Abstract
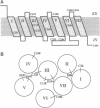
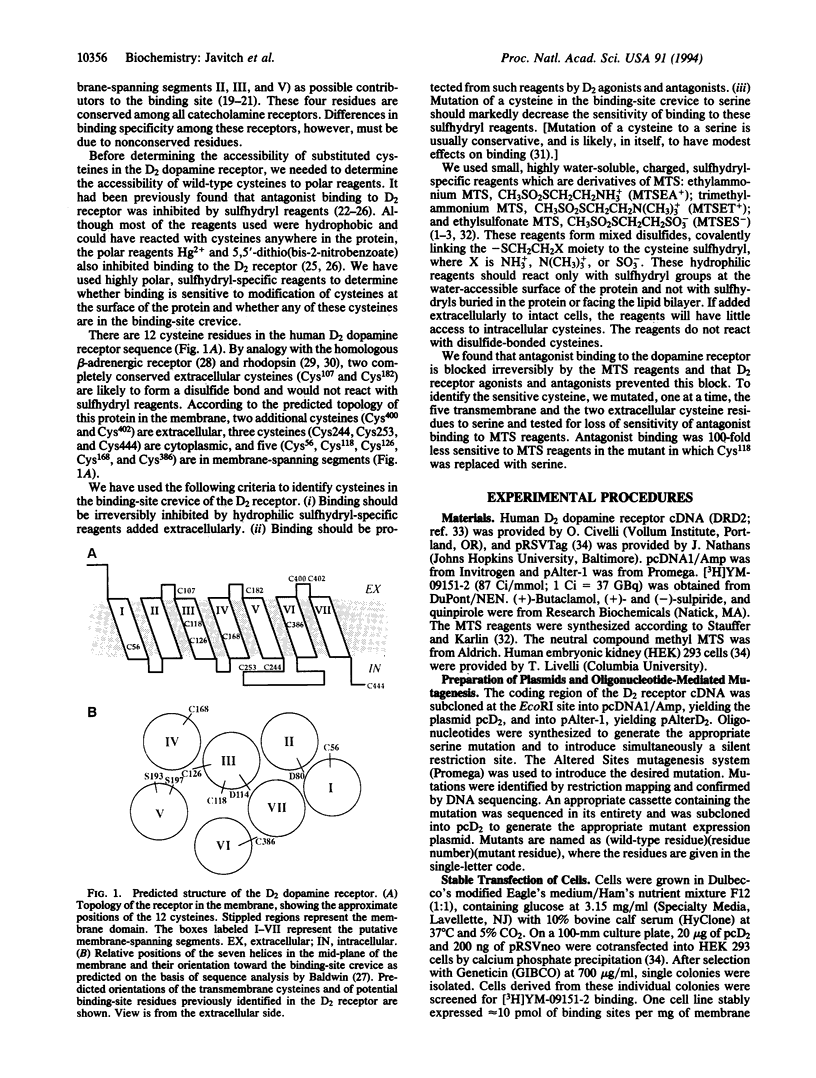
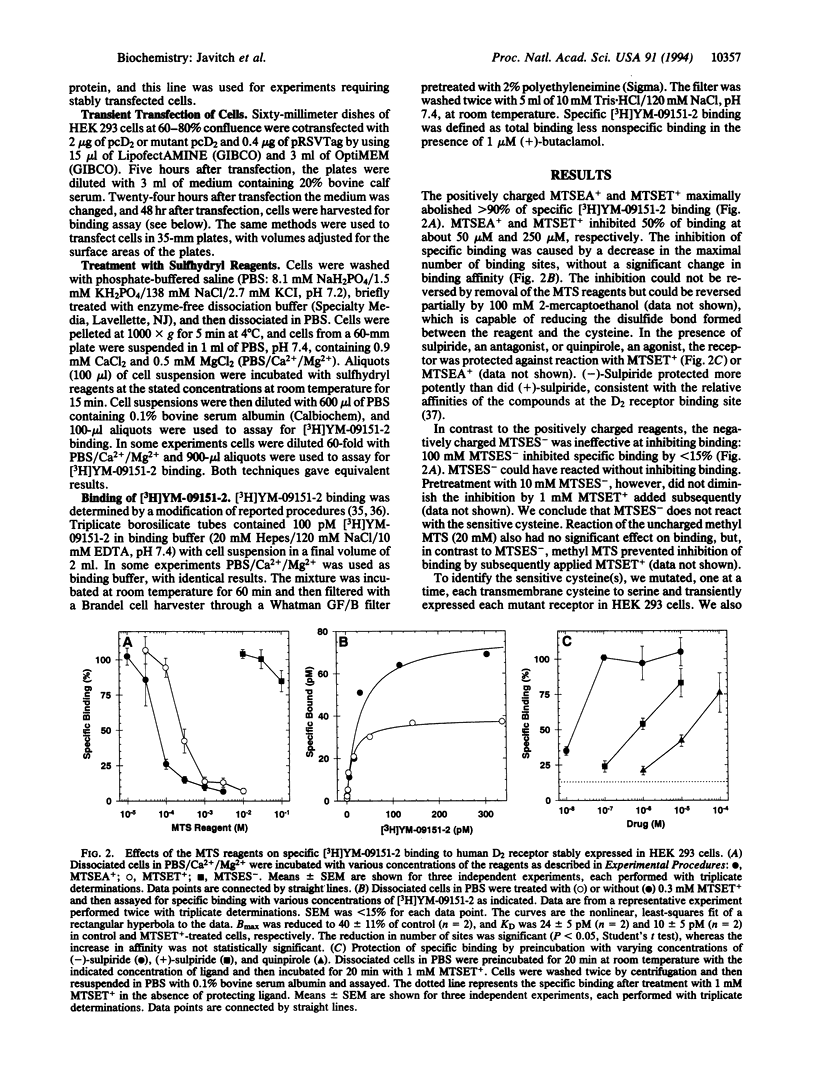
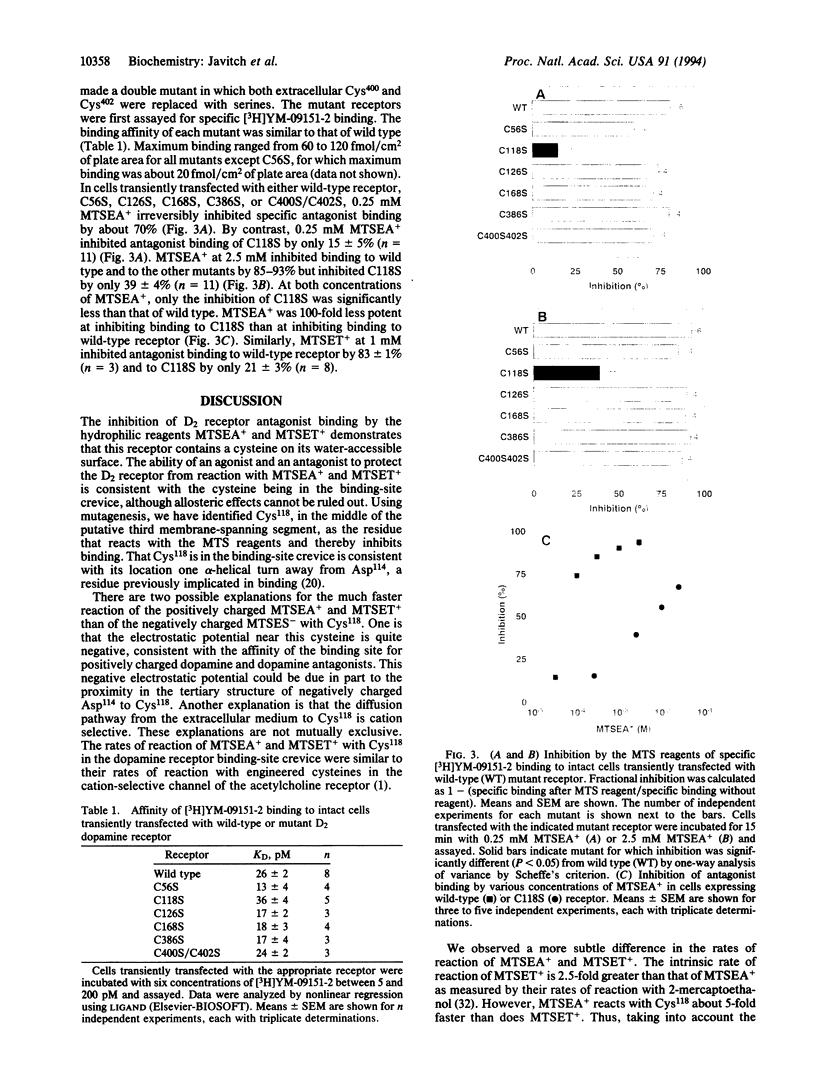
The binding site in G-protein-linked neurotransmitter receptors is formed among their membrane-spanning segments. Because the binding site is in the plane of the bilayer and is accessible to charged, water-soluble agonists, it must lie in a crevice open to the extracellular, aqueous medium. Information about the structure of these receptors can be obtained by identifying the residues in the membrane-spanning segments which face this water-filled crevice. Human D2 dopamine receptor was expressed in human embryonic kidney 293 cells. Small, charged, sulfhydryl-specific methanethiosulfonate (MTS) derivatives irreversibly inhibited the binding of the D2-specific antagonist [3H]YM-09151-2 to these cells. The highly polar MTS derivatives should react with cysteine sulfhydryl groups only at the water-accessible surface of the receptor, which includes the surface of the binding-site crevice. In contrast, these reagents will have little access to sulfhydryls facing the lipid bilayer or buried in the protein interior. Positively charged MTS reagents irreversibly inhibited binding several hundredfold faster than a negatively charged MTS reagent, consistent with the affinity of the binding site for positively charged dopamine agonists and antagonists. Furthermore, both agonists and antagonists of the D2 receptor protected against irreversible inhibition by the MTS reagents. To identify the susceptible cysteine, we mutated, one at a time, five transmembrane and two extracellular cysteine residues to serine. Only the mutation of Cys118 to serine decreased the susceptibility of antagonist binding to irreversible inhibition by the MTS reagents. Thus, Cys118, a residue in the middle of the third membrane-spanning segment, is exposed in the D2 receptor binding-site crevice.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akabas M. H., Kaufmann C., Cook T. A., Archdeacon P. Amino acid residues lining the chloride channel of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1994 May 27;269(21):14865–14868. [PubMed] [Google Scholar]
