Abstract
Many psychiatric illnesses are associated with early mortality and with an increased risk of developing physical diseases that are more typically seen in the elderly. Moreover, certain psychiatric illnesses may be associated with accelerated cellular aging, evidenced by shortened leukocyte telomere length (LTL), which could underlie this association. Shortened LTL reflects a cell’s mitotic history and cumulative exposure to inflammation and oxidation as well as the availability of telomerase, a telomere-lengthening enzyme. Critically short telomeres can cause cells to undergo senescence, apoptosis or genomic instability, and shorter LTL correlates with poorer health and predicts mortality. Emerging data suggest that LTL may be reduced in certain psychiatric illnesses, perhaps in proportion to exposure to the psychiatric illnesses, although conflicting data exist. Telomerase has been less well characterized in psychiatric illnesses, but a role in depression and in antidepressant and neurotrophic effects has been suggested by preclinical and clinical studies. In this article, studies on LTL and telomerase activity in psychiatric illnesses are critically reviewed, potential mediators are discussed, and future directions are suggested. A deeper understanding of cellular aging in psychiatric illnesses could lead to re-conceptualizing them as systemic illnesses with manifestations inside and outside the brain and could identify new treatment targets.
Keywords: aging, telomeres, telomerase, inflammation, oxidative stress, stress, early life adversity, depression, major depressive disorder, bipolar affective disorder, manic-depression, post-traumatic stress disorder, anxiety, schizophrenia, psychosis, disease, mortality, antidepressant, neurotrophic, leukocytes
1. Introduction
Many psychiatric disorders such as major depressive disorder (MDD), bipolar disorder (BD), post-traumatic stress disorder (PTSD) and schizophrenia, are associated with an increased risk of serious medical illnesses (O'Donovan et al., 2011a; Penninx et al., 2013; Viron and Stern, 2010) and premature mortality from natural causes (Viron and Stern, 2010). Although lifestyle and socioeconomic factors play a role, the psychiatric condition itself may be an independent risk factor (Viron and Stern, 2010). The particular medical illnesses that are more frequent in these psychiatric conditions are those that are more commonly seen with advanced age, e.g., cardiovascular disease (CVD), stroke, dementia, cancer, obesity, type II diabetes mellitus, osteoporosis (Evans et al., 2005). This raises the possibility that certain psychiatric illnesses are associated with accelerated biological aging at the organism or even cellular level. Whereas chronological age is measured in calendar units, biological age is defined physiologically and is more closely associated with disease processes. “Accelerated biological aging” occurs when biological age outpaces chronological age.
An important aspect of biological aging, and the one primarily focused on in this review, is aging at the cellular level. An emerging marker of cellular aging is telomere length (TL), often measured in leukocytes (as LTL) or peripheral blood mononuclear cells (PBMCs) (Fig. 1). Telomeres are DNA-protein complexes that cap the chromosomal DNA ends, protecting chromosomes from damage. Telomeres shorten with repeated cell divisions in somatic cells due to incomplete replication of the telomere ends (Fig. 1), replication- and nuclease-associated telomeric DNA damage, and/or chronic exposure to oxidation, certain cytotoxins or inflammation and possibly chronic exposure to the stress hormones, cortisol and catecholamines (Wolkowitz et al., 2011b). When telomeres reach a critically short length, cells undergo replicative senescence or can become genomically unstable. Senescent cells malfunction in cell-specific ways. For example, the tumor suppressor protein, p53, may be activated, which inhibits oxidative defense mechanisms (such as peroxisome proliferator-activated receptor-gamma coactivator [PGC] 1-α and β, promoting mitochondrial damage and senescence or apoptosis (Sahin et al., 2011) (Fig. 2). In addition, failure of checkpoints in pre-cancerous cells with critically short telomeres can lead to genomic instability through DNA end-to-end fusions and can promote cancer progression (Maser and DePinho, 2002). Rare Mendelian human mutations compromising telomere maintenance or protection genes cause early death and diseases involving a wide variety of tissues and organ systems (Armanios and Blackburn, 2012).
Fig. 1. Telomeres and Telomerase.
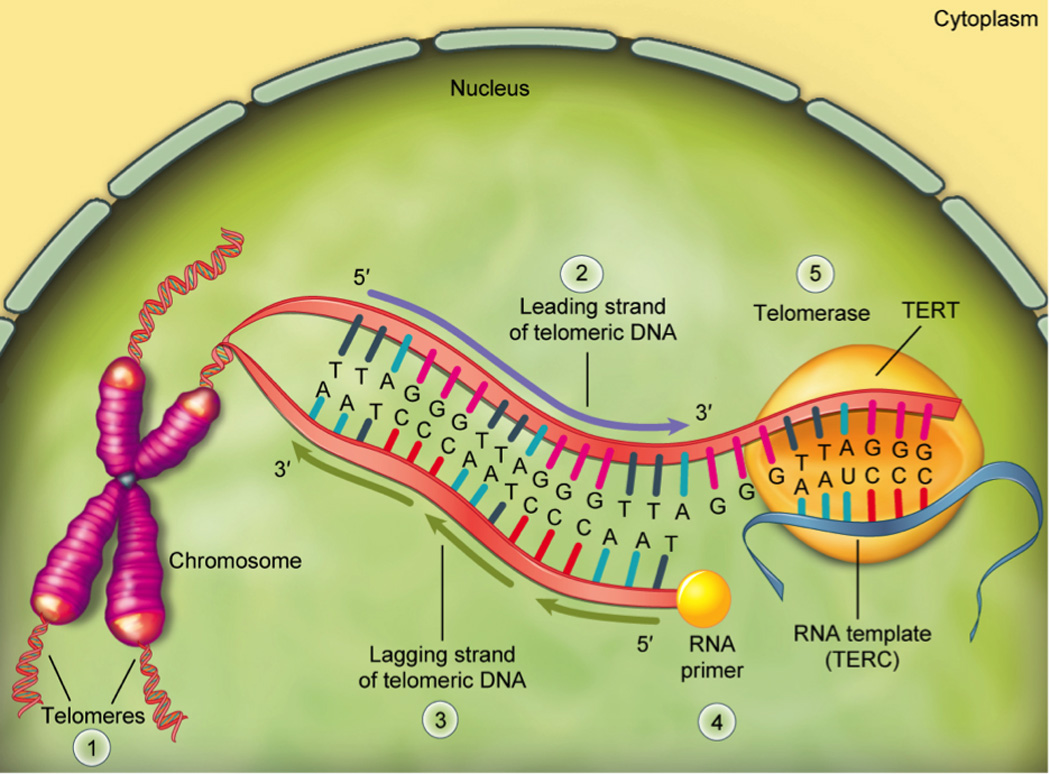
Telomeres [1] are protective caps at the ends of linear DNA strands. In humans, telomeres are comprised of multiple non-coding repeats of the nucleotide sequence, TTAGGG, and at birth, human telomeres are approximately 10,000 nucleotides long (Okuda et al., 2002). Telomeres lose approximately 50–100 nucleotides per DNA replication cycle (unless acted upon by telomerase) due to the so-called “end-replication problem” and can lose even more due to oxidative damage. The end replication problem arises during DNA replication or extension because DNA polymerase can only synthesize DNA in one direction (5’ → 3’). On the 5’ → 3’ leading strand [2], this route is continuous, but on the lagging strand [3], it is discontinuous, synthesized in fragments that require a RNA primer molecule [4] to provide a 5’ initiation point. As each fragment on the lagging strand (called “Okazaki fragments”) is completed, the RNA primer translocates to initiate the synthesis of additional fragments. Since the RNA primer must always attach prior to the synthesis of the lagging strand fragments, and since the RNA primer must base pair to complementary nucleotides on the leading strand, the 5’ end of lagging strand will always be shorter than the 3’ end of the leading strand, and thus is incompletely replicated. Shortened telomeres can be rebuilt by telomerase [5], which is comprised of the telomerase reverse transcriptase (TERT) enzyme and a telomerase RNA component (TERC) that serves as a template for new complementary telomeric DNA construction along the leading strand. As telomerase advances along the leading telomeric DNA strand, new nucleotides are added to it, providing additional room for extension of the lagging strand (Chakhparonian and Wellinger, 2003).
Fig. 2. Critical Shortening of Telomeres Can Lead to Apoptosis, Cell Cycle Arrest or Genomic Instability.
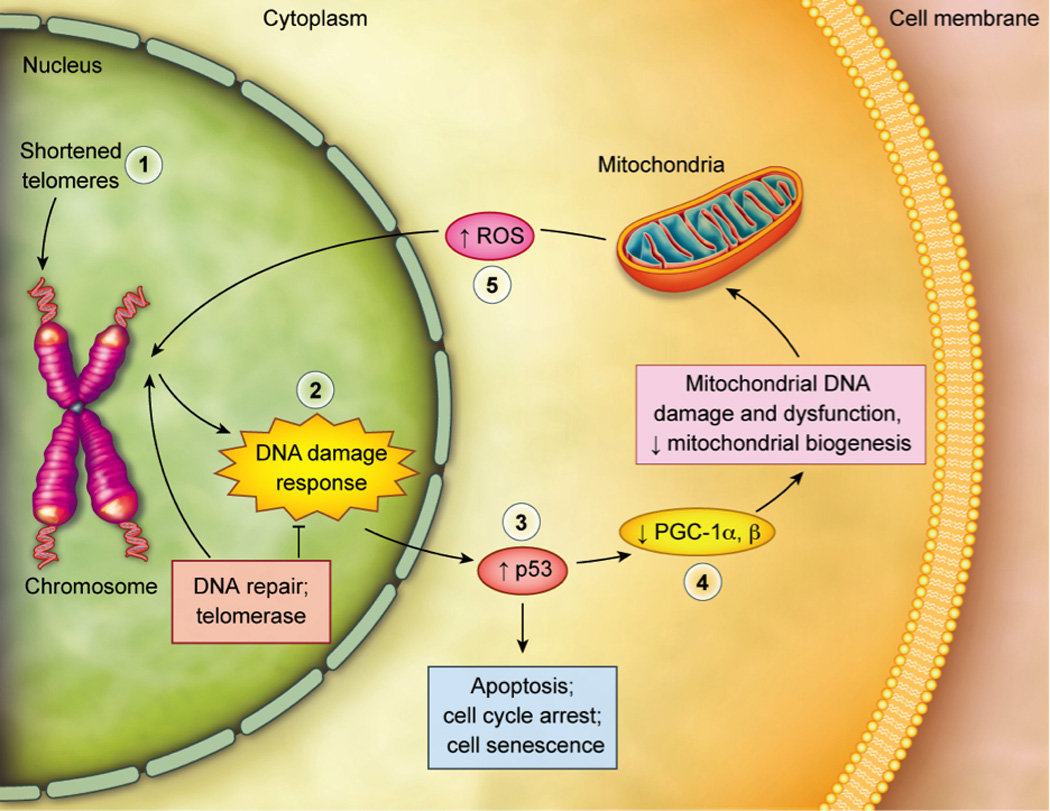
When telomere length is sufficiently shortened [1] or when telomere integrity is sufficiently challenged, classic DNA damage responses (DDR’s) [2] are initiated. A major effector of the DDR is the tumor suppressor protein p53 [3], which is activated upon telomere damage. This can lead to cell cycle arrest (“replicative senescence”), cellular senescence and apoptosis; this is most likely to affect cells turning over rapidly, such as blood cells (Sahin et al., 2011). Cellular death and senescence can give rise to stem cell dysfunction, degenerative diseases and tissue death. Were it not for p53 activation, telomere-damaged cells could survive, and their genomic instability could give rise to cancerous cells. Activation of p53 can also damage cells turning over slowly, such as those in heart and brain, by directly decreasing the expression of peroxisome proliferator-activated receptor gamma, coactivator-1 α and β (PGC-1α and PGC-1β) [4] the master regulators of mitochondrial function and biogenesis (Sahin et al., 2011). Such effects on mitochondrial number and function can also decrease cellular viability by decreasing cellular energy production and by releasing excessive amounts of free radicals such as reactive oxygen species (ROS) [5], which further damage telomeres and other cellular components.
Figure adapted from Kelly et al. (Kelly, 2011), commentary on Sahin et al. (Sahin et al., 2011).
Telomeres are dynamic structures, and LTL is in part genetically programmed, with heritability estimates ranging from 0.36–0.84 (Aviv, 2012). In a recent meta-analysis, TL heritability was estimated to be 0.7 (Broer et al., 2013). Telomeres are also subject to epigenetic influences and to modifications acquired over the lifespan (Aviv, 2012). Leukocyte telomere length generally decreases progressively over the lifespan, with estimates of average attrition rate ranging between 14 to 103 base pairs (bp) per year (weighted mean of 21.9 bp per year) in cross-sectional studies, and between 32.2 to 45.5 bp per year (weighted mean of 40.7 bp per year) in longitudinal studies (Muezzinler et al., 2013). Some individuals maintain and may even lengthen average LTL for some periods (Epel, 2012; Muezzinler et al., 2013). The reasons for this are unknown, but relatively long telomeres tend to shorten over time, and relatively short telomeres tend to lengthen over time, possibly due to the preferential recruitment of reparative mechanisms such as telomerase activity (TA) (Epel, 2012).
Short LTL in humans has been associated with serious medical illnesses with some consistency according to metaanalyses, including cardiovascular disease, diabetes, and cancer (D'Mello et al., 2014; Weischer et al., 2012 Willeit et al, 2014; Wentzensen et al, 2011). There are some studies linking shorter LTL with premature mortality (Cawthon et al., 2003; Fitzpatrick et al., 2011; Weischer et al., 2012) and a reduction in years of healthy living (Njajou et al., 2009). While most studies have noted such patterns, there are also negative reports. For example, Svensson et al. found no association between LTL and all-cause mortality or mortality due to cancer or CVD in a cohort of elderly men (Svensson et al., 2014). Epidemiological stiudies in elderly populations must be interpreted cautiously, however, due to the potential for selective dropout of the more biologically aged individuals due to earlier mortality. In several studies, not only did baseline LTL correlate with medical illnesses cross-sectionally but predicted the subsequent development of cancers, CVD, diabetes or mortality (Cawthon et al., 2003; Willeit et al 2010; Zhao et al., 2014). Also, prospective shortening of LTL over relatively short periods of time (2.5 years) has predicted long-term (12 year) cardiovascular mortality in men in one study (Epel et al., 2009a), although not all studies have found this (Weischer et al., 2014).
It is unknown whether the shortened telomeres are causally involved in these illnesses, or whether they merely reflect the underlying disease process (Masi et al., 2012), or a combination of both. A recent genome-wide meta-analysis of over 37,000 individuals identified several loci associated with mean LTL and containing genes associated with telomere biology, which, combined, showed a strong linkage with coronary artery disease (CAD) (Codd et al., 2013); CAD risk increased 21% for every standard deviation decrease in LTL, supporting a direct relationship between TL variation and certain age-related diseases like CAD (Codd et al., 2010). Because this large meta-analysis showed that the risk of cardiovascular disease is influenced by common variations in a defined set of genes known molecularly to act specifically in telomere maintenance and protection (Codd et al., 2013), this finding supports a causal role for impaired telomere maintenance in partially contributing to cardiovascular disease. Apart from this population-based genetic study, it can be difficult to determine causality, since many of the factors that shorten telomeres likely play an independent role in disease pathophysiology. For example, LTL can be conceptualized as a “canary in the coal mine” (Effros, 2009) by providing an index of the cell’s cumulative mitotic history (for example, immune cell divisions in response to pathogens), and of its cumulative exposure to cytotoxic environments (e.g., chronic inflammation and oxidation). On the other hand, there may be a more direct linkage between short telomeres and disease onset. Since accelerated cell death due to telomere shortening can deplete populations of dividing stem cells and progenitor cells (e.g., hematopoietic stem cells, endothelial progenitor cells and neural stem cells/ neural progenitor cells) (Nalapareddy et al., 2008), cellular replacement and repair processes can become limited. In addition, immune cells that have become senescent due to critical telomere shortening (e.g., CD8+CD28− T lymphocytes) hyper-secrete pro-inflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α (Effros, 2009), which can lead to a vicious cycle of further inflammation, oxidative stress and telomere shortening.
1.1. Caveats in interpreting LTL in psychiatric illnesses
Important caveats must be considered in interpreting LTL (Aviv et al., 2006; Epel, 2012), e.g., (a) distinguishing between telomere shortening in the individual leukocyte vs. “apparent” telomere shortening when examining average LTL, due to a re-distribution of leukocyte cell types having different telomere lengths, such as naïve vs. memory T cells; (b) the relationship of LTL to TL in other tissues has not been well established, and TL varies by tissue (although TL is generally positively correlated across certain, but not all, tissues within individuals) (Daniali et al., 2013; Dlouha et al., 2014; Friedrich et al., 2000; Gadalla et al., 2010; Lukens et al., 2009; Mitchell et al., 2014; Nakamura et al., 2002; Takubo et al., 2002; Takubo et al., 2010; Thomas et al., 2008); (c) different results may derive from different DNA extraction and assay methods and different laboratories (Aubert et al., 2012; Aviv et al., 2011; Cunningham et al., 2013; Montpetit et al., 2014; Nieratschker et al., 2013); and (d) even slight DNA degradation can yield spurious TL measuements (Dlouha et al., 2014). Further, many subject-level variables, such as age, sex, genetic polymorphisms, “resiliency,” education, history of early life adversities, parental responsiveness, socioeconomic status, health behaviors, diet, and latent or active viral infections (e.g., cytomegalovirus or herpes virus) may affect LTL, independent of the disease process being studied (Adler et al., 2013; Asok et al., 2013; Aviv et al., 2011; Effros, 2011; Eitan et al., 2014; Epel, 2012; Epel, 2009; Gardner et al., 2014; Gutierrez-Rodrigues et al., 2014; Jacobs et al., 2013; Lung et al., 2005; Nieratschker et al., 2013; Price et al., 2012; Puterman et al., 2013; Puterman et al., 2010; Rizzo et al., 2013; Spyridopoulos et al., 2009; Wikgren et al., 2012a).
Other potentially important confounds in interpreting LTL in psychiatric illnessess are psychiatric and medical comorbidities. Many psychiatric diagnoses has a high comorbidity with secondary psychiatric illnessess such as substance abuse, various anxiety disorders, and PTSD (Kessler et al., 1994; Lamers et al., 2011). For example MDD has a 50% comorbidity with anxiety disorders (Fava et al., 1997), making it difficult to ascribe telomere effects to single diagnosis. Moreover, several preliminary reports suggest that alcohol and certain types of substance abuse may be associated with altered LTL (Pavanello et al., 2011; Yang et al., 2013). Due to the high commorbidty between alcohol and substance abuse and major psychiatric illnessess this may also confound or complicate the interpretation of LTL in psychiatric lllnessess.
In addition to psychiatric and substance-related comorbidities, many serious comorbid medical illnesses may also be associated with decreased LTL (D'Mello et al., 2014; Weischer et al., 2012 Willeit et al, 2014; Wentzensen et al, 2011), and presumably this is independent of the effect of the psychiatric diagnosis. Among the studies reviewed here, most excluded serious or uncontrolled medical illnesses, although several either did not, or else specifically focused on medically ill populations, making it is difficult to tease apart the role of concurrent medical illnesses in predicting LTL. Similarly, a number of medications, both psychiatric (discussed below) and non-psychiatric, may have significant effects on LTL, especially if these medications are not matched across groups or statistically controlled for. For example, there is preliminary evidence that statins (Olivieri et al., 2012), certain hormones (Lin et al., 2011), and caffeine (Romano et al., 2013) may impact LTL.
1.2 Summary
In summary, LTL is an index and predictor of physical health and of processes associated with biological aging. To the extent leukocyte telomeres are relatively short in psychiatric illnesses, shortening of LTL may help explain the excess medical morbidity associated with serious psychiatric illnesses.
2. Telomere Length In Psychiatric Illnesses
2.1. MedLine Search Strategy
In the following sections, we review the literature on LTL and PBMC TA in major psychiatric illnesses, but we direct the reader to other papers for discussion of cell aging in chronic psychological stress (Kiecolt-Glaser et al., 2011; Schutte and Malouff, 2014a; Starkweather et al., 2014) and adverse childhood experiences (Moffitt, 2012; Price et al., 2013; Shalev et al., 2013; Starkweather et al., 2014; Tyrka et al., 2010), both of which are common in individuals with psychatric illness and may confound data interpretation. Our review strategy started with MedLine searches (through January, 2015) for articles containing the key words “telomeres” or “telomerase” along with any of the following key words: “depression,” “depressive”, “bipolar,” mania”, “manic”, anxiety”, “obsessive”, ”compulsive”, “panic”, “phobia”, “post-traumatic stress”, “psychosis”, “psychotic”, or “schizophrenia”. The relevant articles were reviewed for design, study population and control group, means of diagnostic assessment, sample size and demographics, telomere assay methodology, main findings, effect sizes and possible confounds, although this is not meant to be a formal meta-analysis. We primarily focused on studies that used structured diagnoses to characterize their participants (e.g., categorical diagnoses from the DSM (APA, 2013)), since these presumably yield greater diagnostic reliability, have minimum criteria for specific symptoms and for duration of symptoms, and have criteria for functional impairment, compared to studies characterizing their participants only by dimensional scales of recent symptom severity. However, where applicable, we briefly review the latter group of studies in the text of this review, but they are not included in the summary Tables.
