Abstract
The purpose of this research was to determine whether there were differences in estimated means and rates of change in BMC, bone area, BMD and measures of bone geometry among men (n = 544) from three distinct populations (Hutterite [rural], rural non-Hutterite, non-rural), and whether activity levels or calcium intake explain these population differences. Men were enrolled in the South Dakota Rural Bone Health Study and followed for 7.5 years to estimate means and rates of change in bone mass, density, size and geometry. Femoral neck (FN) and spine measurements were obtained every 18 months by DXA and distal radius (4% and 20%) measurements by pQCT. Activity measurements and calcium intake were obtained quarterly for the first 3 years and at 54, 72, and 90 months. Rural men had greater percent time in moderate plus vigorous activity (mean ± SD: 22 ± 10 vs. 15 ±8%, p < 0.001) and greater lean mass (69 ± 9 vs. 66 ± 10 kg, p = 0.05) than non-rural men. Both rural populations (Hutterite and rural men) had larger femoral neck (FN) bone area and greater 20% radius cross-sectional area than non-rural men ([least square means ± SE] FN area: 5.90 ± 0.02 and 5.86 ± 0.02 vs. 5.76 ± 0.03 cm2, p < 0.001 and p = 0.03 respectively and cross-sectional area: 171.0 ± 1.3 and 165.5 ± 1.5 vs. 150.3 ± 1.6 mm2, both p < 0.001). Despite lower cortical vBMD in Hutterite and rural men compared to non-rural men (1182 ± 2 and 1187 ± 2 vs. 1192 ± 2 mm2, p < 0.001 and p = 0.06 respectively), bone strength (pSSI) was greater (429 ± 5 and 422 ± 5 vs. 376 ± 6 mm3, both p < 0.001). The rates of change in femoral neck BMC and aBMD and trabecular vBMD also differed by rural lifestyle, with greater losses among non-rural men in their 20s and 60s compared to both Hutterite and rural populations (time-by-age-by-group interactions, both p < 0.01). Physical activity was not found to be a potential mediator of population differences. Baseline calcium intake was associated with FN aBMD (p = 0.04), and increases in calcium intake were associated with spine BMC (p = 0.04) and inversely associated with cortical area (p = 0.02). There was some evidence for mediation by either baseline calcium intake or changes in calcium intake over the study period, but the influence on population differences were negligible. We speculate that rural–non-rural differences in bone occur earlier in life or are a result of factors that have not yet been identified.
Keywords: Diet, Motor activity, Rural population, Life style, Human, Male, Mediation
1. Introduction
Osteoporosis and osteoporosis-related fractures occur more frequently in women, with a female-to-male ratio of 1.6; however, the estimated lifetime risk of an osteoporotic fracture in men over 50 years of age is substantial at 30% [1]. Overall fracture-related mortality also is greater in men than women aged 60 years and older, with 20% mortality in the first 12 months following a hip fracture [2].
Fracture rates have been reported to be lower among individuals residing in rural areas compared to urban areas [3–7]. Some investigators have attributed these differences to a higher areal bone mineral density (aBMD) or bone mineral content (BMC) in individuals residing in rural areas [4,8,9]. However, most of these studies categorized individuals based on their residence rather than their individual lifestyle. Gardsell and coworkers reported a higher forearm BMC in rural vs. urban men in southern Sweden, and the BMC difference became greater when they limited their population to men who lived their entire life in a city or never lived in a city [4]. Despite rural–urban differences that have been reported, little is known about the determinants of these differences although physical activity and diet have been suggested as possibilities [7,9–11]. Additionally, these reports were based on cross-sectional studies. There are no reports of longitudinal changes in BMC or aBMD in rural vs. urban populations.
Longitudinal studies are better than cross-sectional studies for determining rates of bone loss with aging. Evaluation of rates of change from cross-sectional studies is potentially subject to bias from cohort effects, and data from cross-sectional studies can both overestimate and underestimate rates of change observed in longitudinal studies [12,13]. The reason for these discrepancies is the difference in methods used to estimate rates of change in cross-sectional vs. longitudinal studies. In cross-sectional studies, the estimated rate of change is based on a regression using single measurements from multiple individuals to provide data across an age range. In longitudinal studies, the average estimated rate of change is based on multiple measurements for an individual.
The South Dakota Rural Bone Health Study (SDRBHS) is a longitudinal study of three populations distinguished by lifestyle (Hutterite, rural, non-rural) that have been followed 7.5 years. Both Hutterite and rural populations live a similar rural farming lifestyle but with significant differences in social structure (Hutterites have a religion-based communal lifestyle). The non-rural population never lived on a working farm and would be expected to have lower physical activity levels [10]. Enrollment of participants from these three distinct populations is helpful in determining whether it is the rural vs. non-rural lifestyle or genetic differences that influence rates of bone loss with aging. The purpose of the current analysis was to determine whether there were population differences in estimated means and rates of change in DXA BMC, bone area and areal BMD (aBMD) and pQCT measures of volumetric bone mineral density (vBMD) and bone geometry among men aged 20 to 66 years at enrollment. It was hypothesized that Hutterite and rural men would have greater BMC, aBMD and bone size than non-rural men and these differences would be maintained across the different ages. Whether physical activity levels or dietary calcium intake could explain the population differences also was determined.
