptor [PR] −, ER +/PR −, ER −/PR +, ER +/PR +, and ER and/or PR unknown); primary treatment for initial BC (mastectomy, breast conserving surgery with radiation, breast conserving surgery without radiation); endocrine therapy for the incident BC (yes/no, time-varying); body mass index (BMI) at diagnosis (<25.0, 25.0–29.9, 30.0–34.9, 35+ kg/m2); smoking status at diagnosis (current, past, never/unknown); menopausal status at diagnosis (peri- or premenopausal, postmenopausal); Charlson co-morbidity score (<2, 2+, time-varying); statin use (yes/no, time-varying); prescription non-steroidal anti-inflammatory medication use, Cox-2 inhibitors, and aspirin (yes/no, time-varying); and receipt of screening mammogram in the 12 months prior to events (yes/no, time-varying).
We did not observe any associations between oral diabetes medications and risk of SBCE (Figure 2). Use of metformin but not sulfonylureas was associated with a decreased risk of all-cause mortality (HR=0.55;95% CI,0.38–0.79).
Discussion
In a large cohort of women diagnosed with an early stage BC, we found an increased risk of BC recurrence among women with diabetes compared to those without. We did not find an association between diabetes diagnosis and BC-specific mortality, although estimates were suggestive of an increased risk of all-cause mortality. Among women with diabetes, insulin use was associated with increased risks of both recurrence and all-cause mortality. We did not observe a statistically significant association between use of metformin or sulfonylureas with risk of SBCE, although metformin users had a reduced risk of all-cause mortality compared to non-users.
Our finding of significantly increased risks of BC recurrence and all-cause mortality among insulin users warrants further studies in larger populations powered to investigate possible differences in incident and prevalent insulin users, duration of insulin use, and length of time with diagnosed diabetes. Multiple other explanations could underlie worse prognosis among BC survivors with diabetes and using insulin. For example, insulin use could be a surrogate for more advanced diabetes or poorer control of diabetes [43] which may lead to higher mortality. So while we found a significantly increased risk of breast cancer recurrence associated with insulin use, confounding by indication (i.e., diabetes severity) makes this observed association less clear [44].
If insulin users do have more severe diabetes and greater complications (e.g., neuropathy, end-organ damage), otherwise appropriate adjuvant chemotherapy treatment may be used less [45]. In our cohort, a slightly smaller proportion of women who were ever insulin users were treated with chemotherapy compared to non-users (21.5% versus 28.6%). Women with severe diabetes and the greatest degree of hyperinsulinemia and insulin resistance would also likely have very reduced IGF binding proteins and high bioavailability of IGF-I, a growth factor and gonadotrophic factor in BC [46]. He, et al. [41] evaluated different classes of diabetes medications in relation to BC-specific mortality and all-cause mortality in a cohort of 154 women with diabetes and stages II-IV HER2+ BC. When comparing insulin users to non-users, no association was found with risk of BC-specific mortality (HR=1.31;95% CI,0.64–2.69) and all-cause mortality (HR=1.38;95% CI,0.72–2.67) in multivariable models. Some considerable differences between the He et al. study and our study include their selection of higher stage, HER2+ BC cases only, classification of medication use at BC presentation only, not differentiating by type of insulin analogues, and a smaller sample size for comparison (48 insulin users versus 106 non-users). While the smaller proportion of certain types of insulin analogues (i.e., long-acting) is reflected in our wider confidence intervals, our associations for increased risks of BC recurrence and all-cause mortality remained statistically significant when evaluating type of insulin use among women with diabetes.
Our observed reduction in risk of all-cause mortality among BC survivors with diabetes that used metformin is in agreement with findings from some epidemiologic studies [41,40] but not others [39,38]. In a Danish registry-based cohort of 1,058 women with BC and diabetes that were treated with diabetes medications [40], current metformin users compared to non-users had a significant reduction in all-cause mortality (HR=0.74;95% CI,0.58–0.96). Other studies reported a beneficial effect of metformin in BC survivors including reduced BC-specific mortality in HER2+ women [41] and reduced risk of distant metastases in triple receptor-negative BC [38]. Another population-based cohort study of 2,361 BC survivors ages ≥ 66 years with diabetes [39] found no association between cumulative duration of past metformin use with risks of all-cause mortality (HR=0.97;95% CI,0.92–1.02) and BC-specific mortality (HR=0.91;95% CI,0.81–1.03). However, this study lacked information on important clinical data and did not account for some important potential confounders, including BMI, family history, tumor stage, and recurrence.
