Abstract
The incidence of AIDS-defining cancers (ADCs) -- Kaposi sarcoma, primary central nervous system lymphoma, non-Hodgkin lymphoma, and cervical cancer -- although on the decline since shortly after the introduction of highly active antiretroviral therapy (HAART), has continued to be greater even in treated HIV-infected persons than in the general population. While the survival of newly infected people living with HIV/AIDS now rivals that of the general population, morbidity and mortality associated with non-AIDS-defining cancers (NADCs) such as lung, liver, anal and melanoma are significant and also continue to rise. Increasing age (i.e., longevity) is the greatest risk factor for NADCs, but longevity alone is not sufficient to fully explain these trends in cancer epidemiology. In this review, we briefly review the epidemiology and etiology of cancers seen in HIV/AIDS, and in this context, discuss preclinical research and broad treatment considerations. Investigation of these considerations provides insight into why malignancies continue to be a major problem in the current era of HIV/AIDS care.
Introduction
In late 1995, the first generation of HIV protease inhibitors became commercially available. Within a few months, clinicians were combining these novel drugs with nucleoside and non-nucleoside reverse transcriptase inhibitors. The beneficial effects of what soon became known as highly active antiretroviral therapy (HAART) were immediate and profound. In just a few years, the number of cases of newly diagnosed AIDS, AIDS-related deaths, and AIDS-defining cancers (ADCs), had decreased by greater than 70% [1-3]. However, even as AIDS-related mortality has continued to decline, the rate of new HIV infections has remained constant [4-6]. Consequently, the number of people living with HIV/AIDS (PLWHA) has increased by a factor of four [4-6]. Although HAART affords PLWHA a longer life expectancy, it also leaves them increasingly vulnerable to the same array of cancers associated with aging that are seen in the general population [7-12]. These non-AIDS-defining cancers (NADCs) include those associated with viral infections (e.g., anal [Human Papilloma Virus (HPV)], liver [Hepatitis C (HCV) and Hepatitis B Viruses (HBV)], head and neck [HPV]) and those not associated with viral infections (e.g., lung and melanoma). In industrial nations, the number of cases of NADCs now equals or exceeds the number of cases of ADCs, and NADCs are a leading cause of mortality for PLWHA [3, 7-10, 13-16]. Age and immune status, however, are insufficient to fully explain these trends in cancer risk. For PLWHA, even those with normal CD4+ T cell counts, the risk for many NADCs remains greater than for their age-matched HIV-sero-negative counterparts [8-10, 13, 14]. Furthermore, compared to the general population, PLWHA present with more aggressive and advanced disease at the time of cancer diagnosis [17-20]. These changes in cancer epidemiology are not well understood. We include preclinical research findings and discuss the epidemiology and etiology of both NADC and ADCs. We also briefly examine cancer treatment in the context of HAART-chemotherapy interactions.
Epidemiology
AIDS-defining Cancers
In 1982, the United States Center for Disease Control and Prevention expanded the case definition of AIDS to include HIV-infected individuals diagnosed with Kaposi Sarcoma (KS) and primary central nervous system lymphoma (PCNSL) [2]. Cervical cancer and intermediate- and high-grade forms of non-Hodgkin lymphoma (NHL) were added to the list of ADCs shortly thereafter [2]. The risk of cancer in PLWHA can be defined by the standard incidence ratio (SIR) [21]. For malignancies in HIV, the SIR compares the rate of cancers in the HIV/AIDS population to the number expected in the general population at any given time [2, 7-10, 22, 23]. In the HAART era, the SIR has decreased for all ADCs, with the exception of invasive cervical carcinoma [2, 7-10, 22, 23]. However, even now the risk for each of the ADCs remains above that of the general population (Table 1) [2, 7-10, 22, 23].
Table 1. Standard incidence ratios for common AIDS-defining and non-AIDS-defining cancers in the early and later HAART era and in the context of tumor-associated oncogenic viruses.
| HIV-associated Malignancies | SIR Pre-HAART (1990-1995) | SIR Early-HAART ERA 1996-2002) |
|---|---|---|
| ADCs | ||
| Kaposi Sarcoma | 22,100 | 3,640 |
| PCNSL | 5,000 | >1,020 |
| Burkitt's Lymphoma | 52 | 49 |
| DLBCL | 64 | 29.6 |
| All NHLs | 79 | 22.6 |
| Cervical Carcinoma | 4.2 | 5.3 |
| NADCs | ||
| Hodgkin Lymphoma | 8.1 | 14 |
| Anal Carcinoma | 18.3 | 33 |
| Lung Carcinoma | 2.5 | 2.2-6.6 |
| Head and Neck Carcinoma | 1.2 | 1-4 |
| Prostate Cancer | N/A | 4 |
| Hepatocellular Carcinoma | 19 | 7-35 |
| Melanoma | N/A | 3 |
| ALL NADCs | 1.8 | 1.7-2 |
ADCs (AIDS-defining cancers), DLBCL (diffuse large B cell lymphoma), EBV (Epstein-Barr virus), HPV (human papillomavirus), N/A (not available), NADCs (non-AIDS-defining cancers), NHL (non-Hodgkin lymphoma), PCNSL (primary central nervous system lymphoma), SIR (standard incidence ratio)
Prior to the emergence of HIV, KS was a rare disorder. Between 1987 and 1993, KS incidence increased 66-fold from 0.5 to 33 patients per 100,000-patient years in PLWHA [1]. During this period, NHL incidence increased by a more modest three-fold. Based the complex epidemiology and histologic interpretation of lymphomas, nine subtypes were defined by the World Health Organization as associated with HIV infection (Table 2) [24]. Among these nine, all are considered ADCs except low-grade lymphomas, extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT), and Hodgkin lymphoma (HL) [2, 21, 24]. The most common AIDS-defining NHLs are of B cell origin, present with advanced-stage disease, and follow an aggressive clinical course [25]. These AIDS-defining NHLs include PCNSL, Burkitt's lymphoma (BL), diffuse large B cell lymphoma (DLBCL), plasmablastic lymphoma, and primary effusion lymphoma (PEL) [24, 25]. In the pre-HAART era, DLBCL and PCNSL were the most common NHLs in PLWHA, presenting with an incidence of 453 and 233 cases per 100,000 patient-years, respectively [23, 25]. Between 2001 and 2007, the most common AIDS-defining NHL remained DLBCL although its incidence had decreased to 120 cases per 100,000 patient-years [23]. During this same period, and with an incidence of 32 cases per 100,000 patient-years, BL supplanted PCNSL as the second most common AIDS-defining NHL [23].
Table 2. WHO classification of lymphoid malignancies associated with HIV infection.
| Lymphoma Type | Comments |
|---|---|
| Burkitt's Lymphoma Burkitt's-like Lymphoma |
Presents with a higher CD4+ T cell count; 100% associated with cMYC translocation |
| Diffuse Large B Cell Lymphoma Centroblastic Immunoblastic (associated with PCNSL) | Centroblastic 30% EBV [28, 29] Immunoblastic 90% EBV and associated with primary CNS lymphoma [28, 29] |
| Extranodal MALT Lymphoma | Non-AIDS-defining cancer; rare |
| Peripheral T Cell Lymphoma | Rare |
| Primary Effusion/Body Cavity Lymphoma | Rare; presents in patients in the late stages of HIV; associated 100% with HHV-8 and EBV [28, 30] |
| Plasmablastic Lymphoma of the Oral Cavity | Rare; associated with HHV-8 50% and EBV 50% [29] |
| Polymorphic B cell Lymphoma (PTLD-like) | Rare |
| Hodgkin Lymphoma | Non-AIDS-defining lymphoma; may presents in patients with elevated CD4 + T cell count; EBV 80-100% [54] |
DLBCL (diffuse large B cell lymphoma); EBV (Epstein-Barr virus), HHV-8 (human herpesvirus 8), MALT (marginal zone lymphoma of mucosa-associated lymphoid tissue), PCNSL (primary central nervous system lymphoma), PTLD (post-transplant lymphoproliferative disorder), WHO (world health organization).
Together, KS and NHL accounted for 99% percent of all ADCs in the pre-HAART era [1]. From 1991-1995 to 2001-2005 when ART was introduced, the number of KS cases declined by 84% and the number of NHL cases declined by 54%. During this same interval, the incidence of all ADCs decreased by 70% [1, 3]. In the HAART era, however, ADCs continue to be a major problem. Between 2001 and 2005, more than 2,000 cases per year were diagnosed in the United States, and these malignancies currently account for 15-19% of all deaths in PLWHA [14, 16]. One of the most important risk factors for NHL and KS is immune suppression as reflected by the CD4+ T cell count [7, 26, 27]. In retrospective analyses, the SIR for NHL increased from 35.8 to 145 and for KS from 76 to 571 when the CD4+ T cell count decreased from 500 to less than 100 cells/mm3. In contrast, the incidence of BL increased from 9.6 to 30.7 per 100,000 person-years as the CD4+ T cell count increased from less than 50 to greater than 250 cells/mm3 [28-30]. This paradoxical issue is further discussed in the Etiology section below.
