Abstract
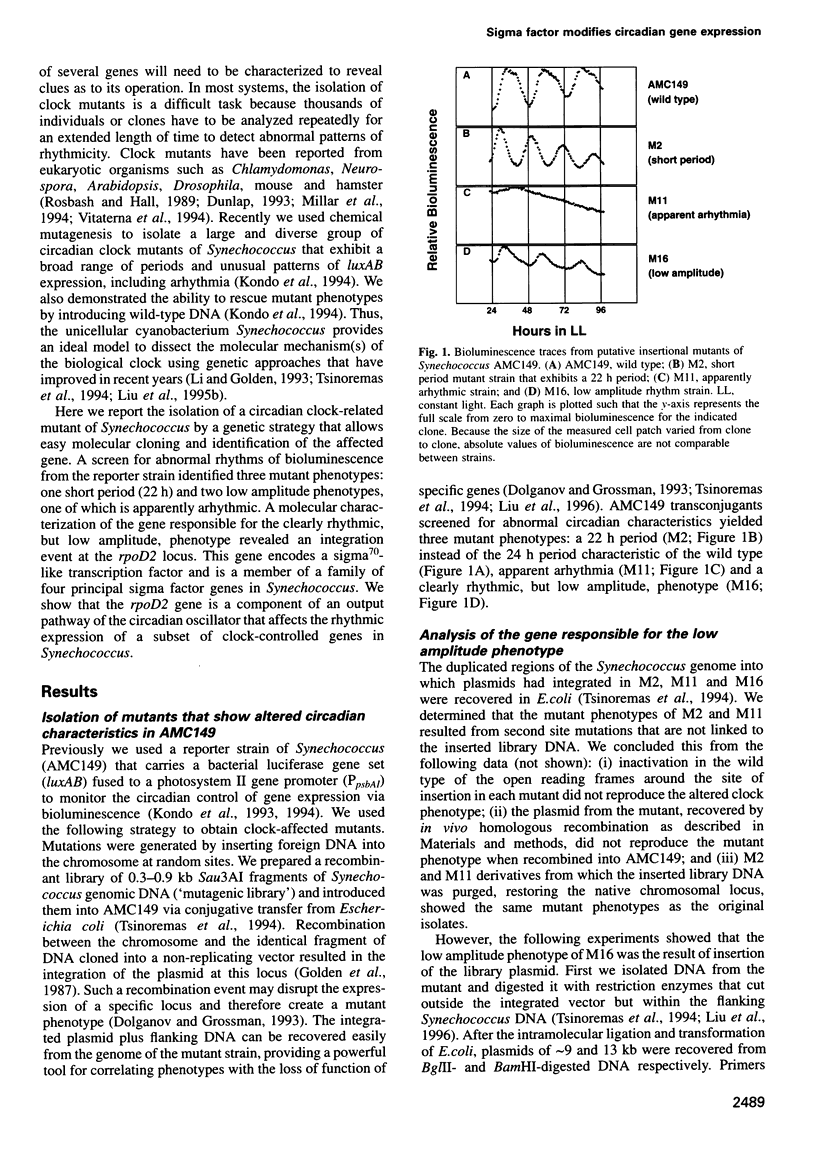
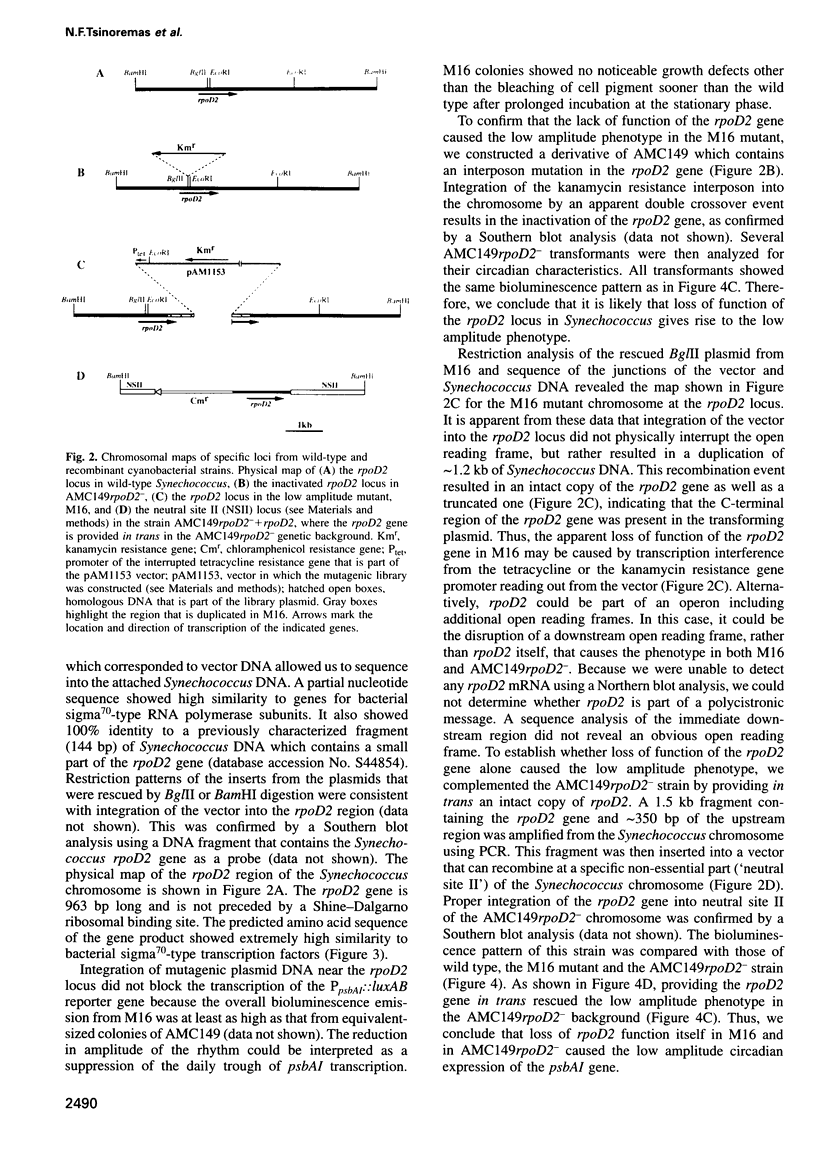
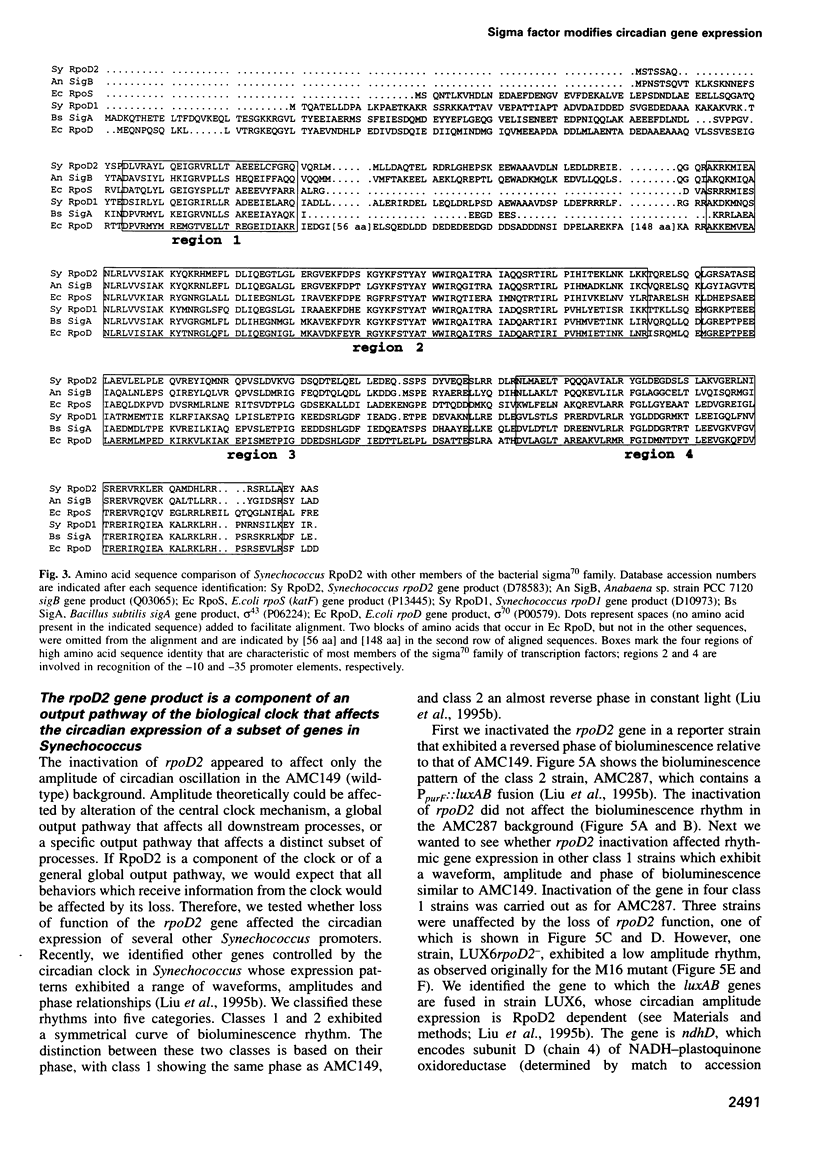
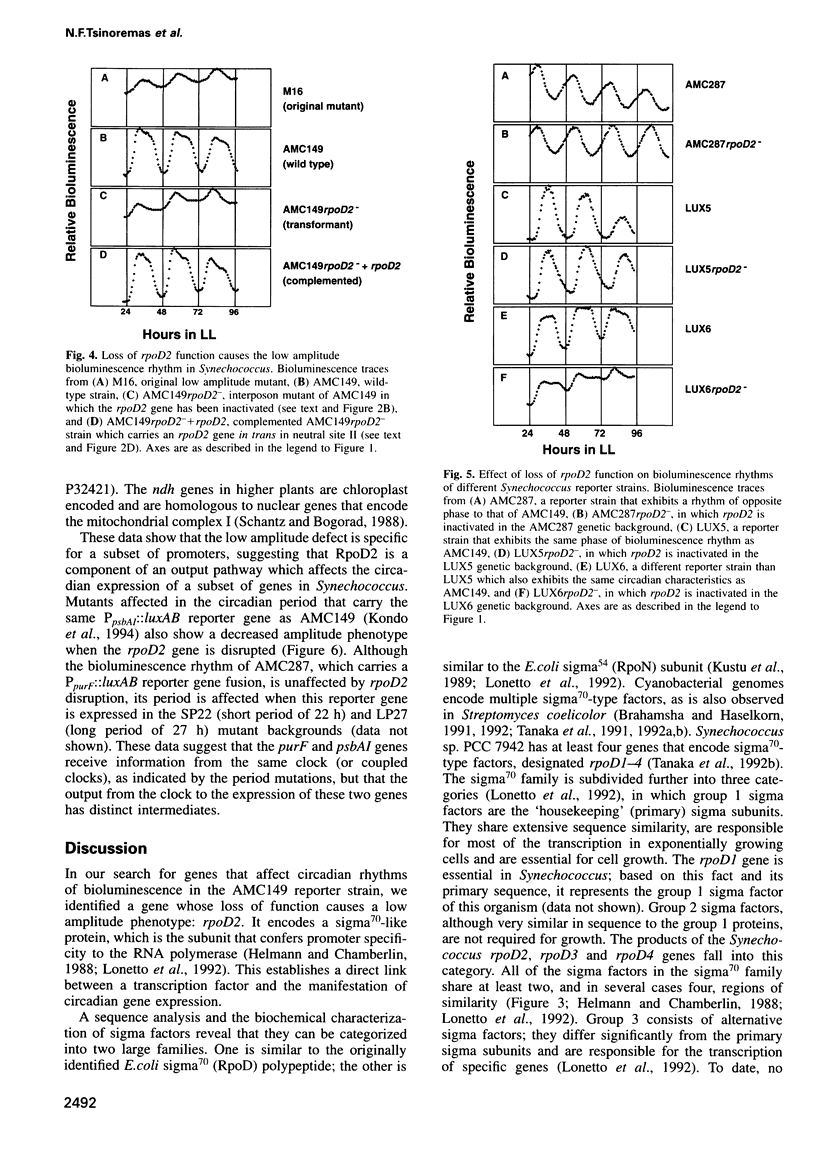
We isolated mutants affected in the circadian expression of the psbAI gene in Synechococcus sp. strain PCC 7942 using a strategy that tags the genomic locus responsible for the mutant phenotype. The search identified one short period (22 h) mutant (M2) and two low amplitude mutants, one of which showed apparent arhythmia (M11) and one that was still clearly rhythmic (M16). We characterized the disrupted locus of the low amplitude but still rhythmic mutant (M16) as the rpoD2 gene, a member of a gene family that encodes sigma70-like transcription factors in Synechococcus. We also inactivated rpoD2 in a number of reporter strains and showed that the circadian expression of some genes is not modified by the loss of this sigma factor. Therefore, we conclude that rpoD2 is a component of an output pathway of the biological clock that affects the circadian expression of a subset of genes in Synechococcus. This work demonstrates a direct link between a transcription factor and the manifestation of circadian gene expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Brahamsha B., Haselkorn R. Identification of multiple RNA polymerase sigma factor homologs in the cyanobacterium Anabaena sp. strain PCC 7120: cloning, expression, and inactivation of the sigB and sigC genes. J Bacteriol. 1992 Nov;174(22):7273–7282. doi: 10.1128/jb.174.22.7273-7282.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahamsha B., Haselkorn R. Isolation and characterization of the gene encoding the principal sigma factor of the vegetative cell RNA polymerase from the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1991 Apr;173(8):2442–2450. doi: 10.1128/jb.173.8.2442-2450.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos S. A., Golden S. S. Light-regulated expression of the psbD gene family in Synechococcus sp. strain PCC 7942: evidence for the role of duplicated psbD genes in cyanobacteria. Mol Gen Genet. 1992 Mar;232(2):221–230. doi: 10.1007/BF00280000. [DOI] [PubMed] [Google Scholar]