Our study has several strengths that include: use of a well-characterized and population-based cohort of BC survivors; ascertainment of incident BC cases through a validated registry; detailed information on incident BC characteristics and treatment with extensive follow-up; review of medical charts to determine recurrences and obtain characteristics on recurrent cancer; healthcare utilization including medications dispensed and breast services; comprehensive information on comorbidities to examine confounding; and application of analytic methods to address potential competing risks and informative censoring in a robust manner. Also, we were able to collect date of diabetes diagnosis and dispensing of all diabetes medications during the year prior to the incident BC through the end of study follow-up, allowing us to update and evaluate the effects of diabetes and diabetes medication classes during the entire follow-up period.
Findings from our study should be interpreted in the context of the following limitations. First, COMBO uses data from a single health plan and includes an insured, educated, and primarily Caucasian population. This may limit generalizability. Loss to follow-up is a possible source of bias with 18% censored due to disenrollment from the health plan.
Second, women with diabetes in our cohort were classified as medication users based on time-varying exposure to diabetes medications from pharmacy dispensing records following incident BC diagnosis. However, data from COMBO only went back one year prior to incident BC diagnosis, so we could not distinguish prevalent and incident medication users nor did we have complete information on total length of time with diabetes prior to the study period. We also could not distinguish women with Type I versus Type II diabetes. Another limitation to our classification of “ever/never” use of diabetes medications was the inability to examine subcategories of duration or cumulative use.
Third, despite our rigorous study design and statistical approach, residual confounding is possible. We ascertained and considered the majority of potential confounders, but lacked information on certain modifiable lifestyle factors such as diet, physical activity, over-the-counter (OTC) aspirin/NSAID use, and alcohol intake. People who fill a prescription but do not ingest the medication and who obtain medications at non-GH pharmacies with no claim submitted to GH may be misclassified, but GH enrollees obtain almost all of their prescription medications at GH pharmacies or contracting pharmacies and any misclassification is likely non-differential [26–28].
Finally, due to limited statistical power, we did not evaluate other hypotheses related to risk of SBCE such as the interaction between medication classes or differences in risk within subgroups according to medication adherence, clinical control of diabetes, BC stage and BMI. This also limited the ability to test our hypotheses within restricted groups, such as only women with diabetes prior to BC. Our median follow-up was 6.3 years overall and 3.3 years in women that developed SBCE. Given the small number of second primary BC and BC-specific deaths, additional studies in larger and more diverse populations are warranted to better understand the effects of diabetes medications on these BC outcomes.
Given the biological plausibility and results of our and other studies, it appears likely that women with diabetes have worse BC outcomes than similar women without diabetes. This is of public health importance. The relationship between diabetes and BC is complex, with one affecting the other in potentially dramatic ways; yet, optimal tailoring of treatment for these patients is unclear. Our results should be used to spur discussions about the importance of BC screening and surveillance but also prompt questions on whether there may be differences in treatment management that help explain differences in health outcomes. For instance, recent studies report that greater diabetes severity is associated with lower concordance with National Comprehensive Cancer Network guidelines for locoregional BC treatment among middle-aged women and adjuvant chemotherapy [45]. Studies such as ours that provide additional information on differences in outcomes by diabetes treatments can aide in the decision making process of appropriately managing diabetes while also optimizing cancer outcomes. Additional studies are necessary to confirm the possible adverse influences of insulin therapies on risk of BC recurrence and all-cause mortality, as well as the potential benefit of metformin therapy to reduce all-cause mortality in women with BC and diabetes. If confirmed, then future interventions to improve patient-centered outcomes like diabetes management and medication adherence as part of ongoing cancer prevention efforts would be well motivated.
