Abstract
Objective
The aim of this study was to compare oral radiologic abnormalities associated with systemic sclerosis (SSc) against abnormalities in the general population.
Study Design
Patients with SSc and healthy controls were enrolled in a multi-site cross-sectional study. Included in the radiology examination were a panoramic radiograph, four bitewings, and an anterior mandibular periapical radiograph. Radiographs were evaluated by two oral and maxillofacial radiologists tested for interobserver and intraobserver reliability. Chi-squared tests, Fisher exact tests, and Mann Whitney U tests were used to summarize the radiologic manifestations of patients and controls.
Results
We assessed 163 SSc patients and 231 controls. Widening of the periodontal ligament space (PLS) (P < .001), with higher percentage of teeth with PLS widening (P < .001), was significantly more frequent in patients with SSc than in controls. The most significant differences between the two groups were found in the molars and premolars (P < .001). Moreover, 26% of the patients with SSc had a periapical PLS greater than 0.19 mm compared with 13% of the controls (P = .003). Patients with SSc had significantly more erosions compared with controls (14.5% vs. 3.6%; P < .001), mostly in the condyles (P = .022), coronoid processes (P = .005) and other locations (P = .012).
Conclusion
Patients with SSc had more teeth with PLS widening and erosions of the mandible compared with controls.
Systemic sclerosis (SSc) is a multi-system disorder of connective tissue characterized clinically by thickening and fibrosis of the skin and involvement of internal organs. Prevalence estimates for SSc are between 240 per million1 and 276 per million2 in an American population. The incidence rate of SSc is about 20 new cases per million per year.3 It affects mainly women in their third or fourth decade of life and is associated with significant morbidity and increased mortality. No treatment has been shown to alter the natural history of SSc.4 SSc is characterized by fibroblast dysfunction, which leads to fibrosis of the skin, internal organs, or both;5 autoantibodies (anticentromere, anti—topoisomerase I, anti—RNA polymerase III); and vasculopathy.5,6 SSc is usually categorized into limited cutaneous SSc (lcSSc), diffuse cutaneous SSc (dcSSc), and SSc without skin involvement.6 Although several candidate genes and regions of the genome have been identified as potentially being involved in SSc, these are also associated with other autoimmune conditions. SSc is a complex disease, where no single polymorphism is known to cause the complete manifestation of the condition; it has been suggested that it may be the combined action of several single nucleotide polymorphisms in different genes that may result in the complex traits of the disease.7
We compared patients with SSc with a control population and demonstrated that oral problems in patients with SSc include decreased oral opening, decreased saliva production, increased number of missing teeth, and worse periodontal disease.8–10
The radiographic manifestations in the jaws, considered characteristic of SSc, are well-defined bony erosions at the sites of muscle attachment in the mandible and increasing width of periodontal ligaments around teeth.11 Only a few studies, however, have estimated the prevalence of these radiographic abnormalities, and none has included more than 47 patients with SSc. To our knowledge, only six previous studies have compared patients with SSc to normal controls,12–17 whereas a seventh study used controls with rheumatoid arthritis and systemic lupus erythematosus.18
The purpose of this carefully performed study in a large sample of patients with SSc was to assess all the previously described individual radiographic manifestations of SSc and to compare the prevalence of these radiographic manifestations with those in the general population. The aim was to present the radiologic results of a heterogeneous population of patients with scleroderma, involving miscellaneous cohorts throughout Canada against a matched general population.
METHODS
This study is the radiographic component of a large multi-site cross-sectional study looking at the orofacial manifestations and oral health—related quality of life in patients with SSc previously reported by Baron et al.8–10 In this study, 163 patients with SSc were recruited from 7 of the 15 Canadian Scleroderma Research Group sites. All patients had a diagnosis of SSc made by the participating rheumatologists. Over 95% of the patients in this study met the recent American College of Rheumatology/The European League Against Rheumatism (ACR/EULAR) classification criteria for SSc.5,19 Another 231 new patients presenting for mechanical joint disease (e.g., osteoarthritis) were recruited as controls. Patients and controls were matched for gender and age. Patients’ ages were between 46 and 67 years, and those of controls between 47 and 68 years. Orthodontic cases were excluded. This study is in compliance with the Helsinki Declaration and was approved by the research ethics board of each site. Informed consent was obtained from all study patients.