Non-AIDS-Defining Cancers
In the United States, NADC incidence increased greater than three-fold, from 3,193 cases to 10,059 cases when comparing the intervals 1991-1995 and 2001-2005 [3]. In resource-rich countries in the pre-HAART era, NADCs accounted for approximately 8% to 38% of all HIV malignancies [3, 31, 32]. In the HAART era, this number has increased to 50-58% [3, 13, 22, 31-34]. Depending on the malignancy, the SIR for most NADCs ranges from 2 to 35 (Table 1) [13, 23, 31-38]. The most important risk factors for NADCs are advancing age and the length of time one has been infected with HIV [7, 13, 39]. Additional important factors that impact NADC incidence are exposure to cigarette smoke and oncogenic viruses [38, 40-42]. Less clear risk factors include a low CD4+ T cell count and the use of anti-HIV therapy [7, 22, 43, 44]. In a multivariate analysis, two risk factors for NADCs emerge: a CD4+ cell count less than 200 cells/mm3 and the use of HAART [10]. However, the link between low CD4+ T cell count and NADCs is conflicting and is disease- and time-dependent. The SIR for anal cancer increases from 22 to 68 when the CD4+ T count remains less than 200 cells/mm3 for more than five years compared to two years [42]. In contrast, the SIR for HL increases from 5 to 14 as the CD4+ T cell count rises from 50 to 200 cells/mm3 [3, 35, 38]. When PLWHA are compared to recipients of solid organ transplants, both populations have similar risks for KS and NHL as well as certain NADCs including HL, anal, liver and lung cancer [43]. In the case of lung cancer, even when correcting for cigarette smoking, the incidence remains greater in PLWHA than in the general population, although no correlation between low CD4+ T cell count and lung cancer risk has been identified [40, 41].
The role of HAART as a possible contributor to malignancy has not been validated by all studies [13, 45, 46]. NADCs are prevalent among long-term surviving HIV-infected patients not requiring therapy or in developing countries without access to therapy [47]. Therefore, HAART may be a risk factor due to its ability to increase longevity rather than its direct carcinogenic or anti-carcinogenic potential. Of interest, statins (like HAART) have, through alternative modes of action, anti-inflammatory properties that reduce immune activation. In a recent retrospective analysis, they were associated with a 57% decrease in NADCs [48]. Additional studies are needed to better understand the role of chronic inflammation as a mediator of cancer risk.
The effects of both NADCs and ADCs are profound and 26-30% of PLWHA will die from these malignancies [14, 15]. In the United States, more than 4,000 new cases of cancer are diagnosed in PLWHA each year [3]. The median age of PLWHA in the United States now exceeds 50 years. Developing robust strategies to screen this group for preventable cancers will become increasingly important. Unfortunately, standard guidelines for cancer screening in this group do not exist. More research and algorithms are needed to improve cancer detection rates while also examining behaviors that can influence risk factors for cancer such as unsafe sex, heavy alcohol consumption, and cigarette smoking [49, 50].
Etiology
The etiological factors that contribute to both ADCs and NADCs are multifactorial and an entire review could be devoted to this topic alone. Thus, we describe the major factors that may be most relevant to malignancies in HIV/AIDS, including those that have been less described or previously not recognized. Further, as these factors are well-represented across the spectrum of lymphomas, we focus on AIDS-defining NHLs and HIV-associated HL as models of these factors.
PLWHA are susceptible to infection by oncogenic viruses. Kaposi sarcoma-associated herpes virus (KSHV) or human herpes virus 8 (HHV-8), Epstein Barr Virus (EBV), HPV, and hepatitis B and C viruses are implicated in various ADCs and NADCs and are more prevalent in PLWHA (Table 3) than the general population [28, 29, 51-55]. These viruses can alter mechanisms of apoptosis and cell cycle regulation, activate oncogenes, and inhibit tumor suppressor genes [56-62]. Viruses associated with ADCs and NADCs can also express micro-ribonucleic acids (miRNAs), which are small non-coding RNAs that act as negative regulators of protein synthesis by covalently binding to single-stranded mRNA [63-66]. HHV-8, HPV, and EBV together express over 40 miRNAs that play varied roles in promoting cancer [64, 66-68]. EBV-expressed miRNAs include MiR-BHRF1-1, which inhibits the tumor suppressor gene p53 and miR-BART1, which activates BCL-2, an anti-apoptotic protein [59, 66-69]. HIV, although clearly not a direct oncogenic virus, has also been implicated in inhibiting the p53 tumor suppressor gene, altering cell cycle regulation, and activating proto-oncogenes that can lead to cellular transformation [56, 62].
Table 3. Common AIDS-defining and non-AIDS-defining and tumor-associated oncogenic viruses.
| HIV-associated Malignancies | Associated Oncogenic Virus |
|---|---|
| Kaposi Sarcoma | 100% HHV-8 [61] |
| PCNSL | 100% EBV [29] |
| Burkitt's Lymphoma | 20-40% EBV [29] |
| DLBCL | Centroblastic 30% EBV [29] Immunoblastic 90% EBV [29] |
| PEL | 100% HHV-8 [30] 100% EBV [28, 30] |
| Plasmablastic [28, 29] | 50% HHV-8 50% EBV |
| Cervical | 100% HPV [61] |
| Hodgkin Lymphoma | 80-100% EBV [28, 29] |
| Anal Carcinoma | 100% HPV [55] |
| Head and Neck Carcinoma | HPV [61] EBV [61] |
| Hepatocellular Carcinoma |
HBV [61] HCV [61] |
DLBCL (diffuse large B-cell lymphoma), EBV (Epstein-Barr virus), HBV (hepatitis B virus), HCV (hepatitis C virus), HHV-8 (human herpesvirus 8), HPV (human papillomavirus), NADC (non-AIDS-defining cancer), NHL (non-Hodgkin lymphoma), PCNSL (primary central nervous system lymphoma), PEL (primary effusion lymphoma).
In terms of lymphoma, EBV and HHV-8 are the most common viruses associated with AIDS-defining NHLs and HIV-associated HL (Table 3) [28, 29, 54, 70, 71]. Forty percent of BLs and as many as 80-100% of immunoblastic B cell lymphomas, HLs, and PCNSLs are linked to EBV infection in the HIV/AIDS setting [28, 29, 54]. PEL, a rare lymphoma subtype with a peculiar tropism for involving serous cavities, is most often associated with both HHV-8 and EBV [28, 29, 54]. Chronic antigenic stimulation of B cells and macrophages induced by EBV, HIV, and HHV-8 elicits cytokine and growth factor release which promote B cell proliferation and the outgrowth of a monoclonal B cell population [71, 72]. In addition, AIDS-defining NHLs are often associated with characteristic molecular abnormalities. For BL and Burkitt's-like lymphomas this includes a translocation of the cMYC gene with the immunoglobulin heavy and light chains [73, 74]. In addition, the tumor suppressor genes p53 and BCL-6 are mutated in 30% or more of DLBCL and BL cases. Both of these genes, if mutated or overexpressed, contribute to lymphoma genesis by preventing cell cycle regulation, inhibiting B cell apoptosis (via p53 mutation), and preventing B cell terminal differentiation (via BCL-6 overexpression)[73, 74]. The extent to which overexpression of various proteins, translocation of important genes, and oncogene activation by various viruses play in this intricate process of lymphoma genesis remains an area of active investigation.
PLWHA and persons with low CD4+ cell counts are at greatest risk for DLBCL although in the HAART era the absolute CD4+ cell count at diagnosis is greater than in the pre-HAART era. In contrast, patients with BL and HL are usually diagnosed with somewhat higher CD4+ T cell counts [35, 54, 75]. CD4+ T cells modulate B cells and antibodies directed at extracellular organisms, and cell-mediated responses, which involve CD8+ T cell defense against intercellular organisms [76-81]. HIV can readily infect these CD4+ T cells and without HAART, they are destined to be depleted [76, 79]. CD4+ T cells produce cytokines that help regulate B and T cell responses [76, 79]. In addition to immune regulation, these cytokines can also influence B and T cell survival [79]. This effect could account for the disruption (in patients with chronic HIV infection) of germinal centers, the areas in the lymph nodes and lymphoid tissues where B cells proliferate, differentiate, class switch, and augment antibody production in response to infection [77, 80, 81]. The B cells that reside in the germinal centers but are not exposed to CD4+ T cell survival signals (due to elimination of CD4+ T cells by HIV), are destined to undergo premature apoptosis [76-79, 81]. BL is a cancer derived from germinal center B cells. If the germinal center is not present or disrupted (e.g., due to lack of CD4+ T cells), then BL may not develop. This might help explain the paradox of why BL incidence increases as the CD4+ T cell count rises [32].
The cancer cell associated with HL, the Hodgkin Reed Sternberg (hRS) cell, is also of B cell origin [82]. Studies of both primary HL cultures and hRS cell lines reveal that these cells secrete some of the same cytokines produced by CD4+ T cells, including interleukin-5 (IL-5), IL-10, IL-12, IL-13 [78, 83-89]. These ILs not only affect B, T, and eosinophil cell activation, proliferation, and survival but also act as trophic factors promoting hRS cell survival via the Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT) signal transduction pathway [85, 87, 88]. The hRS cell also secretes chemokines that attract CD4+ T cells to their “microenvironment,” which further augments hRS survival [72, 78, 83, 84, 86].