2. Methods
2.1. Subjects
The SDRBHS is a 7.5-year longitudinal study of 1271 healthy adults aged 20 to 66 years who were enrolled between 2001 and 2004. Of the 1271 participants, 585 (226 males) were Hutterites, 350 (184 males) were classified as rural, and 336 (134 males) were classified as non-rural. Hutterites are an Anabaptist religious group who believes in isolated communal living and self-sufficiency through technologically advanced agricultural-based rural lifestyle. To be classified as Hutterite, an individual had to be of Hutterite descent (originating in Tyrol region of Germany and Austria in the 1500s) and currently residing on a Hutterite colony. Both rural and non-rural populations were recruited from eight counties that included at least one participating colony. All eight of these counties are considered to be rural, non-metro counties. To be considered rural, the subject had to have reported at the time of enrollment spending ≥75% of their life on a working farm while working <1040 h/year off the farm. In order to be considered non-rural, the subject could never have spent time living on a working farm. Both rural and non-rural participants were recruited by calling every tenth phone number from local phone books. This process was repeated once for one of the 8 counties that had a large population of non-rural residents. In addition, individuals whose names were on County Zoning Office phone lists of people owning land zoned agricultural in these 8 counties were called. Difficulty was encountered in locating sufficient numbers of participants who had never lived on a working farm and eventually recruitment was opened to anyone interested in participating as long as they met the criteria for being non-rural. Twenty-one percent of the non-rural participants were recruited through open enrollment [10]. SDSU Institutional Review Board approved the protocol and written informed consent was obtained from all participants.
2.2. Body composition and bone measurements
Body composition and bone measurements of femoral neck (FN), hip and spine were measured every 18 months using a DXA densitometer (Hologic QDR 4500A/Discovery, Bedford, MA) (Table 1). Hip scans were done on the left hip, and aBMD Z-scores for femoral neck, hip and spine were obtained from the manufacturers' software (Version 12.3). Due to decreased accuracy in spine scans obtained on obese patients, all scans with an epoxy equivalent thickness greater than 12 in., as determined by the Hologic software, were deleted per manufacturer recommendations (33 spine scans from 16 men [7 Hutterite, 4 rural, 5 non-rural] were omitted). Coefficients of variation (CV) were analyzed from triplicate scans on 15 adults with repositioning between each scan and were <2% for hip and spine BMC and bone area. Quality control and quantification procedures outlined by Orwoll et al. were used to monitor and adjust DXA results for alterations in scanner performance [14].
Table 1.
Timeline of assessments.
| Study month |
||||||
|---|---|---|---|---|---|---|
| 0 | 18 | 36 | 54 | 72 | 90 | |
| Consent & baseline demographics | X | |||||
| Bone (pQCT, DXA) & body composition (DXA) | X | X | X | X | X | X |
| Anthropometrics | X | X | X | X | X | X |
| 24-hour diet recall | Quarterlya | |||||
| Food frequency questionnaire | X | X | X | |||
| 7-day physical activity recall | Quarterlya | X | X | X | ||
Records collected at 0, 3 & 6 months were used for baseline estimate; 15, 18 & 21 months for 18-month visit estimate; and 30, 33 & 36 months for 36-month visit estimate. Research staff completed all recalls during an interview with the participant.
pQCT measurements of the left radius were obtained using a Norland-Stratec XCT2000 densitometer (Pforzheim, Germany). Arm length was measured from the elbow to the ulna styloid process and a scout view was taken to identify the end of the radius. Slices were obtained at the 4% and 20% of the measured arm length from the distal radius using a voxel size of 0.4 mm and scan speed of 30 mm/s with a 1-block rotation. The slices were analyzed using ContMode2, Peel Mode 2 and a threshold of 400 mg/cm3 to obtain trabecular volumetric bone mineral density (vBMD) (4% site only). Cortical bone was identified using a density threshold of 710 mg/cm3 at the 20% site, and a threshold of 280 mg/cm3 for pSSI. The polar stress strain index (pSSI) at the 20%, a measure of torsional bone strength, is based on structural and material properties obtained by pQCT:
where ri is the voxel position from the center, a is the area of the pixel, rmax is the maximum distance of the voxel to the bone center, CD is the measured cortical density of the voxel, and ND is the normal physiological cortical density (1200 mg/ccm) [15]. CVs from duplicate scans obtained on 9 adults following repositioning and with a scout view were 0.5%, 1.2% and 0.5% for cortical vBMD, cortical thickness and total bone cross-sectional area at the 20% site and 2.4% for trabecular vBMD at the 4% distal site.
2.3. Anthropometric measurements
Height without shoes, recorded to the nearest 0.5 cm, was measured every 18 months in duplicate using a Seca stadiometer (Chino, CA) and repeated if measurements differed by more than 0.5 cm. Weight with light clothing was measured to the nearest 0.1 kg using a Seca digital scale (Model 770, Chino, CA).
