Abstract
Background
Calcium disorders are common in both intensive care units and in patients with chronic kidney disease and are associated with increased morbidity and mortality. It is unknown whether calcium abnormalities in unselected emergency department admissions have an impact on in-hospital mortality.
Methods
This cross-sectional analysis included all admissions to the Emergency Department at the Inselspital Bern, Switzerland from 2010 to 2011. For hyper- and hypocalcaemic patients with a Mann-Whitney U-test, the differences between subgroups divided by age, length of hospital stay, creatinine, sodium, chloride, phosphate, potassium and magnesium were compared. Associations between calcium disorders and 28-day in-hospital mortality were assessed using the Cox proportional hazard regression model.
Results
8,270 patients with calcium measurements were included in our study. Overall 264 (3.2%) patients died. 150 patients (6.13%) with hypocalcaemia and 7 patients with hypercalcaemia (6.19%) died, in contrast to 104 normocalcaemic patients (1.82%). In univariate analysis, calcium serum levels were associated with sex, mortality and pre-existing diuretic therapy (all p<0.05). In multivariate Cox regression analysis, hypocalcaemia and hypercalcaemia were independent risk factors for mortality (HR 2.00 and HR 1.88, respectively; both p<0.01).
Conclusion
Both hypocalcaemia and hypercalcaemia are associated with increased 28-day in-hospital mortality in unselected emergency department admissions.
Introduction
Although electrolyte disorders are frequent in emergency departments, there have been few studies of the prevalence and outcome of hypercalcaemia and hypocalcaemia in emergency department populations [1,2].
In contrast, hypocalcaemia is known to be extremely common in the intensive care unit setting (up to 88% prevalence) and correlated with the severity of illness and mortality. No correlation with a specific illness has yet been found [3,4].
In critically ill patients in intensive care units increased mortality is associated with hypocalcaemia, but not hypercalcaemia [4,5]. In most of these patients it was not possible to identify a single cause for the hypocalcaemia [4].
In patients with chronic kidney disease (CKD), a prospective cohort study in Asia found that low serum calcium is an independent prognostic marker of the need for renal transplantation, as well as being a prognostic marker for the renal function of patients with stage 3–4 CKD. In this study, no association was observed between high serum calcium and renal outcome [6].
Case reports suggest that hypocalcaemia is associated with heart failure in both children and adults, perhaps due to a decline in myocardial contractility [7–9]. One study showed that hypocalcaemia is an independent predictive factor for left ventricular diastolic dysfunction in patients with CKD [10].
In patients with acute ST-elevation myocardial infarction, a recent study suggests that serum calcium levels on admission and in-hospital mortality are associated [11].
Moreover, a recent German study suggested that calcium disorders might also be of concern in trauma patients. This study found that hypocalcaemia on the third day after moderate or severe traumatic brain injury is a prognostic factor of early mortality [12].
In the present retrospective study, we have now tested whether abnormal calcium serum levels and in-hospital mortality are associated in unselected emergency department admissions.
Material, Methods and Patients
This cross-sectional study included all patients admitted to the Emergency Department of the Inselspital between January 1, 2009, and December 31, 2010. Multiple presentations to our department during the study period were excluded and only the first presentation counted. The emergency department of the university hospital of Bern, Inselspital, is treating the whole spectrum of traumatic and non-traumatic emergency patients and is a Level 1 major trauma centre, stroke centre and specialised centre for cardiovascular emergencies. Within this period 22,239 patients were monitored. 8,270 patients with calcium measurement at emergency department admission were included in our study. Patients who had at least one cardiovascular, neurological or metabolic, nephrologic, intestinal or hepatic comorbidity alongside the primary reason for admission received a blood test including calcium concentration. Data from the medical records were collected on age, sex, ethnic group, nationality, outcome, length of hospital stay and blood tests (creatinine, eGFR, sodium, chloride, phosphate, calcium, potassium, magnesium, osmolality). The study protocol for the present cross-sectional study of emergency admissions was approved by the Ethics Committee of the Canton Bern, Switzerland.
In addition, the Ethics Committee of the Canton Bern, Switzerland waived the need for informed consent for the present study due to the retrospective design.
The results are presented as medians and 25–75% interquartile ranges.
For hyper- and hypocalcaemic patients with a Mann-Whitney U-test, the differences between subgroups divided by age, length of hospital stay, creatinine, sodium, chloride, phosphate, potassium and magnesium were compared. With a Pearson`s Chi-Square Test, associations of calcium levels with different parameters (sex, hospitalisation, mortality, ethnic group and diuretic therapy) were tested in univariate analysis.