How oncogenic viruses, HIV, and CD4+ T cell lymphocytes interact to promote these malignancies remains an area of active investigation. As discussed above, without these signals, as is the situation in AIDS, the microenvironment may be inadequate to support the genesis of malignancies. Another explanation may be that CD4+ T cell counts may not sufficiently indicate malignancy potential at the time of cancer diagnosis, as by the time that CD4+ T cell counts drop, the cancer has been developing for some time undetected. Some studies, instead, have linked the nadir CD4+ T cell count to future pro-malignancy potential [9, 10]. Lastly, total CD4+ T cell counts (nadir or at the time of cancer diagnosis) alone may not adequately provide an indication of the potential of certain CD4+ T cell subsets in helping or regulating anti-tumor responses. In HIV/AIDS particularly, CD4+ T cells have important dual impact on both anti-HIV and anti-tumor responses. First, CD4+ T cells are important providers of CD8+ T cell help and, in their absence, long-term CD8+ T cell responses require alternative forms of co-stimulation [90-92]. Second, CD4+ T cells are a prime target and reservoir of HIV infection. A growing number of CD4+ T cell subsets have been recognized with roles ranging from regulation (inhibition) to propagation of immune responses based on their cytokine profile, transcription factor requirements, and immune regulatory functions [79, 93-103]. While the role of traditional subsets of CD4+ T cells (T helper type 1 [Th1], Th2, Th17, and regulator T [Treg] cells) has been investigated, little to no basic science or clinical information exists regarding novel subsets (Th9, Th17, Th22, natural [nTreg], inducible Treg, IL-35-producing [Tr35] and IL-35-inducible Treg [iTr35], and cytotoxic CD4+ T cells) in HIV/AIDS and/or in the context of malignancies in HIV/AIDS. In addition while total CD4+ T cell counts are used as a marker of disease progression or treatment efficacy (as CD4+ T cell counts recover with HAART), it is unknown whether all subsets recover proportionately, or whether certain subsets or proportions of subsets leave HIV/AIDS patients resistant to infection but more susceptible to malignancies.
Life style choices also clearly impact cancer risk. Cigarette smoking is common in the HIV population. Greater than 50% of PLWHA smoke compared to just 18% of the United States general population older than 18 years [104, 105]. PLWHA who smoke are at higher risk for ADCs and NADCs, including those that involve the lung, esophagus, kidney, bladder, head and neck, breast, and cervix [104, 106, 107]. The correlation between smoking and colon and hepatocellular cancer is not as strong [107]. Alcohol addiction is also more common in PLWHA. Cancer mortality is three times higher in patients who smoke than in non-smokers [108]. In addition, when assessing all causes of death, AIDS-related and non-AIDS-related mortality rates are five times higher in PLWHA who smoke compared to those who do not [136]. In the United States, rates of heavy alcohol consumption among PLWHA are twice as great as they are in the general population [106]. Alcohol consumption is also associated with cancers of the mouth, esophagus, pharynx, larynx, and liver [109].
Differences in exposure to oncogenic viruses, CD4+ T cell recovery, and lifestyle may strongly influence the incidence of certain cancers in PLWHA, but why such individuals are often diagnosed with more advanced-stage tumors than the general population requires further study [25, 41, 42]. Disparities in health care and how PLWHA access medical care, as well as the higher incidence of AIDS and cancer in minority populations, are additional important points when considering the incidence of various cancers in PLWHA [110-112].
Treatment
In the HAART era, HIV-infected patients are increasingly offered chemotherapy options for malignancies that were once restricted to non-immunosuppressed patients with cancer or cancer patients who were HIV sero-negative. Safer, more convenient, and better-tolerated HAART options, improved supportive care strategies, and incremental refinements in cancer treatments have all contributed to improvements in overall survival (OS) and a decrease in therapy-associated toxicity in select HIV-cancer groups. From the pre-HAART to the HAART era, the OS for patients with HL improved from 45% at two years to 76%-81% at five years [113-115]. Similarly, DLBCL OS improved from just eight months in the pre-HAART era to a current five-year OS of 60%-80% [116-119]. HIV-infected patients with anal carcinoma now have similar treatment-associated toxicities, including risk of colostomy, and OS compared to their non-HIV-infected counterparts [120, 121]. While the specific treatments for each cancer type are outside the scope of this review, here we focus on the interactions of HIV medications and chemotherapeutic agents and briefly assess immunotherapeutic options.
Many chemotherapeutic drugs and HIV medications are metabolized through the cytochrome p450 (CYP) enzyme system of the liver. HAART can augment or inhibit the clearance of chemotherapy agents by the up regulation or inhibition of the CYP system (Table 4) [122-126]. This can lead to either increased chemotherapy-associated toxicity or a decrease in treatment efficacy [122-124]. In addition, several HIV medications and chemotherapy agents have overlapping toxicities [123-127] (Table 5). Zidovudine can cause myelosupression in 8% of treated patients, didanosine and stavudine can cause peripheral neuropathy, and HIV protease inhibitors can cause nausea and vomiting. Nucleotide analogs can contribute to nephrotoxicity while protease inhibitors and non-nucleoside reverse transcriptase inhibitors can cause hepatotoxicity [122-124]. Such known side effects and their frequencies need to be considered when combining HAART with specific chemotherapy agents that have a high risk of cytopenias, nephrotoxicity, peripheral neuropathy, hepatotoxicity, and unwanted gastrointestinal side effects.
Table 4. Interactions between chemotherapeutics and antiretroviral agents.
| Chemotherapeutic Agents | P450 System Responsible for Metabolism | Antiretroviral Inhibitor1 | Antiretroviral Inducer2 | Associated Cancers (being treated) |
|---|---|---|---|---|
| Vinblastine Vincristine |
CYP 3A4 | Delavirdine, Ritonavir, Amprenavir, Atazanavir, Indinavir Lopinavir Nelfinavir, Saquinavir | Nivarapine Efavirenz | HL, NHL, ALL |
| Paclitaxel Docetaxol |
CYP 2C8 CYP 3A4 |
Same as above | Nivarapine Efavirenz | KS, Breast, Lung, Cervical |
| Etoposide | CYP 3A4 CYP 2E1 |
Same as above | NHL, Lung | |
| Ifosphamide cyclophosphamide | CYP 3A4 CYP 2C9 CYP 2B6 |
Efavirenz Amprenavir | Nivarapine Efavirenz | NHL, Lung, Breast, Sarcoma |
| Dacarbazine | CYP 2E1 | Ritonavir | HL |
ALL (acute lymphocytic leukemia), cytochrome P450 (CYP), HAART (highly active antiretroviral therapy), HL (Hodgkin lymphoma), KS (Kaposi Sarcoma), NHL (non-Hodgkin lymphoma),
Inhibitors increase the concentration of the active metabolite,
Inducers decrease the concentration of the active metabolite.
Table 5. Major overlapping toxicities observed with HIV medications and chemotherapy.
| Chemotherapy Agent | Adverse Events | HIV Medication/Class |
|---|---|---|
| Common with most chemotherapeutic classes. (i.e., Anthracyclines, Taxanes Vinca Alkaloids, Platinums Alkylating agents, camptothecans, etoposide, and antimetabolites [methotrexate and 5FU]) | Myelosupression | Zidovidine |
| All Taxanes All Vinca Alkaloids Oxaliplatin Bortezomib |
Neuropathy (motor and/or peripheral) | Didanosine Stauvadine |
| Cisplatin Carboplatin |
Nephrotoxicity | Tenofovir Indinavir |
| Common with most chemotherapeutic classes. | Nausea/Vomiting | Protease Inhibitors Zidovidine |
| Vinca Alcaloids | Constipation | |
| Common with most chemotherapeutic classes. (Chemotherapeutic agents that can be administered without any dose reductions in the setting of liver toxicity include cisplatin, gemcitabine, and bleomycin) [143] | Hepatotoxicity | Protease Inhibitors Nucleoside and Non-nucleoside Reverse Transcriptase Inhibitors |
| Irinotecan Topotecan Flurouracil |
Diarrhea | Nelfinovir Lopinavir |
The HIV protease inhibitor ritonavir is a particularly potent inhibitor of the CYP system. Its effect on CYP3A4 leads to diminished clearance of vinca alcaloids, taxanes, and alkylating agents [123, 124, 126]. Consequently, ritonavir is associated with increased toxicity when combined with vincristine- and even more so when combined with vinblastine-based chemotherapies (used to treat NHL and HL, respectively) [128-130]. Ritonavir should be avoided during vinblastine-based HL treatment as the rates of febrile neutropenia, neuropathy, and neutropenia are considerably elevated when these agents are combined [128-130]. Fluconazole is also associated with increased toxicity (e.g., neutropenia and neuropathy) when combined with the vinca alkaloids through a similar mechanism, as it too inhibits the CYP3A4 system [130]. Thus, care should be taken to identify chemotherapy-HAART interactions as well as HAART interactions with other medications that are used to treat or to prevent opportunistic infections or to mitigate chemotherapy side effects, including anti-emetic agents. In light of these complicated interactions, we recommend treating cancer patients in consultation with infectious disease specialists and pharmacists who are aware of potential and complicated drug-drug interactions, and if possible, with hematologist/oncologists who have special expertise in the treatment of patients with HIV and cancer.
