Abstract
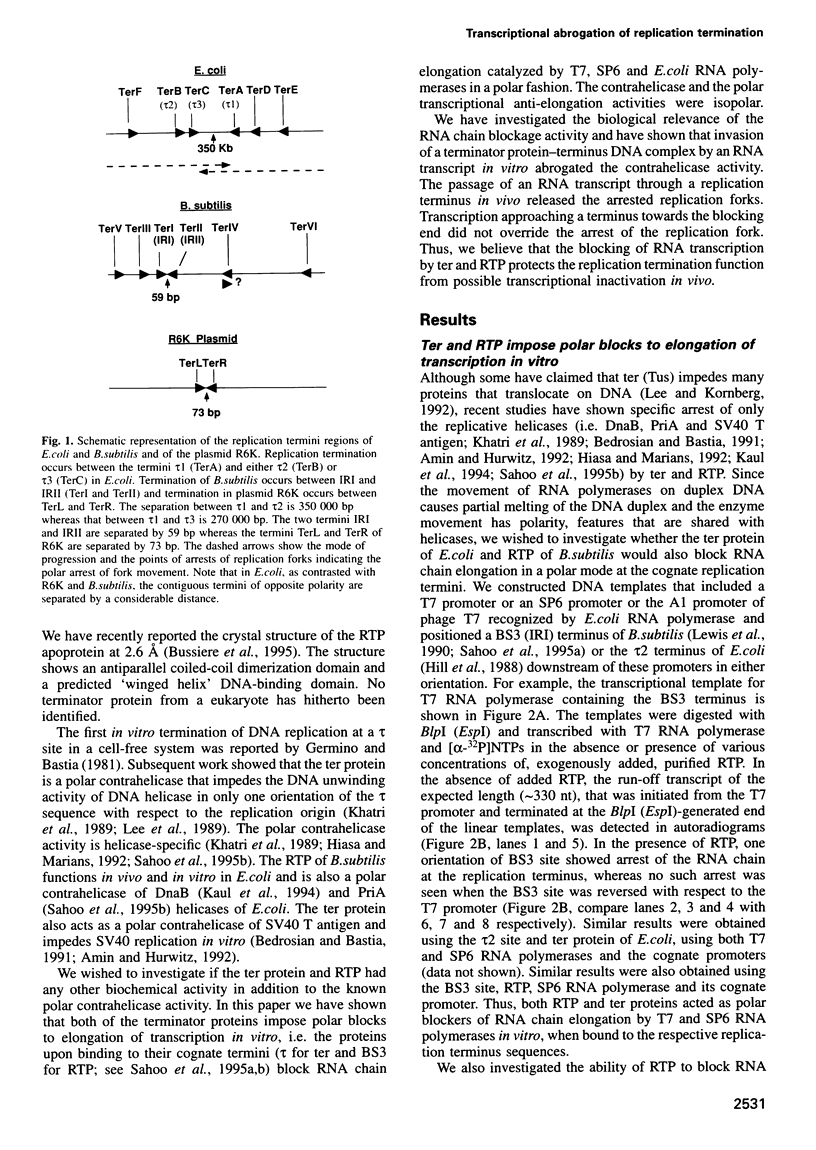
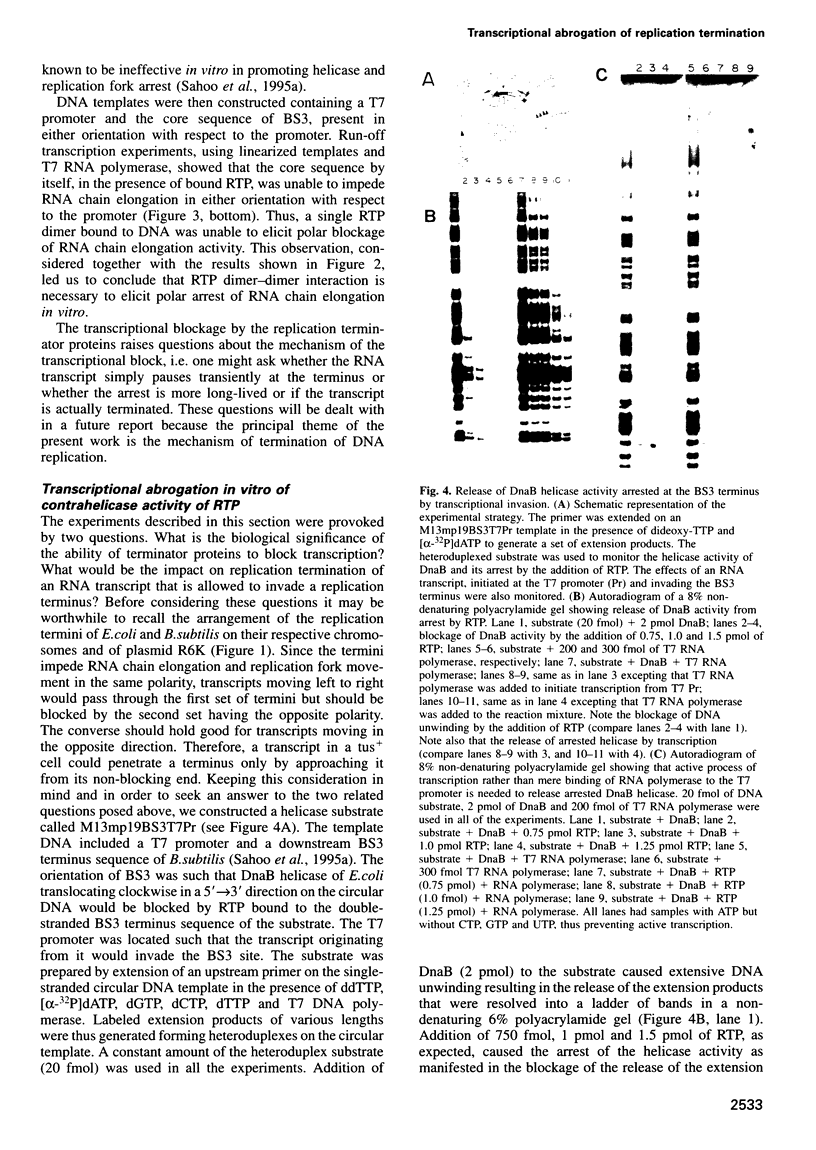
In Escherichia coli and Bacillus subtilis replication fork arrest occurs in the terminus at sequence-specific sites by the binding of replication terminator proteins to the fork arrest sites. The protein-DNA complex causes polar arrest of the replication forks by inhibiting the activity of the replicative helicases in only one orientation of the terminus with respect to the replication origin. This activity has been named as polar contrahelicase. In this paper we report on a second novel activity of the terminator proteins of E.coli and B.subtilis, namely the ability of the proteins to block RNA chain elongation by several prokaryotic RNA polymerases in a polar mode. The replication terminator proteins ter and RTP of E.coli and B.subtilis respectively, impeded RNA chain elongation catalyzed by T7, SP6 and E.coli RNA polymerases in a polar mode at the replication arrest sites. The RNA chain anti-elongation and the contrahelicase activities were isopolar. Whereas one monomer of ter was necessary and sufficient to block RNA chain elongation, two interacting dimers of RTP were needed to effect the same blockage. The biological significance of the RNA chain anti-elongation activity is manifested in the functional inactivation of a replication arrest site by invasion of RNA chains from outside, and the consequent need to preserve replication arrest activity by restricting the passage of transcription through the terminus-terminator protein complex.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amin A. A., Hurwitz J. Polar arrest of the simian virus 40 tumor antigen-mediated replication fork movement in vitro by the tus protein-terB complex of Escherichia coli. J Biol Chem. 1992 Sep 15;267(26):18612–18622. [PubMed] [Google Scholar]
- Bastia D., Germino J., Crosa J. H., Ram J. The nucleotide sequence surrounding the replication terminus of R6K. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2095–2099. doi: 10.1073/pnas.78.4.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian C. L., Bastia D. Escherichia coli replication terminator protein impedes simian virus 40 (SV40) DNA replication fork movement and SV40 large tumor antigen helicase activity in vitro at a prokaryotic terminus sequence. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2618–2622. doi: 10.1073/pnas.88.7.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer B. J., Fangman W. L. A replication fork barrier at the 3' end of yeast ribosomal RNA genes. Cell. 1988 Nov 18;55(4):637–643. doi: 10.1016/0092-8674(88)90222-x. [DOI] [PubMed] [Google Scholar]
- Brewer B. J., Fangman W. L. The localization of replication origins on ARS plasmids in S. cerevisiae. Cell. 1987 Nov 6;51(3):463–471. doi: 10.1016/0092-8674(87)90642-8. [DOI] [PubMed] [Google Scholar]
- Brewer B. J., Lockshon D., Fangman W. L. The arrest of replication forks in the rDNA of yeast occurs independently of transcription. Cell. 1992 Oct 16;71(2):267–276. doi: 10.1016/0092-8674(92)90355-g. [DOI] [PubMed] [Google Scholar]