2.2. Major Depressive Disorder
Major depressive disorder (MDD) has been considered a syndrome of “premature aging” (Heuser, 2002), and this notion has been reiterated in several recent reviews (Kinser and Lyon, 2013; Luca et al., 2013; Wolkowitz et al., 2011b). Further, MDD has been associated with neuroanatomic/neurodevelopmental as well as brain transcriptome evidence of accelerated brain aging (Douillard-Guilloux et al., 2013; Koutsouleris et al., 2014). In 2006, Simon and colleagues (Simon et al., 2006) were the first to examine LTL in a combined group of chronically ill mood disorder subjects. Fifteen of them had MDD without any comorbid anxiety disorders; the remainder had bipolar disorder with or without anxiety disorders. The mean LTL of the entire mood disorder group was 660 base pairs (bp) shorter than in healthy controls. In the MDD group alone, mean LTL was 770 bp shorter than in healthy controls. According to the authors’ calculations, this represents a very large effect, representing approximately 10 years of accelerated cellular aging in the combined mood disorder group. While ground-breaking, this study had certain limitations, including lack of structured diagnostic interviews in the controls and lack of information regarding several potential confounds, including current and historical medication use. In the largest longitudinal clinical cohort study to date (the Netherlands Study of Depression and Anxiety; NESDA), currently depressed and remitted MDD groups both showed significantly shorter LTL than the healthy controls, whereas LTL in the currently depressed and remitted MDD groups did not significantly differ from each other (Verhoeven et al., 2014a). The difference in LTL between the depressed groups and the control group persisted after co-varying for age, sex, education, alcohol use, BMI, symptom duration, depression severity, co-morbid anxiety and alcohol dependence disorders, somatic diseases, and exercise. In the currently depressed subjects, LTL was inversely associated with severity of depression and with the duration of depression over the preceding four years (although longer-term depressive history was not available), suggesting a “dose-response” relationship (see below in sections 2 and 3). The lack of difference in LTL between active and remitted depressed subjects suggested to the authors that MDD episodes leave a lasting “imprint” on LTL (Verhoeven et al., 2014a; Verhoeven et al., 2014b). However, it is not possible to rule out the possibility that LTL is already short before the first depressive episode, perhaps even representing a risk factor. This possibility is supported by a study by Gotlib and colleagues in which they measured LTL of girls at risk for depression (due to having mothers with depression) and in girls at lower risk and found shorter LTL in the girls at risk for depression (Gotlib et al., 2014).
The “dose-response” hypothesis is supported by the longitudinal study of Shalev et al. (2014), which found, in men, that duration of “internalizing disorders” (including MDD) between the ages of 11– 38 years old predicted LTL at age 38 years, in a dose-response manner. Three other studies did not support this “dose-response” hypothesis, although they do not appear to have had accurate measures of cumulative exposure to the disorder in years. Jodczyk et al. (2014) reported that diagnoses of MDD between age 16 and 25 did not predict shorter LTL at age 28– 30. Hoen et al. (2011) found, in a large cohort of patients with coronary heart disease, that baseline MDD did not predict LTL five years later, although it is possible that the association of coronary heart disease with LTL obscured an association with MDD. In another report, Hoen et al. (2013), found that baseline anxiety disorders in a population-based sample, but not depressive disorders, predicted LTL two years later.
In total, 11 studies have investigated LTL in MDD (Table 1). Of these, seven found significantly shorter LTL in currently depressed individuals (or in individuals with mixed mood disorders including MDD) compared to controls (Garcia-Rizo et al., 2013; Hartmann et al., 2010; Hoen et al., 2011; Lung et al., 2007; Simon et al., 2006; Verhoeven et al., 2014a; Wikgren et al., 2012b), while four did not (Needham et al., 2014; Schaakxs et al., 2015; Teyssier et al., 2012; Wolkowitz et al., 2011a). Of studies having greater than 40 depressed subjects, all except two (Needham et al., 2014; Schaakxs et al., 2015) found significantly shorter LTL in MDD (Hartmann et al., 2010; Hoen et al., 2011; Lung et al., 2007; Simon et al., 2006; Verhoeven et al., 2014a; Wikgren et al., 2012b).
Table 1.
Studies of Leukocyte Telomere Length in Major Depressive Disorder (MDD)
| Referenc e |
Study Population Diagnosis; (Method of Diagnostic Assessment) |
Sample Size: Psychiatric subjects/ Healthy Controls (% Female) |
Mean Age (Yrs): Psychiatri c subjects /Healthy Controls |
Mean Duration of illness in psychiatri c subjects (Yrs) |
Mean telomere length; psychiatric subjects/healt hy controls (Telomere Assay Method) |
Effect Size: Cohen’s d |
Main Findings |
Co-morbidity | Notes and Limitations |
|---|---|---|---|---|---|---|---|---|---|
|
Simon etal., 2006 |
Chronic MDD/BD with or without Anxiety Disorder (Patients by SCID-DSM- IV; Controls by simple questioning) |
44(48%)/ 44(43%) |
51/ 51 |
32 |
6.98/7.64 (kb) (Southern blot) |
0.73 |
Shorter LTL in mood disorders |
Psychiatric: Chronic MDD/BD with or without Anxiety Disorder Somatic: Excluded “all active diseases” |
Analyses reported on the combined mood disorder group, not the MDD sample alone. No data were given regarding possible associations between telomere length and duration of illness. DNA was collected from banked samples collected for other studies, and the MDD and control subjects’ DNA may have derived from different DNA repositories. The authors did not have data to control for trauma, stressful life events, socioeconomic status, obesity, medications, or stress levels. No structured diagnostic interviews were conducted for the control subjects – some subjects with mood disorders may have been included in the control group, according to the authors. Subjects were primarily Caucasian. |
|
Lung etal., 2007 |
MDD (Patients by SCID-DSM- IV; Controls by undocumente d method) |
253 (64%) /411 (57%) |
44 /45 |
Unknown |
8.17/9.13 kbp (Southern blot) |
0.78 |
Shorter LTL in MDD |
Psychiatric: None Somatic: not reported |
The sample was not characterized with regards to number of depressive episodes or duration of illness. No data on medications or somatic co-morbidity. |
|
Hartmann et al., 2010 |
MDD inpatients (Patients by DSM-IV; Controls by undocumente d method) |
54 (61%)/ 20 (45%) |
49/ 49 |
15 | 7.20/7.55 (kb) (Southern blot) |
0.59 |
Shorter LTL in MDD |
Psychiatric: This study excluded subjects with manic, mixed or hypomanic episodes, schizoaffective or dysthymic disorders, and dementia. But they did include patients with other (unspecified) psychiatric disorders. Somatic: This study included subjects with neurological, and (unspecified) somatic disorders and patients with substance abuse in the past |
No significant correlations between LTL and duration of illness, number of hospital stays, severity of depressive symptoms or current antidepressant doses. None of the subjects were untreated (medication or ECT) at the time of study. Duration of illness was defined as length of time from anamnestic onset until blood sampling without excluding intervening periods of euthymia. The authors did not control for potential confounds such as obesity, stress, socioeconomic status, somatic disorders. Past substance abuse or other psychiatric diagnoses (except for manic/hypomanic/mix ed episodes, schizoaffective syndrome, dysthymia, and dementia) were not exclusion criteria. All patients were inpatients, and all were Caucasian. |
|
Hoen etal., 2011 |
MDD in outpatients with stable CHD (MDD by CDIS-IV- DSM-IV. Controls had stable coronary heart disease without current MDD. Past history of MDD was not assessed in cases or controls). |
206 (31%)/ 746 (15%) |
62/ 68 |
Unknown |
0.86/0.90 (T/S) (Q-PCR) |
0.15 |
Significantly shorter LTL in MDD, controlling for age and sex; trend after controlling for additional covariates, |
Psychiatric: Approximately 1/3 (in both groups) used alcohol on a regular basis. Somatic: Subjects had a history of myocardial infarction or coronary revascularizatio n, angiographic evidence of at least 50% stenosis in at least one coronary vessel, or a diagnosis of CHD. Exclusion was a history of myocardial infraction in past 6 months, unable to walk one block. |
The study sample was comprised of stable coronary heart disease patients and mainly older men, which may limit generalizability. The association between LTL and depression may have been confounded by greater cardiac disease severity in the depression group, per the authors. The sample had relatively low depression severity. When full covariates were entered, LTL difference just missed significance (p=0.06) |
|
Wolkowitz et al., 2011a |
MDD, unmedicated outpatients (Patients and controls by SCID-DSM- IV) |
18 (67%)/17 (65%) |
37/ 37 |
13 |
5101/5141 (bp) (Q-PCR) |
0.11 |
No difference in LTL across all MDD subjects. Shorter LTL was observed in MDD subjects with more chronic MDD (those with lifetime depression exposure greater than the median for the sample) |
Psychiatric: No psychiatric co- morbidity except for co- morbid anxiety disorders (except PTSD) when MDD was considered the primary diagnosis. 39% of MDD subjects had co-morbid anxiety disorder.. Somatic: no uncontrolled medical illness; no illnesses or medications that could affect variables. Free of psychiatric medication for at least 6 weeks. |
LTL was inversely correlated with lifetime days of untreated depression. LTL was inversely correlated with peripheral inflammatory cytokines and oxidative stress markers. Mean duration of illness was defined as lifetime years of active depression, excluding intervening periods of euthymia. The study had a relatively small sample size. |
|
Wolkowitz et al., 2012 |
MDD, “severe depression phenotype” (Patients by DIGS/FIGS- DSM-IV). 90% had melancholia |
91 (60%)/ 451 (50%) |
60/59 |
28 |
5261/5538 (bp) (Q-PCR) |
0.40 |
Shorter LTL in MDD |
Psychiatric: Bipolar disorders, dysthymia, substance abuse, alcohol abuse, organic brain disorder, neurologic disorder, PTSD and anxiety disorder were excluded. Somatic: Not reported |
LTL was not significantly related to basal cortisol levels but was directly correlated with post- dexamethasone cortisol levels. This was interpreted as short LTL being associated with overly sensitive HPA axis negative feedback and with hypocortisolism. LTL was not significantly correlated with duration or severity of depression Duration of illness was defined as time from anamnestic onset until the time of blood collection. |
|
Teyssier et al., 2012 |
MDD patients by SCID- DSM-IV and MINI) |
17 (100%) / 16 (100%) |
40/38 |
12 of the MDD subjects were first episode. Mean duration: 11.4 years, range 0– 32 years) |
13.42/13.60 (mean Ct) (Q-PCR) |
0.58 |
No significant difference in LTL. |
Psychiatric: Comorbid psychiatric disorders were excluded, except for “anxiety symptoms” Somatic: Somatic pathology was excluded, especially cardiovascular and metabolic |
In MDD subjects, there was increased expression of p16INK4a and stathmin (STMN1) genes, which are associated with telomere dysfunction, cell senescence, microtubule dynamics, biological aging and regulation of cell cycle dynamics. Moreover, MDD subjects displayed increased expression of OGG1, a DNA/telomere oxidative damage- repairing enzyme, consistent with exposure to oxidative stress. Small sample. All female, all Caucasian, most were relatively recent onset depressive episode (< 6 months in all cases). No subjects reported early life stress, but this was not systematically assessed. 70% received antidepressant medication. |
|
Garcia-Rizo etal., 2013 |
MDD, medication- naïve (patients and controls by SCID-DSM- IV) |
9/48 (unknown gender distribution but reported as “similar”) |
Unknown for the subset in which telomere data were available. In the entire study: 31/ 28 |
Unknown, all subjects were newly diagnosed |
89.0/103.7 (telomere content) (Fluorimetric assay) |
0.98 |
Lower telomere content in MDD compared to controls |
Psychiatric: MDD subjects had no other axis 1 disorders Somatic: no history of diabetes or other conditions associated with glucose intolerance or insulin resistance |
MDD subjects were first episode, medication-naïve. Homogeneous sample of middle/upper class subjects. Very small MDD sample. Important information such as age, sex, symptom severity were not reported. |
|
Verhoeven et al., 2014a |
MDD patients from the Netherlands Study of Depression and Anxiety (NESDA), longitudinal cohort study (MDD by CIDI-DSM-IV) |
1095 current MDD (67% women) + 802 remitted MDD (70% women)/51 0 controls (60% women) |
41 (current MDD)/44 (remitted MDD)/41 (controls) |
Remitted MDD: 11 months of depressio n during the past 4– 5 years. Current MDD: 21 months during the past 4–5 years. |
5474 (current MDD)/5433 (remitted MDD)/5553 (controls) (bp) (Q-PCR) |
0.13 (current MDD vs controls) 0.14 (remitted MDD vs controls) |
Shorter LTL in current and remitted MDD compared to controls |
Psychiatric: Other severe psychiatric conditions, such as bipolar disorder, obsessive– compulsive disorder, severe substance use disorder or psychotic disorder were excluded.. Somatic: Between 33% controls) and 46% (current MDD) had at least one comorbid somatic disorder. |
LTL was similar in current and remitted MDD. The number of years in remission and the current use of antidepressants were not correlated with TL. Within the current MDD subjects, both higher depression severity and longer symptom duration (in the past 4–5 years) were associated with shorter LTL. Group differences were significant even after adjusting for somatic co-morbidity. |
|
Needham et al., 2014 |
MDD (Composite International Diagnostic inventory) |
75 MDD (58.6%*)/96 6 controls (56.0%) |
30.3*/29.2 |
Not reported |
1.12/1.14 (T/S) (Q-PCR) |
0.06 |
No overall group effect on LTL, but among subjects taking antidepressant s, those with MDD had shorter LTL than controls |
Psychiatric: Significant co- morbidity with anxiety disorders . Somatic: No information regarding somatic co- morbidity or substance abuse |
Relatively young sample (age range 20–39 years, mean of approximately 30 years). The study oversampled for low income and Mexican American and African American participants. There was along duration between DNA extraction and analyses. |
|
Shalev etal. 2014 |
Longitudinal study in a complete birth cohort (the Dunedin Multidisciplina ry Development Study). Subjects had “internalizing disorders” including MDD, generalized anxiety disorder and PTSD. Subjects were combined due to high co- morbidity of these diagnoses. Diagnoses by the Diagnostic Interview Schedule for Children and life history calendars. |
Analysis plan 1: Internalizin g disorder from age 11 to 38: 455 (58%)/ 372 (65%) Number of MDD not specified. Analysis plan 2: Internalizin g disorder between age 26 to 38: 234 (58%)/ 524 (45%) 193 had MDD |
Longitudin al study from 11– 38 years old. |
Not reported |
Estimated from graphs in (T/S, PCR). Analysis 1 (based on phases of internalizing disorder): 0 phases (men=1.075, women=1.09), 1 phase (men=1.03, women=1.08), 2 phases (men=1.0, women=1.0), 3 phases (men=0.98, women=1.0), 4 phases (men=0.9, women=1.14), 5+ phases (men=0.78, women=1.03) Analysis 2: No diagnosis (men=1.05, women=1.05), any diagnosis (men=0.97, women=1.035), MDD (men=0.97, women=1.025), GAD (men=0.94, women=1.045), PTSD (men=0.98, women=1.05) |
Insufficien t informatio n |
The persistence of “internalizing disorder” diagnoses between ages 11– 38 predicted shorter LTL at age 38 in a dose-response manner in men, but not in women. LTL assessed at ages 26 and 38 showed an accelerated rate of LTL shortening in men (but not women) with “internalizing disorder” diagnoses in the interim. |
Psychiatric: High co- morbidity between different internalizing disorders although exact numbers are not given. Somatic: Approximately 75% had exceeded clinical cut-off for one or more of the following physical health indicators: Metabolic abnormalities, cardiorespirator y fitness, pulmonary function, periodontal disease, and systemic inflammation. |
The sample was primarily Caucasian – limited generalizability? Approximately 1/3 took psychiatric medications There was no information on other treatments. |
|
Schaakxs et al., 2015 |
Late-life depression (defined by current age, not by age at onset): MDD or dysthymia (DSM-IV, CIDI) |
355 depressed (within past 6 months) (66.2%)/12 8 never depressed (61.7%) |
70.6/70.1 (Range 60–93) |
Not specified, but mean age at onset= 49.1 yrs (range 4– 86 yrs) |
5,036 bp/ 5055 bp (unadjusted) (Q-PCR) |
0.04 |
No significant difference in LTL |
Psychiatric: Primary dementia was excluded. 4% in the depression group and 9% in the control group were “heavy drinkers”. Somatic: Chronic somatic diseases not excluded |
In this elderly sample, age and the number of chronic medical diseases were significantly inversely correlated with LTL, but depression diagnosis, depression severity, number of depressive episodes, and duration of longest depressive episode were not. Controlling for medication use, chronic medical illnesses, lifestyle factors and depression onset before or after 50 or 60 years old did not change the results. |
Note: Studies of individuals with depressive symptoms but without MDD diagnoses are not included here, but they are briefly discussed in the text.
This includes MDD subjects and subsyndromal depression
ABBREVIATIONS I
BD=Bipolar Disorder
BP=base pairs
CDIS=Computerized Diagnostic Interview Schedule
CHD=Coronary Heart Disease
CIDI= Composite International Diagnostic Interview
Ct=cycle threshold for telomeric signal relative to cycle threshold for single copy gene
DIGS=Diagnostic Interview for Genetic Studies
DSM=Diagnostic and Statistical Manual of Mental Disorders
FIGS= The Family Interview for Genetic Studies
LTL=Leukocyte Telomere Length
MDD=Major Depressive Disorder
MINI= The Mini International Neuropsychiatric Interview
PCR=Polymerase chain reaction
PTSD=Post-traumatic stress disorder
SCID= the Structural Clinical Interview for DSM
Four studies found no significant difference in LTL in MDD. One of these studies (Schaakxs et al., 2015) was exclusively in late-life depression, and the authors (who had previously found significantly shorter LTL in a younger MDD population (Verhoeven et al., 2014a)), hypothesized that their negative finding might be due to the larger heterogeneity of late-life depression (including vascular pathologies), the cumulative lifetime occurrence of other LTL-shortening factors possibly overriding the effects of MDD on LTL, and the possibility of premature loss to the study of elderly depressed individuals with advanced cell aging, who may have already died (Schaakxs et al., 2015). Somewhat arguing against this latter explanation, a study in anxiety disorder subjects (reviewed below in section 2.5) found significant LTL shortening only in older subjects (ages 48– 87 years old) (Kananen et al., 2010). The negative study by Needham et al. (2014) found that individuals with MDD who were receiving antidepressants did have significantly shorter LTL than controls, but depressed individuals not taking antidepressants did not, and the authors speculated that the former group may have had more serious depressions that required medication treatment. A small-scale negative study (17 MDD and 16 controls) found no significant difference between subjects with MDD and controls, although the MDD sample was largely comprised of recently diagnosed subjects whose current episodes were all less than six months (Teyssier et al., 2012). Despite finding no difference in mean LTL, however, the latter study did find increased expression of p16INK4a and stathmin (STMN1) genes in the depressed sample, which are markers of cellular senescence, telomere dysfunction, microtubule dynamics and biological aging, and are regulators of cell cycle dynamics (Teyssier et al., 2012). The remaining small-scale study that did not find overall differences in LTL between 18 individuals with MDD and 17 controls, did find, in an exploratory analysis, significantly shorter LTL in the more chronically depressed individuals (above the median cumulative duration of active MDD of 9.2 years) (Wolkowitz et al., 2011a). Across the MDD group, lifetime untreated depression duration was significantly inversely correlated with LTL, consistent with a “dose-response” relationship, although the pilot sample size was too small too draw meaningful conclusions.
The studies on LTL in MDD are summarized in Table 1, where it is seen that the effect sizes for group differences in LTL ranged from 0.04–0.98 (mean Cohen’s d= 0.41; weighted mean Cohen’s d= 0.23). The smallest effect size was seen in the study of late-life depression (Schaakxs et al., 2015).
Among the studies examining the relationship of LTL to dimensional depression ratings in the absence of specific MDD diagnosis, three studies found significant correlations or between-group differences (Hassett et al., 2012; Karabatsiakis et al., 2014; Liu et al., 2014). Hassett et al (2012) found significant correlations between LTL and depression ratings but not an overall between-group difference in LTL. Twelve studies did not report significant results (Canela et al., 2007; Epel et al., 2013; Georgin-Lavialle et al., 2013; Huzen et al., 2010; Ladwig et al., 2013; Lin et al., 2015; Parks et al., 2009; Phillips et al., 2013; Rius-Ottenheim et al., 2012; Shaffer et al., 2012; Surtees et al., 2011; Yen and Lung, 2013). The reasons for overall difference between the studies using categorical diagnostic inclusion criteria versus those using dimensional symptom ratings are not known, but could be due to the use of only short-term symptom rating scales (generally 1–2 weeks), lack of illness duration or severity requirements, lack of functional impairment requirements and generally including subjects with milder symptomatology in the dimensional depression studies. Cumulatively, the bulk of evidence suggests that LTL is decreased in individuals with full syndromal diagnoses of MDD, especially in those with more chronic or severe depression, although the effect size is small..
2.3. Bipolar Disorder
Bipolar disorder (BD) may also be characterized by “accelerated aging” (Rizzo et al., 2014; Sodhi et al., 2012; Yatham et al., 2009 (but see Gildengers et al., 2013)). Six studies have examined LTL in BD subjects (type I, type II or rapid cycling) (Table 2). Elvsåshagen and colleagues (Elvsashagen et al., 2011) found the percentage of telomeres shorter than 3,000 bp (“short telomeres”) was increased in BD type II subjects (p= 0.04) and a trend for shortened absolute LTL in the BD type II subjects (p=0.08), although those statistical tests were one-tailed and would not be considered statistically significant by two-tailed criteria. The rationale for defining “short telomeres” as < 3,000 bp was somewhat unclear but would be consistent with subsequent accurate telomere measurements in human lymphocytes using the “STELA” technique that suggest that the telomeric DNA length cannot fall below about 3800 bp without causing telomeric instability (Lin et al., 2010). Elvsåshagen et al found that the total lifetime number of depressive episodes (corrected for age), but not hypomanic episodes, was significantly correlated with the percentage of telomeres shorter than 3,000 bp (by two tailed criteria), consistent with a “dose-response” relationship with depression (Elvsashagen et al., 2011). Rizzo et al. (2013) found significantly shorter PBMC TL in euthymic women with BD type I (men were not studied). In that study, IgG antibodies to cytomegalovirus (CMV) were significantly elevated in the BD group, and these levels were inversely correlated with LTL, suggesting that exposure to this virus might have played a role in immunosenescence and telomere shortening in the BD group. Years of bipolar illness (corrected for age) were not significantly correlated with LTL. Martinsson et al. (2013) reported significantly increased LTL in lithium-treated BD subjects compared to controls. The cumulative amount of time receiving lithium over the preceding 30 months was associated with increased LTL, and lithium responders had longer LTL than lithium non-responders. The authors suggested that lithium may exert a protective effect against telomere shortening, especially when therapeutically efficacious, and that lithium-induced telomerase activation might be involved, although TA was not measured (Martinsson et al., 2013). This possibility is further discussed under section 5.3 (Effects of Psychotropic Medication on Telomerase Activity) below. The different results in the Elvsåshagen et al. (2011) and Martinsson et al. (2013) studies might, therefore, be explained by lithium treatment. All of the subjects in the Martinsson et al. study (2013), but only two of 28 subjects in the Elvsåshagen et al. study (2011), received lithium treatment. Subjects in the Rizzo et al. study (Rizzo et al., 2013) all received psychotropic medication, which in some subjects included lithium. Most recently, Lima et al. (2014) corroborated shorter LTL in BD, although they lacked data on duration of illness and medication treatment. These and the other studies in BD are summarized in Table 2. Overall, the studies of LTL in BD are inconclusive, perhaps due to the effects of medication on LTL.