- Bustos S. A., Schaefer M. R., Golden S. S. Different and rapid responses of four cyanobacterial psbA transcripts to changes in light intensity. J Bacteriol. 1990 Apr;172(4):1998–2004. doi: 10.1128/jb.172.4.1998-2004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. H., Chen T. L., Hung L. M., Huang T. C. Circadian Rhythm in Amino Acid Uptake by Synechococcus RF-1. Plant Physiol. 1991 Sep;97(1):55–59. doi: 10.1104/pp.97.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolganov N., Grossman A. R. Insertional inactivation of genes to isolate mutants of Synechococcus sp. strain PCC 7942: isolation of filamentous strains. J Bacteriol. 1993 Dec;175(23):7644–7651. doi: 10.1128/jb.175.23.7644-7651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap J. C. Closely watched clocks: molecular analysis of circadian rhythms in Neurospora and Drosophila. Trends Genet. 1990 May;6(5):159–165. doi: 10.1016/0168-9525(90)90151-u. [DOI] [PubMed] [Google Scholar]
- Dunlap J. C. Genetic analysis of circadian clocks. Annu Rev Physiol. 1993;55:683–728. doi: 10.1146/annurev.ph.55.030193.003343. [DOI] [PubMed] [Google Scholar]
- Elhai J., Wolk C. P. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol. 1988;167:747–754. doi: 10.1016/0076-6879(88)67086-8. [DOI] [PubMed] [Google Scholar]
- Eskin A. Circadian system of the Aplysia eye: properties of the pacemaker and mechanisms of its entrainment. Fed Proc. 1979 Nov;38(12):2573–2579. [PubMed] [Google Scholar]