When chemotherapy and HAART are given concomitantly during NHL treatment, CD4+ cell counts typically decline by greater than 50% [131]. These values usually return to normal at six months to one year post therapy [131]. For anal cancer patients who receive pelvic radiation, the CD4+ T cell count may fall even more severely and may not readily recover to pre-treatment values [132]. Pelvic radiation is myelosuppressive since the major source of bone marrow is also radiated. In addition, scatter of radiation may also affect the gut, which is also an important compartment for CD4+ T cells [127, 133, 134]. For HIV patients with malignancies, we recommend that Pneumocystis jiroveci prophylaxis be initiated regardless of the CD4+ T cell count at the time of chemotherapy initiation. Prophylactic medications to minimize the risk of other opportunistic infections, including thrush and herpes simplex virus are also warranted during treatment [132]. Granulocyte colony-stimulating agents to minimize the effects of chemotherapy-induced neutropenia and antibiotic prophylaxis to further reduce infectious complications are also routinely implemented during the treatment of HIV/AIDS-related lymphomas and other cancers on a risk-assessment basis [113, 116, 118, 119].
In theory, all patients with HIV/AIDS-related malignancies, for whom HAART would be prescribed in the absence of cancer, should be maintained on HAART during chemotherapy. Reasons for HAART discontinuation during treatment include concerns of drug interactions with chemotherapy and poor patient adherence because of nausea or vomiting. Due to HAART-chemotherapy interactions, investigators from the National Cancer Institute (NCI) have examined the discontinuation of HIV therapy during a short four- to six-month treatment period for AIDS-related lymphomas [116, 118]. As anticipated, the HIV viral load increased and the CD4+ T cell count declined, but once HIV therapy was reinitiated at the completion of chemotherapy, both improved over the following 6 to 12 months [116, 118]. At five years, the OS was 68% and appears comparable to results that have been reported when HIV-infected patients with AIDS-related lymphomas were treated with concurrent chemotherapy and HAART. In the absence of head-to-head comparisons, offering to continue HAART or to hold HAART until systemic chemotherapy is completed are both reasonable options [116, 118, 119].
The chimeric anti-CD20 monoclonal antibody rituximab offers substantial benefit when used with combination chemotherapy for treatment of CD20+ aggressive B cell lymphomas [135]. The role of rituximab in the treatment of AIDS-associated lymphomas has been more controversial based on findings from two studies, which linked rituximab with a significant risk of infection complications [135, 136]. The first study was a phase 3 trial of The AIDS Malignancy Consortium (AMC) comparing the chemotherapy regimen cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) with CHOP plus rituximab (CHOP-R) in the treatment of aggressive AIDS-defining lymphomas [136]. In this trial, the rituximab arm was associated with a modest benefit in efficacy but patients who received monoclonal therapy had a significantly higher incidence of treatment-related infectious deaths (14% versus 2%; p=0.027) [136]. This finding was particularly true for those patients who went into treatment with CD4+ T cell counts less than 50 cells/mm3. A second study was a retrospective analysis of three phase 2 trials of cyclophosphamide, doxorubicin, etoposide, and rituximab (CDE-R) that also showed an 8% elevation in treatment-related infectious deaths [135]. More recently, studies of CHOP-R for the treatment of AIDS-associated lymphomas demonstrated few infectious complications and a two-year OS rate of 75%. In one recent study of 52 evaluated patients, only one death was due to infection [137].
Three subsequent phase 2 studies for AIDS-associated lymphomas, one by the AMC and two by the NCI also confirmed the safety and efficacy of rituximab [116, 118, 119]. Based on these results, rituximab is now typically combined with chemotherapy, and should not be withheld for patients with CD4+ T cell counts less than 50 cells/mm3. Caution should be exercised when treating patients with severe immunosuppression however, as these patients have an elevated risk for infection. Studies by the AMC are now being undertaken to evaluate immunotherapy for HIV-associated HL. Brentuximab vedotin, is an antibody-drug conjugate which specifically binds to the hRS cell receptor, CD30, thus initiating targeted tumor death. Remarkably, non-HIV infected patients with refractory HL, who participated in a phase 1 study of Brentuximab as a single agent, 46% of patients achieved a complete response (CR) [138].
Recently other modes of immunomodulation have emerged as successful mainstream therapies for cancer. Among these, sipuleucel-T (which utilizes loading of patient dendritic cells with prostate tumor antigens fused with an immunomodulatory factor granulocyte macrophage colony-stimulating factor [GM-CSF]) and ipilimumab (which blocks T cell inhibitory surface protein CTLA-4) are FDA-approved for use in the treatment of castration-resistant prostate cancer and melanoma, respectively [139, 140]. The major direct or indirect end result of these immunotherapies and others in late-stage clinical trials (including stimulation with IL-2 and blockade of PD-1) is likely the rescue of CD8+ T cells. Based on the critical role of CD8+ T cells in both viral infection and cancer, and the dual dysfunction of CD8+ T cell responses against both HIV and malignancies, consideration should be given to the use of similar immunotherapy for treatment of malignancies in HIV/AIDS.
Conclusions
More than 30 years after cases of HIV/AIDS were finally globally recognized and more than 55 years since the first proven case of HIV infection [141], HIV/AIDS continues to be a deadly epidemic. Despite medical, social, and political interventions, the number of people living with HIV (approximately 34.0 million as of 2011), the number of people newly-infected each year with HIV (2.5 million in 2011), and the number of people dying of HIV (1.7 million people in 2011) continue to increase [5]. While medical interventions (mainly in the form of HAART) have resulted in a decrease in AIDS-defining diseases (including infections and cancers), longevity of the HIV/AIDS population has also allowed for the emergence of aging-related pathologies, including NADCs (Figure 1). However, age alone cannot explain the rise in NADCs, especially since NADCs present earlier, more often with metastases, and exhibit a more rapidly progressive course even in adequately treated, medication-compliant HIV/AIDS patients [142]. The paucity in basic science, translational, and clinical research towards an understanding of the etiology and best course of treatment for malignancies in HIV/AIDS raises the need for further investment of time, effort, and funds in these fields. Enrolling patients into clinical trials who are diagnosed with HIV/AIDS is of the utmost importance, as without structured research protocols, questions regarding treatment, pharmacology, etiology, and screening will be more difficult to answer. We also recommend a multidisciplinary approach to HIV-cancer care, including a primary care physician, infectious disease specialist, a hematologist/oncologist, and pharmacist all specializing in HIV/AIDS care (see table 6 for general treatment recommendations). Current epidemiological and etiological data strongly suggest that HIV/AIDS patients should be under increased surveillance not only for ADCs, but likewise for NADCs, even years earlier than the general population. In addition, earlier or more monitored treatment of oncogenic viruses and avoidance of cancer-associated lifestyles must be considered. The HIV/AIDS population appears to be more susceptible to almost all cancers. While great strides have been made towards increasing the longevity and survival of patients with HIV/AIDS and of patients with malignancies, new considerations must be made and new actions taken to overcome the challenge of preventing and treating malignancies in HIV/AIDS.
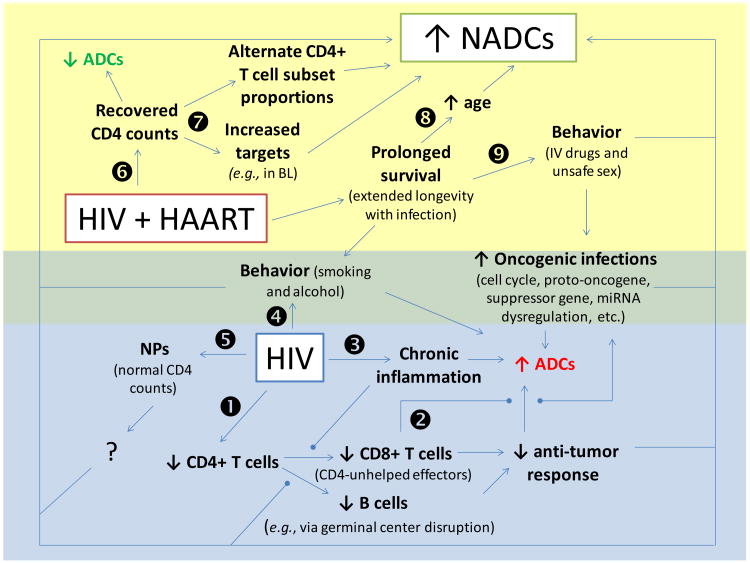
Figure 1. Summary of AIDS-defining cancers (ADC) and non-AIDS-defining cancer (NADC) etiology in the context of HIV and HAART.
- HIV infection results in the depletion of CD4 cells leading to ineffective CD4-unhelped CD8+ T effector and B cells, which results in decreased anti-tumor responses and a resultant increase in ADCs.
- Lack of CD4 help leads to a lack of responses against oncogenic infections, leading to cellular and molecular dysregulation and a resultant increase in ADCs and NADCs.