- Akabas M. H., Stauffer D. A., Xu M., Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992 Oct 9;258(5080):307–310. doi: 10.1126/science.1384130. [DOI] [PubMed] [Google Scholar]
- Altenbach C., Greenhalgh D. A., Khorana H. G., Hubbell W. L. A collision gradient method to determine the immersion depth of nitroxides in lipid bilayers: application to spin-labeled mutants of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1667–1671. doi: 10.1073/pnas.91.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenbach C., Marti T., Khorana H. G., Hubbell W. L. Transmembrane protein structure: spin labeling of bacteriorhodopsin mutants. Science. 1990 Jun 1;248(4959):1088–1092. doi: 10.1126/science.2160734. [DOI] [PubMed] [Google Scholar]
- Baldwin J. M. The probable arrangement of the helices in G protein-coupled receptors. EMBO J. 1993 Apr;12(4):1693–1703. doi: 10.1002/j.1460-2075.1993.tb05814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzow J. R., Van Tol H. H., Grandy D. K., Albert P., Salon J., Christie M., Machida C. A., Neve K. A., Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988 Dec 22;336(6201):783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- Chazot P. L., Strange P. G. Importance of thiol groups in ligand binding to D2 dopamine receptors from brain and anterior pituitary gland. Biochem J. 1992 Jan 15;281(Pt 2):377–380. doi: 10.1042/bj2810377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B. A., Henningsen R. A., Spanoyannis A., Neve R. L., Neve K. A. Contributions of conserved serine residues to the interactions of ligands with dopamine D2 receptors. J Neurochem. 1992 Aug;59(2):627–635. doi: 10.1111/j.1471-4159.1992.tb09416.x. [DOI] [PubMed] [Google Scholar]
- Dal Toso R., Sommer B., Ewert M., Herb A., Pritchett D. B., Bach A., Shivers B. D., Seeburg P. H. The dopamine D2 receptor: two molecular forms generated by alternative splicing. EMBO J. 1989 Dec 20;8(13):4025–4034. doi: 10.1002/j.1460-2075.1989.tb08585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlman H. G., Caron M. G., DeBlasi A., Frielle T., Lefkowitz R. J. Role of extracellular disulfide-bonded cysteines in the ligand binding function of the beta 2-adrenergic receptor. Biochemistry. 1990 Mar 6;29(9):2335–2342. doi: 10.1021/bi00461a018. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Falke J. J., Dernburg A. F., Sternberg D. A., Zalkin N., Milligan D. L., Koshland D. E., Jr Structure of a bacterial sensory receptor. A site-directed sulfhydryl study. J Biol Chem. 1988 Oct 15;263(29):14850–14858. [PubMed] [Google Scholar]