Conclusion
Future studies are warranted in larger and more diverse populations that are powered to examine differences by length of time with diabetes or disease severity and the influence of diabetes medications on BC-specific mortality in women with diabetes. Questions related to the potential benefit of diabetes medications such as metformin in the adjuvant BC therapy setting and among women without diabetes will be further clarified in ongoing randomized trials of metformin versus placebo on risk of recurrence and survival [47]. Nevertheless, we believe that our study contributes to the limited evidence available to understand diabetes medication safety in relation to SBCE and the potential benefits of some diabetes medications in early stage BC survivors with diabetes.
Supplementary Material
Table 3.
Risk of SBCE, recurrence, and all-cause mortality in relation to diabetes medication classes among women with diabetes in the COMBO cohort (insulin analogues stratified by type)
| SBCE | Recurrence | All-cause mortality | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Diabetes medication classes | ||||||
| Long-acting insulin only | 7.10 | (0.85–59.2) | 13.9 | (1.58–122) | 4.95 | (1.10–22.2) |
| Short-/rapid-acting insulin only | 1.58 | (0.91–2.75) | 2.07 | (1.12–3.81) | 1.93 | (1.38–2.71) |
| Both long- and short-/rapid-acting insulin | 2.23 | (0.64–7.75) | 3.62 | (1.02–12.9) | 3.24 | (1.83–5.73) |
| Metformin | 1.00 | (0.57–1.77) | 1.08 | (0.56–2.06) | 0.59 | (0.40–0.85) |
| Sulfonylureas | 1.00 | (0.59–1.70) | 0.99 | (0.54–1.81) | 1.10 | (0.79–1.53) |
Abbreviations: SBCE second breast cancer event (recurrence and second primary BC), HR hazard ratio, CI confidence interval
Note: all hazard ratios for individual diabetes medication classes are adjusted for other medication classes of interest (ever/never use, time-varying); age at diagnosis (18–49, 50–59, 60–69, 70–79, 80+ years); diagnosis year (1990–1994, 1995–1999, 2000–2004, 2005–2008); AJCC stage (I, IIA, IIB); hormone receptor status (estrogen receptor [ER]−/progesterone receptor [PR]−, ER +/PR−, ER−/PR +, ER +/PR +, and ER and/or PR unknown); primary treatment for initial BC (mastectomy, breast conserving surgery with radiation, breast conserving surgery without radiation); endocrine therapy for the incident BC (yes/no, time-varying); body mass index (BMI) at diagnosis (<25.0, 25.0–29.9, 30.0–34.9, 35+ kg/m2); smoking status at diagnosis (current, past, never/unknown); menopausal status at diagnosis (peri- or premenopausal, postmenopausal); Charlson co-morbidity score (<2, 2+, time-varying); statin use (yes/no, time-varying); prescription non-steroidal anti-inflammatory medication use, Cox-2 inhibitors, and aspirin (yes/no, time-varying); and receipt of screening mammogram in the 12 months prior to events (yes/no, time-varying).
Acknowledgments
This manuscript was supported by a grant from the National Cancer Institute (R01 CA120562 to D.M.B.) at Group Health Research Institute. Collection of cancer incidence data used in this study was supported by the Cancer Surveillance System of the Fred Hutchinson Cancer Research Center (N01-CN-67009 and N01-PC-35142) from the Surveillance, Epidemiology and End Results Program of the National Cancer Institute with additional support from the State of Washington. The National Institutes of Health Cancer Prevention Training Grant in Nutrition, Exercise and Genetics (R25 CA094880) at the University of Washington and Fred Hutchinson Cancer Research Center supported author G.S.C.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Results from this study were presented, in part, at the 47th annual meeting of the Society for Epidemiologic Research in a Concurrent Contributed Session, Seattle, WA, June 24–27, 2014.