2.4. Activity levels
Physical activity was measured every 18 months using a 7-day physical activity recall [16], which requires the participant to determine the average amount of time spent per day sleeping, sitting, or in vigorous or moderate activity during the previous week. The remaining time was classified as light activity. Vigorous activity was considered as any activity that leads to an increase in heart rate or heavy breathing and included such activities as running, brisk walking, shoveling, etc. Moderate activity was considered as an activity that required significant movement but did not noticeably increase heart rate or result in heavy breathing. Activity patterns for weekdays and weekend days, as well as the number of days per week considered weekend days, were obtained. The average percent of time spent in moderate plus vigorous activity was then calculated. Records collected at 0, 3 & 6 months were used for baseline estimate; 15, 18 & 21 months for 18-month visit estimate; and 30, 33 & 36 months for 36-month visit estimate. Research staff completed all recalls during interviews with the participants.
2.5. Calcium intake
Calcium intakes from 24-hour diet recalls obtained quarterly during the first 3 years of the study (Table 1) were determined using Nutritionist V software (First Data Bank, San Bruno, CA). Records collected at 0, 3 & 6 months were used for baseline estimate; 15, 18 & 21 months for 18-month visit estimate; and 30, 33 & 36 months for 36-month visit estimate. Research staff completed all recalls during interviews with the participants. The food frequency questionnaires (FFQ) obtained at 54, 72 and 90 months were used to assess calcium intake over the previous year [17] and was previously validated in rural populations [18]. Calcium intake from both the 24-hour recalls and the FFQs included calcium supplements.
2.6. Statistical analyses
Analyses were carried out using SAS statistical software (v 9.3, SAS Institute, Inc., Cary, NC). ANOVA was used to evaluate population differences in potential covariates at baseline, with Tukey adjustment for multiple comparisons.
A linear mixed effects model was used to evaluate population differences adjusting for potential covariates, allowing for simultaneous evaluation of both cross-sectional associations at baseline and longitudinal associations [19]. Random error was modeled among and within individuals, thereby accounting for correlated repeated measures within an individual.
For each bone outcome, the mixed model (referred to as base model) included population group, age, age2, height, lean mass, fat mass, change in lean mass, change in fat mass, age-by-group, age2-by-group, time-by-age, time-by-age2, time-by-group, time-by-age-by-group, and time-by-age2-by-group. The age2 term was included since most relationships with age were non-linear. Lean mass and fat mass were used rather than weight or BMI due to the opposite influences that lean and fat mass may have on bone size, BMC, aBMD, and vBMD [20,21]. Changes in lean and fat mass were calculated as the difference of a follow-up measurement from the corresponding baseline measurement.
To investigate whether activity levels or calcium intake mediates the relationship between population group and bone measurements, the step method described by Baron and Kenny was used [22]. The steps require 1) the total effect of the predictor of interest (population group) on the dependent variable (bone) must be significant; 2) the relationship between the independent variable (population group) and the mediator (activity or calcium intake) must be significant; and 3) the relationship from the mediator (activity or calcium intake) to the dependent variable (bone) must be significant. The effect of a mediator is to attenuate the beta coefficient, or the association of the predictor of interest with the dependent variable, in a regression model containing both the mediator and predictor of interest. If the associations in the three steps described above were observed, the beta coefficient for population group from the base model, or the highest-order interaction that was statistically significant and included population group, was considered a measure of the overall effect (OE) [23]. The beta coefficients obtained for the significant highest order interaction term in a model also containing a similar interaction that included the mediator provided an estimate of the direct effect (DE) of population group on the bone measure [24]. We then calculated the percent of the beta coefficient explained by the potential mediator as ([OE – DE] / OE) * 100, which is used as a summary measure of the indirect effect of the mediator on the bone outcome measure. We also determined the least square means of the main effect of population group or the highest order interaction after including the potential mediator in the statistical model.
Least square means and annual percent change in bone outcomes are graphed at different ages for illustrative purposes although the actual analysis was done on the rate of change as described above using age as a continuous variable. Least square means are the means after adjusting for all the covariates in the statistical model. Denominator degrees of freedom were determined using the Satterthwaite approximation. Data are means ± standard deviations unless otherwise noted.
3. Results
Baseline characteristics of the 544 men are shown by population group in Table 2. There was a 94% (n = 510) completion rate after 3 years and 80% (n = 433) completion rate after 7.5 years. Hutterite men were younger, shorter, had greater percent time in moderate plus vigorous activity, and lower calcium intake than rural and non-rural men. aBMD Z-scores of the hip and spine were greater in Hutterite men than non-rural men with rural men being intermediate between the two other populations. Mixed model analysis was done to simultaneously determine the cross-sectional and longitudinal associations, as well as rates of change, among population groups controlling for the covariates described in the Statistical Analysis section. Each covariate was a significant predictor of several bone measurements (see Supplemental Table 1): greater lean mass was associated with greater FN and spine BMC, BA, and aBMD, and radial cross-sectional area, cortical thickness, cortical area, pSSI and trabecular vBMD, while there was a negative relationship between lean mass and cortical vBMD. Fat mass was negatively associated with FN and spine BMC and BA, and radial cross-sectional area, cortical area and pSSI. Non-linear relationships were observed between age and FN and spine BMC and aBMD, and radial cross-sectional area and cortical vBMD. Taller men had larger FN bone area with only slightly greater FN BMC leading to a lower aBMD compared to shorter men. Taller men also had greater cross-sectional area and a greater pSSI, but smaller cortical thickness and lower trabecular vBMD. Results described below are from the models containing all covariates.