The patients were examined by eight dentists at the seven sites. The examination consisted of obtaining odontograms delineating number of teeth, missing teeth, caries, and periodontal indices.10 SSc duration was measured as the time between the onset of the first non-Raynaud symptom and the oral health study visit. Limited cutaneous SSc (lcSSc) was defined as skin involvement distal to the elbows and knees.14 Diffuse cutaneous SSc (dcSSc) was defined as skin involvement proximal to the elbows and knees, with or without truncal involvement.20
All patients underwent radiographic examination to obtain a panoramic radiograph, four bitewings, and a periapical radiograph of the mandibular incisors if the teeth were present. The eight dentists attended a calibration workshop at one site before the start of the survey to standardize radiographic technique; two of seven sites used digital radiography, whereas all the other sites used analog radiography. For all sites using argentic film, a test radiograph of a plaster model of teeth was taken at one site (SMBD-JGH) and became the exposure standard for intraoral radiographs. Exposure times at the different sites were adjusted by comparing with the standard.21 All radiographs were developed in automatic processors at each site under standardized conditions, according to manufacturers’ recommendations. All films were digitized using an Epson Expression 1680 scanner (Epson America, Inc. Long Beach, CA) at 1600 × 3200 dpi optical resolution and Adobe Photoshop (Adobe System Incorporated, San Jose, CA). Adobe Photoshop is already widely used in the dental and oral maxillofacial disciplines for measurement.22,23 Intraoral radiographs were scanned at 1600 dpi, and panoramic radiographs were scanned at 300 dpi and saved as jpeg images.
The radiographs were evaluated by two trained experienced oral and maxillofacial radiologists (DM, MD) tested for interobserver and intraobserver reliability;24 the observers performed the assessment on all digital and scanned radiographs under the same conditions.24 Differences between digital and scanned images were deemed clinically insignificant.25 Observers were blinded to the names of the patients, sources of the radiographs, and the subjects being patients with SSc or controls. Radiographs judged nondiagnostic to perform the required tasks were rejected by the observers.
An adjudicator (MG) was used for binary variables in case of disagreement between the observers. The advantage of using an adjudicator is that his interpretation is completely independent and not influenced by personality or previous scores.
Bone erosions
Bone erosions were recorded using panoramic radiographs in the following sites: right and left condyles, coronoid processes, digastric regions, posterior rami, and other regions. The presence of erosion was rated by using a Likert-type scale from 1 to 5, from “definitely no erosion” to “definitely erosion.” Flattening of the condyles suggestive of degenerative joint disease was rated as erosion.
Widening of the periodontal ligament space
The presence of widening of the periodontal ligament space (PLS) was recorded by using the panoramic radiographs. Our definition of PLS widening included widening near the coronal portion of the root or in the periapical region on one or both sides of the root.
Measurement of the PLS
The width of the PLS was measured digitally in Adobe Photoshop on the periapical radiograph of the mandibular incisor region to validate the finding of Alexandridis and White,12 that it predicts the presence of SSc. The PLS was measured in four places: at one third and two thirds of the distance between the cementoenamel junction (CEJ) and the apex of the tooth on each side.12 For each of the four measurements, we averaged the measurements of the two observers. Then, we averaged the measurements of the four sites. The measurements were made on tooth 3.2. Tooth 4.2 was used when tooth 3.2 was absent or nondiagnostic.
Statistical analysis
Descriptive statistics were used to summarize the demographic characteristics, radiologic manifestations of the patients with SSc and the controls. Chi-squared tests, Fisher’s exact tests, and Mann Whitney U tests were used, as appropriate.