References
- 1.American Cancer Society. Breast Cancer Facts & Figures 2013–2014. Atlanta: American Cancer Society, Inc; 2013. [Google Scholar]
- 2.Ritchie CS, Kvale E, Fisch MJ. Multimorbidity: an issue of growing importance for oncologists. J Oncol Pract. 2011;7 (6):371–374. doi: 10.1200/JOP.2011.000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33 (7):1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf I, Sadetzki S, Catane R, Karasik A, Kaufman B. Diabetes mellitus and breast cancer. Lancet Oncol. 2005;6 (2):103–111. doi: 10.1016/S1470-2045(05)01736-5. [DOI] [PubMed] [Google Scholar]
- 5.DeCensi A, Gennari A. Insulin breast cancer connection: confirmatory data set the stage for better care. J Clin Oncol. 2011;29 (1):7–10. doi: 10.1200/JCO.2010.32.3022. [DOI] [PubMed] [Google Scholar]
- 6.Jiralerspong S, Kim ES, Dong W, Feng L, Hortobagyi GN, Giordano SH. Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol. 2013;24 (10):2506–2514. doi: 10.1093/annonc/mdt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li CI, Daling JR, Tang MT, Malone KE. Relationship between diabetes and risk of second primary contralateral breast cancer. Breast cancer research and treatment. 2011;125 (2):545–551. doi: 10.1007/s10549-010-1035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peairs KS, Barone BB, Snyder CF, Yeh HC, Stein KB, Derr RL, Brancati FL, Wolff AC. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol. 2011;29 (1):40–46. doi: 10.1200/JCO.2009.27.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chlebowski RT, McTiernan A, Wactawski-Wende J, Manson JE, Aragaki AK, Rohan T, Ipp E, Kaklamani VG, Vitolins M, Wallace R, Gunter M, Phillips LS, Strickler H, Margolis K, Euhus DM. Diabetes, metformin, and breast cancer in postmenopausal women. J Clin Oncol. 2012;30 (23):2844–2852. doi: 10.1200/JCO.2011.39.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruiter R, Visser LE, van Herk-Sukel MP, Coebergh JW, Haak HR, Geelhoed-Duijvestijn PH, Straus SM, Herings RM, Stricker BH. Risk of cancer in patients on insulin glargine and other insulin analogues in comparison with those on human insulin: results from a large population-based follow-up study. Diabetologia. 2012;55 (1):51–62. doi: 10.1007/s00125-011-2312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lind M, Fahlen M, Eliasson B, Oden A. The relationship between the exposure time of insulin glargine and risk of breast and prostate cancer: an observational study of the time-dependent effects of antidiabetic treatments in patients with diabetes. Primary care diabetes. 2012;6 (1):53–59. doi: 10.1016/j.pcd.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Suissa S, Azoulay L, Dell’Aniello S, Evans M, Vora J, Pollak M. Long-term effects of insulin glargine on the risk of breast cancer. Diabetologia. 2011;54 (9):2254–2262. doi: 10.1007/s00125-011-2190-9. [DOI] [PubMed] [Google Scholar]
- 13.Chang CH, Toh S, Lin JW, Chen ST, Kuo CW, Chuang LM, Lai MS. Cancer risk associated with insulin glargine among adult type 2 diabetes patients--a nationwide cohort study. PloS one. 2011;6 (6):e21368. doi: 10.1371/journal.pone.0021368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morden NE, Liu SK, Smith J, Mackenzie TA, Skinner J, Korc M. Further exploration of the relationship between insulin glargine and incident cancer: a retrospective cohort study of older Medicare patients. Diabetes Care. 2011;34 (9):1965–1971. doi: 10.2337/dc11-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52 (9):1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Angulo AM, Meric-Bernstam F. Metformin: a therapeutic opportunity in breast cancer. Clin Cancer Res. 2010;16 (6):1695–1700. doi: 10.1158/1078-0432.CCR-09-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, Hsu L, Hung MC, Hortobagyi GN, Gonzalez-Angulo AM. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27 (20):3297–3302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonanni B, Puntoni M, Cazzaniga M, Pruneri G, Serrano D, Guerrieri-Gonzaga A, Gennari A, Trabacca MS, Galimberti V, Veronesi P, Johansson H, Aristarco V, Bassi F, Luini A, Lazzeroni M, Varricchio C, Viale G, Bruzzi P, Decensi A. Dual effect of metformin on breast cancer proliferation in a randomized presurgical trial. J Clin Oncol. 2012;30 (21):2593–2600. doi: 10.1200/JCO.2011.39.3769. [DOI] [PubMed] [Google Scholar]
- 19.Blin P, Lassalle R, Dureau-Pournin C, Ambrosino B, Bernard MA, Abouelfath A, Gin H, Le Jeunne C, Pariente A, Droz C, Moore N. Insulin glargine and risk of cancer: a cohort study in the French National Healthcare Insurance Database. Diabetologia. 2012;55 (3):644–653. doi: 10.1007/s00125-011-2429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurtzhals P, Schaffer L, Sorensen A, Kristensen C, Jonassen I, Schmid C, Trub T. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes. 2000;49 (6):999–1005. doi: 10.2337/diabetes.49.6.999. [DOI] [PubMed] [Google Scholar]
- 21.Sommerfeld MR, Muller G, Tschank G, Seipke G, Habermann P, Kurrle R, Tennagels N. In vitro metabolic and mitogenic signaling of insulin glargine and its metabolites. PloS one. 2010;5 (3):e9540. doi: 10.1371/journal.pone.0009540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer D, Chantelau E. Treatment with insulin glargine (Lantus) increases the proliferative potency of the serum of patients with type-1 diabetes: a pilot study on MCF-7 breast cancer cells. Archives of physiology and biochemistry. 2010;116 (2):73–78. doi: 10.3109/13813451003631439. [DOI] [PubMed] [Google Scholar]
- 23.Boudreau DM, Yu O, Chubak J, Wirtz HS, Bowles EJ, Fujii M, Buist DS. Comparative safety of cardiovascular medication use and breast cancer outcomes among women with early stage breast cancer. Breast cancer research and treatment. 2014;144 (2):405–416. doi: 10.1007/s10549-014-2870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wirtz HS, Buist DS, Gralow JR, Barlow WE, Gray S, Chubak J, Yu O, Bowles EJ, Fujii M, Boudreau DM. Frequent antibiotic use and second breast cancer events. Cancer Epidemiol Biomarkers Prev. 2013;22 (9):1588–1599. doi: 10.1158/1055-9965.EPI-13-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surveillance Epidemiology and End Results (SEER) Program Overview of the SEER Program. http://seer.cancer.gov/about/overview.html.
- 26.Saunders KW, Davis RL, Stergachis A. Group Health Cooperative. In: Strom BL, editor. Pharmacoepidemiology. 4. J. Wiley, Chichester; Hoboken, NJ: 2005. pp. 223–239. [Google Scholar]
- 27.Boudreau DM, Doescher MP, Jackson JE, Fishman PA, Saver BG. Impact of healthcare delivery system on where HMO-enrolled seniors purchase medications. Ann Pharmacother. 2004;38 (7–8):1317–1318. doi: 10.1345/aph.1D569. [DOI] [PubMed] [Google Scholar]
- 28.Buist DS, LaCroix AZ, Brenneman SK, Abbott T., 3rd A population-based osteoporosis screening program: who does not participate, and what are the consequences? J Am Geriatr Soc. 2004;52 (7):1130–1137. doi: 10.1111/j.1532-5415.2004.52311.x. [DOI] [PubMed] [Google Scholar]
- 29.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen PI, Clark GM, Edge SB, Hayes DF, Hughes LL, Hutter RV, Morrow M, Page DL, Recht A, Theriault RL, Thor A, Weaver DL, Wieand HS, Greene FL. Staging system for breast cancer: revisions for the 6th edition of the AJCC Cancer Staging Manual. The Surgical clinics of North America. 2003;83 (4):803–819. doi: 10.1016/S0039-6109(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 30. [Accessed June 15, 2011];Group Health Breast Cancer Surveillance Registry. http://www.grouphealthresearch.org/surveillanceproject.