Table 2.
Baseline characteristics by population.
| Hutterite (n = 226) | Rural (n = 184) | Non-rural (n = 134) | P value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (y) | 38.2 (12.3)ab | 44.4 (13.6)a | 42.5 (11.9)b | <0.001 |
| Height (cm) | 176.5 (5.7)ab | 178.7 (7.8)a | 179.0 (7.4)b | <0.001 |
| Weight (kg) | 92.3 (6.7) | 94.9 (18.0) | 91.2 (18.1) | NS |
| BMI (kg/m2) | 29.6 (4.9) | 29.7 (5.3) | 28.4(5.3) | 0.05a |
| Lean mass (kg) | 67.3 (8.3) | 68.8 (8.6)a | 66.4(9.5)a | 0.05 |
| Fat mass (kg) | 22.3 (8.6) | 23.5 (9.6) | 22.0 (9.8) | NS |
| % time in mod + vig activity | 25.7 (8.2)ab | 22.1 (10.3)ac | 15.3 (8.4) bc | <0.001 |
| Dietary calcium (mg/day) | 912 (405) ab | 1179 (577)a | 1059 (468)b | <0.001 |
| Baseline aBMD Z-scores | ||||
| Femoral neck | 0.47 (0.90) | 0.37 (0.91) | 0.30 (0.90) | NS |
| Hip | 0.63 (0.85)a | 0.46 (0.91) | 0.38 (0.85)a | 0.02 |
| Spine | 0.50 (1.39)a | 0.24(1.41) | −0.01 (1.17)a | 0.002 |
Data are mean (SD). Mod + Vig = moderate plus vigorous; aBMD = areal bone mineral density.
Similar superscripts within a row indicate significant difference between means (Tukey HSD, p < 0.05).
No means differed by Tukey HSD.
3.1. Cross-sectional associations
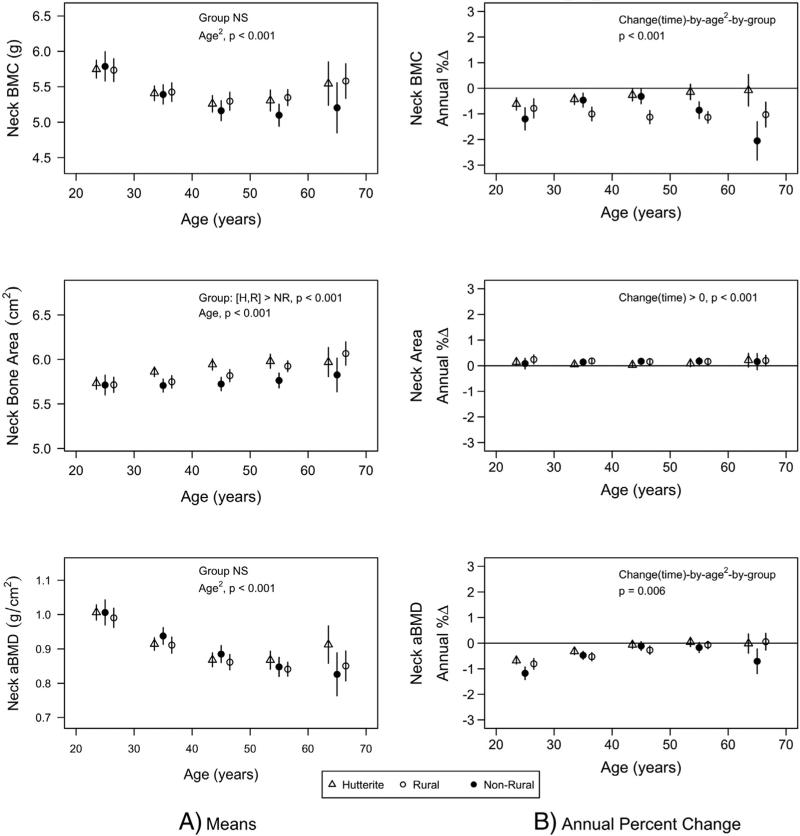
Hutterite and rural men had greater FN bone area than non-rural men (least square means ± SE: 5.90 ± 0.02 and 5.86 ± 0.02 vs. 5.76 ± 0.03 cm2, p < 0.001 and p = 0.03 respectively), but there were no population differences in FN BMC or aBMD (Fig. 1; similar differences were observed for total hip [data not presented]). There were no population differences in spine bone area (70.2 ± 0.33 vs. 70.2 ± 0.36 vs. 69.3 ± 0.41 cm2, all p > 0.05), but Hutterite men had greater spine BMC and aBMD than both rural and non-rural men (80 ± 1 vs. 76 ± 1 and 75 ± 1 g, p = 0.01 and p < 0.001 respectively for BMC and 1.141 ± 0.010 vs. 1.084 ± 0.011 and 1.077 ± 0.013 g/cm2, both p < 0.001) for aBMD (data not shown graphically).