Interrater reliability was calculated for continuous variables (number of teeth with PLS widening and number of molars, premolars and anteriors with PLS widening using periapical and panoramic radiographs), and for binary variables (erosion at each location). Interrater reliability was measured with intraclass correlation (ICC) coefficients for the continuous variables of interest. ICC was calculated by using the variance component estimates of linear and generalized linear mixed models. Interrater reliability was measured by Kappa for the binary variables of interest. ICC and Kappa were interpreted using the following guidelines: 0 = poor; 0.01 to 0.20 = slight; 0.21 to 0.40 = fair; 0.41 to 0.60 = moderate; 0.61 to 0.80 = substantial; and 0.81 to 1 = almost perfect.24
Generalized linear mixed models that use negative binomial distribution for count data and generalized linear mixed models that use binary distribution for binary variables were used to examine the association between SSc and the outcomes of interest, namely, the number of teeth with widening of the PLS, patients with PLS widening of at least one tooth, and patients with at least one site with erosion. Models were adjusted for age, gender, ethnicity (Caucasian vs. other), education (greater vs. less than high school) and current smoking.8 In addition, the number of teeth was assessed as an offset term variable in the models for the number of teeth with widening. The study site was added as a random effect in all mixed models.
P values < .05 were considered statistically significant. All statistical analyses were performed using SAS v.9.2 (SAS Institute, Cary, NC).
RESULTS
Calibration
The interobserver reliability was almost perfect for the measurement of the PLS width (ICC = 0.90) and was fair for the number of teeth with PLS widening (ICC = 0.40). The interobserver reliability for the number of molars, premolars, and anteriors with PLS widening was fair (ICC = 0.25), substantial (ICC = 0.66), and almost perfect (ICC = 1.00), respectively. The agreement between observers was perfect for each site and general erosions (κ = 1).
Study results
Among the 163 patients with SSc and 231 controls included in the study, 4 patients with SSc and 9 controls had to be excluded because of missing or poor-quality radiographs. There were 381 patients in this radiology study. There were no significant differences between patients with SSc and controls with regards to age, gender, ethnicity, and smoking status (Table I). There was a strong trend, however, to a lower level of education in the patients with SSc (P = .058). Patients with SSc included in the radiology study had a mean disease duration of 13.7 years (SD 8.4) and 28.3% (N = 45) had dcSSc.9,10 Patients with SSc were significantly more likely to be edentulous and had a trend to have more missing teeth compared with controls (Table II).
Table I.
Baseline characteristics
| SSc (N = 159)
|
Controls (N = 222)
|
P value | |||
|---|---|---|---|---|---|
| % or mean | N or SD | % or mean | N or SD | ||
| Female | 90.57% | 144 | 90.54% | 201 | .993 |
| Age, years | 56.31 | 10.43 | 57.97 | 10.73 | .117 |
| Caucasian | 91.82% | 146 | 89.19% | 198 | .392 |
| Education >high school | 49.69% | 79 | 59.46% | 132 | .058 |
| Current smoker | 9.62% | 15 | 12.33% | 27 | .412 |
SD, standard deviation.
Table II.
Radiographic oral manifestations in patients with SSc and controls
| N = 381 | SSc (N = 159)
|
Controls (N = 222)
|
P value | ||
|---|---|---|---|---|---|
| % or mean | N or SD | % or mean | N or SD | ||
| Edentulous patients | 13.21% | 21 | 2.70% | 6 | <.001 |
| Number of missing teeth | 7.96 | 9.46 | 5.71 | 7.05 | .097 |
| Widening of the periodontal ligament* | |||||
| Number of teeth with widening of the periodontal ligament | 1.08 | 2.57 | 0.16 | 0.49 | <.001 |
| Number of molars with widening | 0.58 | 1.15 | 0.13 | 0.40 | <.001 |
| Number of premolars with widening | 0.34 | 0.89 | 0.02 | 0.18 | <.001 |
| Number of anteriors with widening | 0.20 | 1.19 | 0.02 | 0.17 | .038 |
| Patients with widening of the periodontal ligament at least one tooth | 37.96% | 52 | 11.11% | 24 | <.001 |
| Erosion | |||||
| Patients with at least one site with erosion | 14.47% | 23 | 3.60% | 8 | <.001 |
| Patients with erosion at each site | |||||
| Condyle | 6.29% | 10 | 1.80% | 4 | .022 |
| Coronoid process | 5.03% | 8 | 0.45% | 1 | .005 |
| Digastric region | 2.53% | 4 | 0.90% | 2 | .239 |
| Posterior ramus | 5.06% | 8 | 1.36% | 3 | .058 |
| Other erosion | 3.16% | 5 | 0.00% | 0 | .012 |
SD, standard deviation.