The effects of different variables on survival were investigated using the Cox proportional hazard regression model. The Kaplan-Meier method was used to compare the 28-day survival and the log-rank test to compare the differences in survival between subgroups. The 28-day mortality was identified through our hospital records.
All calculations were performed with the SPSS Statistics 21 (IBM Coorp.) program. The authors performing the data analysis were not involved in the data acquisition. A two tailed p value of less than 0.05 was considered statistically significant.
Plasma calcium concentrations between 2.20 and 2.55 mmol/l were defined as normal, in accordance with our laboratory reference values.
Results
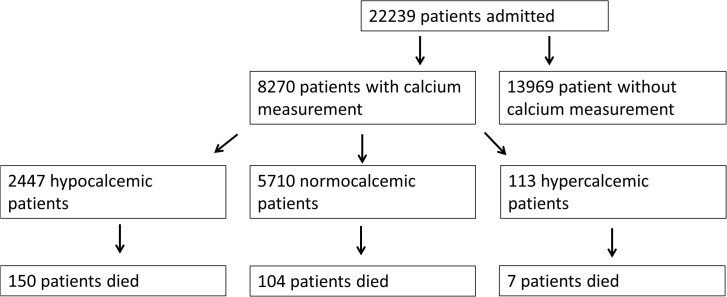
Calcium measurements were performed in the emergency department for 8,270 of 22,239 screened patients (Fig 1).
Fig 1. Flowchart.
Patient flowchart.
The baseline characteristics of the 8,270 patients included are shown in Table 1.
Table 1. Baseline characteristics of all patients with calcium measurement at hospital admission (n = 8,270).
| Parameter | Median (Interquartile range) |
|---|---|
| Age (years) | 58 (41;72) |
| Length of stay (days) | 2 (0;7) |
| Creatinine (μmol/l) | 72 (60;90) |
| eGFR (MDRD) (ml/min/1,73m2) | 95 (72;116) |
| Sodium (mmol/l) | 139 (137;141) |
| Osmolality (mosm/kg) | 291 (284;306) |
| Chloride (mmol/l) | 103 (100;106) |
| Phosphate (mmol/l) | 1.01 (0.83;1.19) |
| Calcium (mmol/l) | 2.26 (2.18;2.34) |
| Potassium (mmol/l) | 3.9 (3.7;4.2) |
| Magnesium (mmol/l) | 0.81 (0.73;0.88) |
In our cohort, 2,447 patients were hypocalcaemic, 5,710 normocalcaemic and 113 hypercalcaemic.
150 hypocalcaemic patients (6.13%) and 7 hypercalcaemic patients (6.19%) died, in comparison to 104 normocalcaemic patients (1.82%).
Compared with normocalcaemic patients, patients with calcium disorders were markedly older, had to stay longer in hospital and had lower eGFR values (all p<0.05, Table 2). Hypocalcaemic patients also had lower sodium and magnesium levels (p<0.05, Table 2).
Table 2. Comparison of patient groups with hypocalcaemia (n = 2,447) and hypercalcaemia (n = 113) with normocalcaemic patients (n = 5,710).
Medians and interquartile ranges. (Mann-Whitney U, p<0.05*)
| Parameter | Hypocalcaemia | Normocalcaemia | Hypercalcaemia |
|---|---|---|---|
| Age | 63 (47;76)* | 55 (38;70) | 66 (50;75) * |
| Length of stay | 4 (0;10)* | 1 (0;6) | 3 (0;10) * |
| Creatinine | 83 (68;114) * | 71 (60;86) | 74 (58;102) * |
| eGFR (MDRD) | 90 (60;117) * | 96 (76;116) | 72 (47;96) * |
| Sodium | 138 (136;141) * | 139 (138;141) | 139 (136;141) |
| Osmolality | 292 (283;311) | 291 (285;301) | 296 (282;312) |
| Chloride | 103 (99;107) | 103 (100;106) | 100 (94;108) |
| Phosphate | 1.01 (0.82;1.24) | 1.00 (0.84;1.16) | 1.08 (0.85;1.40) |
| Calcium | 2.12 (2.05;2.16) * | 2.31 (2.25;2.37) | 2.63 (2.58;2.76) * |
| Potassium | 3.9 (3.6;4.2) | 3.9 (3.7;4.2) | 4 (3.6;4.3) |
| Magnesium | 0.79 (0.70;0.87)* | 0.82 (0.75;0.88) | 0.83 (0.73;0.91) |
In univariate analysis, calcium levels were associated with sex, mortality and pre-existing diuretic therapy (all p<0.05, Table 3).