- 31.Washington State Department of Health, Center for Health Statistics. [Accessed June 15, 2011];Death Data. http://www.doh.wa.gov/DataandStatisticalReports/VitalStatisticsData/DeathData.
- 32.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM. Identifying persons with diabetes using Medicare claims data. American journal of medical quality : the official journal of the American College of Medical Quality. 1999;14 (6):270–277. doi: 10.1177/106286069901400607. [DOI] [PubMed] [Google Scholar]
- 33.Newton KM, LaCroix AZ, Heckbert SR, Abraham L, McCulloch D, Barlow W. Estrogen therapy and risk of cardiovascular events among women with type 2 diabetes. Diabetes Care. 2003;26 (10):2810–2816. doi: 10.2337/diacare.26.10.2810. [DOI] [PubMed] [Google Scholar]
- 34.Newton KM, Wagner EH, Ramsey SD, McCulloch D, Evans R, Sandhu N, Davis C. The use of automated data to identify complications and comorbidities of diabetes: a validation study. J Clin Epidemiol. 1999;52 (3):199–207. doi: 10.1016/s0895-4356(98)00161-9. [DOI] [PubMed] [Google Scholar]
- 35.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B American Diabetes Association, European Association for Study of Diabetes . Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32 (1):193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman JA, Sparano JA, Hunsberger S, Enos RA, Gelber RD, Zujewski JA. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25 (15):2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 37.Varadhan R, Weiss CO, Segal JB, Wu AW, Scharfstein D, Boyd C. Evaluating health outcomes in the presence of competing risks: a review of statistical methods and clinical applications. Med Care. 2010;48 (6 Suppl):S96–105. doi: 10.1097/MLR.0b013e3181d99107. [DOI] [PubMed] [Google Scholar]
- 38.Bayraktar S, Hernadez-Aya LF, Lei X, Meric-Bernstam F, Litton JK, Hsu L, Hortobagyi GN, Gonzalez-Angulo AM. Effect of metformin on survival outcomes in diabetic patients with triple receptor-negative breast cancer. Cancer. 2012;118 (5):1202–1211. doi: 10.1002/cncr.26439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lega IC, Austin PC, Gruneir A, Goodwin PJ, Rochon PA, Lipscombe LL. Association between metformin therapy and mortality after breast cancer: a population-based study. Diabetes Care. 2013;36 (10):3018–3026. doi: 10.2337/dc12-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peeters PJ, Bazelier MT, Vestergaard P, Leufkens HG, Schmidt MK, de Vries F, De Bruin ML. Use of metformin and survival of diabetic women with breast cancer. Current drug safety. 2013;8 (5):357–363. doi: 10.2174/15680266113136660069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He X, Esteva FJ, Ensor J, Hortobagyi GN, Lee MH, Yeung SC. Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2+ breast cancer. Ann Oncol. 2012;23 (7):1771–1780. doi: 10.1093/annonc/mdr534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45 (6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 43.Calip GS, Hubbard RA, Stergachis A, Malone KE, Gralow JR, Boudreau DM. Adherence to oral diabetes medications and glycemic control during and following breast cancer treatment. Pharmacoepidemiology and drug safety. 2015;24 (1):75–85. doi: 10.1002/pds.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chubak J, Boudreau DM, Wirtz HS, McKnight B, Weiss NS. Threats to validity of nonrandomized studies of postdiagnosis exposures on cancer recurrence and survival. J Natl Cancer Inst. 2013;105 (19):1456–1462. doi: 10.1093/jnci/djt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sabatino SA, Thompson TD, Wu XC, Fleming ST, Kimmick GG, Trentham-Dietz A, Cress R, Anderson RT. The influence of diabetes severity on receipt of guideline-concordant treatment for breast cancer. Breast cancer research and treatment. 2014;146 (1):199–209. doi: 10.1007/s10549-014-2998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, Rosner B, Speizer FE, Pollak M. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351 (9113):1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 47.Goodwin PJ ClinicalTrials.gov. A phase III randomized trial of metformin versus placebo on recurrence and survival in early stage breast cancer NCT01101438. [Accessed Feb 18, 2014]; [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.