- Fraser C. M. Site-directed mutagenesis of beta-adrenergic receptors. Identification of conserved cysteine residues that independently affect ligand binding and receptor activation. J Biol Chem. 1989 Jun 5;264(16):9266–9270. [PubMed] [Google Scholar]
- Freedman S. B., Poat J. A., Woodruff G. N. Influence of sodium and sulphydryl groups on [3H]sulpiride binding sites in rat striatal membranes. J Neurochem. 1982 May;38(5):1459–1465. doi: 10.1111/j.1471-4159.1982.tb07926.x. [DOI] [PubMed] [Google Scholar]
- Grandy D. K., Marchionni M. A., Makam H., Stofko R. E., Alfano M., Frothingham L., Fischer J. B., Burke-Howie K. J., Bunzow J. R., Server A. C. Cloning of the cDNA and gene for a human D2 dopamine receptor. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9762–9766. doi: 10.1073/pnas.86.24.9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakes K. S., Abrams C. K., Finkelstein A., Slatin S. L. Alteration of the pH-dependent ion selectivity of the colicin E1 channel by site-directed mutagenesis. J Biol Chem. 1990 Apr 25;265(12):6984–6991. [PubMed] [Google Scholar]
- Javitch J. A., Kaback J., Li X., Karlin A. Expression and characterization of human dopamine D2 receptor in baculovirus-infected insect cells. J Recept Res. 1994 Feb;14(2):99–117. doi: 10.3109/10799899409066999. [DOI] [PubMed] [Google Scholar]
- Jung K., Jung H., Wu J., Privé G. G., Kaback H. R. Use of site-directed fluorescence labeling to study proximity relationships in the lactose permease of Escherichia coli. Biochemistry. 1993 Nov 23;32(46):12273–12278. doi: 10.1021/bi00097a001. [DOI] [PubMed] [Google Scholar]
- Karnik S. S., Khorana H. G. Assembly of functional rhodopsin requires a disulfide bond between cysteine residues 110 and 187. J Biol Chem. 1990 Oct 15;265(29):17520–17524. [PubMed] [Google Scholar]
- Karnik S. S., Sakmar T. P., Chen H. B., Khorana H. G. Cysteine residues 110 and 187 are essential for the formation of correct structure in bovine rhodopsin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8459–8463. doi: 10.1073/pnas.85.22.8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick B. F., De Lean A., Caron M. G. Dopamine receptor of the porcine anterior pituitary gland. Effects of N-ethylmaleimide and heat on ligand binding mimic the effects of guanine nucleotides. Mol Pharmacol. 1982 Sep;22(2):298–303. [PubMed] [Google Scholar]