Table 3. Associations of calcium levels with different parameters in univariate analysis.
(Pearson’s Chi-Square Test (p<0.05*)).
| Parameter | p-value |
|---|---|
| Sex | 0.002* |
| Hospitalisation | <0.001* |
| Mortality | <0.001* |
| Ethnic group | 0.419 |
| Diuretic therapy | <0.001* |
Multivariate Cox regression analysis showed that hypocalcaemia and hypercalcaemia were independent risk factors for mortality (all p<0.05, Tables 4 and 5).
Table 4. Multivariate Cox regression analysis for 28 day mortality in hypocalcaemic patients (p<0.05*).
| Parameter | Hazard ratio (Confidence interval) | p-value |
|---|---|---|
| Hypocalcaemia | 2.000 (1.459; 2.741) | <0.001* |
| Age | 1.029 (1.019; 1.040) | <0.001* |
| Sex | 1.045 (0.762; 1.433) | 0.783 |
| Creatinine | 1.001 (1.000; 1.002) | 0.031 |
| Diuretic therapy | 0.687 (0.470; 1.003) | 0.052 |
Table 5. Multivariate Cox regression analysis for 28 day mortality in hypercalcaemic patients (p<0.05*).
| Parameter | Hazard ratio (Confidence interval) | p-value |
|---|---|---|
| Hypercalcaemia | 1.881 (1.184; 2.989) | 0.007* |
| Age | 1.030 (1.014; 1.047) | <0.001* |
| Sex | 1.004 (0.634; 1.591) | 0.986 |
| Creatinine | 1.003 (1.001; 1.004) | <0.001* |
| Diuretic therapy | 0.883 (0.052; 1.499) | 0.645 |
Age per se is also a risk factor for mortality in these patients (p<0.05, Tables 4 and 5).
Creatinine was only a risk factor for mortality in thepatients with hypercalcaemia (p<0.05, Table 5).
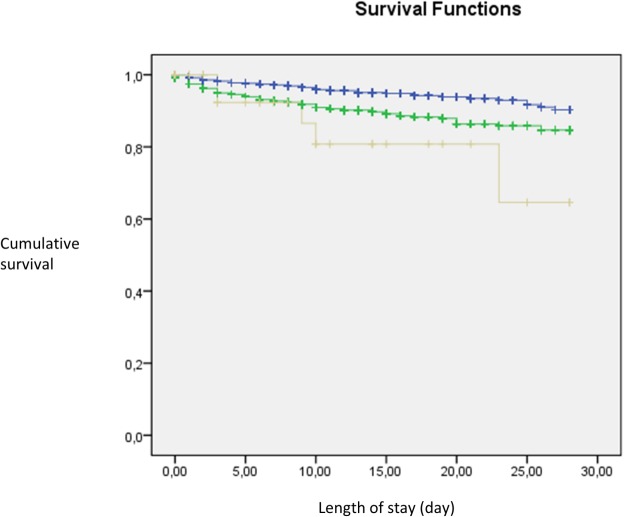
The Kaplan Meier plot shows the decrease in survival in both patient groups with abnormal calcium levels in comparison with normocalcaemic patients (both p<0.05, Fig 2). The marked decrease can be seen from day 5 (Fig 2).
Fig 2. Kaplan Meier plot.
Kaplan Meier plot for 28 day in-hospital mortality in patients with normocalcaemia (blue line), hypocalcaemia (green line) and hypercalcaemia (grey line) (Log rank test (p<0.05)).
Discussion
Our study showed that both hypercalcaemia and hypocalcaemia are independent new risk factors for mortality in emergency departments aside from classical risk factors (creatinine and age). Patients with calcium disorders were older, stayed longer in hospital and had lower eGFR values.
Many factors are known to contribute to increased mortality in chronic and acute kidney disease [13, 14]. Not only end stage kidney disease but also impairment of kidney function have been demonstrated to increase mortality [13]. In our study, creatinine was an independent risk factor for mortality, which is comparable with other study results [15–17]. Overall cardiovascular mortality accounts for nearly half of deaths in patients with chronic kidney disease [9]. As kidney disease progresses, macrovascular disease becomes less important than microvascular problems and electrolyte disorders [18].