Fig. 1.
Femoral neck least square means (A) and annual percent change (B) by population group (triangle = Hutterite; solid circle = non-rural; empty circle = rural). Least square means were estimated from mixed models that included population group, age, age2, height, lean mass, fat mass, change in lean mass, change in fat mass, age-by-group, age2-by-group, time-by-age, time-by-age2, time-by-group, time-by-age-by-group, and time-by-age2-by-group. Least square means are given at specific ages for presentation purposes. Bars represent 95% confidence intervals. Only the significance of the highest order interaction is given.
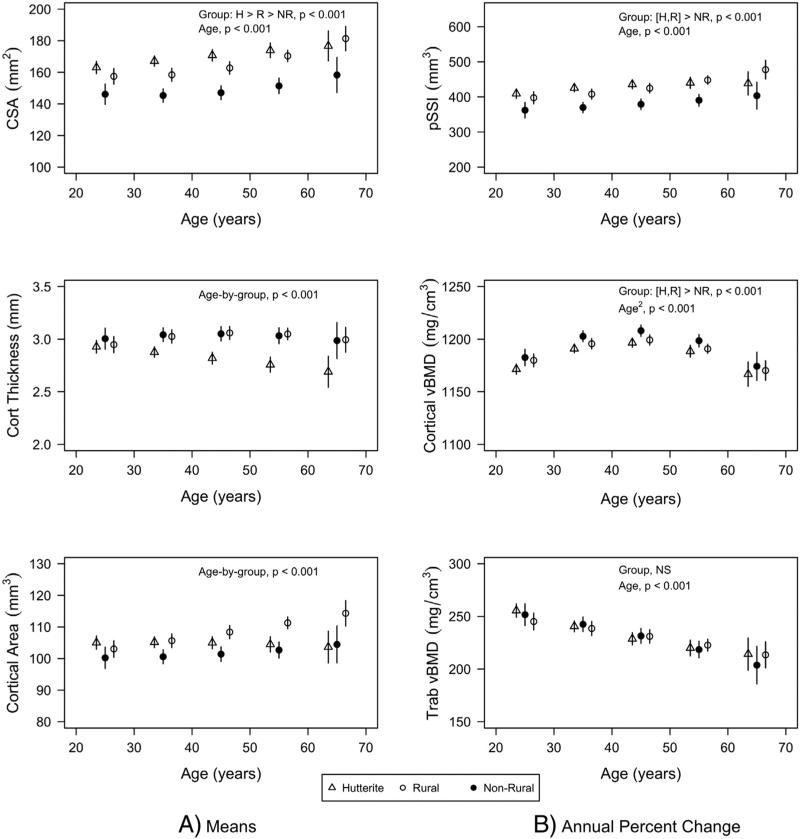
Hutterite and rural men had greater cross-sectional area at the 20% distal radius than non-rural men (171.0 ± 1.3 and 165.5 ± 1.5 vs. 150.3 ± 1.6 mm2, both p < 0.001) resulting in both Hutterite and rural men having greater pSSI than non-rural men (429 ± 5 and 422 ± 5 vs. 376 ± 6 mm3, both p < 0.001) despite lower cortical vBMD (1182 ± 2 and 1187 ± 2 vs. 1192 ± 2 mm2, p < 0.001 and p = 0.06 respectively) (Fig. 2). Cortical thickness was similar among population groups at younger ages, but smaller at older ages among Hutterites compared to rural and non-rural men (age-by-group interaction, p < 0.001). Cortical area was similar among population groups at younger ages, but was greater with increasing age in rural men compared to Hutterites and non-rural men (age-by-group interaction, p < 0.001).
Fig. 2.
Least square means for pQCT bone measures by population group (triangle = Hutterite; solid circle = non-rural; empty circle = rural). All measurements except trabecular vBMD (4% distal radius) were made at the 20% distal radius. Least square means were estimated from mixed models that included population group, age, age2, height, lean mass, fat mass, change in lean mass, change in fat mass, age-by-group, age2-by-group, time-by-age, time-by-age2, time-by-group, time-by-age-by-group, and time-by-age2-by-group. Least square means are given at specific ages for presentation purposes. Bars represent 95% confidence intervals. Only the significance of the highest order interaction is given.