Bold values represent significant results (P < .05).
Edentulous patients were excluded.
Table II shows that PLS widening of at least one tooth was noted in 38% of the patients with SSc compared with 11.1% of the controls (P < .001). Figures 1 and 2 show examples of widened PLS. This significant difference was confirmed by multivariate analysis adjusting for age, gender, ethnicity, education, and current smoking status (OR 6.56; 95% CI 3.52–12.21). There were significantly more teeth with PLS widening in patients with SSc than in controls in univariate (1.08 [SD 2.57] vs. 0.16 [SD 0.49]; P < .001) and multivariate (RR 7.72; 95% CI 4.45–13.38) analyses. The most significant differences between the two groups were found in the molars and premolars (P < .001) (see Table II).
Fig. 1.
Panoramic radiograph of scleroderma patient with significant PLS widening, particularly in the mandibular and maxillary posterior teeth.
Fig. 2.

Periapical radiographs showing periodontal ligament space widening in lower anteriors.41–31
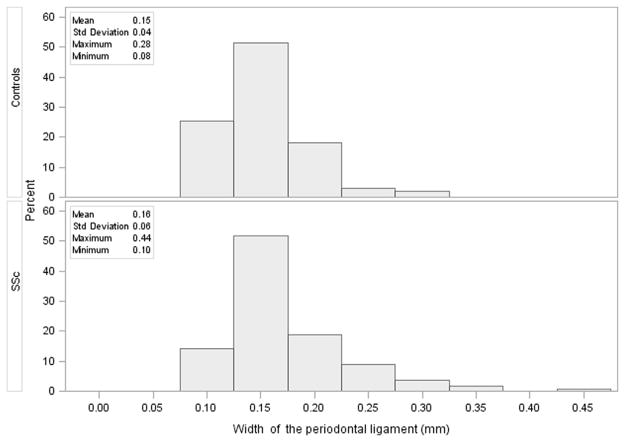
The distribution of the measurement of the PLS width at 3.2 (or 4.2 if 3.2 is missing) in SSc and controls is shown in Figure 3. The two groups had very similar values (mean ± SD: 0.16 ± 0.06 mm in SSc vs. 0.15 ± 0.04 mm in controls; median [interquartile range]: 0.15 [0.13–0.18] in SSc vs. 0.14 [0.12–0.16] in controls), but 26% of the patients with SSc had a periapical PLS greater than 0.19 mm (mean of controls + 1 SD) compared with 13% of the controls (P = .003).
Fig. 3.
Distribution of the width of the periodontal ligament measured at 3.2 (4.2 if 3.2 is missing) in controls and in patients with SSc.
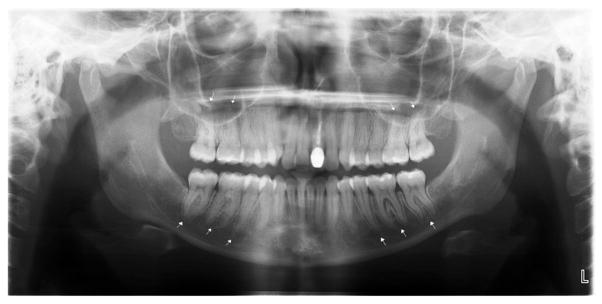
There were significantly more patients with SSc with at least one site in the mandible with erosion compared with controls (14.5% vs. 3.6%; P < .001) (see Table II), and this was confirmed by multivariate analysis (OR 4.49; 95% CI 1.92–10.53). Figure 4 shows an example of mandibular erosions. The difference was most notable in the condyle (6.3% vs. 1.8%; P = .022), the coronoid process (5.0% vs. 0.5%; P = .005), and the other type of erosion (3.2% vs. 0%; P = .012). Results were not significant for the digastric region (P = .239). There was a trend toward more erosions in the posterior ramus in patients with SSc compared with controls (5.1% vs. 1.4%; P = .058) (see Table II). With regard to the mean of the patients with SSc with erosions, 60.9% had more than one erosion in their mouths, and the mean number of erosions was 2.09 (SD 1.16).