- Lidow M. S., Goldman-Rakic P. S., Rakic P., Innis R. B. Dopamine D2 receptors in the cerebral cortex: distribution and pharmacological characterization with [3H]raclopride. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6412–6416. doi: 10.1073/pnas.86.16.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A., Meng F., Meador-Woodruff J. H., Taylor L. P., Civelli O., Akil H. Site-directed mutagenesis of the human dopamine D2 receptor. Eur J Pharmacol. 1992 Oct 1;227(2):205–214. doi: 10.1016/0922-4106(92)90129-j. [DOI] [PubMed] [Google Scholar]
- Nathans J. Determinants of visual pigment absorbance: role of charged amino acids in the putative transmembrane segments. Biochemistry. 1990 Jan 30;29(4):937–942. doi: 10.1021/bi00456a013. [DOI] [PubMed] [Google Scholar]
- Neve K. A., Cox B. A., Henningsen R. A., Spanoyannis A., Neve R. L. Pivotal role for aspartate-80 in the regulation of dopamine D2 receptor affinity for drugs and inhibition of adenylyl cyclase. Mol Pharmacol. 1991 Jun;39(6):733–739. [PubMed] [Google Scholar]
- Niznik H. B., Grigoriadis D. E., Pri-Bar I., Buchman O., Seeman P. Dopamine D2 receptors selectively labeled by a benzamide neuroleptic: [3H]-YM-09151-2. Naunyn Schmiedebergs Arch Pharmacol. 1985 Jun;329(4):333–343. doi: 10.1007/BF00496365. [DOI] [PubMed] [Google Scholar]
- Pakula A. A., Simon M. I. Determination of transmembrane protein structure by disulfide cross-linking: the Escherichia coli Tar receptor. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4144–4148. doi: 10.1073/pnas.89.9.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst W. C., Snyder L. A., Schuster D. I., Brosius J., Sealfon S. C. Sequence alignment of the G-protein coupled receptor superfamily. DNA Cell Biol. 1992 Jan-Feb;11(1):1–20. doi: 10.1089/dna.1992.11.1. [DOI] [PubMed] [Google Scholar]