3.2. Population and age differences in rates of change
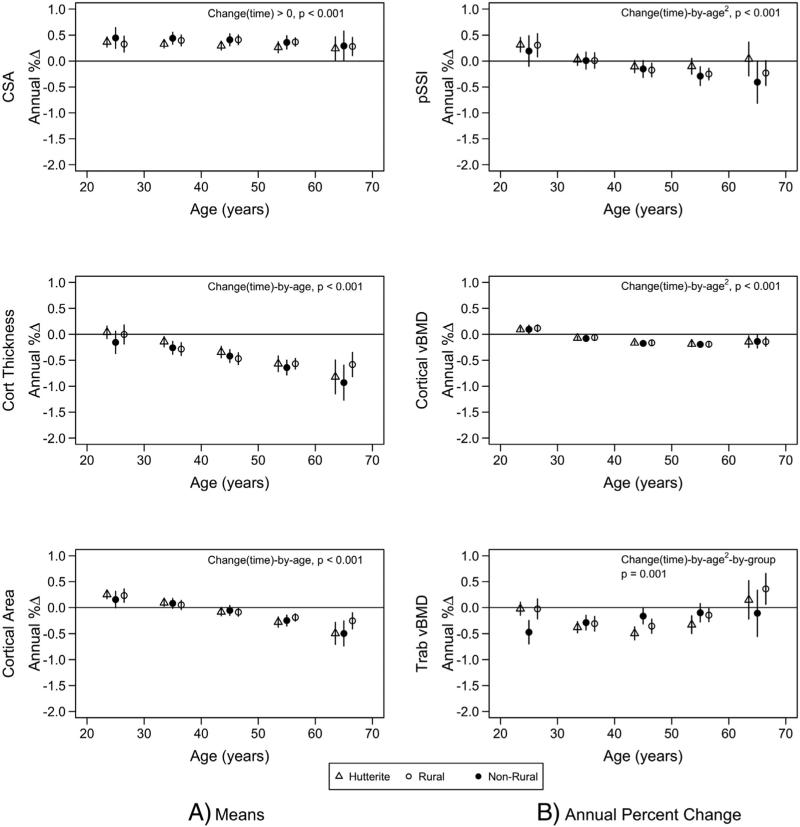
The rates of change in FN BMC and aBMD indicated bone loss that was non-linear with age and varied by population. Greater rates of loss of FN BMC and aBMD were apparent in non-rural men at younger and older ages compared to Hutterite and rural men (time-by-age2-by-group interactions, both p < 0.01; Fig. 1). There was a positive rate of change in FN bone area (p < 0.001) that was similar across all ages (time-by-age interaction not significant) and among all population groups (time-by-group not significant). Greater positive rates of change with increasing age were found for spine BMC and aBMD (time-by-age interactions, both p < 0.01). The rate of gain in bone area decreased with increasing age (time-by-age interaction, p = 0.01) and was lower in Hutterite men compared to rural men (least square means [±sem] of 0.04 ± 0.04 and 0.21 ± 0.05% change per year respectively, p = 0.01).
Changes in pQCT measurements included a positive rate of change in cross-sectional area at all ages that did not differ by population group (time-by-group interaction not significant) (Fig. 3). There were increasing rates of loss in cortical thickness, cortical area, cortical vBMD, and pSSI at older ages (all time-by-age interactions p < 0.001). Rates of change in cross-sectional area, cortical thickness, cortical area, cortical vBMD, and pSSI did not differ among population groups. There was an increased rate of loss in trabecular vBMD among non-rural men in their 20s and 60s compared to Hutterite and rural men; whereas middle-aged Hutterite and rural men had greater rates of loss compared to middle-aged non-rural men (time-by-age-by-group interaction, p < 0.001).
Fig. 3.
Annual percent change for pQCT bone measures by population group (triangle = Hutterite; solid circle = non-rural; empty circle = rural). Least square means were estimated from mixed models that included population group, age, age2, height, lean mass, fat mass, change in lean mass, change in fat mass, age-by-group, age2-by-group, time-by-age, time-by-age2, time-by-group, time-by-age-by-group, and time-by-age2-by-group. Least square means are given at specific ages for presentation purposes. Bars represent 95% confidence intervals. Only the significance of the highest order interaction is given.
3.3. The role of physical activity and calcium intake on explaining population differences in cross-sectional results and rates of change
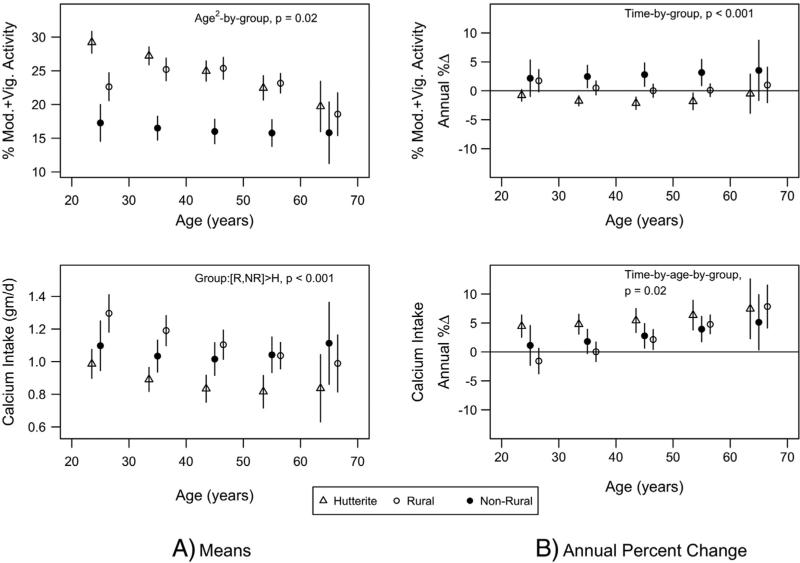
Least square means and rates of change in percent time in moderate plus vigorous activity and calcium intake are shown in Fig. 4. Mean percent time in moderate plus vigorous activity was greater in Hutterite and rural men than non-rural men, especially at younger ages (age2-by-group interaction, p = 0.02). Non-rural men had greater percent increases in activity levels, while Hutterite and rural men did not (time-by-group interaction, p < 0.001).