- Brewer B. J. When polymerases collide: replication and the transcriptional organization of the E. coli chromosome. Cell. 1988 Jun 3;53(5):679–686. doi: 10.1016/0092-8674(88)90086-4. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Bussiere D. E., Bastia D., White S. W. Crystal structure of the replication terminator protein from B. subtilis at 2.6 A. Cell. 1995 Feb 24;80(4):651–660. doi: 10.1016/0092-8674(95)90519-7. [DOI] [PubMed] [Google Scholar]
- Carrigan C. M., Pack R. A., Smith M. T., Wake R. G. Normal terC-region of the Bacillus subtilis chromosome acts in a polar manner to arrest the clockwise replication fork. J Mol Biol. 1991 Nov 20;222(2):197–207. doi: 10.1016/0022-2836(91)90206-l. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chan C. L., Landick R. The Salmonella typhimurium his operon leader region contains an RNA hairpin-dependent transcription pause site. Mechanistic implications of the effect on pausing of altered RNA hairpins. J Biol Chem. 1989 Dec 5;264(34):20796–20804. [PubMed] [Google Scholar]
- Crosa J. H., Luttropp L. K., Falkow S. Mode of replication of the conjugative R-plasmid RSF1040 in Escherichia coli. J Bacteriol. 1976 Apr;126(1):454–466. doi: 10.1128/jb.126.1.454-466.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davanloo P., Rosenberg A. H., Dunn J. J., Studier F. W. Cloning and expression of the gene for bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2035–2039. doi: 10.1073/pnas.81.7.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar V., Mager D., Iqbal A., Schildkraut C. L. The coordinate replication of the human beta-globin gene domain reflects its transcriptional activity and nuclease hypersensitivity. Mol Cell Biol. 1988 Nov;8(11):4958–4965. doi: 10.1128/mcb.8.11.4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks A. H., Griffiths A. A., Wake R. G. Identification and characterization of new DNA replication terminators in Bacillus subtilis. Mol Microbiol. 1995 Jul;17(1):13–23. doi: 10.1111/j.1365-2958.1995.mmi_17010013.x. [DOI] [PubMed] [Google Scholar]
- Gahn T. A., Schildkraut C. L. The Epstein-Barr virus origin of plasmid replication, oriP, contains both the initiation and termination sites of DNA replication. Cell. 1989 Aug 11;58(3):527–535. doi: 10.1016/0092-8674(89)90433-9. [DOI] [PubMed] [Google Scholar]
- Germino J., Bastia D. Termination of DNA replication in vitro at a sequence-specific replication terminus. Cell. 1981 Mar;23(3):681–687. doi: 10.1016/0092-8674(81)90431-1. [DOI] [PubMed] [Google Scholar]
- Greenfeder S. A., Newlon C. S. Replication forks pause at yeast centromeres. Mol Cell Biol. 1992 Sep;12(9):4056–4066. doi: 10.1128/mcb.12.9.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodberg J., Dunn J. J. ompT encodes the Escherichia coli outer membrane protease that cleaves T7 RNA polymerase during purification. J Bacteriol. 1988 Mar;170(3):1245–1253. doi: 10.1128/jb.170.3.1245-1253.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández P., Lamm S. S., Bjerknes C. A., Hof J. V. Replication termini in the rDNA of synchronized pea root cells (Pisum sativum). EMBO J. 1988 Feb;7(2):303–308. doi: 10.1002/j.1460-2075.1988.tb02813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiasa H., Marians K. J. Differential inhibition of the DNA translocation and DNA unwinding activities of DNA helicases by the Escherichia coli Tus protein. J Biol Chem. 1992 Jun 5;267(16):11379–11385. [PubMed] [Google Scholar]