Fig. 4.
Panoramic radiograph of scleroderma patient with severe erosions at (A) the coronoid processes, (B) the angles of the mandible. Also shown: PLS widening in (C) maxillary posterior teeth, and (D) mandibular posterior teeth.
DISCUSSION
In our study of 163 patients with SSc and 231 controls, we found that patients with SSc tend to have significantly more teeth with widening of the PLS and more mandibular erosions (see Figures 1 and 4). Our findings add substantially to the knowledge in the literature, as none of the previous studies of the estimates of the prevalence of radiographic abnormalities in the jaws in SSc systematically studied all suspected SSc radiographic manifestations (Table III). In addition, the number of patients examined in these studies was generally small, between 15 and 47,12–14,17,18,26–29 and controls were used in only seven studies.12–18 Another limitation of the previous studies is that the controls were either selected from patients attending the dental clinic,14 hospital staff,14 or patients with inflammatory diseases, such as rheumatoid arthritis and systemic lupus erythematosus.18 Prevalence estimates had been documented by using a convenience sample in some studies; consequently, selection and information bias may have affected the internal validity of these results. This may have biased these studies against finding abnormalities in the patients with SSc, potentially underestimating the true increased prevalence of oral disease compared with the general population (see Table III).
Table III.
Literature review of radiographic oral manifestations of scleroderma
| Author | Year | Design | Sample size | Mandibular erosions | % of patients with widening of the periodontal ligament at least one tooth |
|---|---|---|---|---|---|
| Leung et al16 | 2011 | Case-Control | 36/36 | Not assessed | Mean width of PLS greater in cases, but % not reported |
| Vincent et al26 | 2010 | Cross-sectional | 30 | 6.67% | 33.3% |
| Marcucci and Abdala13 | 2009 | Case-control | 15/10 | 46.7% | Not assessed |
| Rout et al27 | 1996 | Cross-sectional | 21 | 9.5% | 33% |
| Wood and Lee12 | 1988 | Case-control | 24/28 | 29%/Not assessed | Mean width of PLS greater in cases, but % not reported |
| Janssens et al18 | 1987 | Case-control | 47/359 Controls were 324 rheumatoid arthritis (RA) and 35 systemic lupus erythematosus (SLE) patients |
Not assessed | 59% and 4% for RA controls and 0% for SLE control |
| Alexandridis and White15 | 1984 | Case-control | 26/26 | Not assessed | 65% |
| White et al17 | 1977 | Case-control | 35 | 8.6% | 37% |
| Marmary et al28 | 1981 | Cross-sectional | 21/8 | 19% | 100% |
| Rowell and Hopper14 | 1977 | Case-control | 30/30 | Not assessed | 70% |
| Seifert et al29 | 1975 | Cross-sectional | 16 | 31% | Not assessed |
Our finding of mandibular erosions in 14.5% of SSc is within the range of reported results (6.6%–46.7%).12,13,17,26–29 Bone resorption has been noted in patients with more advanced disease27 and can occur in areas of muscle attachment in the mandible. Muscles involved in such areas are the masseter at the angle of the mandible, the lateral pterygoids at the condylar head, and the temporalis the coronoid process. With resorption at the condylar head and the coronoid process, the sigmoid notch becomes flatter and wider (Figure 4). Muscle contraction, ischemia due to atrophy, or both may be responsible for the resorption,29 as well as rigidity and pressure from overlying skin.29 Resorption of the mandible is an incidental finding and causes no symptoms.