Table 2.
Studies on telomere length (TL) in subjects with psychiatric disorders other than MDD
| Reference | Study Population (Diagnostic Method) |
Sample Size: Psychiatric subjects/ Healthy Controls (% Female) |
Mean Age (Yrs): Psychiatric subjects /Healthy Controls |
Mean Duration of illness in psychiatric subjects (Yrs) |
Mean Telomere Length: Psychiatric Subjects/ Healthy Controls (Telomere Assay Method) |
Effect Size: Cohen’s d |
Main Findings |
Co-morbidity | Notes and Limitations |
|---|---|---|---|---|---|---|---|---|---|
| Bipolar disorder (BD) | |||||||||
| Simon et al., 2006 | BD with or without concurrent Anxiety Disorder (SCID) |
Total sample: 44 /44 total. Of these, 15 had MDD (53% female), 15 had BD with a concurrent anxiety disorder (47% female), and 14 had BD with no anxiety disorders (43% female). In the control group there were 43% female |
BD + Anxiety: 51.6/ All controls: 50.5 BD alone: 51.5 / All controls: 50.5 |
BD + Anxiety: 33.5 ± 6.3 BD alone: 36.7 ± 11.8 |
BD + Anxiety: 7100 ± 860 bp BD alone: 6960 ± 810 bp Controls (all): 7640 ± 1100 bp (Southern blot) |
0.55 (BD + anxiety vs controls) 0.70 (BD alone vs controls) |
Across all mood disorder subjects, LTL was significantly shorter than in controls (Individual mood disorder groups not reported separately) |
Psychiatric: Chronic MDD/BD with or without Anxiety Disorder Somatic: Excluded “all active diseases” |
Results are not reported separately for BD or BD plus Anxiety Disorder groups, so the effects of BD alone cannot be inferred. Lifetime psychiatric history of the controls was assessed by unstructured interview. No data on potential confounders of lifetime stress exposure, medication use, subjective stress levels or BMI. Sample was primarily Caucasian. |
| Elvsåshagen et al., 2011 | BD II, outpatient sample. Sixteen subjects were euthymic and 12 were mildly- moderately depressed. (MINI) |
28 (68) / 28 (68) |
35/35 | 19 (time from anamnestic onset until the time of blood withdrawal) |
Percentage of “short: telomeres: Bipolar II: 15.04%, vs. Controls: 13.48%. Telomere length: BD II: 10,067 bp, vs. Controls: 10,619 bp (High- throughput quantitative fluorescence in situ hybridization with automated fluorescence) |
Insufficient information to calculate |
The load of short telomeres (<3000 bp) was significantly greater in the bipolar subjects, compared to the controls, using one- tailed testing (p<0.04). The difference in telomere length was not statistically significant (p=0.08, one- tailed). |
Psychiatric: Social phobia and panic disorder were frequent comorbid psychiatric disorders. One subject met the criteria for current alcohol abuse. Somatic: Neurological and severe chronic somatic disorders excluded. |
Load of short telomeres was defined as the number of telomeres below 3,000 base pairs. Significance tests used one-tailed tests. Small sample size. No control for early life stress. The load of short telomeres and telomere length were not significantly correlated with illness duration (current age minus age at onset, including euthymic periods), but the load of short telomeres was significantly positively correlated with the number of depressive (but not hypomanic) episodes. The number of depressive episodes was negatively correlated with telomere length at trend level (p= 0.08). |
| Mansour et al., 2011 | BD I (SCAN). | 108/114 | 24.9 (50%)/ 27.5 (44%) |
Unknown | 0.95 ± 0.40/ 0.97 ± 0.40 (T/S) (PCR) |
0.05 | No significant differences in TL between cases and controls |
No information on co-morbid conditions, somatic or psychiatric. |
Subjects had high rates of consanguinity, with higher rates in the cases vs. the controls. Subjects were generally younger than those in the other studies. No data were available to co-vary for medications, comorbid illnesses, BMI, lifestyle factors or early life stress. |
| Rizzo et al., 2013 | BD I (SCID) | 22/17 | 39.5 (100)/44.6(100) |
9.45 (range 1–25) |
0.71±0.20 (T/S) vs. 0.90±0.19 (T/S) (q-PCR) |
0.97 | BD subjects had significantly shorter LTL than controls. |
Psychiatric: Psychotic disorders, mood disorders, anxiety disorder, or substance-related disorder were exclusion criteria. Somatic: Other exclusion criteria were a history of brain injury or severe medical illness or neurological disorders; use of substances that might induce immune or endocrine changes |
The sample was female only. CMV IgG levels were higher in BD subjects than in controls. CMV IgG levels were associated with expansion of senescent CD8+CD28− T cells and with shorter LTL |
| Martinsson et al., 2013 | BD (SCAN) | 202/135 | Samples were matched for age and sex (age range 33–77). Mean age for psychiatric subjects/controls not reported |
Not reported for whole sample |
Exact values not reported (Q-PCR) |
N/A | LTL was significantly longer in BD compared to controls. Lithium treated BD subjects had longer TL than controls (p<0.0005). |
No information on co-morbid conditions, somatic or psychiatric. |
TL correlated significantly and positively with lithium treatment duration of >30 months (p=0.031) and was negatively associated with number of depressive episodes (p<0.007). Lithium responders had significantly longer TL than lithium non- responders (p=0.047). This study did not control for smoking, obesity, inflammation and somatic disorders. |
| Lima et al 2014 | BD type 1 and type 2 (MINI Plus 5.0) |
85 (25) / 95 (37) |
39/38 | Information not available |
Absolute values not given. (PCR) |
0.36 | BD subjects had significantly shorter TL than controls (p<0.001). No significant difference between BD subtype. Short LTL was associated with panic disorder co- morbidity |
Psychiatric: Relatively high co- morbidity for GAD, Panic Disorder, Borderline, Alcoholism, Drug abuse, Eating disorder, and OCD. Somatic co- morbidity not reported. |
No information regarding illness duration was provided. Smoking, BMI, medication use and somatic illnesses were not controlled for. |
| Psychotic disorders | |||||||||
|
Kao et al., 2008 (two analyses; S1 & S2) |
Schizophrenia, outpatients (13 from cohort A and 18 from cohort B. Cohort A: SCID interviews. Cohort B: structured modified Schedule for Affective Disorders and Schizophrenia combined with Structured Interview for DSM-III-R Personality Disorders and Diagnostic Interview for Genetic Studies. |
S1 (schizophreni a subjects from cohorts A and B): 31(23)/41(32) S2 (all male sample matched or age, all were from cohort A): 33(0)/26(0) The two analyses were analyzed separately in independent laboratories but using same protocol |
S1: 39/26 S2: 36/33 |
Approximately 20 years |
Telomere length given in T/S: S1: 1.14/1.51 S2: 1.09/1.50 (PCR) |
1.17 (S1 vs controls) 0.72 (S2 vs controls) |
Significantly shorter TL in schizophrenia in S1 (p=0.002) and S2 (p=0.008) |
No information on co-morbid conditions, somatic or psychiatric. |
No significant correlations between current or lifetime antipsychotic dose and TL. No information on co-morbidity (psychiatric or somatic) was given, nor information regarding health behaviors such as exercise or smoking. |
| Yu et al., 2008 | Schizophrenia, inpatients (SCID). Schizophrenia subjects were subdivided into good vs poor responders for TL analysis |
68 /76 | 38 (78) /38 (72) | 16 | 7.41 + 0.97 (poor responders)/8.8 8 0.90 (good responders) 8.91 + 1.36 (controls). (Terminal restriction fragment assays were used) |
1.22 (poor responder s vs. controls). 0.03 (good responders vs controls) |
Significantly shorter TL in schizophrenics with poor treatment response compared to controls (p<0.001) and schizophrenia subjects with good treatment response (p<0.001). There was no significant difference in TL between good responders and controls (p>0.05) |
Psychiatric: No DSM-IV diagnosis of alcohol or substance abuse or neurodegenerative disorder Somatic: Physically healthy with normal laboratory parameters. |
It is unclear if all schizophrenia subjects as a group differed significantly from controls in terms of TL. No information was given on potential confounders such as smoking and BMI. |
| Fernandez-Egea et al., 2009 | “Non-affective psychosis” (SCID) |
41(32)/41(32) | 29/28 | 0 | Telomere content: 93.1%/101.9% (relative to a reference DNA standard (fluorometric assay) |
N/A | Psychotic patients had significantly decreased telomere content compared to controls (p=0.011) |
Psychiatric: no lifetime diagnosis of schizophrenia or MDD, or a current diagnosis of adjustment disorder. Substance abuse data not reported. Somatic: no diabetes or other serious medical or neurological condition associated with glucose intolerance or insulin resistance and |
No antipsychotic use 30 days prior to the study. The participants had maximum lifetime antipsychotic exposure of 1 week. Small sample size. Information regarding diet and health behaviors was not available. |
| Mansour et al., 2011 | Schizophrenia or schizoaffectiv e disorder (SCAN). |
60 / 60 | 28.2 (35%)/ 27.0 (35%) |
Unknown | 0.89 ± 0.30/ 0.87 ± 0.26 (T/S) (PCR) |
0.07 | No significant differences in TL between cases and controls |
No information on co-morbid conditions, somatic or psychiatric. |
Subjects had high rates of consanguinuity, with higher rates in the cases vs. the controls. Subjects were generally younger than those in other studies. No data were available to co-vary for medications, comorbid illnesses, BMI, lifestyle factors or early life stress. |
| Nieratschker et al., 2013 | Schizophrenia (SCID, the Operational Criteria Checklist for Psychotic Illness, medical records, family history) |
539/519 | 36.9 (44)/39.1 (49) |
Not reported | Not reported (q-PCR) |
N/A | LTL was significantly longer in schizophrenia subjects than controls |
No information on co-morbid conditions, somatic or psychiatric. |
The authors excluded outliers more than 3 SD from the mean, which resulted more schizophrenia subjects being excluded than controls. All subjects received medications, which the authors suggest may account for their findings. |
| Malaspina et al., 2014 | Schizophrenia (DIGS) |
53 (40%)/ 20 (45%) |
42/37 | Not available | Schizophrenia: males: 1.98 T/S, females: 1.80; Controls: males: 1.92, females: 1.65 (PCR) |
Males: 0.08; Females: 0.24 |
No significant difference in LTL between schizophrenic subjects and controls or between males and females. No significant diagnosis × gender interaction. |
No information on co-morbid conditions, somatic or psychiatric. |
Small control sample size. Healthy control group only verified as having no Axis I diagnosis for the previous 2 years. Schizophrenia subjects were on stable doses of antipsychotic medication. Schizophrenia group was significantly older and had significantly more current tobacco use than controls. Paternal age was positively correlated with LTL in male cases but was negatively correlated with paternal age in female cases. |
| Kota et al., In press | Schizophrenia (DSM-IV). Unremitted or remitted |
71 (36 unremitted, 35 remitted) (38%)/ 73 (53%) |
31.7/ 32.1 | 5.5 | Schizophrenia: 0.59 T/S (Remitted: 0.56 T/S; Unremitted: 0.42 T/S); Controls: 0.85 T/S (PCR) |
Not available |
Mean LTL shorter in schizophrenia compared to controls. This difference was significant in the unremitted schizophrenia subjects but not in the remitted ones. |
No information on co-morbid conditions, somatic or psychiatric. |
Schizophrenia subjects had been treated with antipsychotic medication for at least six months before blood sampling. Analyses controlled for age and sex but not for tobacco use. |
| Anxiety disorders | |||||||||
| Kananen et al., 2010 | Anxiety disorders and “sub-threshold” disorders (Including Panic disorder, Generalized Anxiety Disorder, Social Phobia, Agoraphobia, and Phobia Not Otherwise Specified). Diagnoses were determined with the Munich Composite International Diagnostic Interview. |
321(63)/653 (64) |
50 /50 | Not reported | Not reported (Q-PCR) |
Not available |
No significant differences in TL between cases and controls in the entire sample. Significant difference in TL between cases and controls only in individuals>48 years (p=0.013) |
Psychiatric: 28% of the anxiety disorder subjects had concurrent MDD diagnosis. Comorbid alcohol use disorder in 22% of cases. Somatic: Frequency of somatic co- morbidity was not reported |
LTL was significantly associated with childhood adversities but not with current perceived stress, psychiatric co- morbidity, or the use of psychotropic medication. Psychiatric diagnoses were only obtained for the preceding 12 months. |
| Hoen et al., 2013 | Mixed anxiety disorders (panic disorder, GAD, social phobia, agoraphobia); Mixed depressive disorders (MDD and dysthymia) (CIDI, self- report computerized) |
Anxiety disorders: 108 (63%)/ 970 (53%) Depressive disorders: 97 (64%)/ 980 (53%) |
Anxiety disorders: 52/ 54 Depressive disorders: 51/ 54 |
Not reported | Not reported (PCR) |
Not available |
The presence of an anxiety disorder diagnosis (panic disorder, agoraphobia or social phobia, but not GAD) over the preceding year significantly predicted shorter LTL 2.2 years later. Depressive disorder diagnoses did not significantly predict LTL. |
Psychiatric: 3.3% of all subjects had both an anxiety and a depressive disorder. Somatic: The study was performed in a cohort investigating risk factors for renal and cardiovascular disease. The cohort was oversampled for albuminuria. |
This was a longitudinal study. Diagnoses were heterogeneous, and the depressive disorder group included dysthymia. Diagnoses were not based on clinician interview and were based on a one year window; therefore controls may have had psychiatric diagnoses prior to that one year period. . Diagnosis and LTL measurement were not contemporaneous (separated by an average of 2.2 years). Psychiatric patients were from a general population, and may have been of mild severity. |
| O`Donovan et al., 2011a | PTSD (SCID and CAPS) |
42/46 | 30 (48%)/ 31 (54%) |
>3 months | 6,594 ± 528.1 bp /6,798 ± 528.3 bp (q-PCR) |
0.39 | LTL significantly shorter in PTSD, but this was accounted for by the significant correlation between cumulative exposure to childhood trauma, which was seen only in the PTSD group. |
Psychiatric: Exclusion criteria included alcohol abuse or dependence in the previous 2 years; substance abuse or dependence in the previous year; any psychiatric disorder with psychotic features; bipolar disorder or obsessive-compulsive disorder; and pregnancy. Somatic: Medically healthy and medication- free. Exclusion criteria included neurologic disorders or systemic illness; use of psychiatric, anticonvulsant, antihypertensive, sympathomimetic, estrogen replacement therapy, steroidal, statin or other prescription medications; obesity (BMI > 30); |
The control sample lacked individuals with childhood trauma, leaving unanswered the question of whether PTSD or childhood trauma accounted for the shorter LTL. |
| Ladwig et al., 2013 |
PTSD (partial and full), Post- Traumatic Diagnostic Scale; Impact of Event Scale |
Partial PTSD: 262; Full PTSD: 51; Controls: 2687 |
Partial PTSD: 52.5 (61.8%); Full PTSD: 54.5 (62.7%); Controls: 56.5 (50.5%) |
Not reported | Partial PTSD: 1.85 + 0.29 T/S Full PTSD: 1.78 + 0.29 T/S Controls: 1.85 ± 0.33 T/S (PCR) |
0.0 (partial PTSD vs controls) 0.23 (full PTSD vs controls) |
Although raw TL values were nearly identical, both PTSD groups had significantly shorter TL than controls when age was co-varied. |
Psychiatric: Between 17% (no PTSD) and 24% full PTSD) had high alcohol consumption. Somatic: History of MI, diabetes, stroke, or cancer between 17% (no PTSD) and 23% (partial PTSD). Hypertension between 24% (partial PTSD) and 32% (no PTSD). |
Age-adjusted “full PTSD” had shorter TL than age-adjusted subjects with “partial PTSD,” but the statistical significance of this difference was not reported. Controlling for depressive symptoms (PHQ- 9) did not alter the main findings. |
| Zhang et al. 2014 |
“Probable PTSD” in US Army Special Operations Units (self- report PCL and Life Events Checklist) |
84/566. An age-matched control group (N=84) was also compared to the PTSD group. |
29.2 ± 7.3 (not separated by group) (12.9%) |
Not reported | Nor reported (PCR) |
N/A | Significantly shorter LTL in PTSD vs. controls |
No information on co-morbid conditions, somatic or psychiatric. |
Analyses did not co-vary for age, sex, BMI, and tobacco use, but the significant LTL finding remained in an age-matched sub-sample. Duration of illness was not reported. Scant details were provided about the subjects. Childhood trauma was not correlated with LTL. |
| Needham et al., 2014 |
General Anxiety Disorder, and Panic Disorder (Composite International Diagnostic Interview) |
52 (55.7*)/952 (56.6) |
30.0*/29.3 |
Not reported | 1.12*/1.14 (T/S ratio) Q-PCR |
0.06 | No overall significant difference between groups. However, females with GAD or panic disorder had shorter TL than the controls. |
Psychiatric: Significant co- morbidity with MDD Somatic: No information regarding somatic co-morbidity or substance abuse |
Relatively young sample (age range 20–39 years, mean of approximately 30 years). The study oversampled for low income and Mexican American and African American participants. There was along duration between DNA extraction and analyses. |
| Verhoeven et al., in press |
NESDA; DSM-IV Included: panic +/− agoraphobia, social phobia, GAD and agoraphobia. Excluded: OCD, PTSD, bipolar, substance abuse, psychosis |
128 current anxiety d/o; 459 remitted anxiety d/o/ 582 controls; 67% female |
Mean 41.7 +/− 13.1 Range 18–65 y.o. |
Not reported | 5431 bp (Current) vs. 5506 bp (Control); 5499 bp (Remitted) qPCR |
0.12– 0.22 | Current anxiety disorder subjects had shorter LTL compared to controls and to remitted anxiety disorder subjects. Remitted anxiety disorder subjects’ LTL did not differ from that in controls. |
Psychiatric: Excluded primary diagnosis of other severe psychiatric conditions, such as bipolar disorder, obsessive– compulsive disorder, severe substance use disorder or psychotic disorder. Somatic: Somatic disorders not excluded but covaried for in the analyses |
Lifestyle, health variables and all demographics were controlled. LTL was not associated with symptom duration in the past 4 years. The time since remission was positively correlated with LTL in the remitted subjects. Subjects in remission for over 10 years had significantly longer LTL than subject in remission for only 6 months- 9 years, raising the possibility (per the authors) that cellular aging associated with anxiety disorders may eventually be reversible. The anxiety/ control difference in LTL persisted even after excluding comorbid MDD diagnoses. Among the current anxiety disorders, panic with agoraphobia, social phobia and generalized anxiety disorder (but not agoraphobia or panic disorder without agoraphobia) were associated with significantly shorter LTL. Symptom clusters that correlated with shorter LTL across the whole sample included: anxiety arousal, social phobic symptoms and worrying. |
| Jergovic et al., 2014 |
PTSD, ICD-9 by MINI. and CAPS |
28 combat- PTSD/ 17 non-combat- exposed controls Middle-aged men (0% female) |
45.9 + 1.12/ 47.2 + 1.7 |
Not reported | 0.86 + 0.03 / 1.03 + 0.04 |
Unknown |
PTSD, compared to age-matched controls, had significantly decreased LTL. No difference in PBMC basal TA |
Psychiatric: Only four patients were without any comorbid psychiatric condition, 24 (80%) had major depression, 13 (43%) had panic disorder, 9 (30%) had obsessive compulsive disorder, and 7 (23%) were diagnosed with social phobia. 65% of healthy controls and 20% of PTSD subjects used alcohol. Somatic: Substance abuse, acute or chronic physical illnesses were exclusion criteria. 60% of the subjects took NSAID, 10% opiod analgesics, 10% hypollipidemics, 10% antihypertensives, 3% proton pump inhibitors. |
PTSD subjects were described as severely traumatized in war and having had “several forms of psychiatric treatment”Many of the PTSD subjects were taking opioid analgesics, non-steroidal anti-inflammatory drugs, statins, psychotropics, or anti-hypertensive drugs. 80% of PTSD subjects had comorbid MDD. MDD was accounted for in the regression models, and the significant difference in TL remained. There was no assessment of early life adversity. LTL and TA did not significantly correlate with CAPS severity or sub-scales or with depressive symptoms. |
This includes subsyndromal anxiety symptoms and DSM verified anxiety disorders
ABBREVIATIONS
BD=Bipolar Disorder
BMI=Body Mass Index
BP=base pairs
CAPS=Clinician Administered PTSD Scale
CMV=Cytomegalovirus
DIGS=Diagnostic Interview for Genetic Studies
DSM=Diagnostic and Statistical Manual of Mental Disorders
GAD=Generalized Anxiety Disorder
LTL=Leukocyte Telomere Length
MDD=Major Depressive Disorder
MINI= The Mini International Neuropsychiatric Interview
PCR=Polymerase chain reaction
PCL= PTSD Checklist
PBMC=Peripheral Blood Mononuclear Cell
PTSD=Post-traumatic stress disorder
SCAN= Schedule for Assessment in Neuropsychiatry
SCID= the Structural Clinical Interview for DSM
TA=Telomerase activity
2.4. Psychotic Disorders
Schizophrenia, like MDD and BD, may also be associated with premature biological aging (Anthes, 2014; Jeste et al., 2011; Kirkpatrick et al., 2008; Kochunov et al., 2013; Koutsouleris et al., 2014; Okusaga, 2014; Shivakumar et al., 2014). Seven studies have assessed LTL in schizophrenia or other psychotic disorders (Fernandez-Egea et al., 2009; Kao et al., 2008; Kota et al., In press; Malaspina et al., 2014 Mansour et al., 2011; Nieratschker et al., 2013; Yu et al., 2008) (Table 2). In one study, LTL was significantly shortened in individuals with schizophrenia and was unrelated to antipsychotic use or duration of illness (Kao et al., 2008). A potential limitation of this study was that no information regarding co-morbidity (somatic or psychiatric) or health behaviors (such as smoking and exercise) was given. In another study, newly diagnosed, antipsychotic-naïve individuals with non-affective psychoses also showed shortened LTL (Fernandez-Egea et al., 2009). Yu et al. (2008) found shorter LTL in individuals with schizophrenia who responded poorly to treatment but not in the schizophrenia group as a whole. Smoking and BMI were not examined as potential confounds. Similarly, Kota et al. (In press) reported that “unremitted,” but not “remitted,” schizophrenia was associated with short LTL compared to controls. The three remaining studies failed to detect short LTL in schizophrenia. Mansour et al. (2011), studying a relatively young, highly inbred population, found no significant LTL difference in schizophrenia vs. controls, but LTL was confounded by the extent of inbreeding in the schizophrenia population, and no data were available regarding comorbid diagnoses, medications or treatment response. Most recently, Malaspina et al. (2014) reported no significant difference in LTL between medicated individuals with schizophrenia compared to controls, but the control sample size was small, and the psychiatric history of the controls was assessed only for the preceding two years. The largest study to date (comprising 539 schizophrenia subjects and 519 controls) (Nieratschker et al., 2013) reported an unexpected increase in LTL in individuals with schizophrenia in comparison to healthy controls, especially in the younger subjects. A possible confounder is that an unequal number of “outlier” data points (> 3 SD from the mean) were excluded from analysis (23 data points from the schizophrenia sample and four data points from the control sample). Since all of the individuals with schizophrenia were receiving psychotropic medication, the authors suggested a similarity of their findings with those of Savolainen et al. (2012) in a mixed inpatient psychiatric sample, which found that telomere lengthening in certain psychiatric patients may be moderated by psychotropic medication usage. This explanation is similar to the finding with lithium treatment reviewed above (Martinsson et al., 2013), although no clinical studies have yet directly assessed the effect of psychotropic medications on LTL. In summary, data on LTL in schizophrenia and other psychotic disorders are mixed, and it is possible that age, medication exposure, treatment response, comrbidities and confounds may explain some of the variability in findings. Studies in psychotic disorders are summarized in Table 2.