- HIV infection results in a state of chronic inflammation leading to a subsequent increase in ADCs and NADCs through mechanisms that are not sufficiently understood.
- Behaviors increased in HIV/AIDS (e.g., smoking and alcohol overconsumption) lead to an increase in ADCs and NADCs.
- Despite normal CD4 counts in non-progressors (NPs; i.e., HIV patients not requiring treatment while maintaining low/undetectable HIV viral loads for extended periods of time), NADCs continue to be increased above the level found in the non-HIV infected population.
- CD4 counts recover in HIV patients treated with HAART, and a resultant decrease in ADCs is observed, but ADCs remain increased above the level found in the non-HIV infected population.
- Despite recovery of total CD4 counts, the CD4+ T cell subset distribution (Th1, Th2, Th9, Th17, Th35, Treg cells, etc.) may not return to normal proportions, and increased numbers of CD4+ T cells allow for outgrowth of CD4-involved/dependent cancers.
- Increased survival with HAART results in extended longevity in the context of infection leading to increased age (i.e., time for NADC outgrowth).
- Increased survival with HAART results in extended time involving behaviors associated HIV/AIDS (e.g., smoking, alcohol overconsumption, IV drug use, and unsafe sex leading to oncogenic infections) and resulting in a subsequent increase in ADCs and NADCs.
Table 6. General guidelines for the treatment of HIV-associated cancers.
| Optimal treatment of patients with HIV-associated malignancies includes input from a multidisciplinary team consisting of a pharmacist, an infectious disease specialist, and a hematologist/oncologist. |
| HIV medications can inhibit the Cyt p450 system, potentially augmenting toxicities by preventing chemotherapy metabolism (see Table 4 as a guide). |
| Multiple overlapping toxicities are seen with HIV medications and chemotherapy agents (see Table 5 as a guide). |
| Supportive care medications can also augment chemotherapy toxicities (i.e., azole antifungals and vincristine). |
| Avoid the use of ritonavir when combined with vinblastine in the setting of Hodgkin's lymphoma. |
| Rituximab offers substantial benefit when used with combination chemotherapy for treatment of CD20+ aggressive B cell lymphomas. Rituximab should not be withheld for patients with CD4+ T cell counts less than 50 cells/mm3. But care should be taken, as patients with low CD4+ T cell counts are more prone to infectious complications.. |
| CD4+ T cell counts can decrease during chemotherapy and or in the setting of pelvic radiation. Thus prophylaxis during therapy for opportunistic infections is often warranted despite a normal CD4+ T cell count at therapy onset. |
| Granulocyte colony-stimulating agents and antibiotic prophylaxis are strongly encouraged to minimize the effects of chemotherapy-induced neutropenia during the treatment of AIDS-related lymphomas. |
Acknowledgments
Paul G. Rubinstein and David M. Aboulafia are members of the AIDS Malignancy Consortium (AMC) and their effort was supported in part by NIH Grant U01CA121947. We thank Adriana B. Ferreira and David N. Schwartz for their critical reading of the manuscript.
References
- 1.Eltom MA, Jemal A, Mbulaiteye SM, Devesa SS, Biggar RJ. Trends in Kaposi's sarcoma and non-Hodgkin's lymphoma incidence in the United States from 1973 through 1998. Journal of the National Cancer Institute. 2002;94:1204–1210. doi: 10.1093/jnci/94.16.1204. [DOI] [PubMed] [Google Scholar]
- 2.Schneider E, Whitmore S, Glynn KM, Dominguez K, Mitsch A, McKenna MT. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years--United States, 2008. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 2008;57:1–12. [PubMed] [Google Scholar]
- 3.Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, et al. Cancer burden in the HIV-infected population in the United States. Journal of the National Cancer Institute. 2011;103:753–762. doi: 10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. The New England journal of medicine. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Global summary of the AIDS epidemic. 2011 http://www.who.int/hiv/data/2012_epi_core_en.png.
- 6.Hall HI, Song R, Rhodes P, Prejean J, An Q, Lee LM, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. Journal of the National Cancer Institute. 2005;97:425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 8.Herida M, Mary-Krause M, Kaphan R, Cadranel J, Poizot-Martin I, Rabaud C, et al. Incidence of non-AIDS-defining cancers before and during the highly active antiretroviral therapy era in a cohort of human immunodeficiency virus-infected patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:3447–3453. doi: 10.1200/JCO.2003.01.096. [DOI] [PubMed] [Google Scholar]
- 9.Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Annals of internal medicine. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 10.Powles T, Robinson D, Stebbing J, Shamash J, Nelson M, Gazzard B, et al. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:884–890. doi: 10.1200/JCO.2008.19.6626. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann C, Horst HA, Weichenthal M, Hauschild A. Malignant melanoma and HIV infection -- aggressive course despite immune reconstitution. Onkologie. 2005;28:35–37. doi: 10.1159/000082291. [DOI] [PubMed] [Google Scholar]
- 12.Lanoy E, Dores GM, Madeleine MM, Toro JR, Fraumeni JF, Jr, Engels EA. Epidemiology of nonkeratinocytic skin cancers among persons with AIDS in the United States. AIDS. 2009;23:385–393. doi: 10.1097/QAD.0b013e3283213046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgi A, Brodine S, Wegner S, Milazzo M, Wallace MR, Spooner K, et al. Incidence and risk factors for the occurrence of non-AIDS-defining cancers among human immunodeficiency virus-infected individuals. Cancer. 2005;104:1505–1511. doi: 10.1002/cncr.21334. [DOI] [PubMed] [Google Scholar]
- 14.Gill J, M M, Lewden C. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996-2006: collaborative analysis of 13 HIV cohort studies. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50:1387–1396. doi: 10.1086/652283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewden C, May T, Rosenthal E, Burty C, Bonnet F, Costagliola D, et al. Changes in causes of death among adults infected by HIV between 2000 and 2005: The ‘Mortalite 2000 and 2005’ surveys (ANRS EN19 and Mortavic) Journal of acquired immune deficiency syndromes. 2008;48:590–598. doi: 10.1097/QAI.0b013e31817efb54. [DOI] [PubMed] [Google Scholar]
- 16.Rodger AJ, L Rebecca, Schechter Mauro, Deeks Steven, Amin Janaki, Gilson Richard, Paredes Roger, Bakowska Elzbieta, Engsig Frederik N, Phillips Andrew. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS. 2013;27:973–979. doi: 10.1097/QAD.0b013e32835cae9c. [DOI] [PubMed] [Google Scholar]
- 17.Biggar RJ, Engels EA, Ly S, Kahn A, Schymura MJ, Sackoff J, et al. Survival after cancer diagnosis in persons with AIDS. J Acquir Immune Defic Syndr. 2005;39:293–299. doi: 10.1097/01.qai.0000164033.02947.e3. [DOI] [PubMed] [Google Scholar]
- 18.Brock MV, Hooker CM, Engels EA, Moore RD, Gillison ML, Alberg AJ, et al. Delayed diagnosis and elevated mortality in an urban population with HIV and lung cancer: implications for patient care. J Acquir Immune Defic Syndr. 2006;43:47–55. doi: 10.1097/01.qai.0000232260.95288.93. [DOI] [PubMed] [Google Scholar]
- 19.Glaser SL, Clarke CA, Gulley ML, Craig FE, DiGiuseppe JA, Dorfman RF, et al. Population-based patterns of human immunodeficiency virus-related Hodgkin lymphoma in the Greater San Francisco Bay Area, 1988-1998. Cancer. 2003;98:300–309. doi: 10.1002/cncr.11459. [DOI] [PubMed] [Google Scholar]
- 20.Puoti M, Bruno R, Soriano V, Donato F, Gaeta GB, Quinzan GP, et al. Hepatocellular carcinoma in HIV-infected patients: epidemiological features, clinical presentation and outcome. AIDS. 2004;18:2285–2293. doi: 10.1097/00002030-200411190-00009. [DOI] [PubMed] [Google Scholar]
- 21.Jones ME, Swerdlow AJ. Bias in the standardized mortality ratio when using general population rates to estimate expected number of deaths. American journal of epidemiology. 1998;148:1012–1017. doi: 10.1093/oxfordjournals.aje.a009567. [DOI] [PubMed] [Google Scholar]
- 22.Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, et al. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20:1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 23.Shiels MS, Pfeiffer RM, Hall HI, Li J, Goedert JJ, Morton LM, et al. Proportions of Kaposi sarcoma, selected non-Hodgkin lymphomas, and cervical cancer in the United States occurring in persons with AIDS, 1980-2007. JAMA : the journal of the American Medical Association. 2011;305:1450–1459. doi: 10.1001/jama.2011.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swerdlow SH, Jaffe ES International Agency for Research on Cancer., World Health Organization. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: International Agency for Research on Cancer; 2008. [Google Scholar]