The association we found between hypocalcaemia and hyponatraemia and hypomagnesaemia is consistent with other studies [3, 9], for example in intensive care unit patients with sepsis [4].
Hypocalcaemia is often seen after thyroidectomy, common in patients in intensive care units and often of multifactorial aetiology [4]. It has been shown to be a predictor of poor renal outcome and mortality in patients with CKD [4, 5]. Vroonhof et al. found that lower calcium values were associated with increased mortality in unselected emergency department admissions [19]. This increased mortality is confirmed in our study with emergency department patients. In intensive care unit patients, this mortality is not associated with a specific illness but is thought to be part of the severe illness reaction [4]. It is not yet clear whether this accounts for patients admitted to emergency departments. Calcium is necessary for the cardiac myocyte contraction. Whether this is the major cause for the increased mortality remains unclear and needs further research [10].
The increased mortality in hypercalcaemia is consistent with an Asian study on calcium disorders in an emergency department [2]. Hypercalcaemia is often associated with diseases such as cancer [20].
One of the best known causes of hypercalcemia is Parathyroid hormone related peptide (PTHrP) associated hypercalcemia, which is most often found in patients with solid organ malignancy and is associated with poor outcome [21].
In a recent study, the cause of mild hypercalcaemia was only identified in 21% of patients [22]. In addition, only the minority of patients had a follow up or workup plan at discharge [22]. In contrast, the vast majority of these patients, symptoms related to hypercalcaemia could be found [22]. Another study demonstrated that in patients with malignancy nearly half of patients showed symptoms of hypercalcaemia but in none of the medical reports was hypercalcaemia mentioned as the reason for hospitalization [23]. Two Asian studies had higher numbers of hypercalcaemic patients [2, 24]. A possible explanation for this discrepancy might be that calcium was only measured in a third of our population, which might include some selection bias. Due to the retrospective design of our study no additional routine calcium measurements were conducted. This should be topic of further research to exclude selection bias. It appears to be unlikely that additional calcium measurements would have changed the presented results, as general calcium measurements are likely to be within the normal range.
One limitation of our study is a vast heterogeneity of discharge diagnoses. Therefore, associations with endpoints of our study could not be detected. The same limitation exists for the increased mortality shown by Vroonhof et al. for hypocalcaemia [19]. However, registry studies carry that limitation in general. In addition, large discrepancies exist between the initial suspected diagnosis (leading to calcium analysis) and the final discharge diagnosis.
In summary we could show that both hypocalcaemia and hypercalcaemia are associated with increased 28-day in-hospital mortality in unselected emergency department admissions. More attention should be paid to abnormal calcium levels beside classical risk factors and metabolic disorders.
Data Availability
The study contains patient data of emergency department admissions and is therefore protected by privacy/ethics committee. Data are available from the Bernese Institutional Data Access / Ethics Committee for researchers who meet the criteria for access to confidential data. Thomas Sauter, Inselspital, Bern, (Thomas.sauter@insel.ch) is responsible for the contact and can be contacted at any time.
Funding Statement
The authors have no support or funding to report.
References
- 1. Lindner G, Felber R, Schwarz C, Marti G, Leichtle AB, Fiedler GM et al. Hypercalcemia in the ED: prevalence, etiology, and outcome. Am J Emerg Med 2013;31:657–660. 10.1016/j.ajem.2012.11.010 [DOI] [PubMed] [Google Scholar]
- 2. Lee CT, Yang CC, Lam KK, Kung CT, Tsai CJ, Chen HC. Hypercalcemia in the emergency department. Am J Med Sci 2006;331:119–123. [DOI] [PubMed] [Google Scholar]
- 3. Zivin JR, Gooley T, Zager RA, Ryan MJ. Hypocalcemia: a pervasive metabolic abnormality in the critically ill. Am J Kidney Dis 2001;37:689–698. [DOI] [PubMed] [Google Scholar]
- 4. Desai TK, Carlson RW, Geheb MA. Prevalence and clinical implications of hypocalcemia in acutely ill patients in a medical intensive care setting. Am J Med 1988;84:209–214. [DOI] [PubMed] [Google Scholar]