2.5. Anxiety Disorders
In the largest population-based study to date on cell aging in anxiety disorders, Verhoeven et al. (In press) reported that subjects with current anxiety disorder diagnoses (including panic with agoraphobia, social phobia and generalized anxiety disorder) had significantly shorter LTL than controls, even after accounting for health, demographics, lifestyle factors and concurrent MDD. Remitted anxiety disorder subjects, however, especially those in remission for 10 years or more, showed no significant difference in LTL compared to controls. This differed from this group’s finding, using the same study population, that remitted MDD was still associated with shortened LTL (Verhoeven et al., 2014a), and they suggested that LTL shortening with anxiety disorders may be more reversible than that associated with MDD. Needham et al. (2014) found, in a large-scale study, that shorter LTL was significantly associated with a diagnosis of generalized anxiety disorder and panic disorder in women but not in men. Kananen et al. (2010), found no overall significant difference in LTL between a mixed group of anxiety disorder subjects (panic disorder, generalized anxiety disorder, social phobia, agoraphobia and phobia not otherwise specified, plus cases of “sub-threshold” anxiety disorders not meeting DSM-IV criteria for anxiety disorder diagnosis) and controls. However, LTL was significantly shorter among the older anxiety disorder subjects (48–87 years of age) compared to controls, and the authors suggested that more prolonged exposure to anxiety disorder-related stress might be needed for accelerated telomere shortening to be detected (Kananen et al., 2010). It is also possible, however, that age (as distinct from chronicity) directly interacts with anxiety in predicting LTL. In their study, sub-threshold cases, not meeting the full DSM criteria for anxiety disorders, were included to increase the sample size, which may also have had an impact on the results. In a large-scale study, while phobic anxiety was not overall significantly correlated with LTL, there was evidence of a threshold or “dose-response” effect, with more severe phobia being associated with shorter LTL (Okereke et al., 2012). However, phobic anxiety disorder in this study was rated dimensionally, rather than syndromally per accepted diagnostic standards, and this study, therefore, is not included in Table 2. This study was also limited by the use of an all-female sample, the presence of certain severe medical ilnesses (although the main findings withstood controlling for medical illnesses), and the lack of information regarding illness duration, illness onset and treatments or the presence of comorbid depression. In summary, the data are mixed, but the evidence tends to support LTL shortening in anxiety disorders, especially with more severe anxiety disorders (meeting full diagnostic criteria) and with longer exposure to the anxiety disorder. Studies in anxiety disorders are summarized in Table 2.
2.6. Post-Traumatic Stress Disorder (PTSD)
PTSD has been considered to have aspects of “accelerated aging” (Bremner and Narayan, 1998; Miller and Sadeh, 2014; Moreno-Villanueva et al., 2013; Torgashov et al., 2013; Yehuda et al., 2005). One study found significantly shorter LTL in PTSD, but found that this effect was largely explained by early life stress, which is a risk factor for developing PTSD (O'Donovan et al., 2011a). In another study, involving combat deployed soldiers, individuals with PTSD had significantly shorter LTL compared to combat-deployed soldiers without PTSD (Zhang et al., 2014). That study did not detect a relationship between early life trauma and LTL, although the childhood trauma measure used was brief and non-validated. Potential limitations of the latter study were that the analyses did not co-vary for age, sex, BMI, or tobacco use, although the significant LTL finding remained in an age-matched sub-sample. Another study reported significantly shorter LTL in individuals with partial and full PTSD (Ladwig et al., 2013). Controlling for comorbid depressive symptoms did not attenuate the effect of PTSD in that study. A study in men with severe combat-related PTSD found significantly shorter LTL in the men with PTSD, although the control group had no combat exposure, and most subjects were prescribed a variety of medications and had comorbid diagnoses, making interpretation difficult (Jergovic et al., 2014). Apart from these studies of cross-sectional correlations of PTSD with LTL, a recent longitudinal study found that the development of combat-related PTSD (changes in PTSD symptom severity from pre-combat to post-combat exposure) was associated with an unexpected lengthening in LTL (from pre-combat to post-combat exposure), although structured PTSD diagnostic criteria were not applied, and cross-sectional post-combat (as opposed to “change”) data were not reported (Boks et al., 2015). Therefore, this study is not included in Table 2. In summary, although the number of studies is small, the existing evidence base is consistent with LTL shortening in PTSD, although several confounds, especially early life adversity, exist, making conclusions difficult. To the extent LTL is decreased in individuals with PTSD, it remains to be determined whether the telomere shortening is a direct consequence of PTSD or, rather, if the development of PTSD and the shortening of LTL are both the consequence of increased stress sensitivity of the affected individuals (Zhang et al., 2014) or if telomere shortening might even represent a risk factor for acquiring PTSD (Malan et al., 2011). Studies in PTSD are summarized in Table 2.
As seen from the above reviews, there are inconsistent reports of LTL associations with psychiatric illnesses with the strongest evidence seen in MDD. There is a modest preponderance of evidence suggesting that shorter LTL is seen across these illnesses, although methodological differences and, in many cases, small sample sizes preclude more definitive conclusions. Nonetheless, it is apparent that LTL shortening, when it occurs, is not confined to specific traditional diagnostic categories. Therefore, it is unlikely that LTL measurement, used alone, will come into clinical practice as a diagnostic biomarker, let alone a diagnostic aid in psychiatric diagnoses. On the other hand, it is possible that LTL may reflect underlying pathophysiological processes that span traditional diagnoses, e.g. inflammation, oxidative stress, lymphocyte proliferation in the face of chronic antigen presentation, and, perhaps, long-term biological changes induced by early life adversity. Potential physiologic mechanisms that may affect LTL are reviewed below in section 4.
3. Is Telomere Shortening Related to The Duration and Severity of the Psychiatric Illness?
To the extent LTL reflects cumulative exposure to inflammation and oxidative stress, which are often features accompanying serious mental illnesses (Pandya et al., 2013; Rosenblat et al., 2014; Smyth and Lawrie, 2013), longer and/or more severe exposure to the illnesses might result in accelerated telomere shortening (i.e., a “dose-response” relationship). On the other hand, if LTL shortening antedates, or is even a risk factor for, the psychiatric illness (Gotlib et al., 2014; Malan et al., 2011), there might be a fixed degree of LTL shortening regardless of the degree of exposure (“premature” as opposed to “accelerated” telomere shortening). These hypotheses are not mutually exclusive, and it is possible that susceptible individuals have shortened telomeres prior to the onset of psychiatric illness and show further acceleration of telomere shortening with greater exposure to the illness.
In the studies reviewed here, the evidence for a “dose-response” relationship is suggestive but mixed. In the MDD study by Verhoeven et al. (2014a), the severity of depression, as well as the duration of depression over a four-year period, were inversely correlated with LTL. Consistent with this, the longitudinal study by Shalev et al. (2014), found, in men but not in women, that that persistence of “internalizing disorders” (including MDD) between the ages of 11– 38 years old predicted LTL at age 38 years in a dose-response manner. Also, in a small-scale MDD study, Wolkowitz et al. (2011a) found that LTL was inversely correlated with lifetime duration of MDD, especially untreated depression. Finally, in a study in BD, Martinsson et al. (2013) found that the number of prior depressive episodes, but not the number of prior manic episodes, was associated with shorter LTL.
On the other hand, Hartmann et al. (2010) did not find a relationship between MDD chronicity or severity and LTL. In a very small study, Garcia-Rizo et al. (2013) found decreased telomere content even in first-episode, never-medicated individuals with MDD (N= 15). This could either argue against a “dose-response” relationship or could suggest that telomere shortening is seen even early in the course of MDD, perhaps even preceding the onset of MDD, but this does not preclude further shortening in a “dose-response” manner.
The evidence regarding a “dose-response” relationship in anxiety disorders is also mixed. In the largest study, Verhoeven et al. (In press) reported that LTL was inversely proportional to the severity of anxiety across the entire sample (anxiety subjects and controls) but was not related to the duration of anxiety over a preceding four-year period. In another large-scale study, while phobic anxiety was not significantly correlated with LTL (p=0.15), there was evidence of a threshold or “dose-response” effect, with more severe phobia being associated with shorter LTL (p=0.02) (Okereke et al., 2012). Two studies in PTSD, both of which found significant decreases in LTL in the PTSD subjects, differed in their assessment of whether a ”dose-response” relationship existed. Ladwig et al. (2013), noted a greater degree of LTL shortening in “full” PTSD than in “partial” PTSD, representing an estimated five years of accelerated cell aging in the latter compared to an estimated 10 years in the former. Although the authors suggested this represents a “dose-response” effect of PTSD severity on LTL, the LTL differences between “partial” and “full” PTSD, relative to controls, were not statistcially significant. The other study found that LTL was not significnatly correlated with the severity of PTSD symptoms (Jergovic et al., 2014).
4. Potential Mediators of Telomere Shortening in Psychiatric Illness
4.1. Overview
Biological abnormalities seen in certain psychiatric conditions (e.g. inflammation, oxidative stress and perhaps changes in steroids or in biogenic amine activity) are associated with, and may cause, telomere shortening (Wolkowitz et al., 2011a; Wolkowitz et al., 2011b). Since such biochemical abnormalities cut across traditional psychiatric diagnoses (Pandya et al., 2013; Rosenblat et al., 2014; Smyth and Lawrie, 2013), telomere shortening may be related to specific biological processes or endophenotypes more than to specific diagnostic categories, although this remains to be adequately tested. This could help explain the inconsistency of LTL findings in specific diagnostic groups and the heterogeneity of findings among different diagnostic groups. It is also possible that certain psychiatric illnesses and LTL shortening are related to third factors common to both, rather than being directly causally related (e.g., poor sleep, poor nutrition, insufficient exercise, cigarette smoking) (Prather et al., 2014; Puterman and Epel, 2012; Puterman et al., 2014; Shalev et al., 2013). Indeed, lifestyle changes that often accompany certain psychiatric illnesses may secondarily lead to LTL shortening (Lin et al., 2012; Puterman et al., 2013; Puterman et al., 2015). As mentioned above, it is also possible that short LTL precedes certain psychiatric illnesses, rather than being a consequence of them, and may even represent a risk factor for them (Gotlib et al., 2014; Malan et al., 2011). Such temporal causality is clearer in dyskeratosis congenita, which is caused by defective telomere maintenance genes, resulting in shortened telomeres. Patients with dyskeratosis congenita show not only multiple somatic signs of premature senescence (Armanios and Blackburn, 2012) but also an increased incidence of neuropsychiatric conditions compared to other chronically ill patients (Rackley et al., 2012).
4.2. Inflammation, Oxidation and Increased Cell Turnover
Continuing cell division in the absence of adequate TA is a major cause of shortened telomeres in mitotic cells, such as leukocytes and stem/progenitor cells as well as mitotic cells in the brain, such as microglia and cells in the dentate gyrus and subventricular zone. An increasingly recognized correlate of shortened LTL is chronic viral infection, such as cytomegalovirus (CMV), which likely leads to LTL shortening due to clonal expansion of leukocytes and an increased preponderance of senescent T cells (e.g., CD8+CD28−) (van de Berg et al., 2010). Two important drivers of LTL shortening, apart from that resulting from incomplete DNA end replication in cells undergoing frequent mitoses, are inflammation and oxidative stress (Wolkowitz et al., 2011a; von Zglinicki 2002), which are often increased in certain psychiatric illnesses (Wolkowitz et al., 2011a; Wolkowitz et al., 2011b). Thus, even post-mitotic cells, including mature neurons, can acquire a senescent phenotype if exposed to these conditions (Jurk et al., 2012). Inflammation coupled with increased oxidation can become mutually reinforcing and may be especially damaging and likely to foster accelerated cell aging (Rawdin et al., 2013). The effect of inflammation on LTL is potentially caused by its association with increased immune cell replication during inflammation, as described above, as well as by pathways leading from inflammation to oxidation (Rawdin et al., 2013). Pro-inflammatory cytokine concentrations are inversely correlated with LTL in MDD (Wolkowitz et al., 2011a), in individuals with histories of early life stress (Kiecolt-Glaser et al., 2011), and in healthy individuals with high C-reactive protein levels or high cumulative inflammatory load (O'Donovan et al., 2011b; Revesz et al., 2013). Oxidative stress may have a more intrinsic role in telomere shortening, since telomeric DNA is particularly sensitive to oxidative damage (von Zglinicki, 2002; Wolkowitz et al., 2011b), and since repair of oxidative damage is relatively inefficient in telomeres (De Meyer, 2011; von Zglinicki, 2002). Oxidative stress markers are inversely correlated with LTL in MDD (Wolkowitz et al., 2011a) and in healthy pre-menopausal women (Epel et al., 2004).
4.3. Stress Hormones (Cortisol and Catecholamines/ Sympathetic Nervous System Activity) and Anabolic Hormones
Shortened LTL has been associated with increased urinary catecholamine concentrations or increased sympathetic nervous system activity (Epel et al., 2006; Revesz et al., 2013). Leukocyte telomere length associations with resting cortisol concentrations are less clear, with reports of an inverse correlation (Epel et al., 2006) and of no significant correlation (Parks et al., 2009). In Cushing’s syndrome, LTL and cortisol levels were not significantly related cross-sectionally, but LTL significantly lengthened after remission from the active disease (Aulinas et al., 2013a; Aulinas et al., 2014; Aulinas et al., 2013b). Studies are more, but not always, consistent in showing inverse relationships between LTL and dynamic aspects of cortisol secretion (e.g., waking-associated increases in cortisol or cortisol responses provoked by psychological stress) (Gotlib et al., 2014; Kroenke et al., 2011; Revesz et al., 2013; Tomiyama et al., 2012) as opposed to basal, resting or even circadian cortisol levels. Individuals with increased inflammation, higher cortisol awakening responses, and increased heart rates displayed progressively shorter telomeres as the number of such dysregulations increased (Revesz et al., 2013). However, short LTL has also been associated with hypocortisolism in MDD, at least as defined by a high degree of cortisol suppression after a dexamethasone challenge (Wikgren et al., 2012b), which the authors propose may reflect HPA axis “burnout” due to chronic stress (Wikgren et al., 2012b). Alternatively, this may reflect a heightened sensitivity of glucocorticoid receptors, which could result in greater negative feedback and greater dexamethasone suppression (Raison and Miller, 2003; Wolkowitz et al., 2011b). Lastly, certain anabolic hormones may be related to LTL. Stress-stimulated salivary testosterone levels were positively correlated with buccal cell TL, but resting, basal and circadian testosterone levels were not (Drury et al., 2014). Also, higher anabolic/ catabolic ratios (higher dehydroepiandrosterone sulfate and insulin-like growth factor-I levels, along with lower cortisol, catecholamine and IL-6 levels) in elderly subjects are associated with relatively longer LTL (Epel, 2009; Vasunilashorn and Cohen, 2014).
4.4. Effect of Psychotropic Medications on Leukocyte Telomere Length
Two studies in MDD (Verhoeven et al., 2014a; Wikgren et al., 2012b) and one in BD type II (Elvsåshagen et al., 2011) found no significant difference in LTL between those who were currently receiving psychoactive medication compared to those who were not, and one study found no difference between those on high dose versus low dose antidepressants (Hartmann et al., 2010). These findings must be interpreted cautiously, however, since only current or recent medication use was assessed, not the cumulative duration of prior medication use. Savolainen et al. (2012) reported that a mixed group of patients who had had psychiatric illnesses severe enough to require hospitalization had longer LTL than controls, but only if they had been prescribed psychotropic medication. This finding is difficult to interpret, however, since patients did not necessarily have current mental illnesses (patients were included if they had been psychiatrically hospitalized over approximately the preceding four decades), since patients were not randomized to medication, and since there may have been a survivor selection bias. As reviewed above, Martinsson et al. (2013) reported significantly increased LTL in individuals with BD treated with lithium compared to controls, and they hypothesized that lithium may increase TA, but this has not yet been empirically tested in humans. To our knowledge, only one unpublished study has yet directly assessed the impact of antipsychotic medication on TL in animals. Mice, administered atypical antipsychotics for two weeks (from the age of 8 weeks on) had lengthened hippocampal TL compared to untreated mice; typical antipsychotic medications did not share this effect (Toriumi et al., unpublished results).
5. Telomerase Activity (TA) in Psychiatric Illness
5.1. Overview
The ribonucleoprotein enzyme telomerase provides the major mechanism for telomere preservation and length replenishment (Armanios and Blackburn, 2012; Chakhparonian and Wellinger, 2003) (Fig 1). Insufficient TA in dividing or damaged cells reduces the cell’s ability to restore telomere length, conferring susceptibility to replicative senescence, apoptosis, cell death or genomic instability, as described above (Fig 2) (Armanios and Blackburn, 2012; Kelly, 2011; Sahin et al., 2011). Most normal human somatic cells have very little, if any, detectable TA, explaining their susceptibility to finite limits on cellular division. By contrast, germ-lineage cells, stem cells, progenitor cells, many rapidly dividing cells and cancerous cells typically have high TA.
Clinical and preclinical studies have demonstrated the importance of appropriate TA for organismic health and successful aging. Inherited telomerase deficiencies resulting in a two-fold drop in gene dosage are directly linked to malignancies and several other diseases in humans (Armanios and Blackburn, 2012). However, excessive levels of TA can also be detrimental. Mutations that increase expression of telomerase reverse transcriptase (TERT; the catalytic subunit of telomerase) by two-fold, cause large increases in risks of certain cancers (Horn et al., 2013; Huang et al., 2013). This evidence of a “just right” level of TA highlights the importance of its appropriate regulation throughout human life (Zalli et al., 2014). Indeed, more recent studies have begun examining the ratio of TA to LTL, since higher ratios, especially in the setting of lower telomere length, may indicate active cell stress and/or an unsuccessful compensatory attempt of telomerase to lengthen or maintain LTL (Brydon et al., 2012; Damjanovic et al., 2007; Jacobs et al., 2014; Kroenke et al., 2012; Wolkowitz et al., 2012; Zalli et al., 2014).
Apart from its role in telomere preservation and elongation, telomerase and TERT may have significant but poorly understood roles in cell protection via alternative mechanisms such as angiogenesis, mitochondrial protection, neurogenesis, neuronal survival and differentiation and blocking apoptosis and excitotoxicity (Bar et al., 2014; Li et al., 2011; Saretzki, 2014; Sahin et al., 2011), although nearly all of this evidence is from preclinical models, and the significnace for humans is uncertain. Adult mice completely deficient for telomerase displayed short, dysfunctional telomeres and a degenerative somatic phenotype (Jaskelioff et al., 2011). Remarkably, experimental re-activation of telomerase for as little as 4 weeks in these mice extended telomeres, reduced DNA damage signaling and reversed degenerative phenotypes across multiple organs, including the brain, where signs of neurodegeneration were reversed (Jaskelioff et al., 2011). Such findings raise the possibility that consequences of telomerase action may include not only the repair of certain types of age-associated cellular damage but also their reversal. Preclinical studies have also highlighted a number of aspects of brain TA that, together, may be relevant to depression, the brain and hippocampal integrity (Zhou et al., 2011): In mice, (1) chronic mild stress (CMS) lowered hippocampal TA; (2) fluoxetine treatment reversed the CMS-induced decreases in hippocampal TA; (3) inhibition of TA, either systemically or intra-hippocampally, resulted in “depression-like” behaviors in the mice and impaired hippocampal neurogenesis; (4) in contrast, overexpressing intra-hippocampal TA up-regulated neurogenesis, produced “antidepressant-like” behaviors and prevented CMS-induced behavioral changes; and (5) irradiation ablation of the dentate gyrus (the part of the hippocampus that retains the capacity for neurogenesis throughout adulthood) prevented the “antidepressantlike” effects of telomerase overexpression. These preclinical studies suggest that hippocampal TA in mice is involved in the regulation of “depression-like” behaviors and possibly “antidepressant-like” mechanisms, perhaps by regulating adult neurogenesis in the dentate gyrus (Zhou et al., 2011). A role of telomerase in neurogenesis is further supported by evidence that telomerase mediates neurotrophic effects of brain-derived neurotrophic factor (BDNF) in embryonic and early post-mitotic hippocampal neurons in rats (Fu et al., 2002). However, most of these studies have not yet been replicated. Another important caveat is that telomeres and telomerase are regulated differently in rodents and humans (Prowse and Greider, 1995), so caution is necessary in extrapolating these animal findings to man.