- 25.Cote TR, Biggar RJ, Rosenberg PS, Devesa SS, Percy C, Yellin FJ, et al. Non-Hodgkin's lymphoma among people with AIDS: incidence, presentation and public health burden. AIDS/Cancer Study Group. International journal of cancer Journal international du cancer. 1997;73:645–650. doi: 10.1002/(sici)1097-0215(19971127)73:5<645::aid-ijc6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 26.Appleby P, Beral V, Newton R, et al. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. Journal of the National Cancer Institute. 2000;92:1823–1830. doi: 10.1093/jnci/92.22.1823. [DOI] [PubMed] [Google Scholar]
- 27.Frisch M, Biggar RJ, Engels EA, Goedert JJ. Association of cancer with AIDS-related immunosuppression in adults. JAMA : the journal of the American Medical Association. 2001;285:1736–1745. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 28.Ambinder RF. Epstein-Barr virus associated lymphoproliferations in the AIDS setting. European journal of cancer. 2001;37:1209–1216. doi: 10.1016/s0959-8049(01)00123-x. [DOI] [PubMed] [Google Scholar]
- 29.Carbone A, Tirelli U, Gloghini A, Volpe R, Boiocchi M. Human immunodeficiency virus-associated systemic lymphomas may be subdivided into two main groups according to Epstein-Barr viral latent gene expression. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1993;11:1674–1681. doi: 10.1200/JCO.1993.11.9.1674. [DOI] [PubMed] [Google Scholar]
- 30.Nador RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, Sald J, et al. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood. 1996;88:645–656. [PubMed] [Google Scholar]
- 31.Bedimo R, Chen RY, Accortt NA, Raper JL, Linn C, Allison JJ, et al. Trends in AIDS-defining and non-AIDS-defining malignancies among HIV-infected patients: 1989-2002. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;39:1380–1384. doi: 10.1086/424883. [DOI] [PubMed] [Google Scholar]
- 32.Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. International journal of cancer Journal international du cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 33.Biggar RJ, Kirby KA, Atkinson J, McNeel TS, Engels E. Cancer risk in elderly persons with HIV/AIDS. Journal of acquired immune deficiency syndromes. 2004;36:861–868. doi: 10.1097/00126334-200407010-00014. [DOI] [PubMed] [Google Scholar]
- 34.Hessol NA, Pipkin S, Schwarcz S, Cress RD, Bacchetti P, Scheer S. The impact of highly active antiretroviral therapy on non-AIDS-defining cancers among adults with AIDS. American journal of epidemiology. 2007;165:1143–1153. doi: 10.1093/aje/kwm017. [DOI] [PubMed] [Google Scholar]
- 35.Biggar RJ, Jaffe ES, Goedert JJ, Chaturvedi A, Pfeiffer R, Engels EA. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108:3786–3791. doi: 10.1182/blood-2006-05-024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bini EJ, Green B, Poles MA. Screening colonoscopy for the detection of neoplastic lesions in asymptomatic HIV-infected subjects. Gut. 2009;58:1129–1134. doi: 10.1136/gut.2008.165985. [DOI] [PubMed] [Google Scholar]
- 37.Shiels MS, Goedert JJ, Moore RD, Platz EA, Engels EA. Reduced risk of prostate cancer in U.S. Men with AIDS. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:2910–2915. doi: 10.1158/1055-9965.EPI-10-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spina M, Carbone A, Gloghini A, Serraino D, Berretta M, Tirelli U. Hodgkin's Disease in Patients with HIV Infection. Advances in hematology. 2011;2011 doi: 10.1155/2011/402682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aberg JA. The changing face of HIV care: common things really are common. Annals of internal medicine. 2006;145:463–465. doi: 10.7326/0003-4819-145-6-200609190-00011. [DOI] [PubMed] [Google Scholar]
- 40.Engels EA, Brock MV, Chen J, Hooker CM, Gillison M, Moore RD. Elevated incidence of lung cancer among HIV-infected individuals. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:1383–1388. doi: 10.1200/JCO.2005.03.4413. [DOI] [PubMed] [Google Scholar]
- 41.Kirk GD, Merlo C, OD P, Mehta SH, Galai N, Vlahov D, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;45:103–110. doi: 10.1086/518606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piketty C, Selinger-Leneman H, Bouvier AM, Belot A, Mary-Krause M, Duvivier C, et al. Incidence of HIV-related anal cancer remains increased despite long-term combined antiretroviral treatment: results from the french hospital database on HIV. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:4360–4366. doi: 10.1200/JCO.2012.44.5486. [DOI] [PubMed] [Google Scholar]
- 43.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 44.Silverberg MJ, Neuhaus J, Bower M, Gey D, Hatzakis A, Henry K, et al. Risk of cancers during interrupted antiretroviral therapy in the SMART study. AIDS. 2007;21:1957–1963. doi: 10.1097/QAD.0b013e3282ed6338. [DOI] [PubMed] [Google Scholar]
- 45.Kowalska JD, Reekie J, Mocroft A, Reiss P, Ledergerber B, Gatell J, et al. Long-term exposure to combination antiretroviral therapy and risk of death from specific causes: no evidence for any previously unidentified increased risk due to antiretroviral therapy. AIDS. 2012;26:315–323. doi: 10.1097/QAD.0b013e32834e8805. [DOI] [PubMed] [Google Scholar]
- 46.Rodger AJ, Lodwick R, Schechter M, Deeks S, Amin J, Gilson R, et al. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS. 2013;27:973–979. doi: 10.1097/QAD.0b013e32835cae9c. [DOI] [PubMed] [Google Scholar]
- 47.Pantanowitz L. Overview of non-AIDS-defining malignancies in HIV infection. In: Dezube BK, editor. UpToDate. Waltham, MA: 2013. [Google Scholar]
- 48.Overton ET, Kitch D, Benson CA, Hunt PW, Stein JH, Smurzynski M, et al. Effect of statin therapy in reducing the risk of serious non-AIDS-defining events and nonaccidental death. Clin Infect Dis. 2013;56:1471–1479. doi: 10.1093/cid/cit053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sigel K, Dubrow R, Silverberg M, Crothers K, Braithwaite S, Justice A. Cancer screening in patients infected with HIV. Current HIV/AIDS reports. 2011;8:142–152. doi: 10.1007/s11904-011-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tyerman Z, Aboulafia DM. Review of screening guidelines for non-AIDS-defining malignancies: evolving issues in the era of highly active antiretroviral therapy. AIDS Rev. 2012;14:3–16. [PubMed] [Google Scholar]
- 51.Ateenyi-Agaba C. Conjunctival squamous-cell carcinoma associated with HIV infection in Kampala, Uganda. Lancet. 1995;345:695–696. doi: 10.1016/s0140-6736(95)90870-6. [DOI] [PubMed] [Google Scholar]
- 52.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273 e1261. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goedert JJ, Cote TR, Virgo P, Scoppa SM, Kingma DW, Gail MH, et al. Spectrum of AIDS-associated malignant disorders. Lancet. 1998;351:1833–1839. doi: 10.1016/s0140-6736(97)09028-4. [DOI] [PubMed] [Google Scholar]
- 54.Guech-Ongey M, Simard EP, Anderson WF, Engels EA, Bhatia K, Devesa SS, et al. AIDS-related Burkitt lymphoma in the United States: what do age and CD4 lymphocyte patterns tell us about etiology and/or biology? Blood. 2010;116:5600–5604. doi: 10.1182/blood-2010-03-275917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palefsky JM, Holly EA, Hogeboom CJ, Ralston ML, DaCosta MM, Botts R, et al. Virologic, immunologic, and clinical parameters in the incidence and progression of anal squamous intraepithelial lesions in HIV-positive and HIV-negative homosexual men. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1998;17:314–319. doi: 10.1097/00042560-199804010-00004. [DOI] [PubMed] [Google Scholar]
- 56.Amini S, Khalili K, Sawaya BE. Effect of HIV-1 Vpr on cell cycle regulators. DNA and cell biology. 2004;23:249–260. doi: 10.1089/104454904773819833. [DOI] [PubMed] [Google Scholar]
- 57.Corallini A, Sampaolesi R, Possati L, Merlin M, Bagnarelli P, Piola C, et al. Inhibition of HIV-1 Tat activity correlates with down-regulation of bcl-2 and results in reduction of angiogenesis and oncogenicity. Virology. 2002;299:1–7. doi: 10.1006/viro.2002.1459. [DOI] [PubMed] [Google Scholar]
- 58.Guo HG, Pati S, Sadowska M, Charurat M, Reitz M. Tumorigenesis by human herpesvirus 8 vGPCR is accelerated by human immunodeficiency virus type 1 Tat. Journal of virology. 2004;78:9336–9342. doi: 10.1128/JVI.78.17.9336-9342.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harrod R, Nacsa J, Van Lint C, Hansen J, Karpova T, McNally J, et al. Human immunodeficiency virus type-1 Tat/co-activator acetyltransferase interactions inhibit p53Lys-320 acetylation and p53-responsive transcription. The Journal of biological chemistry. 2003;278:12310–12318. doi: 10.1074/jbc.M211167200. [DOI] [PubMed] [Google Scholar]