Fig. 4.
Least square means (A) and annual percent change (B) for percent time in moderate plus vigorous activity and calcium intake by population group (triangle = Hutterite; solid circle = non-rural; empty circle = rural). Least square means were estimated from mixed models that included population group, age, age2, age-by-group, age2-by-group, time-by-age, time-by-age2, time-by-group, time-by-age-by-group, and time-by-age2-by-group. Least square means are given at specific ages for presentation purposes. Bars represent 95% confidence intervals. Only the significance of the highest order interaction is given.
Calcium intakes were greater in rural and non-rural men compared to Hutterite men (least square means ± SE: 1209 ± 31 and 1146 ± 35 vs. 1038 ± 28 mg/day, p < 0.001 and p = 0.04 respectively). Annual changes in calcium intake were positive and greater at older ages, with Hutterite men having greater increases than rural and non-rural men (time-by-age-by-group interaction, p = 0.02).
We next identified which bone outcomes were associated with these potential mediators. Neither percent time in moderate plus vigorous activity at baseline nor changes in activity levels were associated with any of the bone outcomes and therefore did not meet the criteria for being a mediator. However, baseline calcium intake was associated with FN aBMD (p = 0.04) and increases in calcium intake were associated with spine BMC (p = 0.04) and inversely associated with cortical area (p = 0.02). Inclusion of baseline calcium intake resulted in attenuations of the beta coefficients for rate of change in FN aBMD by age and group (time-by-age-by-group and time-by-age2-by-group interactions) that ranged from 6.9 to 50% depending upon the population and age group. The overall effects, however, on the least square means were small as shown in Table 3 and the time-by-age2-by-group interaction term remained statistically significant (p = 0.006 in base model to p = 0.02 in model with baseline calcium intake). Adding changes in calcium intake to the model predicting spine BMC attenuated the beta coefficient for population group, but the mediating effect of changes in calcium intake was small (2.9%). Changes in the least square means also were negligible (Table 3), and population group differences remained significant (p = 0.03). Inclusion of changes in calcium intake in the model predicting cortical area also reduced the beta coefficients for the age-by-group interaction (range 1.2 to 10%) but the effects were minimal and the age-by-group interaction remained significant (p < 0.001).
Table 3.
Mediating effect of calcium intake on least square means for FN aBMD, spine BMC, and cortical area.a
| Hutterite |
Rural |
Non-rural |
|||||
|---|---|---|---|---|---|---|---|
| Base modelb | Base + calcium intake | Base model | Base + calcium intake | Base model | Base + calcium intake | ||
| FN aBMD | Age (year) | ||||||
| Annual % change | 25 | - 0.68 | -0.68 | -0.81 | -0.82 | -1.17 | -1.13 |
| 35 | - 0.32 | -0.31 | -0.53 | -0.52 | -0.47 | -0.46 | |
| 45 | -0.06 | -0.04 | -0.27 | -0.26 | -0.11 | -0.12 | |
| 55 | 0.05 | 0.08 | -0.07 | -0.06 | -0.17 | -0.18 | |
| 65 | - 0.01 | 0.03 | 0.06 | 0.06 | -0.71 | -0.68 | |
| Spine BMC | |||||||
| Least square means | 80.38 | 80.36 | 76.40 | 76.42 | 74.91 | 74.93 | |
| Cortical area | Age (year) | ||||||
| Least square means | 25 | 105.03 | 104.90 | 103.04 | 3.01 | 100.22 | 100.23 |
| 35 | 105.18 | 105.06 | 105.64 | 105.60 | 100.57 | 100.58 | |
| 45 | 105.00 | 104.83 | 108.38 | 108.31 | 101.39 | 101.38 | |
| 55 | 104.49 | 104.30 | 111.28 | 111.14 | 102.69 | 102.64 | |
| 65 | 103.64 | 103.46 | 114.32 | 114.09 | 104.47 | 104.35 | |
FN aBMD: significant highest order interaction including population group was time-by-age2-group; baseline calcium intake was included as a potential mediator. LS BMC: significant main effect for population group; change in calcium intake was included as a potential mediator. Cortical Area: significant age-by-group interaction; change in calcium intake was included as a potential mediator.
Base model included population group, age, age2, height, lean mass, fat mass, change in lean mass, change in fat mass, age-by-group, age2-by-group, time-by-age, time-by-age2,time-by-group, time-by-age-by-group, and time-by-age2-by-group.