5.2. Telomerase Activity (TA) in Psychiatric Illnesses
While TA in psychiatric illnesses remains poorly studied, it has been better characterized in the context of psychological stress. Epel et al. (2004) reported that highly stressed caregiving mothers (who were generally healthy and not clinically depressed) had lower resting PBMC TA than low-stress mothers. In another study examining TA in resting – that is, unstimulated - PBMCs (“basal TA”), exposure to acute laboratory stress transiently increased the basal TA levels, both in proportion to the cortisol response to the stressor, and (in the low-stress women only) to the degree of anticipatory threat (Epel et al., 2010). Additional studies suggest that PBMC basal TA can be upregulated in stressful situations or in clinical depression. For example, in another caregiver study, but one in which many of in the caregivers had signs of clinical depression, short LTL was accompanied by increased PBMC basal TA (Damjanovic et al., 2007). Possible explanations for these divergent results are, firstly, that the caregiver mothers in the study by Epel et al (2004) were pre-menopausal whereas those in the Damjanovic et al. (2007) study were post-menopausal. Estrogen is a known regulator of TERT, a central telomerase gene. Furthermore, few of the caregiver mother subjects (Epel et al., 2004), but many in the study by Damjanovic et al. (2007) had clinical depression. However, the reasons for the opposite effects on PBMC basal TA are not understood. It has been speculated that the increased TA was “an unsuccessful attempt to compensate for the excessive loss of telomeres” (Damjanovic et al., 2007; Lin et al., 2012). The same explanation was suggested in two other studies (Brydon et al., 2012; Zalli et al., 2014), as well as in a small-scale study in MDD, in which un-medicated individuals with MDD had substantially elevated PBMC basal TA (Wolkowitz et al., 2012). Additionally, Tessyier et al. found that expression of TERT mRNA, while not significantly different in MDD and control groups, was positively correlated with depression and anxiety severity ratings in the combined sample of MDD subjects and controls (Teyssier et al., 2012). The only three other studies of TA in psychiatric illnesses reported that individuals with schizophrenia had nominally significantly decreased PBMC basal TA compared to unaffected individuals (Porton et al., 2008), and that individuals with BD (depressed phase) (Soeirode-Souza et al., 2014) and combat-related PTSD (Jergovic et al., 2014) had unaltered PBMC basal TA compared to controls. Methodological differences between studies, including sample preparation (Wolkowitz et al., 2012), may account for some variability.
While the mechanisms of these changes in PBMC basal TA are unknown, they may include changes in both transcriptional and fast-acting post-translational regulation of TA (Epel et al., 2010). Oxidative stress (Lopez-Diazguerrero et al., 2012; Maeda et al., 2013) and inflammation (Gizard et al., 2011; Goodman and Jain, 2011), in particular, may stimulate TA or expression of TERT, presumably as a compensatory, cell survival-enhancing mechanism (Lin et al., 2012). Cortisol may have significant effects on TA, as well, but the nature of these effects is unclear. In a human laboratory study of acute stress, PBMC basal TA rapidly and transiently increased in direct proportion to increases in psychological stress (threat perception) and to increases in serum cortisol (Epel et al., 2010). However, in a four-month study of a mindfulness intervention in overweight women, changes in PBMC TA across the four months were inversely correlated with changes in anxiety and changes in cortisol (Daubenmier et al., 2012). Preclinical data are also difficult to interpret. In one in vitro study, exposure of human lymphocytes to shortterm cortisol resulted in reduced PBMC TA and decreased expression of TERT (Choi et al., 2008). However, an in vivo study in male rats showed that extended exposure to unpredictable stress (which was associated with increased corticosterone responsivity to acute stress) increased PBMC TA, although correlations between changes in TA and changes in corticosterone were not reported (Beery et al., 2012). In summary, there have not yet been enough studies, especially large-scale ones, in psychiatric populations to assess possible changes in PBMC basal TA, although stress seems a potent regulator of TA, even though the direction and mechanisms of these effects are unknown at this time.
5.3. Effects of Psychotropic Medication on Telomerase Activity (TA)
Some pre-clinical data suggest that SSRIs and lithium can increase TA. For example, in the study reviewed above, fluoxetine increased TA in the hippocampus, and telomerase overexpression was reported to have direct antidepressant-like effects in mice (Zhou et al., 2011). More recently, Wei et al. (In press) reported short telomeres and reduced TERT expression and TA in the hippocampus of Flinders Sensitive Line rats, which are a genetic model of depression, compared to Flinders Resistant Line rats. They also found that lithium administration for 6 weeks significantly increased TERT expression and TA in the hippocampus of the Flinders Sensitive Line rats, thereby normalizing their baseline abnormalities (Wei et al. In press).
Clinical data are sparse and inconclusive. In a small-scale study, Wolkowitz et al. (2012) reported that unmedicated MDD subjects who had relatively low PBMC basal TA at baseline (prior to treatment and compared to the entire MDD group), and who had the greatest increases in PBMC basal TA over the course of treatment, showed superior antidepressant response to eight weeks of sertraline treatment. Across the entire sample (responders and non-responders to treatment), however, antidepressant treatment was not associated with significant changes in PBMC basal TA (Wolkowitz et al., 2012). These findings raise the possibility that depressed individuals with relatively low PBMC basal TA while un-medicated (compared to other depressed individuals) stand to gain the most from exogenous telomerase activation, and that telomerase activation may be a novel mechanism of action of some antidepressants, as also suggested by animal studies (Zhou et al., 2011). However, this finding will require replication with a larger sample, and the relationship between PBMC basal TA and brain/hippocampal TA is unknown. A recently published study in medication-free BD subjects (all in the depressed phase), reported that, while PBMC basal TA did not significantly change after six weeks of lithium monotherapy, lower PBMC basal TA at the end of treatment was associated with superior antidepressant responses to lithium (Soeiro-de-Souza et al., 2014). This latter finding should be interpreted cautiously, since inspection of the figure in that article suggests that one outlying data point contributed to the significant finding. In any event, these clinical studies are not fully comparable, since the former study found significant relationships between change in depression ratings vs. change in PBMC basal TA (Wolkowitz et al., 2012), whereas the latter study found significant relationships between change in depression ratings vs. cross-sectional PBMC basal TA after six-weeks of treatment (Soeiro-de-Souza et al., 2014). Other possible reasons for the discrepancy between these two studies are inadequate sample sizes, differences between unipolar and bipolar depression and, especially, differences between serotonin-specific reuptake inhibitor (SSRI) antidepressants and lithium, which may engage TA differently in achieving their clinical effects. Additional studies are critical to evaluate the role of telomerase in mental illness and mental health, to detect novel mechanisms of action of various treatments, and to address the important question of whether cell aging can be reversed or attenuated.
6. Relationship of Peripheral Cell Aging Markers to the Brain
The obvious question that arises when discussing a peripheral marker in psychiatric illness is whether the marker directly reflects some aspect of brain function relevant to mental illness. It is plausible that LTL is correlated with TL in certain brain tissues, since TL’s are often inter-correlated across some tissues (including skeletal muscle, skin, subcutaneous fat, and cerebral cortex) (Daniali et al., 2013; Gadalla et al., 2010; Mitchell et al., 2014; Takubo et al., 2002), but not necessarily across all tissues (Dlouha et al., 2014) within the same individual, even though the absolute length of the telomeres varies across tissues. The rates of telomere shortening over time are also similar across tissues, at least for leukocytes, skeletal muscle, skin and subcutaneous fat (Daniali et al., 2013). Further, to the extent LTL is shortened by systemic inflammation or oxidative stress, these systemic conditions may affect telomeres in brain cells that are also sensitive to inflammation or oxidative stress. Despite these theoretical possibilities, the actual relationship between LTL and telomere length in brain cells is unknown. Two post-mortem studies of TL in cerebellar gray matter and occipital cortex found no significant differences between MDD subjects and controls, although correlations with LTL were not assessed (Teyssier et al., 2010; Zhang et al., 2010). Cerebellar and occipital gray matter, however, might be less affected by mitosis-related telomere shortening, compared to the areas like the hippocampus, since mitotic neuronal precursor cells (e.g., neuronal stem cells and progenitor cells) are found in the subventricular zone and the dentate gyrus. Apart from neurons and neural stem/ precursor cells, certain other brain cells are particularly sensitive to oxidative stress, such as oligodendrocytes. Szebeni and colleagues, studying the autopsied brains of individuals who had had MDD during their lives, reported decreased TL, decreased TERT expression and decreased antioxidant enzymes in oligodendrocytes in two white matter regions implicated in MDD (Szebeni et al., 2014).
Neuroimaging research is just beginning to assess the relationship between LTL and hippocampal volume in MDD. Wikgren et al found that, while shorter LTL was correlated with greater subcortical atrophy and more white matter hyper-intensities (Wikgren et al., 2013), shorter LTL was correlated with larger hippocampal volume. This was found in non-demented apolipoprotein E ε3/ε3 carriers, but not in non-demented apolipoprotein E ε4 carriers (Wikgren et al., 2012a). The authors interpreted their finding in the non-demented apolipoprotein E ε3/ε3 carriers as being consistent with greater overall cellular proliferation in leukocytes as well as in the hippocampus, which would lead to relatively shorter LTL due to more frequent mitoses but relatively larger hippocampal volume due to more neurogenesis in the dentate gyrus. A more recent MRI study found the opposite relationship, however. In apolipoprotein E ε3/ε3 carriers, LTL was directly correlated with HC volume, which was interpreted as evidence of “coordinated chromosomal and neural aging” (Jacobs et al., 2014). A recent population-based study, not specifically in MDD, found that LTL was positively associated with total cerebral volume, including white and cortical matter gray volume, as well as with hippocampal volume and volumes of several other sub-regions (King et al., 2014). These correlations were generally more robust in relatively older individuals, but remained significant after adjusting for multiple covariates, including age, gender and cardiovascular risk factors
Very few studies have examined relationships between PBMC basal TA and brain TA or brain structural volumes. A study in abstinent heroin users noted significant positive correlations between PBMC basal TA and white and gray matter volume in the right dorsolateral prefrontal cortex (Cheng et al., 2013). The authors suggested that their data in abstinent heroin users suggest premature aging at both the cellular and brain system levels (Cheng et al., 2013). It is not clear from the paper whether other brain regions were also examined. Of potential importance in interpreting these data, the abstinent heroin use group also had significantly higher depression and anxiety ratings than the controls, making attribution of the findings difficult. Another important consideration in interpreting that study is the impact of the heroin use history on this relationship, as these correlations were not seen in the control group (Cheng et al., 2013). To our knowledge, only one study has assessed the relationship of PBMC basal TA to hippocampal volume in MDD. Wolkowitz et al. (In press) reported a significant positive correlation between PBMC basal TA and hippocampal volume in a small group of unmedicated individuals with MDD but not in healthy controls. The authors interpreted these results as being consistent with those of the preclinical study by Zhou et al. (2011), reviewed above.
In summary, peripheral markers of cell aging are the only markers that are practical to obtain in living humans. Without determining their relationship to neural process that are presumably more closely related to psychiatric illnesses, their ultimate informational value will be limited. There are enough promising leads to justify further trials comparing peripheral and central markers using various forms of neuroimaging in living humans, as well as autopsied human brain specimens and animal modeling.
7. Is Cellular Aging Preventable or Reversible?
That shortened LTL may accompany certain psychiatric illnesses raises the intriguing possibility that appropriate treatment (or prevention) of the psychiatric illnesses might slow cell aging or even lead to telomere lengthening (Rizzo et al., 2014; Wolkowitz et al., 2011b), even though causality has yet to be demonstrated. While few pharmacologic studies have examined this, several behavioral and psychological intervention studies in non-psychiatric populations have examined the effects on PBMC basal TA or LTL of interventions ranging from intensive lifestyle modification, mindful eating, mindfulness-based stress reduction and various types of meditation (see Verhoeven et al. 2014b for an overview), although it is unknown if these results would generalize to psychiatric populations. Further, these studies have often been non-randomized and not adequately controlled. These studies have generally found intervention-associated increases in PBMC basal TA (Epel, 2012; Epel et al., 2009b; Epel, 2009; Jacobs et al., 2011; Lengacher et al., 2014; Ornish et al., 2008; Schutte and Malouff, 2014b; Starkweather et al., 2014). A recent metaanalysis of four studies with randomized controlled designs (Jacobs et al., 2011; Daubenmier et al., 2012; Ho et al., 2012; Lavretsky et al., 2013) found an effect size of d= 0.46 for meditation intervention increasing PBMC basal TA (Schutte and Malouff, 2014b). However, it is obviously difficult to double-blind such interventions. In three of these studies, the control condition was a “waitlist” (Jacobs et al., 2011; Daubenmier et al., 2012; Ho et al., 2012) and in the other was “relaxation” (Lavretsky et al., 2013). Nonetheless, several studies reported “dose-response” relationships, with greater improvement in mental well-being or sense of purpose in life or greater adherence to the behavioral intervention, associating with larger increases in PBMC basal TA (Epel, 2012; Jacobs et al., 2011). Jacobs et al., (2011) found that retreat participants, meditating for 6 hours daily for 3 months, had greater PBMC basal TA at the end of the three months than did a waitlist control group. A caveat when interpreting that study, however, is that pre-test PBMC basal TA measures were not available for the meditation group, thus the analyses relied on cross-sectional post-meditation data only (Jacobs et al., 2011). Lengacher et al. (2014) showed, in a sample of breast cancer patients, that mindfulness-based stress reduction for six weeks significantly increased PBMC basal TA compared to a waitlist control group. In this study, baseline PBMC basal TA was controlled for, but potential limitations were that the active group was heterogeneous in terms of treatment received and time since treatment completion. Moreover, the treatment program was relatively short and thus only allowed for short-term assessment of PBMC basal TA. In a pilot non-randomized study in low-risk prostate cancer patients, Ornish et al. (2008) showed that three-months of comprehensive lifestyle changes were associated with significant increases in PBMC basal TA accompanied by a decrease in psychological distress. A potential limitation of this study was the lack of a control group and the fact that only 30 of 126 eligible patients agreed to participate in the study after learning the details and the fact that all participants were male, which may affect generalizability of the findings. A five-year follow-up of ten of the original participants from that study showed that their LTL significantly increased from baseline relative to control men whose prostate cancer was simply followed by active surveillance, although their PBMC basal TA had decreased somewhat compared to the baseline measurement (Ornish et al., 2013). This study was limited by the small sample size (10 subjects in the intervention group and 25 controls) and by the non-randomized study design, which may introduce unknown sources of bias.
The literature on exercise and telomere length is inconsistent but generally suggests that exercise is associated with a telomere-protective phenotype in leukocytes and skeletal muscles (Ludlow et al., 2013). One study found that aspects of “multi-system resiliency” and positive lifestyle (e.g., social support, good emotion regulation, sleep and exercise), collectively but not individually, statistically attenuated the negative relationship between MDD and LTL (Puterman et al., 2013). Analyses in this study were cross-sectional, thus causality cannot be inferred, and data on diet, another lifestyle factor that can influence LTL, were not available. The same group of investigators previously found, in a group of caregiving and non-caregiving post-menopausal women, that highly stressed women have shorter telomeres, but only if they are inactive, suggesting a protective effect of exercise (Puterman et al., 2010). Since that study was cross-sectional, though, it is difficult to draw causal interpretations, especially since the more highly stressed women were less likely to be physically active. Similarly, a prospective study of healthy post-menopausal women followed over the course of one year found that major life stresses during the preceding year were associated with significant telomere shortening over the one-year period, but that this effect was significantly attenuated in women with positive health behaviors (leisure time physical activity, healthy dietary practices and good sleep quality) (Puterman et al., 2015). This study is important, as it is one of the only prospective longitudinal studies to examine stress-related changes in TL and possible moderators of this relationship. However, the measures of stress and of health behaviors were self-reported, possibly influencing the results. Further, health behaviors may have been spuriously associated with LTL if physical diseases (that could, themselves, lessen healthful health behaviors) bore the primary relationship with LTL. One additional small-scale study involving telephone-based psychological stress-reduction counseling in 22 women with cervical cancer (Biegler et al., 2012), found no significant overall change in LTL following four months of counseling, but did find that changes in distress ratings over that time period were inversely correlated with changes in LTL (Biegler et al., 2012). This study, however, had no control group, and four months may be too short to discern significant changes in LTL.
The behavioral/ psychological/ lifestyle intervention literature relating these interventions to PBMC basal TA and LTL is intriguing and points in the hypothesized direction, but it is limited by the small-scale, non-randomized, non-blinded design of the studies, as well as, in most cases, the short duration of the interventions. The putative effects of certain of these interventions on cellular aging may be mediated by improvements in stress arousal and lessening of threat cognitions and ruminative thought (Epel et al., 2009b), although the biochemical intermediaries remain to be determined.
8. Summary
The study of LTL, PBMC basal TA and other indices of cell aging in psychiatric illnesses is just beginning, but further advances are expected to develop rapidly. A modest preponderance of the evidence reviewed here suggests that several psychiatric illnesses (viz., MDD, anxiety disorders and PTSD) may be associated with accelerated cellular aging, as indexed by short LTL and possibly by altered PBMC basal TA, although conflicting reports exist, and no conclusions can yet be made. Methodological differences between studies, as well as many studies with small sample sizes, make conclusions difficult to draw. Although the reasons for the discrepancies between studies are unknown, possibilities include different subject demographics (e.g., age, gender, race, socioeconomic status, history of childhood adversity (O'Donovan et al., 2011a; Price et al., 2013)), different study designs, differences in duration or severity of the investigated illness (Wolkowitz et al., 2011a), different specimen processing and assay protocols (Aviv et al., 2006; Montpetit et al., 2014; Nieratschker et al., 2013) and, importantly, differences in moderators of LTL and PBMC basal TA that are often not assessed, e.g., genetic risk-alleles (Armanios and Blackburn, 2012; Codd et al 2013), including ApoE status (Jacobs et al., 2013; Takata et al., 2012; Wikgren et al., 2012a), cognitive threat appraisal (O'Donovan et al., 2012), pessimistic outlook (O'Donovan et al., 2009), arousal and regulatory system activation (Epel, 2009) and stress resiliency factors (Puterman et al., 2013). For example, recent data suggest that “high risk” genetic polymorphisms in the serotonin and dopamine systems may interact with early life adversity to affect adult LTL (Mitchell et al., 2014). Short LTL is unlikely to be specific to any one categorical psychiatric illness. If it occurs, it is more likely related to underlying trans-diagnostic biological abnormalities or behavioral dimensions/ phenotypes (Wolkowitz et al., 2010). It is not yet known whether combining LTL measures with psychiatric disorder status may inform clinical practices. For example, a recent study found that the combination of short telomere length with depression was (compared with either measure alone) a strong prospective predictor of both cancer disease progression and mortality in a cohort of bladder cancer patients (Lin et al., 2015). Further study of cell aging in psychiatric illnesses and of its moderators and mediators may even help define more homogenous diagnoses based on underlying biological aspects of pathophysiology. A significant remaining question is whether peripheral LTL and PBMC basal TA reflect brain processes that are relevant to mental illness.
Evidence of significant LTL shortening in psychiatric illness would be cause for concern, since shorter LTL has been linked to current and future medical illnesses and to premature mortality, although causality has not been demonstrated. More research is needed to define possible roles of telomerase and TERT in psychiatric illnesses, but initial preclinical and clinical findings are provocative and suggest possible roles in hippocampal neurogenesis and in the actions of antidepressants. Risk factors and mechanisms for accelerated cell aging in humans are just beginning to be understood, and longitudinal studies will be needed to infer causality as well as to address the important questions of timing, prevention and reversibility of cell aging. If LTL attrition is related to psychiatric disease in a “dose-response” relationship, it will be important to determine whether lessening the “dose” of the disease by adequate treatment will help preserve LTL or reverse telomere attrition. In an otherwise concerning picture, findings that certain positive lifestyle changes are correlated with lowered degrees of cell aging (as indexed by LTL and/or PBMC basal TA) (Epel, 2009; Epel, 2012; Hoge et al., 2013; Puterman et al., 2010; Puterman et al., 2015) provide some encouragement, although many of the studies were small-scale, open-label or inadequately controlled. Thus, more prospective longitudinal, large-scale, well-controlled studies are required. Since subjective stress ratings and the anticipation of threat bear closer relationships to cell aging than do objective stressors (Epel et al., 2004; O'Donovan et al., 2012), it is conceivable that psychotherapy and stress coping mechanisms might also attenuate stressassociated cell aging (Biegler et al., 2012), but this has not been well-studied.
Measuring LTL and PBMC basal TA may someday prove to be useful biomarkers in personalized medicine for staging disease progression and disease risk and for selecting treatments (Hochstrasser et al., 2012), but there is certainly insufficient research yet to support this. Further, insufficient calibration of assay methods across labs and the lack of accepted “normal ranges” for LTL and PBMC basal TA make it premature for cell aging markers to enter clinical use at this time (Martin-Ruiz et al., In press). The relatively small effect sizes reported in reviewed positive studies, as well as the lack of diagnostic specificity of LTL and PBMC basal TA changes, also argue against the use of such markers as diagnostic tools, in isolation from other measures, at this time. As the mechanistic relationships between psychiatric illnesses, biological aging and comorbid physical illnesses become clearer, psychiatric illnesses may come to be understood as systemic illnesses with specific mental manifestations rather than as purely mental or even purely brain diseases, thus expanding the range of therapeutic targets and diminishing the stigma associated with these illnesses.
Highlights.
-
▫
Cellular aging is assessed in leukocytes by telomere length and telomerase activity
-
▫
Several psychiatric illnesses may be associated with accelerated cellular aging
-
▫
This may contribute to or reflect an increased risk of medical illness and mortality
-
▫
Further investigation may lead to new treatments and preventative strategies
Acknowledgments
FUNDING
This work was supported by NIMH R01MH083784 (OMW, ESE, SHM), DOD W81XWH-10-1-0021 (OMW), the O’Shaughnessy Foundation and the Tinberg Family, The Bernard Osher Foundation (EHB and JL), NIA R01AG030424-01A1 (ESE & OMW), NWO-VICI 91811602 (Netherlands; BWP, JV, DR); The Swedish Society of Medicine (DL), the Sjobring Foundation (DL), OM Persson Foundation (DL) and the province of Scania (Sweden) state grants (ALF; DL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
JL is a consultant to Telomere Diagnostics Inc., formerly Telome Health, and owns stock in the company. The company had no role in this research or in writing this review. The remaining authors report no current disclosures or conflicts of interest.