- Gekakis N., Saez L., Delahaye-Brown A. M., Myers M. P., Sehgal A., Young M. W., Weitz C. J. Isolation of timeless by PER protein interaction: defective interaction between timeless protein and long-period mutant PERL. Science. 1995 Nov 3;270(5237):811–815. doi: 10.1126/science.270.5237.811. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Heintzen C., Melzer S., Fischer R., Kappeler S., Apel K., Staiger D. A light- and temperature-entrained circadian clock controls expression of transcripts encoding nuclear proteins with homology to RNA-binding proteins in meristematic tissue. Plant J. 1994 Jun;5(6):799–813. doi: 10.1046/j.1365-313x.1994.5060799.x. [DOI] [PubMed] [Google Scholar]
- Helmann J. D., Chamberlin M. J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- Huang T. C., Tu J., Chow T. J., Chen T. H. Circadian Rhythm of the Prokaryote Synechococcus sp. RF-1. Plant Physiol. 1990 Feb;92(2):531–533. doi: 10.1104/pp.92.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T., Strayer C. A., Kulkarni R. D., Taylor W., Ishiura M., Golden S. S., Johnson C. H. Circadian rhythms in prokaryotes: luciferase as a reporter of circadian gene expression in cyanobacteria. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5672–5676. doi: 10.1073/pnas.90.12.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T., Tsinoremas N. F., Golden S. S., Johnson C. H., Kutsuna S., Ishiura M. Circadian clock mutants of cyanobacteria. Science. 1994 Nov 18;266(5188):1233–1236. doi: 10.1126/science.7973706. [DOI] [PubMed] [Google Scholar]
- Kustu S., Santero E., Keener J., Popham D., Weiss D. Expression of sigma 54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol Rev. 1989 Sep;53(3):367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Golden S. S. Enhancer activity of light-responsive regulatory elements in the untranslated leader regions of cyanobacterial psbA genes. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11678–11682. doi: 10.1073/pnas.90.24.11678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Golden S. S., Kondo T., Ishiura M., Johnson C. H. Bacterial luciferase as a reporter of circadian gene expression in cyanobacteria. J Bacteriol. 