- Hidaka M., Akiyama M., Horiuchi T. A consensus sequence of three DNA replication terminus sites on the E. coli chromosome is highly homologous to the terR sites of the R6K plasmid. Cell. 1988 Nov 4;55(3):467–475. doi: 10.1016/0092-8674(88)90033-5. [DOI] [PubMed] [Google Scholar]
- Hidaka M., Kobayashi T., Horiuchi T. A newly identified DNA replication terminus site, TerE, on the Escherichia coli chromosome. J Bacteriol. 1991 Jan;173(1):391–393. doi: 10.1128/jb.173.1.391-393.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. M. Arrest of bacterial DNA replication. Annu Rev Microbiol. 1992;46:603–633. doi: 10.1146/annurev.mi.46.100192.003131. [DOI] [PubMed] [Google Scholar]
- Hill T. M., Pelletier A. J., Tecklenburg M. L., Kuempel P. L. Identification of the DNA sequence from the E. coli terminus region that halts replication forks. Cell. 1988 Nov 4;55(3):459–466. doi: 10.1016/0092-8674(88)90032-3. [DOI] [PubMed] [Google Scholar]
- Hill T. M., Tecklenburg M. L., Pelletier A. J., Kuempel P. L. tus, the trans-acting gene required for termination of DNA replication in Escherichia coli, encodes a DNA-binding protein. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1593–1597. doi: 10.1073/pnas.86.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi T., Hidaka M., Akiyama M., Nishitani H., Sekiguchi M. Replication intermediate of a hybrid plasmid carrying the replication terminus (ter) site of R 6K as revealed by agarose gel electrophoresis. Mol Gen Genet. 1987 Dec;210(3):394–398. doi: 10.1007/BF00327188. [DOI] [PubMed] [Google Scholar]
- Kaul S., Mohanty B. K., Sahoo T., Patel I., Khan S. A., Bastia D. The replication terminator protein of the gram-positive bacterium Bacillus subtilis functions as a polar contrahelicase in gram-negative Escherichia coli. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11143–11147. doi: 10.1073/pnas.91.23.11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri G. S., MacAllister T., Sista P. R., Bastia D. The replication terminator protein of E. coli is a DNA sequence-specific contra-helicase. Cell. 1989 Nov 17;59(4):667–674. doi: 10.1016/0092-8674(89)90012-3. [DOI] [PubMed] [Google Scholar]
- Kolter R., Helinski D. R. Activity of the replication terminus of plasmid R6K in hybrid replicons in Escherichia coli. J Mol Biol. 1978 Sep 25;124(3):425–441. doi: 10.1016/0022-2836(78)90180-8. [DOI] [PubMed] [Google Scholar]
- Kuempel P. L., Duerr S. A., Seeley N. R. Terminus region of the chromosome in Escherichia coli inhibits replication forks. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3927–3931. doi: 10.1073/pnas.74.9.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang W. H., Morrow B. E., Ju Q., Warner J. R., Reeder R. H. A model for transcription termination by RNA polymerase I. Cell. 1994 Nov 4;79(3):527–534. doi: 10.1016/0092-8674(94)90261-5. [DOI] [PubMed] [Google Scholar]