REFERENCES
- Adler N, Pantell MS, O'Donovan A, Blackburn E, Cawthon R, Koster A, Opresko P, Newman A, Harris TB, Epel E. Educational attainment and late life telomere length in the Health, Aging and Body Composition Study. Brain Behav Immun. 2013;27:15–21. doi: 10.1016/j.bbi.2012.08.014. 10.1016/j.bbi.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. fifth ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Anthes E. Ageing: Live faster, die younger. Nature. 2014;508:S16–S17. doi: 10.1038/508S16a. 10.1038/508S16a. [DOI] [PubMed] [Google Scholar]
- Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13:693–704. doi: 10.1038/nrg3246. 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asok A, Bernard K, Roth TL, Rosen JB, Dozier M. Parental responsiveness moderates the association between early-life stress and reduced telomere length. Dev Psychopathol. 2013;25:577–585. doi: 10.1017/S0954579413000011. 10.1017/S0954579413000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert G, Hills M, Lansdorp PM. Telomere length measurement-caveats and a critical assessment of the available technologies and tools. Mutat Res. 2012;730:59–67. doi: 10.1016/j.mrfmmm.2011.04.003. 10.1016/j.mrfmmm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulinas A, Ramirez MJ, Barahona MJ, Mato E, Bell O, Surralles J, Webb SM. Telomeres and endocrine dysfunction of the adrenal and GH/IGF-1 axes. Clin Endocrinol (Oxf) 2013a;79:751–759. doi: 10.1111/cen.12310. 10.1111/cen.12310. [DOI] [PubMed] [Google Scholar]
- Aulinas A, Ramirez MJ, Barahona MJ, Valassi E, Resmini E, Mato E, Santos A, Crespo I, Bell O, Surralles J, Webb S. Telomere length analysis in Cushing's syndrome. Eur J Endocrinol. 2014;171:21–29. doi: 10.1530/EJE-14-0098. 10.1530/EJE-14-0098. [DOI] [PubMed] [Google Scholar]
- Aulinas A, Santos A, Valassi E, Mato E, Crespo I, Resmini E, Roig O, Bell O, Webb SM. Telomeres, aging and Cushing's syndrome: are they related? Endocrinol Nutr. 2013b;60:329–335. doi: 10.1016/j.endonu.2012.10.002. 10.1016/j.endonu.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Aviv A. Genetics of leukocyte telomere length and its role in atherosclerosis. Mutat Res. 2012;730:68–74. doi: 10.1016/j.mrfmmm.2011.05.001. 10.1016/j.mrfmmm.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 2011;39:e134. doi: 10.1093/nar/gkr634. 10.1093/nar/gkr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, Valdes AM, Spector TD. Human telomere biology: pitfalls of moving from the laboratory to epidemiology. Int J Epidemiol. 2006;35:1424–1429. doi: 10.1093/ije/dyl169. [DOI] [PubMed] [Google Scholar]
- Bar C, Bernardes de Jesus B, Serrano R, Tejera A, Ayuso E, Jimenez V, Formentini I, Bobadilla M, Mizrahi J, de Martino A, Gomez G, Pisano D, Mulero F, Wollert KC, Bosch F, Blasco MA. Telomerase expression confers cardioprotection in the adult mouse heart after acute myocardial infarction. Nat Commun. 2014;5:5863. doi: 10.1038/ncomms6863. 10.1038/ncomms6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Lin J, Biddle JS, Francis DD, Blackburn EH, Epel ES. Chronic stress elevates TA in rats. Biol Lett. 2012;8:1063–1066. doi: 10.1098/rsbl.2012.0747. 10.1098/rsbl.2012.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegler KA, Anderson AK, Wenzel LB, Osann K, Nelson EL. Longitudinal change in telomere length and the chronic stress response in a randomized pilot biobehavioral clinical study: implications for cancer prevention. Cancer Prev Res (Phila) 2012;5:1173–1182. doi: 10.1158/1940-6207.CAPR-12-0008. 10.1158/1940-6207.CAPR-12-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boks MP, Mierlo HC, Rutten BP, Radstake TR, De Witte L, Geuze E, Horvath S, Schalkwyk LC, Vinkers CH, Broen JC, Vermetten E. Longitudinal changes of telomere length and epigenetic age related to traumatic stress and post-traumatic stress disorder. Psychoneuroendocrinology. 2015;51:506–512. doi: 10.1016/j.psyneuen.2014.07.011. 10.1016/j.psyneuen.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M. The effects of stress on memory and the hippocampus throughout the life cycle: implications for childhood development and aging. Dev Psychopathol. 1998;10:871–885. doi: 10.1017/s0954579498001916. [DOI] [PubMed] [Google Scholar]
- Broer L, Codd V, Nyholt DR, Deelen J, Mangino M, Willemsen G, Albrecht E, Amin N, Beekman M, de Geus EJ, Henders A, Nelson CP, Steves CJ, Wright MJ, de Craen AJ, Isaacs A, Matthews M, Moayyeri A, Montgomery GW, Oostra BA, Vink JM, Spector TD, Slagboom PE, Martin NG, Samani NJ, van Duijn CM, Boomsma DI. Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet. 2013;21:1163–1168. doi: 10.1038/ejhg.2012.303. 10.1038/ejhg.2012.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Lin J, Butcher L, Hamer M, Erusalimsky JD, Blackburn EH, Steptoe A. Hostility and cellular aging in men from the Whitehall II cohort. Biol Psychiatry. 2012;71:767–773. doi: 10.1016/j.biopsych.2011.08.020. 10.1016/j.biopsych.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canela A, Vera E, Klatt P, Blasco MA. High-throughput telomere length quantification by FISH and its application to human population studies. Proc Natl Acad Sci U S A. 2007;104:5300–5305. doi: 10.1073/pnas.0609367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Chakhparonian M, Wellinger RJ. Telomere maintenance and DNA replication: how closely are these two connected? Trends Genet. 2003;19:439–446. doi: 10.1016/S0168-9525(03)00135-5. 10.1016/S0168-9525(03)00135-5. [DOI] [PubMed] [Google Scholar]
- Cheng GL, Zeng H, Leung MK, Zhang HJ, Lau BW, Liu YP, Liu GX, Sham PC, Chan CC, So KF, Lee TM. Heroin abuse accelerates biological aging: a novel insight from telomerase and brain imaging interaction. Transl Psychiatry. 2013;3:e260. doi: 10.1038/tp.2013.36. 10.1038/tp.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Fauce SR, Effros RB. Reduced TA in human T lymphocytes exposed to cortisol. Brain Behav Immun. 2008;22:600–605. doi: 10.1016/j.bbi.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd V, Mangino M, van der Harst P, Braund PS, Kaiser M, Beveridge AJ, Rafelt S, Moore J, Nelson C, Soranzo N, Zhai G, Valdes AM, Blackburn H, Mateo Leach I, de Boer RA, Kimura M, Aviv A, Goodall AH, Ouwehand W, van Veldhuisen DJ, van Gilst WH, Navis G, Burton PR, Tobin MD, Hall AS, Thompson JR, Spector T, Samani NJ Wellcome Trust Case Control, C. Common variants near TERC are associated with mean telomere length. Nat Genet. 2010;42:197–199. doi: 10.1038/ng.532. 10.1038/ng.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd V, Nelson CP, Albrecht E, Mangino M, Deelen J, Buxton JL, Hottenga JJ, Fischer K, Esko T, Surakka I, Broer L, Nyholt DR, Mateo Leach I, Salo P, Hagg S, Matthews MK, Palmen J, Norata GD, O'Reilly PF, Saleheen D, Amin N, Balmforth AJ, Beekman M, de Boer RA, Bohringer S, Braund PS, Burton PR, de Craen AJ, Denniff M, Dong Y, Douroudis K, Dubinina E, Eriksson JG, Garlaschelli K, Guo D, Hartikainen AL, Henders AK, Houwing-Duistermaat JJ, Kananen L, Karssen LC, Kettunen J, Klopp N, Lagou V, van Leeuwen EM, Madden PA, Magi R, Magnusson PK, Mannisto S, McCarthy MI, Medland SE, Mihailov E, Montgomery GW, Oostra BA, Palotie A, Peters A, Pollard H, Pouta A, Prokopenko I, Ripatti S, Salomaa V, Suchiman HE, Valdes AM, Verweij N, Vinuela A, Wang X, Wichmann HE, Widen E, Willemsen G, Wright MJ, Xia K, Xiao X, van Veldhuisen DJ, Catapano AL, Tobin MD, Hall AS, Blakemore AI, van Gilst WH, Zhu H, Consortium C, Erdmann J, Reilly MP, Kathiresan S, Schunkert H, Talmud PJ, Pedersen NL, Perola M, Ouwehand W, Kaprio J, Martin NG, van Duijn CM, Hovatta I, Gieger C, Metspalu A, Boomsma DI, Jarvelin MR, Slagboom PE, Thompson JR, Spector TD, van der Harst P, Samani NJ. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45:422–427. 427e421–422e421. doi: 10.1038/ng.2528. 10.1038/ng.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JM, Johnson RA, Litzelman K, Skinner HG, Seo S, Engelman CD, Vanderboom RJ, Kimmel GW, Gangnon RE, Riegert-Johnson DL, Baron JA, Potter JD, Haile R, Buchanan DD, Jenkins MA, Rider DN, Thibodeau SN, Petersen GM, Boardman LA. Telomere length varies by DNA extraction method: implications for epidemiologic research. Cancer Epidemiol Biomarkers Prev. 2013;22:2047–2054. doi: 10.1158/1055-9965.EPI-13-0409. 10.1158/1055-9965.EPI-13-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello MJ, Ross SA, Briel M, Anand SS, Gerstein H, Pare G. The Association Between Shortened Leukocyte Telomere Length and Cardio-Metabolic Outcomes: A Systematic Review and Meta-Analysis. Circ Cardiovasc Genet. 2014 doi: 10.1161/CIRCGENETICS.113.000485. 10.1161/CIRCGENETICS.113.000485. [DOI] [PubMed] [Google Scholar]
- Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, Zou Y, Beversdorf DQ, Weng NP. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer's disease patients. J Immunol. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, Desai K, Granick M, Aviv A. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013;4:1597. doi: 10.1038/ncomms2602. 10.1038/ncomms2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmier J, Lin J, Blackburn E, Hecht FM, Kristeller J, Maninger N, Kuwata M, Bacchetti P, Havel PJ, Epel E. Changes in stress, eating, and metabolic factors are related to changes in TA in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology. 2012;37:917–928. doi: 10.1016/j.psyneuen.2011.10.008. 10.1016/j.psyneuen.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyer T. Telomere length integrates psychological factors in the successful aging story, but what about the biology? Psychosom Med. 2011;73:524–527. doi: 10.1097/PSY.0b013e31822ed876. 10.1097/PSY.0b013e31822ed876. [DOI] [PubMed] [Google Scholar]
- Dlouha D, Maluskova J, Kralova Lesna I, Lanska V, Hubacek JA. Comparison of the relative telomere length measured in leukocytes and eleven different human tissues. Physiol Res. 2014;63(Suppl 3):S343–S350. doi: 10.33549/physiolres.932856. [DOI] [PubMed] [Google Scholar]
- Douillard-Guilloux G, Guilloux JP, Lewis DA, Sibille E. Anticipated brain molecular aging in major depression. Am J Geriatr Psychiatry. 2013;21:450–460. doi: 10.1016/j.jagp.2013.01.040. 10.1016/j.jagp.2013.01.040 10.1097/JGP.0b013e318266b7ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury SS, Shirtcliff EA, Shachet A, Phan J, Mabile E, Brett ZH, Wren M, Esteves K, Theall KP. Growing up or growing old? Cellular aging linked with testosterone reactivity to stress in youth. Am J Med Sci. 2014;348:92–100. doi: 10.1097/MAJ.0000000000000299. 10.1097/MAJ.0000000000000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB. Kleemeier Award Lecture 2008--the canary in the coal mine: telomeres and human healthspan. J Gerontol A Biol Sci Med Sci. 2009;64:511–515. doi: 10.1093/gerona/glp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB. Telomere/telomerase dynamics within the human immune system: effect of chronic infection and stress. Exp Gerontol. 2011;46:135–140. doi: 10.1016/j.exger.2010.08.027. 10.1016/j.exger.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan E, Hutchison ER, Mattson MP. Telomere shortening in neurological disorders: an abundance of unanswered questions. Trends Neurosci. 2014;37:256–263. doi: 10.1016/j.tins.2014.02.010. 10.1016/j.tins.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvsashagen T, Vera E, Boen E, Bratlie J, Andreassen OA, Josefsen D, Malt UF, Blasco MA, Boye B. The load of short telomeres is increased and associated with lifetime number of depressive episodes in bipolar II disorder. J Affect Disord. 2011;135:43–50. doi: 10.1016/j.jad.2011.08.006. 10.1016/j.jad.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens) 2009;8:7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- Epel ES. How "reversible" is telomeric aging? Cancer Prev Res (Phila) 2012;5:1163–1168. doi: 10.1158/1940-6207.CAPR-12-0370. 10.1158/1940-6207.CAPR-12-0370. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Daubenmier J, Moskowitz JT, Folkman S, Blackburn E. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009b;1172:34–53. doi: 10.1111/j.1749-6632.2009.04414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Lin J, Dhabhar FS, Wolkowitz OM, Puterman E, Karan L, Blackburn EH. Dynamics of TA in response to acute psychological stress. Brain Behav Immun. 2010;24:531–539. doi: 10.1016/j.bbi.2009.11.018. 10.1016/j.bbi.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, Dolbier C, Mendes WB, Blackburn EH. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Epel ES, Merkin SS, Cawthon R, Blackburn EH, Adler NE, Pletcher MJ, Seeman TE. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging. 2009a;1:81–88. doi: 10.18632/aging.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Puterman E, Lin J, Blackburn E, Lazaro A, Mendes W. Wandering minds and aging cells. Clin Psychol Sci. 2013;1:75–83. [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O'Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P, Jr, Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Fava M, Uebelacker LA, Alpert JE, Nierenberg AA, Pava JA, Rosenbaum JF. Major depressive subtypes and treatment response. Biol Psychiatry. 1997;42:568–576. doi: 10.1016/S0006-3223(96)00440-4. [DOI] [PubMed] [Google Scholar]
- Fernandez-Egea E, Bernardo M, Heaphy CM, Griffith JK, Parellada E, Esmatjes E, Conget I, Nguyen L, George V, Stoppler H, Kirkpatrick B. Telomere length and pulse pressure in newly diagnosed, antipsychotic-naive patients with nonaffective psychosis. Schizophr Bull. 2009;35:437–442. doi: 10.1093/schbul/sbn169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Kimura M, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Hardikar S, Aviv A. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2011;66:421–429. doi: 10.1093/gerona/glq224. 10.1093/gerona/glq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanary BE, Streit WJ. Progressive telomere shortening occurs in cultured rat microglia, but not astrocytes. Glia. 2004;45:75–88. doi: 10.1002/glia.10301. 10.1002/glia.10301. [DOI] [PubMed] [Google Scholar]
- Friedrich U, Griese E, Schwab M, Fritz P, Thon K, Klotz U. Telomere length in different tissues of elderly patients. Mech Ageing Dev. 2000;119:89–99. doi: 10.1016/s0047-6374(00)00173-1. [DOI] [PubMed] [Google Scholar]
- Fu W, Lu C, Mattson MP. Telomerase mediates the cell survival-promoting actions of brain-derived neurotrophic factor and secreted amyloid precursor protein in developing hippocampal neurons. J Neurosci. 2002;22:10710–10719. doi: 10.1523/JNEUROSCI.22-24-10710.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadalla SM, Cawthon R, Giri N, Alter BP, Savage SA. Telomere length in blood, buccal cells, and fibroblasts from patients with inherited bone marrow failure syndromes. Aging (Albany NY) 2010;2:867–874. doi: 10.18632/aging.100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rizo C, Fernandez-Egea E, Miller BJ, Oliveira C, Justicia A, Griffith JK, Heaphy CM, Bernardo M, Kirkpatrick B. Abnormal glucose tolerance, white blood cell count, and telomere length in newly diagnosed, antidepressant-naive patients with depression. Brain, Behavior, & Immunity. 2013;28:49–53. doi: 10.1016/j.bbi.2012.11.009. 10.1016/j.bbi.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Bann D, Wiley L, Cooper R, Hardy R, Nitsch D, Martin-Ruiz C, Shiels P, Sayer AA, Barbieri M, Bekaert S, Bischoff C, Brooks-Wilson A, Chen W, Cooper C, Christensen K, De Meyer T, Deary I, Der G, Diez Roux A, Fitzpatrick A, Hajat A, Halaschek-Wiener J, Harris S, Hunt SC, Jagger C, Jeon HS, Kaplan R, Kimura M, Lansdorp P, Li C, Maeda T, Mangino M, Nawrot TS, Nilsson P, Nordfjall K, Paolisso G, Ren F, Riabowol K, Robertson T, Roos G, Staessen JA, Spector T, Tang N, Unryn B, van der Harst P, Woo J, Xing C, Yadegarfar ME, Park JY, Young N, Kuh D, von Zglinicki T, Ben-Shlomo Y Halcyon study, t. Gender and telomere length: systematic review and meta-analysis. Exp Gerontol. 2014;51:15–27. doi: 10.1016/j.exger.2013.12.004. 10.1016/j.exger.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgin-Lavialle S, Moura DS, Bruneau J, Chauvet-Gelinier JC, Damaj G, Soucie E, Barete S, Gacon AL, Grandpeix-Guyodo C, Suarez F, Launay JM, Durieu I, Esparcieux A, Guichard I, Sparsa A, Nicolini F, Gennes CD, Trojak B, Haffen E, Vandel P, Lortholary O, Dubreuil P, Bonin B, Sultan S, Teyssier JR, Hermine O. Leukocyte telomere length in mastocytosis: Correlations with depression and perceived stress. Brain Behav Immun. 2013 doi: 10.1016/j.bbi.2013.07.009. 10.1016/j.bbi.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Gildengers AG, Chisholm D, Butters MA, Anderson SJ, Begley A, Holm M, Rogers JC, Reynolds CF, 3rd, Mulsant BH. Two-year course of cognitive function and instrumental activities of daily living in older adults with bipolar disorder: evidence for neuroprogression? Psychol Med. 2013;43:801–811. doi: 10.1017/S0033291712001614. 10.1017/S0033291712001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizard F, Heywood EB, Findeisen HM, Zhao Y, Jones KL, Cudejko C, Post GR, Staels B, Bruemmer D. Telomerase activation in atherosclerosis and induction of telomerase reverse transcriptase expression by inflammatory stimuli in macrophages. Arterioscler Thromb Vasc Biol. 2011;31:245–252. doi: 10.1161/ATVBAHA.110.219808. 10.1161/ATVBAHA.110.219808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WA, Jain MK. Length does not matter: a new take on telomerase reverse transcriptase. Arterioscler Thromb Vasc Biol. 2011;31:235–236. doi: 10.1161/ATVBAHA.110.220343. 10.1161/ATVBAHA.110.220343. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, LeMoult J, Colich NL, Foland-Ross LC, Hallmayer J, Joormann J, Lin J, Wolkowitz OM. Telomere length and cortisol reactivity in children of depressed mothers. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.119. 10.1038/mp.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Rodrigues F, Santana-Lemos BA, Scheucher PS, Alves-Paiva RM, Calado RT. Direct Comparison of Flow-FISH and qPCR as Diagnostic Tests for Telomere Length Measurement in Humans. PLoS One. 2014;9:e113747. doi: 10.1371/journal.pone.0113747. 10.1371/journal.pone.0113747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann N, Boehner M, Groenen F, Kalb R. Telomere length of patients with major depression is shortened but independent from therapy and severity of the disease. Depress Anxiety. 2010;27:1111–1116. doi: 10.1002/da.20749. 10.1002/da.20749. [DOI] [PubMed] [Google Scholar]
- Hassett AL, Epel E, Clauw DJ, Harris RE, Harte SE, Kairys A, Buyske S, Williams DA. Pain is associated with short leukocyte telomere length in women with fibromyalgia. J Pain. 2012;13:959–969. doi: 10.1016/j.jpain.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Heuser I. Depression, endocrinologically a syndrome of premature aging? Maturitas. 2002;41(Suppl 1):S19–S23. doi: 10.1016/s0378-5122(02)00012-9. [DOI] [PubMed] [Google Scholar]
- Hochstrasser T, Marksteiner J, Humpel C. Telomere length is age-dependent and reduced in monocytes of Alzheimer patients. Exp Gerontol. 2012;47:160–163. doi: 10.1016/j.exger.2011.11.012. 10.1016/j.exger.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoen PW, de Jonge P, Na BY, Farzaneh-Far R, Epel E, Lin J, Blackburn E, Whooley MA. Depression and leukocyte telomere length in patients with coronary heart disease: data from the Heart and Soul Study. Psychosom Med. 2011;73:541–547. doi: 10.1097/PSY.0b013e31821b1f6e. 10.1097/PSY.0b013e31821b1f6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoen PW, Rosmalen JG, Schoevers RA, Huzen J, van der Harst P, de Jonge P. Association between anxiety but not depressive disorders and leukocyte telomere length after 2 years of follow-up in a population-based sample. Psychol Med. 2013;43:689–697. doi: 10.1017/S0033291712001766. 10.1017/S0033291712001766. [DOI] [PubMed] [Google Scholar]
- Hoge EA, Chen MM, Orr E, Metcalf CA, Fischer LE, Pollack MH, De Vivo I, Simon NM. Loving-Kindness Meditation practice associated with longer telomeres in women. Brain Behav Immun. 2013;32:159–163. doi: 10.1016/j.bbi.2013.04.005. 10.1016/j.bbi.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, Schadendorf D, Kumar R. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huzen J, van der Harst P, de Boer RA, Lesman-Leegte I, Voors AA, van Gilst WH, Samani NJ, Jaarsma T, van Veldhuisen DJ. Telomere length and psychological well-being in patients with chronic heart failure. Age Ageing. 2010;39:223–227. doi: 10.1093/ageing/afp256. 10.1093/ageing/afp256. [DOI] [PubMed] [Google Scholar]