We assessed widening of the PLS by using two methods. One was the subjective assessment of the observers of the number of teeth with PLS widening and the other was a measurement of the PLS at one tooth. Measurements of the PLS in our study were done with the use of Adobe Photoshop and were read by two radiologists, increasing the accuracy of the results. Differences between radiologists were resolved by using an adjudicator (MG). Previous studies have used either measurements of magnified periapical radiographs15,28 or subjective assessments.14,17,18,26,27 The two studies that used more than one observer12,15 did not report a method for resolving conflicts. We found PLS widening in at least one tooth in 38% of the patients with SSc but in only 11% of controls (see Table II), in the range of the reports of similar findings of 33% to 100% in previous studies (see Table III). Widening of the PLS in normal persons is known to occur because of periodontal or periapical disease and dental occlusal trauma.30 In the periodontal ligament of patients with SSc, connective tissue involvement may be responsible for widening.15 Occlusal trauma would not necessarily be involved, as the masseter muscles in patients with SSc show signs of atrophy31 and presumably reduced pressure during mastication. Although we could not confirm the findings of Alexandridis and White,15 that mean values for the measurement of the PLS at one tooth showed widening in patients with SSc versus controls, we did note that significantly more patients with SSc than controls had measurements of the PLS greater than 0.19 mm.
This study has some limitations. The number of condylar erosions in our controls may be overestimated, since they were patients recruited from rheumatologists’ offices or physiotherapy clinics with mechanical joint disease or osteoarthritis, conditions which could lead to flattening of the condylar head.32 The interrater reliability was only fair for the number of molars with PLS widening (ICC = 0.25), increasing the chance of misclassification for results pertaining to this measurement. The correlation between disease severity, erosions, and PLS widening and the impact on quality of life will be addressed in future reports. Another limitation is that there were some differences in the comparison between the scanned image and the original analog radiographs (a much larger image than intraoral radiographs), but these differences were deemed clinically insignificant.25
Our study also has a number of strengths. First, subjects were drawn from a large pool of patients with SSc and controls selected from seven sites across Canada, increasing the generalizability of the study results. Second, the recruitment of patients with SSc and controls from the same rheumatology clinics minimized selection bias. Third, the diagnosis of SSc was performed by experts after clinical examination, minimizing diagnosis misclassification, and 95% of the patients with SSc met the 2013 ACR criteria. Fourth, the evaluation of radiographs by blinded experts decreased imaging misclassification. Fifth, the interrater reliability was excellent for measurement of PLS width and was substantial and perfect for PLS widening in premolars and anteriors, respectively, thus minimizing imaging misclassification. Sixth, the large sample size provides sufficient power in the analysis. Seventh, the statistical analysis adjusted for important confounders (e.g., age, gender, ethnicity and smoking status) increased the internal validity of the results.
CONCLUSIONS
Compared with controls, patients with SSc are significantly more likely to have mandibular erosion at the condyles and coronoid processes. These patients also have significantly more teeth with PLS widening. This is particularly true of molars and premolars. Even though we might not have found significant changes that have not been described before, this is a definitive case-control study.
The underlying factors contributing to mandibular erosions and PLS widening are not clear, but in future studies, we will investigate the relationships between these oral abnormalities and various aspects of the disease, as well as their relationship to the altered oral quality of life that we and others have demonstrated in patients with SSc.
Statement of Clinical relevance.
This article describes the radiographic manifestations of systemic sclerosis in a large cohort of patients and the prevalence of these changes is documented. In comparison with the general population, patients with systemic sclerosis have more erosions of condyles and coronoid processes and more teeth with widening of the periodontal ligament.
Acknowledgments
This work was supported by a Canadian Institutes of Health Research Operating Grant (MOP-86613).