- Savarese T. M., Fraser C. M. In vitro mutagenesis and the search for structure-function relationships among G protein-coupled receptors. Biochem J. 1992 Apr 1;283(Pt 1):1–19. doi: 10.1042/bj2830001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuhammer A. M., Cherian M. G. Effects of heavy metal cations, sulfhydryl reagents and other chemical agents on striatal D2 dopamine receptors. Biochem Pharmacol. 1985 Oct 1;34(19):3405–3413. doi: 10.1016/0006-2952(85)90710-5. [DOI] [PubMed] [Google Scholar]
- Sokoloff P., Giros B., Martres M. P., Bouthenet M. L., Schwartz J. C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990 Sep 13;347(6289):146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Stauffer D. A., Karlin A. Electrostatic potential of the acetylcholine binding sites in the nicotinic receptor probed by reactions of binding-site cysteines with charged methanethiosulfonates. Biochemistry. 1994 Jun 7;33(22):6840–6849. doi: 10.1021/bi00188a013. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Dixon R. A. Structural basis of beta-adrenergic receptor function. FASEB J. 1989 May;3(7):1825–1832. doi: 10.1096/fasebj.3.7.2541037. [DOI] [PubMed] [Google Scholar]
- Suen E. T., Stefanini E., Clement-Cormier Y. C. Evidence for essential thiol groups and disulfide bonds in agonist and antagonist binding to the dopamine receptor. Biochem Biophys Res Commun. 1980 Sep 30;96(2):953–960. doi: 10.1016/0006-291x(80)91447-3. [DOI] [PubMed] [Google Scholar]
- Todd A. P., Cong J., Levinthal F., Levinthal C., Hubbell W. L. Site-directed mutagenesis of colicin E1 provides specific attachment sites for spin labels whose spectra are sensitive to local conformation. Proteins. 1989;6(3):294–305. doi: 10.1002/prot.340060312. [DOI] [PubMed] [Google Scholar]
- Van Tol H. H., Bunzow J. R., Guan H. C., Sunahara R. K., Seeman P., Niznik H. B., Civelli O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991 Apr 18;350(6319):610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- Van Tol H. H., Wu C. M., Guan H. C., Ohara K., Bunzow J. R., Civelli O., Kennedy J., Seeman P., Niznik H. B., Jovanovic V. Multiple dopamine D4 receptor variants in the human population. Nature. 1992 Jul 9;358(6382):149–152. doi: 10.1038/358149a0. [DOI] [PubMed] [Google Scholar]
- Xu M., Akabas M. H. Amino acids lining the channel of the gamma-aminobutyric acid type A receptor identified by cysteine substitution. J Biol Chem. 1993 Oct 15;268(29):21505–21508. [PubMed] [Google Scholar]