- Jacobs EG, Epel ES, Lin J, Blackburn EH, Rasgon NL. Relationship between leukocyte telomere length, TA and hippocampal volume in early aging. JAMA Neurol. 2014;71:921–923. doi: 10.1001/jamaneurol.2014.870. 10.1001/jamaneurol.2014.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EG, Kroenke C, Lin J, Epel ES, Kenna HA, Blackburn EH, Rasgon NL. Accelerated cell aging in female APOE-epsilon4 carriers: implications for hormone therapy use. PLoS One. 2013;8:e54713. doi: 10.1371/journal.pone.0054713. 10.1371/journal.pone.0054713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs TL, Epel ES, Lin J, Blackburn EH, Wolkowitz OM, Bridwell DA, Zanesco AP, Aichele SR, Sahdra BK, MacLean KA, King BG, Shaver PR, Rosenberg EL, Ferrer E, Wallace BA, Saron CD. Intensive meditation training, immune cell TA, and psychological mediators. Psychoneuroendocrinology. 2011;36:664–681. doi: 10.1016/j.psyneuen.2010.09.010. 10.1016/j.psyneuen.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadinanos J, Horner JW, Maratos-Flier E, Depinho RA. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102–106. doi: 10.1038/nature09603. 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jergovic M, Tomicevic M, Vidovic A, Bendelja K, Savic A, Vojvoda V, Rac D, Lovric-Cavar D, Rabatic S, Jovanovic T, Sabioncello A. Telomere shortening and immune activity in war veterans with posttraumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:275–283. doi: 10.1016/j.pnpbp.2014.06.010. 10.1016/j.pnpbp.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Wolkowitz OM, Palmer BW. Divergent trajectories of physical, cognitive, and psychosocial aging in schizophrenia. Schizophr Bull. 2011;37:451–455. doi: 10.1093/schbul/sbr026. 10.1093/schbul/sbr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodczyk S, Fergusson DM, Horwood LJ, Pearson JF, Kennedy MA. No Association between Mean Telomere Length and Life Stress Observed in a 30 Year Birth Cohort. PLoS One. 2014;9:e97102. doi: 10.1371/journal.pone.0097102. 10.1371/journal.pone.0097102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurk D, Wang C, Miwa S, Maddick M, Korolchuk V, Tsolou A, Gonos ES, Thrasivoulou C, Saffrey MJ, Cameron K, von Zglinicki T. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell. 2012;11:996–1004. doi: 10.1111/j.1474-9726.2012.00870.x. 10.1111/j.1474-9726.2012.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kananen L, Surakka I, Pirkola S, Suvisaari J, Lonnqvist J, Peltonen L, Ripatti S, Hovatta I. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLoS One. 2010;5:e10826. doi: 10.1371/journal.pone.0010826. 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabatsiakis A, Kolassa IT, Kolassa S, Rudolph KL, Dietrich DE. Telomere shortening in leukocyte subpopulations in depression. BMC Psychiatry. 2014;14:192. doi: 10.1186/1471-244X-14-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- King KS, Kozlitina J, Rosenberg RN, Peshock RM, McColl RW, Garcia CK. Effect of leukocyte telomere length on total and regional brain volumes in a large population-based cohort. JAMA Neurol. 2014;71:1247–1254. doi: 10.1001/jamaneurol.2014.1926. 10.1001/jamaneurol.2014.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao HT, Cawthon RM, Delisi LE, Bertisch HC, Ji F, Gordon D, Li P, Benedict MM, Greenberg WM, Porton B. Rapid telomere erosion in schizophrenia. Mol Psychiatry. 2008;13:118–119. doi: 10.1038/sj.mp.4002105. [DOI] [PubMed] [Google Scholar]
- Kelly DP. Cell biology: Ageing theories unified. Nature. 2011;470:342–343. doi: 10.1038/nature09896. 10.1038/nature09896. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinser PA, Lyon DE. Major depressive disorder and measures of cellular aging: an integrative review. Nurs Res Pract. 2013;2013:469070. doi: 10.1155/2013/469070. 10.1155/2013/469070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR. Is schizophrenia a syndrome of accelerated aging? Schizophr Bull. 2008;34:1024–1032. doi: 10.1093/schbul/sbm140. 10.1093/schbul/sbm140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Glahn DC, Rowland LM, Olvera RL, Winkler A, Yang YH, Sampath H, Carpenter WT, Duggirala R, Curran J, Blangero J, Hong LE. Testing the hypothesis of accelerated cerebral white matter aging in schizophrenia and major depression. Biol Psychiatry. 2013;73:482–491. doi: 10.1016/j.biopsych.2012.10.002. 10.1016/j.biopsych.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota LN, Purushottam M, Moily NS, Jain S. Shortened telomere in unremitted schizophrenia. Psychiatry Clin Neurosci. doi: 10.1111/pcn.12260. In press. 10.1111/pcn.12260. [DOI] [PubMed] [Google Scholar]
- Koutsouleris N, Davatzikos C, Borgwardt S, Gaser C, Bottlender R, Frodl T, Falkai P, Riecher-Rossler A, Moller HJ, Reiser M, Pantelis C, Meisenzahl E. Accelerated Brain Aging in Schizophrenia and Beyond: A Neuroanatomical Marker of Psychiatric Disorders. Schizophr Bull. 2014;40:1140–1153. doi: 10.1093/schbul/sbt142. 10.1093/schbul/sbt142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CH, Epel E, Adler N, Bush NR, Obradovic J, Lin J, Blackburn E, Stamperdahl JL, Boyce WT. Autonomic and adrenocortical reactivity and buccal cell telomere length in kindergarten children. Psychosom Med. 2011;73:533–540. doi: 10.1097/PSY.0b013e318229acfc. 10.1097/PSY.0b013e318229acfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CH, Pletcher MJ, Lin J, Blackburn E, Adler N, Matthews K, Epel E. Telomerase, telomere length, and coronary artery calcium in black and white men in the CARDIA study. Atherosclerosis. 2012;220:506–512. doi: 10.1016/j.atherosclerosis.2011.10.041. 10.1016/j.atherosclerosis.2011.10.041. [DOI] [PubMed] [Google Scholar]
- Ladwig KH, Brockhaus AC, Baumert J, Lukaschek K, Emeny RT, Kruse J, Codd V, Hafner S, Albrecht E, Illig T, Samani NJ, Wichmann HE, Gieger C, Peters A. Posttraumatic stress disorder and not depression is associated with shorter leukocyte telomere length: findings from 3,000 participants in the population-based KORA F4 study. PLoS One. 2013;8:e64762. doi: 10.1371/journal.pone.0064762. 10.1371/journal.pone.0064762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers F, van Oppen P, Comijs HC, Smit JH, Spinhoven P, van Balkom AJ, Nolen WA, Zitman FG, Beekman AT, Penninx BW. Comorbidity patterns of anxiety and depressive disorders in a large cohort study: the Netherlands Study of Depression and Anxiety (NESDA) J Clin Psychiatry. 2011;72:341–348. doi: 10.4088/JCP.10m06176blu. [DOI] [PubMed] [Google Scholar]
- Lengacher CA, Reich RR, Kip KE, Barta M, Ramesar S, Paterson CL, Moscoso MS, Carranza I, Budhrani PH, Kim SJ, Park HY, Jacobsen PB, Schell MJ, Jim HS, Post-White J, Farias JR, Park JY. Influence of Mindfulness-Based Stress Reduction (MBSR) on TA in Women With Breast Cancer (BC) Biol Res Nurs. 2014;16:438–447. doi: 10.1177/1099800413519495. 10.1177/1099800413519495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Tang B, Qu Y, Mu D. Telomerase reverse transcriptase: a novel neuroprotective mechanism involved in neonatal hypoxic-ischemic brain injury. Int J Dev Neurosci. 2011;29:867–872. doi: 10.1016/j.ijdevneu.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Lima IM, Barros A, Rosa DV, Albuquerque M, Malloy-Diniz L, Neves FS, Romano-Silva MA, de Miranda DM. Analysis of telomere attrition in bipolar disorder. J Affect Disord. 2014;172C:43–47. doi: 10.1016/j.jad.2014.09.043. 10.1016/j.jad.2014.09.043. [DOI] [PubMed] [Google Scholar]
- Lin J, Blalock JA, Chen M, Ye Y, Gu J, Cohen L, Cinciripini PM, Wu X. Depressive symptoms and short telomere length are associated with increased mortality in bladder cancer patients Cancer Epidemiol. Biomarkers Prev. 2015;24:336–343. doi: 10.1158/1055-9965.EPI-14-0992. 10.1158/1055-9965.EPI-14-099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Epel E, Blackburn E. Telomeres and lifestyle factors: roles in cellular aging. Mutat Res. 2012;730:85–89. doi: 10.1016/j.mrfmmm.2011.08.003. 10.1016/j.mrfmmm.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Lin J, Kroenke CH, Epel E, Kenna HA, Wolkowitz OM, Blackburn E, Rasgon NL. Greater endogenous estrogen exposure is associated with longer telomeres in postmenopausal women at risk for cognitive decline. Brain Res. 2011;1379:224–231. doi: 10.1016/j.brainres.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TT, Letsolo BT, Jones RE, Rowson J, Pratt G, Hewamana S, Fegan C, Pepper C, Baird DM. Telomere dysfunction and fusion during the progression of chronic lymphocytic leukemia: evidence for a telomere crisis. Blood. 2010;116:1899–1907. doi: 10.1182/blood-2010-02-272104. 10.1182/blood-2010-02-272104. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhang J, Yan J, Wang Y, Li Y. Leucocyte telomere shortening in relation to newly diagnosed type 2 diabetic patients with depression. Oxid Med Cell Longev. 2014;2014:673–959. doi: 10.1155/2014/673959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Diazguerrero NE, Perez-Figueroa GE, Martinez-Garduno CM, Alarcon-Aguilar A, Luna-Lopez A, Gutierrez-Ruiz MC, Konigsberg M. TA in response to mild oxidative stress. Cell Biol Int. 2012;36:409–413. doi: 10.1042/CBI20110308. 10.1042/CBI20110308. [DOI] [PubMed] [Google Scholar]
- Luca M, Luca A, Calandra C. Accelerated aging in major depression: the role of nitro-oxidative stress. Oxid Med Cell Longev. 2013;2013:230–797. doi: 10.1155/2013/230797. 10.1155/2013/230797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens JN, Van Deerlin V, Clark CM, Xie SX, Johnson FB. Comparisons of telomere lengths in peripheral blood and cerebellum in Alzheimer's disease. Alzheimers Dement. 2009;5:463–469. doi: 10.1016/j.jalz.2009.05.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ‘ Lung FW, Chen NC, Shu BC. Genetic pathway of major depressive disorder in shortening telomeric length. Psychiatr Genet. 2007;17:195–199. doi: 10.1097/YPG.0b013e32808374f6. [DOI] [PubMed] [Google Scholar]
- Lung FW, Fan PL, Chen NC, Shu BC. Telomeric length varies with age and polymorphisms of the MAOA gene promoter in peripheral blood cells obtained from a community in Taiwan. Psychiatr Genet. 2005;15:31–35. doi: 10.1097/00041444-200503000-00006. [DOI] [PubMed] [Google Scholar]
- Ludlow AT, Ludlow LW, Roth SM. Do telomeres adapt to physiological stress? Exploring the effect of exercise on telomere length and telomere-related proteins. Biomed Res Int. 2013;2013:601368. doi: 10.1155/2013/601368. 10.1155/2013/601368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Guan JZ, Koyanagi M, Makino N. TA and telomere length distribution in vascular endothelial cells in a short-term culture under the presence of hydrogen peroxide. Geriatr Gerontol Int. 2013;13:774–782. doi: 10.1111/j.1447-0594.2012.00936.x. 10.1111/j.1447-0594.2012.00936.x. [DOI] [PubMed] [Google Scholar]
- Malan S, Hemmings S, Kidd M, Martin L, Seedat S. Investigation of telomere length and psychological stress in rape victims. Depress Anxiety. 2011;28:1081–1085. doi: 10.1002/da.20903. 10.1002/da.20903. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Dracxler R, Walsh-Messinger J, Harlap S, Goetz RR, Keefe D, Perrin MC. Telomere length, family history, and paternal age in schizophrenia. Mol Genet Genomic Med. 2014;2:326–331. doi: 10.1002/mgg3.71. 10.1002/mgg3.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour H, Chowdari K, Fathi W, Elassy M, Ibrahim I, Wood J, Bamne M, Tobar S, Yassin A, Salah H, Elsayed H, Eissa A, El-Boraie H, Ibrahim NE, Elsayed M, El-Bahaei W, Gomaa Z, El-Chennawi F, Nimgaonkar VL. Does telomere length mediate associations between inbreeding and increased risk for bipolar I disorder and schizophrenia? Psychiatry Res. 2011;188:129–132. doi: 10.1016/j.psychres.2011.01.010. 10.1016/j.psychres.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz CM, Baird D, Roger L, Boukamp P, Krunic D, Cawthon R, Dokter MM, van der Harst P, Bekaert S, de Meyer T, Roos G, Svenson U, Codd V, Samani NJ, McGlynn L, Shiels PG, Pooley KA, Dunning AM, Cooper R, Wong A, Kingston A, von Zglinicki T. Reproducibility of telomere length assessment: an international collaborative study. Int J Epidemiol. doi: 10.1093/ije/dyu191. In press. 10.1093/ije/dyu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinsson L, Wei Y, Xu D, Melas PA, Mathe AA, Schalling M, Lavebratt C, Backlund L. Long-term lithium treatment in bipolar disorder is associated with longer leukocyte telomeres. Transl Psychiatry. 2013;3:e261. doi: 10.1038/tp.2013.37. 10.1038/tp.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser RS, DePinho RA. Connecting chromosomes, crisis, and cancer. Science. 2002;297:565–569. doi: 10.1126/science.297.5581.565. 10.1126/science.297.5581.565. [DOI] [PubMed] [Google Scholar]
- Masi S, Nightingale CM, Day IN, Guthrie P, Rumley A, Lowe GD, von Zglinicki T, D'Aiuto F, Taddei S, Klein N, Salpea K, Cook DG, Humphries SE, Whincup PH, Deanfield JE. Inflammation and not cardiovascular risk factors is associated with short leukocyte telomere length in 13- to 16-year-old adolescents. Arterioscler Thromb Vasc Biol. 2012;32:2029–2034. doi: 10.1161/ATVBAHA.112.250589. 10.1161/ATVBAHA.112.250589. [DOI] [PubMed] [Google Scholar]
- Miller MW, Sadeh N. Traumatic stress, oxidative stress and post-traumatic stress disorder: neurodegeneration and the accelerated-aging hypothesis. Mol Psychiatry. 2014;19:1156–1162. doi: 10.1038/mp.2014.111. 10.1038/mp.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, Hobcraft J, McLanahan SS, Siegel SR, Berg A, Brooks-Gunn J, Garfinkel I, Notterman D. Social disadvantage, genetic sensitivity, and children's telomere length. Proc Natl Acad Sci U S A. 2014;111:5944–5949. doi: 10.1073/pnas.1404293111. 10.1073/pnas.1404293111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE the Klaus-Grawe. Think Tank, 2013. Childhood exposure to violence and lifelong health: clinical intervention science and stress-biology research join forces. Dev Psychopathol. 2012;25:1619–1634. doi: 10.1017/S0954579413000801. 10.1017/S0954579413000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montpetit AJ, Alhareeri AA, Montpetit M, Starkweather AR, Elmore LW, Filler K, Mohanraj L, Burton CW, Menzies VS, Lyon DE, Jackson-Cook CK. Telomere length: a review of methods for measurement. Nurs Res. 2014;63:289–299. doi: 10.1097/NNR.0000000000000037. 10.1097/NNR.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Villanueva M, Morath J, Vanhooren V, Elbert T, Kolassa S, Libert C, Burkle A, Kolassa IT. N-glycosylation profiling of plasma provides evidence for accelerated physiological aging in post-traumatic stress disorder. Transl Psychiatry. 2013;3:e320. doi: 10.1038/tp.2013.93. 10.1038/tp.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muezzinler A, Zaineddin AK, Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev. 2013;12:509–519. doi: 10.1016/j.arr.2013.01.003. 10.1016/j.arr.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Izumiyama-Shimomura N, Sawabe M, Arai T, Aoyagi Y, Fujiwara M, Tsuchiya E, Kobayashi Y, Kato M, Oshimura M, Sasajima K, Nakachi K, Takubo K. Comparative analysis of telomere lengths and erosion with age in human epidermis and lingual epithelium. J Invest Dermatol. 2002;119:1014–1019. doi: 10.1046/j.1523-1747.2002.19523.x. [DOI] [PubMed] [Google Scholar]
- Nalapareddy K, Jiang H, Guachalla Gutierrez LM, Rudolph KL. Determining the influence of telomere dysfunction and DNA damage on stem and progenitor cell aging: what markers can we use? Exp Gerontol. 2008;43:998–1004. doi: 10.1016/j.exger.2008.09.002. 10.1016/j.exger.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Needham BL, Mezuk B, Bareis N, Lin J, Blackburn EH, Epel ES. Depression, anxiety and telomere length in young adults: evidence from the National Health and Nutrition Examination Survey. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.89. 10.1038/mp.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieratschker V, Lahtinen J, Meier S, Strohmaier J, Frank J, Heinrich A, Breuer R, Witt SH, Nothen MM, Rietschel M, Hovatta I. Longer telomere length in patients with schizophrenia. Schizophr Res. 2013;149:116–120. doi: 10.1016/j.schres.2013.06.043. 10.1016/j.schres.2013.06.043. [DOI] [PubMed] [Google Scholar]
- Njajou OT, Hsueh WC, Blackburn EH, Newman AB, Wu SH, Li R, Simonsick EM, Harris TM, Cummings SR, Cawthon RM. Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study. J Gerontol A Biol Sci Med Sci. 2009;64:860–864. doi: 10.1093/gerona/glp061. 10.1093/gerona/glp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, Metzler T, Lenoci M, Blackburn E, Neylan TC. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011a;70:465–471. doi: 10.1016/j.biopsych.2011.01.035. 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Lin J, Tillie J, Dhabhar FS, Wolkowitz OM, Blackburn EH, Epel ES. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain Behav Immun. 2009;23:446–449. doi: 10.1016/j.bbi.2008.11.006. 10.1016/j.bbi.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, Cawthon RM, Opresko PL, Hsueh WC, Satterfield S, Newman AB, Ayonayon HN, Rubin SM, Harris TB, Epel ES, Health A Body Composition, S. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PLoS One. 2011b;6:e19687. doi: 10.1371/journal.pone.0019687. 10.1371/journal.pone.0019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Tomiyama AJ, Lin J, Puterman E, Adler NE, Kemeny M, Wolkowitz OM, Blackburn EH, Epel ES. Stress appraisals and cellular aging: a key role for anticipatory threat in the relationship between psychological stress and telomere length. Brain Behav Immun. 2012;26:573–579. doi: 10.1016/j.bbi.2012.01.007. 10.1016/j.bbi.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okereke OI, Prescott J, Wong JY, Han J, Rexrode KM, De Vivo I. High phobic anxiety is related to lower leukocyte telomere length in women. PLoS One. 2012;7:e40516. doi: 10.1371/journal.pone.0040516. 10.1371/journal.pone.0040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K, Bardeguez A, Gardner JP, Rodriguez P, Ganesh V, Kimura M, Skurnick J, Awad G, Aviv A. Telomere length in the newborn. Pediatr Res. 2002;52:377–381. doi: 10.1203/00006450-200209000-00012. 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]
- Okusaga OO. Accelerated aging in schizophrenia patients: the potential role of oxidative stress. Aging Dis. 2014;5:256–262. doi: 10.14336/AD.2014.0500256. 10.14336/AD.2014.0500256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri F, Mazzanti I, Abbatecola AM, Recchioni R, Marcheselli F, Procopio AD, Antonicelli R. Telomere/Telomerase system: a new target of statins pleiotropic effect? Curr Vasc Pharmacol. 2012;10:216–224. doi: 10.2174/157016112799305076. [DOI] [PubMed] [Google Scholar]
- Ornish D, Lin J, Chan JM, Epel E, Kemp C, Weidner G, Marlin R, Frenda SJ, Magbanua MJ, Daubenmier J, Estay I, Hills NK, Chainani-Wu N, Carroll PR, Blackburn EH. Effect of comprehensive lifestyle changes on TA and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14:1112–1120. doi: 10.1016/S1470-2045(13)70366-8. 10.1016/S1470-2045(13)70366-8. [DOI] [PubMed] [Google Scholar]
- Ornish D, Lin J, Daubenmier J, Weidner G, Epel E, Kemp C, Magbanua MJ, Marlin R, Yglecias L, Carroll PR, Blackburn EH. Increased TA and comprehensive lifestyle changes: a pilot study. The lancet oncology. 2008;9:1048–1057. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- Pandya CD, Howell KR, Pillai A. Antioxidants as potential therapeutics for neuropsychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:214–223. doi: 10.1016/j.pnpbp.2012.10.017. 10.1016/j.pnpbp.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks CG, Miller DB, McCanlies EC, Cawthon RM, Andrew ME, DeRoo LA, Sandler DP. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol Biomarkers Prev. 2009;18:551–560. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavanello S, Hoxha M, Dioni L, Bertazzi PA, Snenghi R, Nalesso A, Ferrara SD, Montisci M, Baccarelli A. Shortened telomeres in individuals with abuse in alcohol consumption. Int J Cancer. 2011;129:983–992. doi: 10.1002/ijc.25999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BW, Milaneschi Y, Lamers F, Vogelzangs N. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med. 2013;11:129. doi: 10.1186/1741-7015-11-129. 10.1186/1741-7015-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AC, Robertson T, Carroll D, Der G, Shiels PG, McGlynn L, Benzeval M. Do symptoms of depression predict telomere length? Evidence from the west of Scotland twenty-07 study. Psychosom Med. 2013;75:288–296. doi: 10.1097/PSY.0b013e318289e6b5. 10.1097/PSY.0b013e318289e6b5. [DOI] [PubMed] [Google Scholar]