Footnotes
Ariel Masseto has been a consultant for BMS, UCB, Roche, Amgen, and Pfizer and has received grants for research from Merck (2011). All other authors have declared no conflicts of interest.
The late Dr. Markland, of the University of Saskatchewan College of Medicine, contributed substantially to this manuscript. She was well regarded by her peers for her diagnostic acumen and her concern for the welfare of her patients. She will be sorely missed.
References
- 1.Mayes MD. Scleroderma epidemiology. Rheum Dis Clin North Am. 2003;29:239–254. doi: 10.1016/s0889-857x(03)00022-x. [DOI] [PubMed] [Google Scholar]
- 2.Mayes MD, Lacey JV, Jr, Beebe-Dimmer J, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48:2246–2255. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- 3.Mayes MD. Epidemiology of systemic sclerosis and related diseases. Curr Opin Rheumatol. 1997;9:557–561. doi: 10.1097/00002281-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Lee P, Langevitz P, Alderdice CA, et al. Mortality in systemic sclerosis (scleroderma) Quart J Med. 1992;82:139–148. [PubMed] [Google Scholar]
- 5.van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65:2737–2747. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wollheim FA. Classification of systemic sclerosis. Visions and reality. Rheumatology. 2005;44:1212–1216. doi: 10.1093/rheumatology/keh671. [DOI] [PubMed] [Google Scholar]
- 7.Martin J, Fonseca C. The genetics of scleroderma. Curr Rheumatol Rep. 2011;13:13–20. doi: 10.1007/s11926-010-0139-5. [DOI] [PubMed] [Google Scholar]
- 8.Baron M, Hudson M, Tatibouet S, et al. The Canadian systemic sclerosis oral health study: orofacial manifestations and oral health-related quality of life in systemic sclerosis compared with the general population. Rheumatology. 2014;53:1386–1394. doi: 10.1093/rheumatology/ket441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baron M, Hudson M, Tatibouet S, et al. The Canadian Systemic Sclerosis Oral Health Study II: the relationship between oral and global health-related quality of life in systemic sclerosis. Rheumatology. 2015;54:692–696. doi: 10.1093/rheumatology/keu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baron M, Hudson M, Tatibouet S, et al. The Canadian Systemic Sclerosis Oral Health Study III: relationship between disease characteristics and oro-facial manifestations in systemic sclerosis. Arthritis Care Res. 2014 doi: 10.1002/acr.22490. http://dx.doi.org/10.1002/acr.22490Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 11.Jadu F. Systemic diseases. In: White SC, Pharoah MJ, editors. Oral Radiology: Principles and Interpretations. 7. St. Louis, MO: Mosby; 2014. pp. 465–467. [Google Scholar]
- 12.Wood RE, Lee P. Analysis of the oral manifestations of systemic sclerosis (scleroderma) Oral Surg Oral Med Oral Pathol. 1988;65:172–178. doi: 10.1016/0030-4220(88)90161-2. [DOI] [PubMed] [Google Scholar]
- 13.Marcucci M, Abdala N. Clinical and radiographic study of oro-facial alterations in patients with systemic sclerosis. Brazilian Oral Res. 2009;23:82–88. doi: 10.1590/s1806-83242009000100014. [DOI] [PubMed] [Google Scholar]
- 14.Rowell NR, Hopper FE. The periodontal membrane in systemic sclerosis. Br J Dermatol. 1977;96:15–20. doi: 10.1111/j.1365-2133.1977.tb05179.x. [DOI] [PubMed] [Google Scholar]
- 15.Alexandridis C, White SC. Periodontal ligament changes in patients with progressive systemic sclerosis. Oral Surg Oral Med Oral Pathol. 1984;58:113–118. doi: 10.1016/0030-4220(84)90375-x. [DOI] [PubMed] [Google Scholar]