1995 Apr;177(8):2080–2086. doi: 10.1128/jb.177.8.2080-2086.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Tsinoremas N. F., Johnson C. H., Lebedeva N. V., Golden S. S., Ishiura M., Kondo T. Circadian orchestration of gene expression in cyanobacteria. Genes Dev. 1995 Jun 15;9(12):1469–1478. doi: 10.1101/gad.9.12.1469. [DOI] [PubMed] [Google Scholar]
- Loewen P. C., Hengge-Aronis R. The role of the sigma factor sigma S (KatF) in bacterial global regulation. Annu Rev Microbiol. 1994;48:53–80. doi: 10.1146/annurev.mi.48.100194.000413. [DOI] [PubMed] [Google Scholar]
- Lonetto M., Gribskov M., Gross C. A. The sigma 70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992 Jun;174(12):3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loros J. J., Denome S. A., Dunlap J. C. Molecular cloning of genes under control of the circadian clock in Neurospora. Science. 1989 Jan 20;243(4889):385–388. doi: 10.1126/science.2563175. [DOI] [PubMed] [Google Scholar]
- Marsh J. L., Erfle M., Wykes E. J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984 Dec;32(3):481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- Millar A. J., Carré I. A., Strayer C. A., Chua N. H., Kay S. A. Circadian clock mutants in Arabidopsis identified by luciferase imaging. Science. 1995 Feb 24;267(5201):1161–1163. doi: 10.1126/science.7855595. [DOI] [PubMed] [Google Scholar]
- Myers M. P., Wager-Smith K., Wesley C. S., Young M. W., Sehgal A. Positional cloning and sequence analysis of the Drosophila clock gene, timeless. Science. 1995 Nov 3;270(5237):805–808. doi: 10.1126/science.270.5237.805. [DOI] [PubMed] [Google Scholar]
- Rosbash M., Hall J. C. The molecular biology of circadian rhythms. Neuron. 1989 Oct;3(4):387–398. doi: 10.1016/0896-6273(89)90199-2. [DOI] [PubMed] [Google Scholar]