- Porton B, Delisi LE, Bertisch HC, Ji F, Gordon D, Li P, Benedict MM, Greenberg WM, Kao HT. Telomerase levels in schizophrenia: a preliminary study. Schizophr Res. 2008;106:242–247. doi: 10.1016/j.schres.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather AA, Gurfein B, Moran P, Daubenmier J, Acree M, Bacchetti P, Sinclair E, Lin J, Blackburn E, Hecht FM, Epel ES. Tired telomeres: Poor global sleep quality, perceived stress, and telomere length in immune cell subsets in obese men and women. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.12.011. 10.1016/j.bbi.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: an overview. Biol Psychiatry. 2013;73:15–23. doi: 10.1016/j.biopsych.2012.06.025. 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prowse KR, Greider CW. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc Natl Acad Sci U S A. 1995;92:4818–4822. doi: 10.1073/pnas.92.11.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E, Epel E. An intricate dance: Life experience, multisystem resiliency, and rate of telomere decline throughout the lifespan. Soc Personal Psychol Compass. 2012;6:807–825. doi: 10.1111/j.1751-9004.2012.00465.x. 10.1111/j.1751-9004.2012.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E, Epel ES, Lin J, Blackburn EH, Gross JJ, Whooley MA, Cohen BE. Multisystem resiliency moderates the major depression-telomere length association: findings from the Heart and Soul Study. Brain Behav Immun. 2013;33:65–73. doi: 10.1016/j.bbi.2013.05.008. 10.1016/j.bbi.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E, Lin J, Blackburn E, O'Donovan A, Adler N, Epel E. The power of exercise: buffering the effect of chronic stress on telomere length. PLoS One. 2010;5:e10837. doi: 10.1371/journal.pone.0010837. 10.1371/journal.pone.0010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E, Lin J, Krauss J, Blackburn EH, Epel ES. Determinants of telomere attrition over 1 year in healthy older women: stress and health behaviors matter. Mol Psychiatry. 2015;20:529–535. doi: 10.1038/mp.2014.70. 10.1038/mp.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackley S, Pao M, Seratti GF, Giri N, Rasimas JJ, Alter BP, Savage SA. Neuropsychiatric conditions among patients with dyskeratosis congenita: a link with telomere biology? Psychosomatics. 2012;53:230–235. doi: 10.1016/j.psym.2011.09.003. 10.1016/j.psym.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Rawdin BJ, Mellon SH, Dhabhar FS, Epel ES, Puterman E, Su Y, Burke HM, Reus VI, Rosser R, Hamilton SP, Nelson JC, Wolkowitz OM. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav Immun. 2013;31:143–152. doi: 10.1016/j.bbi.2012.11.011. 10.1016/j.bbi.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revesz D, Verhoeven JE, Milaneschi Y, de Geus EJ, Wolkowitz OM, Penninx BW. Dysregulated physiological stress systems and accelerated cellular aging. Neurobiol Aging. 2013 doi: 10.1016/j.neurobiolaging.2013.12.027. 10.1016/j.neurobiolaging.2013.12.027. [DOI] [PubMed] [Google Scholar]
- Rius-Ottenheim N, Houben JM, Kromhout D, Kafatos A, van der Mast RC, Zitman FG, Geleijnse JM, Hageman GJ, Giltay EJ. Telomere length and mental well-being in elderly men from the Netherlands and Greece. Behav Genet. 2012;42:278–286. doi: 10.1007/s10519-011-9498-6. 10.1007/s10519-011-9498-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo LB, Costa LG, Mansur RB, Swardfager W, Belangero SI, Grassi-Oliveira R, McIntyre RS, Bauer ME, Brietzke E. The theory of bipolar disorder as an illness of accelerated aging: Implications for clinical care and research. Neurosci Biobehav Rev. 2014;42C:157–169. doi: 10.1016/j.neubiorev.2014.02.004. 10.1016/j.neubiorev.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Rizzo LB, Do Prado CH, Grassi-Oliveira R, Wieck A, Correa BL, Teixeira AL, Bauer ME. Immunosenescence is associated with human cytomegalovirus and shortened telomeres in type I bipolar disorder. Bipolar Disord. 2013;15:832–838. doi: 10.1111/bdi.12121. 10.1111/bdi.12121. [DOI] [PubMed] [Google Scholar]
- Romano GH, Harari Y, Yehuda T, Podhorzer A, Rubinstein L, Shamir R, Gottlieb A, Silberberg Y, Pe'er D, Ruppin E, Sharan R, Kupiec M. Environmental stresses disrupt telomere length homeostasis. PLoS genetics. 2013;9:e1003721. doi: 10.1371/journal.pgen.1003721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblat JD, Cha DS, Mansur RB, McIntyre RS. Inflamed moods: A review of the interactions between inflammation and mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2014;53C:23–34. doi: 10.1016/j.pnpbp.2014.01.013. 10.1016/j.pnpbp.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, DePinho RA. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliques S, Teyssier JR, Vergely C, Lorgis L, Lorin J, Farnier M, Donzel A, Sicard P, Berchoud J, Lagrost AC, Touzery C, Ragot S, Cottin Y, Rochette L, Zeller M. Circulating leukocyte telomere length and oxidative stress: a new target for statin therapy. Atherosclerosis. 2011;219:753–760. doi: 10.1016/j.atherosclerosis.2011.09.011. 10.1016/j.atherosclerosis.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Saretzki G. Extra-telomeric Functions of Human Telomerase: Cancer, Mitochondria and Oxidative Stress. Curr Pharm Des. 2014;20:6386–6403. doi: 10.2174/1381612820666140630095606. [DOI] [PubMed] [Google Scholar]
- Savolainen K, Raikkonen K, Kananen L, Kajantie E, Hovatta I, Lahti M, Lahti J, Pesonen AK, Heinonen K, Eriksson JG. History of mental disorders and leukocyte telomere length in late adulthood: the Helsinki Birth Cohort Study (HBCS) J Psychiatr Res. 2012;46:1346–1353. doi: 10.1016/j.jpsychires.2012.07.005. 10.1016/j.jpsychires.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Schaakxs R, Verhoeven JE, Oude Voshaar RC, Comijs HC, Penninx BW. Leukocyte Telomere Length and Late-Life Depression. Am J Geriatr Psychiatry. 2015;23:423–432. doi: 10.1016/j.jagp.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM. The Relationship Between Perceived Stress and Telomere Length: A Meta-analysis. Stress Health. 2014a doi: 10.1002/smi.2607. 10.1002/smi.2607. [DOI] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM. A meta-analytic review of the effects of mindfulness meditation on TA. Psychoneuroendocrinology. 2014b;42:45–48. doi: 10.1016/j.psyneuen.2013.12.017. 10.1016/j.psyneuen.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Shaffer JA, Epel E, Kang MS, Ye S, Schwartz JE, Davidson KW, Kirkland S, Honig LS, Shimbo D. Depressive Symptoms Are Not Associated with Leukocyte Telomere Length: Findings from the Nova Scotia Health Survey (NSHS95), a Population-Based Study. PLoS One. 2012;7:e48318. doi: 10.1371/journal.pone.0048318. 10.1371/journal.pone.0048318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, Epel ES. Stress and telomere biology: A lifespan perspective. Psychoneuroendocrinology. 2013;38:1835–1842. doi: 10.1016/j.psyneuen.2013.03.010. 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Braithwaite AW, Danese A, Fleming NI, Goldman-Mellor S, Harrington HL, Houts RM, Israel S, Poulton R, Robertson SP, Sugden K, Williams B, Caspi A. Internalizing disorders and leukocyte telomere erosion: a prospective study of depression, generalized anxiety disorder and post-traumatic stress disorder. Mol Psychiatry. 2014;19:1163–1170. doi: 10.1038/mp.2013.183. 10.1038/mp.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakumar V, Kalmady SV, Venkatasubramanian G, Ravi V, Gangadhar BN. Do schizophrenia patients age early? Asian J Psychiatr. 2014;10C:3–9. doi: 10.1016/j.ajp.2014.02.007. 10.1016/j.ajp.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Nierenberg AA, Fava M, Wong KK. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Smyth AM, Lawrie SM. The Neuroimmunology of Schizophrenia. Clin Psychopharmacol Neurosci. 2013;11:107–117. doi: 10.9758/cpn.2013.11.3.107. 10.9758/cpn.2013.11.3.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi SK, Linder J, Chenard CA, Miller del D, Haynes WG, Fiedorowicz JG. Evidence for accelerated vascular aging in bipolar disorder. J Psychosom Res. 2012;73:175–179. doi: 10.1016/j.jpsychores.2012.06.004. 10.1016/j.jpsychores.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro-de-Souza MG, Teixeira AL, Mateo EC, Zanetti MV, Rodrigues FG, de Paula VJ, Bezerra JF, Moreno RA, Gattaz WF, Machado-Vieira R. Leukocyte TA and antidepressant efficacy in bipolar disorder. Eur Neuropsychopharmacol. 2014;24:1139–1143. doi: 10.1016/j.euroneuro.2014.03.005. 10.1016/j.euroneuro.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Spyridopoulos I, Hoffmann J, Aicher A, Brummendorf TH, Doerr HW, Zeiher AM, Dimmeler S. Accelerated telomere shortening in leukocyte subpopulations of patients with coronary heart disease: role of cytomegalovirus seropositivity. Circulation. 2009;120:1364–1372. doi: 10.1161/CIRCULATIONAHA.109.854299. [DOI] [PubMed] [Google Scholar]
- Starkweather AR, Alhaeeri AA, Montpetit A, Brumelle J, Filler K, Montpetit M, Mohanraj L, Lyon DE, Jackson-Cook CK. An integrative review of factors associated with telomere length and implications for biobehavioral research. Nurs Res. 2014;63:36–50. doi: 10.1097/NNR.0000000000000009. 10.1097/NNR.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NW, Pooley KA, Luben RN, Khaw KT, Easton DF, Dunning AM. Life stress, emotional health, and mean telomere length in the European Prospective Investigation into Cancer (EPIC)-Norfolk population study. J Gerontol A Biol Sci Med Sci. 2011;66:1152–1162. doi: 10.1093/gerona/glr112. 10.1093/gerona/glr112. [DOI] [PubMed] [Google Scholar]
- Svensson J, Karlsson MK, Ljunggren O, Tivesten A, Mellstrom D, Moverare-Skrtic S. Leukocyte telomere length is not associated with mortality in older men. Exp Gerontol. 2014;57:6–12. doi: 10.1016/j.exger.2014.04.013. 10.1016/j.exger.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Szebeni A, Szebeni K, DiPeri T, Chandley MJ, Crawford JD, Stockmeier CA, Ordway GA. Shortened telomere length in white matter oligodendrocytes in major depression: potential role of oxidative stress. Int J Neuropsychopharmacol. 2014:1–11. doi: 10.1017/S1461145714000698. 10.1017/S1461145714000698. [DOI] [PubMed] [Google Scholar]
- Takata Y, Kikukawa M, Hanyu H, Koyama S, Shimizu S, Umahara T, Sakurai H, Iwamoto T, Ohyashiki K, Ohyashiki JH. Association between ApoE phenotypes and telomere erosion in Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 2012;67:330–335. doi: 10.1093/gerona/glr185. 10.1093/gerona/glr185. [DOI] [PubMed] [Google Scholar]
- Takubo K, Aida J, Izumiyama-Shimomura N, Ishikawa N, Sawabe M, Kurabayashi R, Shiraishi H, Arai T, Nakamura K. Changes of telomere length with aging. Geriatr Gerontol Int. 2010;10(Suppl 1):S197–S206. doi: 10.1111/j.1447-0594.2010.00605.x. [DOI] [PubMed] [Google Scholar]
- Takubo K, Izumiyama-Shimomura N, Honma N, Sawabe M, Arai T, Kato M, Oshimura M, Nakamura K. Telomere lengths are characteristic in each human individual. Exp Gerontol. 2002;37:523–532. doi: 10.1016/s0531-5565(01)00218-2. [DOI] [PubMed] [Google Scholar]
- Teyssier JR, Chauvet-Gelinier JC, Ragot S, Bonin B. Up-regulation of leucocytes genes implicated in telomere dysfunction and cellular senescence correlates with depression and anxiety severity scores. PLoS One. 2012;7:e49677. doi: 10.1371/journal.pone.0049677. 10.1371/journal.pone.0049677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyssier JR, Ragot S, Donzel A, Chauvet-Gelinier JC. Telomeres in the brain cortex of depressive patients. Encephale. 2010;36:491–494. doi: 10.1016/j.encep.2010.04.004. 10.1016/j.encep.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Thomas P, NJ OC, Fenech M. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer's disease. Mech Ageing Dev. 2008;129:183–190. doi: 10.1016/j.mad.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, O'Donovan A, Lin J, Puterman E, Lazaro A, Chan J, Dhabhar FS, Wolkowitz O, Kirschbaum C, Blackburn E, Epel E. Does cellular aging relate to patterns of allostasis? An examination of basal and stress reactive HPA axis activity and telomere length. Physiol Behav. 2012;106:40–45. doi: 10.1016/j.physbeh.2011.11.016. 10.1016/j.physbeh.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgashov MN, Miakotnykh VS, Pal'tsev AI. Age-related aspects of the extent of lipid metabolism and post-traumatic stress disorders among veterans of modern warfare. Adv Gerontol. 2013;26:525–532. [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biol Psychiatry. 2010;67:531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Berg PJ, Griffiths SJ, Yong SL, Macaulay R, Bemelman FJ, Jackson S, Henson SM, ten Berge IJ, Akbar AN, van Lier RA. Cytomegalovirus infection reduces telomere length of the circulating T cell pool. J Immunol. 2010;184:3417–3423. doi: 10.4049/jimmunol.0903442. 10.4049/jimmunol.0903442. [DOI] [PubMed] [Google Scholar]
- Vasunilashorn S, Cohen AA. Stress responsive biochemical anabolic/catabolic ratio and telomere length in older adults. Biodemography Soc Biol. 2014;60:174–184. doi: 10.1080/19485565.2014.950722. 10.1080/19485565.2014.950722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven JE, Revesz D, Epel ES, Lin J, Wolkowitz OM, Penninx BW. Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Mol Psychiatry. 2014a;19:895–901. doi: 10.1038/mp.2013.151. 1038/mp.2013.151. [DOI] [PubMed] [Google Scholar]
- Verhoeven JE, Révész D, van Oppen P, Epel ES, Wolkowitz OM, Penninx BWJH. Anxiety Disorders and Accelerated Cellular Aging. Br J Psychiatry. doi: 10.1192/bjp.bp.114.151027. In press. 10.1192/bjp.bp.114.151027. [DOI] [PubMed] [Google Scholar]
- Verhoeven JE, Revesz D, Wolkowitz OM, Penninx BW. Cellular aging in depression: Permanent imprint or reversible process?: An overview of the current evidence, mechanistic pathways, and targets for interventions. Bioessays. 2014b;36:968–978. doi: 10.1002/bies.201400068. 10.1002/bies.201400068. [DOI] [PubMed] [Google Scholar]
- Viron MJ, Stern TA. The impact of serious mental illness on health and healthcare. Psychosomatics. 2010;51:458–465. doi: 10.1176/appi.psy.51.6.458. 10.1176/appi.psy.51.6.458. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- Wei Y, Backlund L, Wegener G, Mathe AA, Lavebratt C. Telomerase dysregulation in the hippocampus of a rat model of depression. Normalization by lithium. Int J Neuropsychopharmacol. doi: 10.1093/ijnp/pyv002. In press. 10.1093/ijnp/pyv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weischer M, Bojesen SE, Cawthon RM, Freiberg JJ, Tybjaerg-Hansen A, Nordestgaard BG. Short telomere length, myocardial infarction, ischemic heart disease, and early death. Arterioscler Thromb Vasc Biol. 2012;32:822–829. doi: 10.1161/ATVBAHA.111.237271. 10.1161/ATVBAHA.111.237271. [DOI] [PubMed] [Google Scholar]
- Weischer M, Bojesen SE, Nordestgaard BG. Telomere shortening unrelated to smoking, body weight, physical activity, and alcohol intake: 4,576 general population individuals with repeat measurements 10 years apart. PLoS Genet. 2014;10:e1004191. doi: 10.1371/journal.pgen.1004191. 10.1371/journal.pgen.1004191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20:1238–1250. doi: 10.1158/1055-9965.EPI-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikgren M, Karlsson T, Lind J, Nilbrink T, Hultdin J, Sleegers K, Van Broeckhoven C, Roos G, Nilsson LG, Nyberg L, Adolfsson R, Norrback KF. Longer leukocyte telomere length is associated with smaller hippocampal volume among non-demented APOE epsilon3/epsilon3 subjects. PLoS One. 2012a;7:e34292. doi: 10.1371/journal.pone.0034292. 10.1371/journal.pone.0034292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikgren M, Karlsson T, Soderlund H, Nordin A, Roos G, Nilsson LG, Adolfsson R, Norrback KF. Shorter telomere length is linked to brain atrophy and white matter hyperintensities. Age Ageing. 2013;43:212–217. doi: 10.1093/ageing/aft172. 10.1093/ageing/aft172. [DOI] [PubMed] [Google Scholar]
- Wikgren M, Maripuu M, Karlsson T, Nordfjall K, Bergdahl J, Hultdin J, Del-Favero J, Roos G, Nilsson LG, Adolfsson R, Norrback KF. Short telomeres in depression and the general population are associated with a hypocortisolemic state. Biol Psychiatry. 2012b;71:294–300. doi: 10.1016/j.biopsych.2011.09.015. 10.1016/j.biopsych.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Willeit P, Raschenberger J, Heydon EE, Tsimikas S, Haun M, Mayr A, Weger S, Witztum JL, Butterworth AS, Willeit J, Kronenberg F, Kiechl S. Leucocyte telomere length and risk of type 2 diabetes mellitus: new prospective cohort study and literature-based meta-analysis. PloS one. 2014;9:e112483. doi: 10.1371/journal.pone.0112483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstatter A, Kronenberg F, Kiechl S. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304:69–75. doi: 10.1001/jama.2010.897. 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: do stress and depression accelerate cell aging? Depress Anxiety. 2010;27:327–338. doi: 10.1002/da.20686. 10.1002/da.20686. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su Y, Reus VI, Rosser R, Burke HM, Kupferman E, Compagnone M, Nelson JC, Blackburn EH. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress--preliminary findings. PLoS One. 2011a;6:e17837. doi: 10.1371/journal.pone.0017837. 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Epel ES, Lin J, Reus VI, Rosser R, Burke H, Compagnone M, Nelson JC, Dhabhar FS, Blackburn EH. Resting leukocyte TA is elevated in major depression and predicts treatment response. Mol Psychiatry. 2012;17:164–172. doi: 10.1038/mp.2010.133. 10.1038/mp.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Lindqvist D, Epel ES, Blackburn EH, Lin J, Reus VI, Burke H, Rosser R, Mahan L, Mackin S, Yang T, Weiner M, Mueller S. PBMC telomerase activity, but not leukocyte telomere length, correlates with hippocampal volume in major depression. Psychiatry Res Neuroimaging. doi: 10.1016/j.pscychresns.2015.01.007. In press. 10.1016/j.pscychresns.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Mellon SH. Of sound mind and body: depression, disease, and accelerated aging. Dialogues Clin Neurosci. 2011b;13:25–39. doi: 10.31887/DCNS.2011.13.1/owolkowitz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Xian RR, Li Y, Polony TS, Beemon KL. Telomerase reverse transcriptase expression elevated by avian leukosis virus integration in B cell lymphomas. Proc Natl Acad Sci U S A. 2007;104:18952–18957. doi: 10.1073/pnas.0709173104. 10.1073/pnas.0709173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Ye J, Li C, Zhou D, Shen Q, Wu J, Cao L, Wang T, Cui D, He S, Qi G, He L, Liu Y. Drug addiction is associated with leukocyte telomere length. Sci Rep. 2013;3:1542. doi: 10.1038/srep01542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatham LN, Kapczinski F, Andreazza AC, Trevor Young L, Lam RW, Kauer-Sant'anna M. Accelerated age-related decrease in brain-derived neurotrophic factor levels in bipolar disorder. Int J Neuropsychopharmacol. 2009;12:137–139. doi: 10.1017/S1461145708009449. 10.1017/S1461145708009449. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Harvey PD, Stavitsky K, Kaufman S, Grossman RA, Tischler L. Relationship between cortisol and age-related memory impairments in Holocaust survivors with PTSD. Psychoneuroendocrinology. 2005;30:678–687. doi: 10.1016/j.psyneuen.2005.02.007. 10.1016/j.psyneuen.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Yen YC, Lung FW. Older adults with higher income or marriage have longer telomeres. Age Ageing. 2013;42:234–239. doi: 10.1093/ageing/afs122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WY, Chang HW, Lin CH, Cho CL. Short telomeres in patients with chronic schizophrenia who show a poor response to treatment. J Psychiatry Neurosci. 2008;33:244–247. [PMC free article] [PubMed] [Google Scholar]
- Zalli A, Carvalho LA, Lin J, Hamer M, Erusalimsky JD, Blackburn EH, Steptoe A. Shorter telomeres with high TA are associated with raised allostatic load and impoverished psychosocial resources. Proc Natl Acad Sci U S A. 2014;111:4519–4524. doi: 10.1073/pnas.1322145111. 10.1073/pnas.1322145111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Cheng L, Craig DW, Redman M, Liu C. Cerebellar telomere length and psychiatric disorders. Behav Genet. 2010;40:250–254. doi: 10.1007/s10519-010-9338-0. 10.1007/s10519-010-9338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hu XZ, Benedek DM, Fullerton CS, Forsten RD, Naifeh JA, Li X, Li H, Benevides KN, Smerin S, Le T, Choi K, Ursano RJ. The interaction between stressful life events and leukocyte telomere length is associated with PTSD. Mol Psychiatry. 2014;19:855–856. doi: 10.1038/mp.2013.141. 10.1038/mp.2013.141. [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhu Y, Lin J, Matsuguchi T, Blackburn E, Zhang Y, Cole SA, Best LG, Lee ET, Howard BV. Short leukocyte telomere length predicts risk of diabetes in american indians: the strong heart family study. Diabetes. 2014;63:354–362. doi: 10.2337/db13-0744. 10.2337/db13-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QG, Hu Y, Wu DL, Zhu LJ, Chen C, Jin X, Luo CX, Wu HY, Zhang J, Zhu DY. Hippocampal telomerase is involved in the modulation of depressive behaviors. J Neurosci. 2011;31:12258–12269. doi: 10.1523/JNEUROSCI.0805-11.2011. 10.1523/JNEUROSCI.0805-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