- 5. Zhang Z, Xu X, Ni H, Deng H. Predictive value of ionized calcium in critically ill patients: an analysis of a large clinical database MIMIC II. PLoS One 2014;15:e95204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lim LM, Kuo HT, Kuo MC, Chiu YW, Lee JJ, Hwang SJ et al. Low serum calcium is associated with poor renal outcomes in chronic kidney disease stages 3–4 patients. BMC Nephrol 2014;15:183 10.1186/1471-2369-15-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hurley K, Baggs D. Hypocalcemic cardiac failure in the emergency department. J Emerg Med 2005;28:155–159. [DOI] [PubMed] [Google Scholar]
- 8. Kazmi AS, Wall BM. Reversible congestive heart failure related to profound hypocalcemia secondary to hypoparathyroidism. Am J Med Sci 2007;333:226–229. [DOI] [PubMed] [Google Scholar]
- 9. Catalano A, Basile G, Lasco A. Hypocalcemia: a sometimes overlooked cause of heart failure in the elderly. Aging Clin Exp Res 2012;24:400–403. [DOI] [PubMed] [Google Scholar]
- 10. Gromadziński L, Januszko-Giergielewicz B, Pruszczyk P. Hypocalcemia is related to left ventricular diastolic dysfunction in patients with chronic kidney disease. J Cardiol 2014;63:198–204. 10.1016/j.jjcc.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 11. Lu X, Wang Y, Meng H, Chen P, Huang Y, Wang Z et al. Association of admission serum calcium levels and in-hospital mortality in patients with acute ST-elevated myocardial infarction: an eight-year, single-center study in China. PLoS One 2014;13:e99895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vinas-Rios JM, Sanchez-Aguilar M, Sanchez-Rodriguez JJ, Gonzalez-Aguirre D, Heinen C, Meyer F et al. Hypocalcaemia as a prognostic factor of early mortality in moderate and severe traumatic brain injury. Neurol Res 2014;36:102–106. 10.1179/1743132813Y.0000000272 [DOI] [PubMed] [Google Scholar]
- 13. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 14. Ross L, Banerjee D. Cardiovascular complications of chronic kidney disease. Int J Clin Pract. 2013;67:4–5. 10.1111/ijcp.12069 [DOI] [PubMed] [Google Scholar]
- 15. Garg AX, Clark WF, Haynes RB, House AA. Moderate renal insufficiency and the risk of cardiovascular mortality: results from the NHANES I. Kidney Int 2002;61:1486–1494. [DOI] [PubMed] [Google Scholar]
- 16. Muntner P, He J, Hamm L, Loria C, Whelton PK. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol 2002;13:745–753. [DOI] [PubMed] [Google Scholar]
- 17. Drey N, Roderick P, Mullee M, Rogerson MA. Population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis 2003;42:677–684. [DOI] [PubMed] [Google Scholar]
- 18. Kumar S, Bogle R, Banerjee D. Why do young people with chronic kidney disease die early? World J Nephrol. 2014;3:143–155. 10.5527/wjn.v3.i4.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vroonhof K, van Solinge WW, Rovers MM, Huisman A. Differences in mortality on the basis of laboratory parameters in an unselected population at the Emergency Department. Clin Chem Lab Med. 2005;43:536–541 [DOI] [PubMed] [Google Scholar]
- 20. Wysolmerski JJ, Broadus AE. Hypercalcemia of malignancy: the central role of parathyroid hormone-related protein. Annu Rev Med 1994;45:189–200. [DOI] [PubMed] [Google Scholar]
- 21.Donovan PJ, Achong N, Griffin K, Galligan J, Pretorius CJ, McLeod DS. PTHrP-mediated hypercalcemia: causes and survival in 138 patients. J Clin Endocrinol Metab. 2015 Feb 26:jc20144250 [DOI] [PubMed]
- 22. Royer AM, Maclellan RA, Stanley JD, Willingham TB, Giles WH. Hypercalcemia in the emergency department: a missed opportunity. Am Surg 2014;80:732–735. [PubMed] [Google Scholar]
- 23. Lamy O, Jenzer-Closuit A, Burckhardt P. Hypercalcaemia of malignancy: an undiagnosed and undertreated disease. J Intern Med 2001;250:73–79. [DOI] [PubMed] [Google Scholar]
- 24. Shek CC, Natkunam A, Tsang V, Cockram CS, Swaminathan R. Incidence, causes and mechanism of hypercalcemia in a hospital population in Hong Kong. Q J Med 1990;77:1277–1285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study contains patient data of emergency department admissions and is therefore protected by privacy/ethics committee. Data are available from the Bernese Institutional Data Access / Ethics Committee for researchers who meet the criteria for access to confidential data. Thomas Sauter, Inselspital, Bern, (Thomas.sauter@insel.ch) is responsible for the contact and can be contacted at any time.