- 60.Li CJ, Wang C, Friedman DJ, Pardee AB. Reciprocal modulations between p53 and Tat of human immunodeficiency virus type 1. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:5461–5464. doi: 10.1073/pnas.92.12.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McLaughlin-Drubin ME, Munger K. Viruses associated with human cancer. Biochimica et biophysica acta. 2008;1782:127–150. doi: 10.1016/j.bbadis.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wright S, Lu X, Peterlin BM. Human immunodeficiency virus type 1 tat directs transcription through attenuation sites within the mouse c-myc gene. Journal of molecular biology. 1994;243:568–573. doi: 10.1016/0022-2836(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 63.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qi P, Han JX, Lu YQ, Wang C, Bu FF. Virus-encoded microRNAs: future therapeutic targets? Cellular & molecular immunology. 2006;3:411–419. [PubMed] [Google Scholar]
- 65.Visone R, Croce CM. MiRNAs and cancer. The American journal of pathology. 2009;174:1131–1138. doi: 10.2353/ajpath.2009.080794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yeung ML, Bennasser Y, Le SY, Jeang KT. siRNA, miRNA and HIV: promises and challenges. Cell research. 2005;15:935–946. doi: 10.1038/sj.cr.7290371. [DOI] [PubMed] [Google Scholar]
- 67.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 68.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 69.Pfeffer S, Voinnet O. Viruses, microRNAs and cancer. Oncogene. 2006;25:6211–6219. doi: 10.1038/sj.onc.1209915. [DOI] [PubMed] [Google Scholar]
- 70.Besson C, Goubar A, Gabarre J, Rozenbaum W, Pialoux G, Chatelet FP, et al. Changes in AIDS-related lymphoma since the era of highly active antiretroviral therapy. Blood. 2001;98:2339–2344. doi: 10.1182/blood.v98.8.2339. [DOI] [PubMed] [Google Scholar]
- 71.Carbone A. AIDS-related non-Hodgkin's lymphomas: from pathology and molecular pathogenesis to treatment. Human pathology. 2002;33:392–404. doi: 10.1053/hupa.2002.124723. [DOI] [PubMed] [Google Scholar]
- 72.Knowles DM. Etiology and pathogenesis of AIDS-related non-Hodgkin's lymphoma. Hematology/oncology clinics of North America. 2003;17:785–820. doi: 10.1016/s0889-8588(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 73.Carbone A, Gloghini A. AIDS-related lymphomas: from pathogenesis to pathology. British journal of haematology. 2005;130:662–670. doi: 10.1111/j.1365-2141.2005.05613.x. [DOI] [PubMed] [Google Scholar]
- 74.Epeldegui M, Widney DP, Martinez-Maza O. Pathogenesis of AIDS lymphoma: role of oncogenic viruses and B cell activation-associated molecular lesions. Current opinion in oncology. 2006;18:444–448. doi: 10.1097/01.cco.0000239882.23839.e5. [DOI] [PubMed] [Google Scholar]
- 75.Barclay LR, Buskin SE, Kahle EM, Aboulafia DM. Clinical and immunologic profile of AIDS-related lymphoma in the era of highly active antiretroviral therapy. Clin Lymphoma Myeloma. 2007;7:272–279. doi: 10.3816/CLM.2007.n.002. [DOI] [PubMed] [Google Scholar]
- 76.Brenchley JM, Hill BJ, Ambrozak DR, Price DA, Guenaga FJ, Casazza JP, et al. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. Journal of virology. 2004;78:1160–1168. doi: 10.1128/JVI.78.3.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Levesque MC, Moody MA, Hwang KK, Marshall DJ, Whitesides JF, Amos JD, et al. Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS medicine. 2009;6:e1000107. doi: 10.1371/journal.pmed.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.MacLennan IC. Germinal centers. Annual review of immunology. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 79.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annual review of immunology. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 80.Pantaleo G, Graziosi C, Butini L, Pizzo PA, Schnittman SM, Kotler DP, et al. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:9838–9842. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thorbecke GJ, Amin AR, Tsiagbe VK. Biology of germinal centers in lymphoid tissue. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1994;8:832–840. doi: 10.1096/fasebj.8.11.8070632. [DOI] [PubMed] [Google Scholar]
- 82.Kuppers R, Rajewsky K, Zhao M, Simons G, Laumann R, Fischer R, et al. Hodgkin disease: Hodgkin and Reed-Sternberg cells picked from histological sections show clonal immunoglobulin gene rearrangements and appear to be derived from B cells at various stages of development. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:10962–10966. doi: 10.1073/pnas.91.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buri C, Korner M, Scharli P, Cefai D, Uguccioni M, Mueller C, et al. CC chemokines and the receptors CCR3 and CCR5 are differentially expressed in the nonneoplastic leukocytic infiltrates of Hodgkin disease. Blood. 2001;97:1543–1548. doi: 10.1182/blood.v97.6.1543. [DOI] [PubMed] [Google Scholar]
- 84.Kapp U, Yeh WC, Patterson B, Elia AJ, Kagi D, Ho A, et al. Interleukin 13 is secreted by and stimulates the growth of Hodgkin and Reed-Sternberg cells. The Journal of experimental medicine. 1999;189:1939–1946. doi: 10.1084/jem.189.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Klein S, Jucker M, Diehl V, Tesch H. Production of multiple cytokines by Hodgkin's disease derived cell lines. Hematological oncology. 1992;10:319–329. doi: 10.1002/hon.2900100605. [DOI] [PubMed] [Google Scholar]
- 86.Skinnider BF, Elia AJ, Gascoyne RD, Patterson B, Trumper L, Kapp U, et al. Signal transducer and activator of transcription 6 is frequently activated in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood. 2002;99:618–626. doi: 10.1182/blood.v99.2.618. [DOI] [PubMed] [Google Scholar]
- 87.Skinnider BF, Mak TW. The role of cytokines in classical Hodgkin lymphoma. Blood. 2002;99:4283–4297. doi: 10.1182/blood-2002-01-0099. [DOI] [PubMed] [Google Scholar]
- 88.Teruya-Feldstein J, Jaffe ES, Burd PR, Kingma DW, Setsuda JE, Tosato G. Differential chemokine expression in tissues involved by Hodgkin's disease: direct correlation of eotaxin expression and tissue eosinophilia. Blood. 1999;93:2463–2470. [PubMed] [Google Scholar]
- 89.van den Berg A, Visser L, Poppema S. High expression of the CC chemokine TARC in Reed-Sternberg cells. A possible explanation for the characteristic T-cell infiltratein Hodgkin's lymphoma. The American journal of pathology. 1999;154:1685–1691. doi: 10.1016/S0002-9440(10)65424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 91.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zloza A, Kohlhapp FJ, Lyons GE, Schenkel JM, Moore TV, Lacek AT, et al. NKG2D signaling on CD8(+) T cells represses T-bet and rescues CD4-unhelped CD8(+) T cell memory recall but not effector responses. Nat Med. 2012;18:422–428. doi: 10.1038/nm.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, et al. Characterization of CD4(+) CTLs ex vivo. J Immunol. 2002;168:5954–5958. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- 94.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 95.Chaturvedi V, Collison LW, Guy CS, Workman CJ, Vignali DA. Cutting edge: Human regulatory T cells require IL-35 to mediate suppression and infectious tolerance. J Immunol. 2011;186:6661–6666. doi: 10.4049/jimmunol.1100315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 98.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 100.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–257. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 102.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 103.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 104.Burns DN, Hillman D, Neaton JD, Sherer R, Mitchell T, Capps L, et al. Cigarette smoking, bacterial pneumonia, and other clinical outcomes in HIV-1 infection. Terry Beirn Community Programs for Clinical Research on AIDS. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1996;13:374–383. doi: 10.1097/00042560-199612010-00012. [DOI] [PubMed] [Google Scholar]
- 105.Niaura R, Shadel WG, Morrow K, Tashima K, Flanigan T, Abrams DB. Human immunodeficiency virus infection, AIDS, and smoking cessation: the time is now. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2000;31:808–812. doi: 10.1086/314048. [DOI] [PubMed] [Google Scholar]
- 106.Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, et al. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. Journal of studies on alcohol. 2002;63:179–186. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- 107.Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung cancer. 2004;45 Suppl 2:S3–9. doi: 10.1016/j.lungcan.2004.07.998. [DOI] [PubMed] [Google Scholar]
- 108.Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, et al. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis. 2013;56:727–734. doi: 10.1093/cid/cis933. [DOI] [PubMed] [Google Scholar]
- 109.Thun MJ, Peto R, Lopez AD, Monaco JH, Henley SJ, Heath CW, Jr, et al. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. The New England journal of medicine. 1997;337:1705–1714. doi: 10.1056/NEJM199712113372401. [DOI] [PubMed] [Google Scholar]