- Shapira S. K., Chou J., Richaud F. V., Casadaban M. J. New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of beta-galactosidase. Gene. 1983 Nov;25(1):71–82. doi: 10.1016/0378-1119(83)90169-5. [DOI] [PubMed] [Google Scholar]
- Stehle J. H., Foulkes N. S., Molina C. A., Simonneaux V., Pévet P., Sassone-Corsi P. Adrenergic signals direct rhythmic expression of transcriptional repressor CREM in the pineal gland. Nature. 1993 Sep 23;365(6444):314–320. doi: 10.1038/365314a0. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Masuda S., Takahashi H. Multiple rpoD-related genes of cyanobacteria. Biosci Biotechnol Biochem. 1992 Jul;56(7):1113–1117. doi: 10.1271/bbb.56.1113. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Masuda S., Takahashi H. The complete nucleotide sequence of the gene (rpoD1) encoding the principal sigma factor of the RNA polymerase from the cyanobacterium Synechococcus sp. strain PCC7942. Biochim Biophys Acta. 1992 Aug 17;1132(1):94–96. doi: 10.1016/0167-4781(92)90060-d. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Shiina T., Takahashi H. Nucleotide sequence of genes hrdA, hrdC, and hrdD from Streptomyces coelicolor A3(2) having similarity to rpoD genes. Mol Gen Genet. 1991 Oct;229(3):334–340. doi: 10.1007/BF00267453. [DOI] [PubMed] [Google Scholar]
- Tsinoremas N. F., Kutach A. K., Strayer C. A., Golden S. S. Efficient gene transfer in Synechococcus sp. strains PCC 7942 and PCC 6301 by interspecies conjugation and chromosomal recombination. J Bacteriol. 1994 Nov;176(21):6764–6768. doi: 10.1128/jb.176.21.6764-6768.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaterna M. H., King D. P., Chang A. M., Kornhauser J. M., Lowrey P. L., McDonald J. D., Dove W. F., Pinto L. H., Turek F. W., Takahashi J. S. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994 Apr 29;264(5159):719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]