- Lang W. H., Reeder R. H. The REB1 site is an essential component of a terminator for RNA polymerase I in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Jan;13(1):649–658. doi: 10.1128/mcb.13.1.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. H., Kornberg A. Features of replication fork blockage by the Escherichia coli terminus-binding protein. J Biol Chem. 1992 May 5;267(13):8778–8784. [PubMed] [Google Scholar]
- Lee E. H., Kornberg A., Hidaka M., Kobayashi T., Horiuchi T. Escherichia coli replication termination protein impedes the action of helicases. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9104–9108. doi: 10.1073/pnas.86.23.9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A., Autret S., Séror S. J. A checkpoint involving RTP, the replication terminator protein, arrests replication downstream of the origin during the Stringent Response in Bacillus subtilis. Mol Microbiol. 1995 Jan;15(2):287–295. doi: 10.1111/j.1365-2958.1995.tb02243.x. [DOI] [PubMed] [Google Scholar]
- Lewis P. J., Ralston G. B., Christopherson R. I., Wake R. G. Identification of the replication terminator protein binding sites in the terminus region of the Bacillus subtilis chromosome and stoichiometry of the binding. J Mol Biol. 1990 Jul 5;214(1):73–84. doi: 10.1016/0022-2836(90)90147-E. [DOI] [PubMed] [Google Scholar]
- Linskens M. H., Huberman J. A. Organization of replication of ribosomal DNA in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Nov;8(11):4927–4935. doi: 10.1128/mcb.8.11.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little R. D., Platt T. H., Schildkraut C. L. Initiation and termination of DNA replication in human rRNA genes. Mol Cell Biol. 1993 Oct;13(10):6600–6613. doi: 10.1128/mcb.13.10.6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Alberts B. M. Head-on collision between a DNA replication apparatus and RNA polymerase transcription complex. Science. 1995 Feb 24;267(5201):1131–1137. doi: 10.1126/science.7855590. [DOI] [PubMed] [Google Scholar]
- Liu B., Wong M. L., Tinker R. L., Geiduschek E. P., Alberts B. M. The DNA replication fork can pass RNA polymerase without displacing the nascent transcript. Nature. 1993 Nov 4;366(6450):33–39. doi: 10.1038/366033a0. [DOI] [PubMed] [Google Scholar]
- Louarn J. M., Louarn J., François V., Patte J. Analysis and possible role of hyperrecombination in the termination region of the Escherichia coli chromosome. J Bacteriol. 1991 Aug;173(16):5097–5104. doi: 10.1128/jb.173.16.5097-5104.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louarn J., Patte J., Louarn J. M. Evidence for a fixed termination site of chromosome replication in Escherichia coli K12. J Mol Biol. 1977 Sep 25;115(3):295–314. doi: 10.1016/0022-2836(77)90156-5. [DOI] [PubMed] [Google Scholar]
- Lucchini R., Sogo J. M. Replication of transcriptionally active chromatin. Nature. 1995 Mar 16;374(6519):276–280. doi: 10.1038/374276a0. [DOI] [PubMed] [Google Scholar]