- 110.Gebo KA, Fleishman JA, Conviser R, Reilly ED, Korthuis PT, Moore RD, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38:96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 111.Hardy D, Xia R, Liu CC, Cormier JN, Nurgalieva Z, Du XL. Racial disparities and survival for nonsmall-cell lung cancer in a large cohort of black and white elderly patients. Cancer. 2009;115:4807–4818. doi: 10.1002/cncr.24521. [DOI] [PubMed] [Google Scholar]
- 112.Centers for Disease Control and Prevention. HIV Surveillance Report. 2010;20 http://www.cdc.gov/hiv/topics/surveillance/resources/reports. [Google Scholar]
- 113.Hentrich M, Berger M, Wyen C, Siehl J, Rockstroh JK, Muller M, et al. Stage-adapted treatment of HIV-associated Hodgkin lymphoma: results of a prospective multicenter study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:4117–4123. doi: 10.1200/JCO.2012.41.8137. [DOI] [PubMed] [Google Scholar]
- 114.Levine AM, Li P, Cheung T, Tulpule A, Von Roenn J, Nathwani BN, et al. Chemotherapy consisting of doxorubicin, bleomycin, vinblastine, and dacarbazine with granulocyte-colony-stimulating factor in HIV-infected patients with newly diagnosed Hodgkin's disease: a prospective, multi-institutional AIDS clinical trials group study (ACTG 149) Journal of acquired immune deficiency syndromes. 2000;24:444–450. doi: 10.1097/00126334-200008150-00009. [DOI] [PubMed] [Google Scholar]
- 115.Xicoy B, Ribera JM, Miralles P, Berenguer J, Rubio R, Mahillo B, et al. Results of treatment with doxorubicin, bleomycin, vinblastine and dacarbazine and highly active antiretroviral therapy in advanced stage, human immunodeficiency virus-related Hodgkin's lymphoma. Haematologica. 2007;92:191–198. doi: 10.3324/haematol.10479. [DOI] [PubMed] [Google Scholar]
- 116.Dunleavy K, Little RF, Pittaluga S, Grant N, Wayne AS, Carrasquillo JA, et al. The role of tumor histogenesis, FDG-PET, and short-course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood. 2010;115:3017–3024. doi: 10.1182/blood-2009-11-253039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lim ST, Karim R, Nathwani BN, Tulpule A, Espina B, Levine AM. AIDS-related Burkitt's lymphoma versus diffuse large-cell lymphoma in the pre-highly active antiretroviral therapy (HAART) and HAART eras: significant differences in survival with standard chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:4430–4438. doi: 10.1200/JCO.2005.11.973. [DOI] [PubMed] [Google Scholar]
- 118.Little RF, Pittaluga S, Grant N, Steinberg SM, Kavlick MF, Mitsuya H, et al. Highly effective treatment of acquired immunodeficiency syndrome-related lymphoma with dose-adjusted EPOCH: impact of antiretroviral therapy suspension and tumor biology. Blood. 2003;101:4653–4659. doi: 10.1182/blood-2002-11-3589. [DOI] [PubMed] [Google Scholar]
- 119.Sparano JA, Lee JY, Kaplan LD, Levine AM, Ramos JC, Ambinder RF, et al. Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV-associated B-cell non-Hodgkin lymphoma. Blood. 2010;115:3008–3016. doi: 10.1182/blood-2009-08-231613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kachnic LA, Tsai HK, Coen JJ, Blaszkowsky LS, Hartshorn K, Kwak EL, et al. Dose-painted intensity-modulated radiation therapy for anal cancer: a multi-institutional report of acute toxicity and response to therapy. International journal of radiation oncology, biology, physics. 2012;82:153–158. doi: 10.1016/j.ijrobp.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 121.Oehler-Janne C, Huguet F, Provencher S, Seifert B, Negretti L, Riener MO, et al. HIV-specific differences in outcome of squamous cell carcinoma of the anal canal: a multicentric cohort study of HIV-positive patients receiving highly active antiretroviral therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:2550–2557. doi: 10.1200/JCO.2007.15.2348. [DOI] [PubMed] [Google Scholar]
- 122.Antoniou T, Tseng AL. Interactions between antiretrovirals and antineoplastic drug therapy. Clinical pharmacokinetics. 2005;44:111–145. doi: 10.2165/00003088-200544020-00001. [DOI] [PubMed] [Google Scholar]
- 123.Deeken JF, Pantanowitz L, Dezube BJ. Targeted therapies to treat non-AIDS-defining cancers in patients with HIV on HAART therapy: treatment considerations and research outlook. Current opinion in oncology. 2009;21:445–454. doi: 10.1097/CCO.0b013e32832f3e04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rudek MA, Flexner C, Ambinder RF. Use of antineoplastic agents in patients with cancer who have HIV/AIDS. The lancet oncology. 2011;12:905–912. doi: 10.1016/S1470-2045(11)70056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schwartz JD, Howard W, Scadden DT. Potential interaction of antiretroviral therapy with paclitaxel in patients with AIDS-related Kaposi's sarcoma. AIDS. 1999;13:283–284. doi: 10.1097/00002030-199902040-00019. [DOI] [PubMed] [Google Scholar]
- 126.Walubo A. The role of cytochrome P450 in antiretroviral drug interactions. Expert opinion on drug metabolism & toxicology. 2007;3:583–598. doi: 10.1517/17425225.3.4.583. [DOI] [PubMed] [Google Scholar]
- 127.Ling B, Mohan M, Lackner AA, Green LC, Marx PA, Doyle LA, et al. The large intestine as a major reservoir for simian immunodeficiency virus in macaques with long-term, nonprogressing infection. J Infect Dis. 2010;202:1846–1854. doi: 10.1086/657413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rubinstein PG, Braik T, Jain S, et al. American Society of Hematology. Orlando, Florida: 2010. Ritonavir based HIV therapy is associated with severe neurotoxicity when combined with ABVD in the treatment of Hodgkin lymphoma but not with non-Hodgkin lymphoma therapy. A Retrospective Study. Abstract # 2807. [Google Scholar]
- 129.Sandhu S, Gupta S, Messinger M, et al. Suspension of Ritonavir and Zidovadine Prevents Adverse Events During the Treatment of HIV Associated Hodgkin Lymphoma. J Clin Oncol. 2012;(suppl 1) abstr 8085. [Google Scholar]
- 130.Ezzat HM, Cheung MC, Hicks LK, Boro J, Montaner JS, Lima VD, et al. Incidence, predictors and significance of severe toxicity in patients with human immunodeficiency virus-associated Hodgkin lymphoma. Leukemia & lymphoma. 2012;53:2390–2396. doi: 10.3109/10428194.2012.697560. [DOI] [PubMed] [Google Scholar]
- 131.Powles T, Imami N, Nelson M, Gazzard BG, Bower M. Effects of combination chemotherapy and highly active antiretroviral therapy on immune parameters in HIV-1 associated lymphoma. AIDS. 2002;16:531–536. doi: 10.1097/00002030-200203080-00003. [DOI] [PubMed] [Google Scholar]
- 132.Alfa-Wali M, Allen-Mersh T, Antoniou A, Tait D, Newsom-Davis T, Gazzard B, et al. Chemoradiotherapy for anal cancer in HIV patients causes prolonged CD4 cell count suppression. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23:141–147. doi: 10.1093/annonc/mdr050. [DOI] [PubMed] [Google Scholar]
- 133.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 134.Chun TW, Engel D, Mizell SB, Hallahan CW, Fischette M, Park S, et al. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5:651–655. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 135.Spina M, Jaeger U, Sparano JA, Talamini R, Simonelli C, Michieli M, et al. Rituximab plus infusional cyclophosphamide, doxorubicin, and etoposide in HIV-associated non-Hodgkin lymphoma: pooled results from 3 phase 2 trials. Blood. 2005;105:1891–1897. doi: 10.1182/blood-2004-08-3300. [DOI] [PubMed] [Google Scholar]
- 136.Kaplan LD, Lee JY, Ambinder RF, Sparano JA, Cesarman E, Chadburn A, et al. Rituximab does not improve clinical outcome in a randomized phase 3 trial of CHOP with or without rituximab in patients with HIV-associated non-Hodgkin lymphoma: AIDS-Malignancies Consortium Trial 010. Blood. 2005;106:1538–1543. doi: 10.1182/blood-2005-04-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boue F, Gabarre J, Gisselbrecht C, Reynes J, Cheret A, Bonnet F, et al. Phase II trial of CHOP plus rituximab in patients with HIV-associated non-Hodgkin's lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:4123–4128. doi: 10.1200/JCO.2005.05.4684. [DOI] [PubMed] [Google Scholar]
- 138.Rothe A, Sasse S, Goergen H, Eichenauer DA, Lohri A, Jager U, et al. Brentuximab vedotin for relapsed or refractory CD30+ hematologic malignancies: the German Hodgkin Study Group experience. Blood. 2012;120:1470–1472. doi: 10.1182/blood-2012-05-430918. [DOI] [PubMed] [Google Scholar]
- 139.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 141.Zhu T, Korber BT, Nahmias AJ, Hooper E, Sharp PM, Ho DD. An African HIV-1 sequence from 1959 and implications for the origin of the epidemic. Nature. 1998;391:594–597. doi: 10.1038/35400. [DOI] [PubMed] [Google Scholar]
- 142.Engels EA. Non-AIDS-defining malignancies in HIV-infected persons: etiologic puzzles, epidemiologic perils, prevention opportunities. AIDS. 2009;23:875–885. doi: 10.1097/QAD.0b013e328329216a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.King PD, Perry MC. Hepatotoxicity of chemotherapy. Oncologist. 2001;6:162–176. doi: 10.1634/theoncologist.6-2-162. [DOI] [PubMed] [Google Scholar]