- 16.Leung WK, Chu CH, Mok MY, Yeung KW, Ng SK. Periodontal status of adults with systemic sclerosis: case-control study. J Periodontol. 2011;82:1140–1145. doi: 10.1902/jop.2010.100593. [DOI] [PubMed] [Google Scholar]
- 17.White SC, Frey NW, Blaschke DD, et al. Oral radiographic changes in patients with progressive systemic sclerosis (scleroderma) J Am Dent Assoc. 1977;94:1178–1182. doi: 10.14219/jada.archive.1977.0367. [DOI] [PubMed] [Google Scholar]
- 18.Janssens X, Herman L, Mielants H, Verbruggen G, Veys EM. Disease manifestations of progressive systemic sclerosis: sensitivity and specificity. Clin Rheumatol. 1987;6:532–538. doi: 10.1007/BF02330590. [DOI] [PubMed] [Google Scholar]
- 19.Alhajeri H, Hudson M, Fritzler M, et al. The 2013 ACR/EULAR classification criteria for systemic sclerosis out-perform the 1980 criteria. Data from the Canadian Scleroderma Research Group. Arthritis Care Res. 2015;67:582–587. doi: 10.1002/acr.22451. [DOI] [PubMed] [Google Scholar]
- 20.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- 21.Schuller PD, Hatcher DC, Caelli TM, Eggert FM, Yuzyk J. [Accessed February14, 2015];Method and apparatus for improving the alignment of radiographic images. Available at: http://www.google.ca/patents/US4941164.
- 22.Malka VB, Hochscheidt GL, Larentis NL, Grecca FS, Fontanella VR, Kopper PM. A new in vitro method to evaluate radiopacity of endodontic sealers. Dentomaxillofac Radiol. 2015:20140422. doi: 10.1259/dmfr.20140422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang S, Liu DG, Gu Y. Comparison of linear measurements between CBCT orthogonally synthesized cephalograms and conventional cephalograms. Dentomaxillofac Radiol. 2014;43:20140024. doi: 10.1259/dmfr.20140024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 25.Bruntz LQ, Palomo JM, Baden S, Hans MG. A comparison of scanned lateral cephalograms with corresponding original radiographs. Am J Orthod Dentofacial Orthop. 2006;130:340–348. doi: 10.1016/j.ajodo.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 26.Vincent C, Agard C, Barbarot S, et al. Orofacial manifestations of systemic sclerosis: a study of 30 consecutive patients [translation] Rev Stomatol Chir Maxillofac. 2010;111:128–134. doi: 10.1016/j.stomax.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Rout PG, Hamburger J, Potts AJ. Orofacial radiologic manifestations of systemic sclerosis. Dentomaxillofac Radiol. 1996;25:193–196. doi: 10.1259/dmfr.25.4.9084272. [DOI] [PubMed] [Google Scholar]
- 28.Marmary Y, Glaiss R, Pisanty S. Scleroderma: oral manifestations. Oral Surg Oral Med Oral Pathol. 1981;52:32–37. doi: 10.1016/0030-4220(81)90169-9. [DOI] [PubMed] [Google Scholar]
- 29.Seifert MH, Steigerwald JC, Cliff MM. Bone resorption of the mandible in progressive systemic sclerosis. Arthritis Rheum. 1975;18:507–512. doi: 10.1002/art.1780180514. [DOI] [PubMed] [Google Scholar]
- 30.Auluck A. Widening of periodontal ligament space and mandibular resorption in patients with systemic sclerosis. Dentomaxillofac Radiol. 2007;36:441–442. doi: 10.1259/dmfr/82985174. [DOI] [PubMed] [Google Scholar]
- 31.Marcucci M, Abdala N. Analysis of the masseter muscle in patients with systemic sclerosis: a study by magnetic resonance imaging. Dentomaxillofac Radiol. 2009;38:524–530. doi: 10.1259/dmfr/57427474. [DOI] [PubMed] [Google Scholar]
- 32.de Bont LG, Boering G, Liem RS, Eulderink F, Westesson PL. Osteoarthritis and internal derangement of the temporomandibular joint: a light microscopic study. J Oral Maxillofac Surg. 1986;44:634–643. doi: 10.1016/s0278-2391(86)80075-1. [DOI] [PubMed] [Google Scholar]