- Mehta P. P., Bussiere D. E., Hoffman D. W., Bastia D., White S. W. Crystallization and preliminary structural analysis of the replication terminator protein of Bacillus subtilis. J Biol Chem. 1992 Sep 15;267(26):18885–18889. [PubMed] [Google Scholar]
- Natarajan S., Kelley W. L., Bastia D. Replication terminator protein of Escherichia coli is a transcriptional repressor of its own synthesis. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3867–3871. doi: 10.1073/pnas.88.9.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier A. J., Hill T. M., Kuempel P. L. Location of sites that inhibit progression of replication forks in the terminus region of Escherichia coli. J Bacteriol. 1988 Sep;170(9):4293–4298. doi: 10.1128/jb.170.9.4293-4298.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R., Bermúdez-Cruz R. M., Chamberlin M. J. Parameters affecting transcription termination by Escherichia coli RNA polymerase. I. Analysis of 13 rho-independent terminators. J Mol Biol. 1992 Mar 5;224(1):31–51. doi: 10.1016/0022-2836(92)90574-4. [DOI] [PubMed] [Google Scholar]
- Roecklein B. A., Kuempel P. L. In vivo characterization of tus gene expression in Escherichia coli. Mol Microbiol. 1992 Jun;6(12):1655–1661. doi: 10.1111/j.1365-2958.1992.tb00890.x. [DOI] [PubMed] [Google Scholar]
- Sahoo T., Mohanty B. K., Lobert M., Manna A. C., Bastia D. The contrahelicase activities of the replication terminator proteins of Escherichia coli and Bacillus subtilis are helicase-specific and impede both helicase translocation and authentic DNA unwinding. J Biol Chem. 1995 Dec 8;270(49):29138–29144. doi: 10.1074/jbc.270.49.29138. [DOI] [PubMed] [Google Scholar]
- Sahoo T., Mohanty B. K., Patel I., Bastia D. Termination of DNA replication in vitro: requirement for stereospecific interaction between two dimers of the replication terminator protein of Bacillus subtilis and with the terminator site to elicit polar contrahelicase and fork impedance. EMBO J. 1995 Feb 1;14(3):619–628. doi: 10.1002/j.1460-2075.1995.tb07038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sista P. R., Hutchinson C. A., 3rd, Bastia D. DNA-protein interaction at the replication termini of plasmid R6K. Genes Dev. 1991 Jan;5(1):74–82. doi: 10.1101/gad.5.1.74. [DOI] [PubMed] [Google Scholar]
- Sista P. R., Mukherjee S., Patel P., Khatri G. S., Bastia D. A host-encoded DNA-binding protein promotes termination of plasmid replication at a sequence-specific replication terminus. Proc Natl Acad Sci U S A. 1989 May;86(9):3026–3030. doi: 10.1073/pnas.86.9.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. T., Wake R. G. Definition and polarity of action of DNA replication terminators in Bacillus subtilis. J Mol Biol. 1992 Oct 5;227(3):648–657. doi: 10.1016/0022-2836(92)90214-5. [DOI] [PubMed] [Google Scholar]
- Valenzuela M. S., Freifelder D. Lack of a unique termination site for the first round of bacteriophage lambda DNA replication. J Mol Biol. 1976 Apr 15;102(3):569–589. doi: 10.1016/0022-2836(76)90335-1. [DOI] [PubMed] [Google Scholar]