4. Discussion
Although some longitudinal studies have investigated rates of change in bone among men [25–28], the current study is the first to report rates of change in bone from three distinct populations of men living two very different lifestyles. Two of the populations (Hutterite and rural) lived at least 75% of their lives on an active working farm, while the third population (non-rural) never lived on a working farm. The inclusion of these three distinct populations provides insight into whether mean measurements or rates of changes in bone are a result of lifestyle differences (Hutterite and rural men would be similar but different from non-rural men) or a result of possible genetic differences (Hutterite results would be different from rural and non-rural men). For example, greater FN bone area and radial cross-sectional area, cortical area, cortical vBMD and pSSI in Hutterites and rural compared to non-rural men are consistent with an effect of lifestyle differences. However, the greater spine BMC and aBMD among Hutterites compared to both rural and non-rural men, along with a greater rate of change in spine bone area in rural than Hutterite men, is consistent with possible genetic effects.
Several studies have reported decreased fracture risk among individuals residing in rural geographical locations compared to urban locations [3–7]. Some investigators have attributed this difference to a higher aBMD or BMC among rural residents compared to urban residents [4,8,9]. Although we did not find population differences in our cross-sectional analysis of hip BMC and aBMD, we did find significant Hutterite and rural vs. non-rural differences in bone size (femoral neck bone area and radial cross-sectional area). Bone size contributes significantly to bone strength, with a larger bone having greater bone strength than a smaller bone [29,30]. We speculate that perhaps it is the larger bones among rural populations that lead to a lower fracture risk rather than differences in aBMD.
It has been proposed that increases in periosteal apposition and bone area that is observed with aging in men, without an apparent change in BMC, may explain the decrease in aBMD with increasing age [31]. Results of the current study suggest that the annual rate of change in BMC parallels changes in aBMD and should not be disregarded. Our findings of increased rates of loss in femoral neck BMC and aBMD, but not spine aBMD, at younger ages (20s and 30s) are consistent with other reports in young men [25,26]. Greater rates of loss in femoral neck BMC and aBMD and trabecular vBMD in non-rural men at the younger (20–30s) and older (60s) ages compared to Hutterite and rural men suggest that these rates of loss are influenced by environmental or lifestyle differences.
Increased periosteal expansion occurred at all ages in all three populations, thereby maintaining the larger femoral neck bone area and radius cross-sectional bone area in Hutterite and rural men compared to non-rural men. An increased rate of cortical thinning occurred at older ages leading to a loss in cortical area and reduction in bone strength as measured by pSSI. In addition, cortical vBMD was found to decrease with increasing age. Although the rate of change in cortical vBMD was positive in early adulthood, by the mid-forties there was an increased rate of loss, albeit small, in cortical vBMD. The timing of the increased cortical thinning and loss in cortical vBMD is consistent with declining testosterone and bioactive E2 concentrations, which have been shown to be associated with increased endosteal resorption and lower cortical vBMD [32]. Other investigators have reported increased cortical porosity with aging using high resolution pQCT [33,34], which may indicate that the reduced cortical vBMD is due to increased cortical porosity and not a reduction in density of bone material [34]. The increased thinning of the cortical shell in combination with reduced cortical area and vBMD may explain the decreased BMC and aBMD findings that were observed by DXA at the femoral neck.
Riggs et al. also reported decreases in trabecular vBMD in men as young as 30 years of age that was not associated with testosterone and bioactive E2 concentrations [32]. We also found significant rates of loss in trabecular vBMD among all three populations in their 30s. However, our finding of a greater rate of loss in trabecular vBMD in non-rural men in their 20s compared to Hutterite and rural men of similar age suggests that lifestyle factors may be associated with preservation of trabecular vBMD. It is not known what aspect of a rural lifestyle could explain these differences. We speculate that although the recall of activity levels was not found to be associated with bone measures, the type of bone-loading activities among these populations may differ and explain group differences that were observed.
Despite the rural–urban differences that have been reported in fracture risk, BMC and aBMD, little is known about the factors that influence these differences. We investigated both activity levels and dietary calcium intake as possible explanations for population differences. Both rural populations had significantly greater percent time in moderate plus vigorous activity than the non-rural population, especially at younger ages, and calcium intake also varied by population group. However, physical activity was not associated with any of the bone measurements and therefore could not be considered as a mediator. This finding was not expected since we previously reported, in a heritability analysis including Hutterite men [35], that the percent time in moderate plus vigorous activity was significantly associated with higher femoral neck BMC and radius cross-sectional area. It is likely that we previously were able to detect the effect of activity levels on bone because the genetic effect, which explained 40–62% of the residual variation in BMC and aBMD, was taken into account thereby allowing for factors with more subtle effects to be identified. We did, however, find that calcium intake and changes in calcium intake over the 7.5-year study partially mediated the relationship between population group and femoral neck aBMD, spine BMC and cortical area. However, the magnitude of the effects of calcium intake on the least square means or rates of change was quite small and did not explain the population differences in these bone outcomes.
Another possibility is that activity levels early in life are a more important predictor of adult bone than current activity levels [36–38]. We previously investigated the relationship between activity levels early in life on baseline bone measurements in the rural non-Hutterite population by looking at individuals who were raised on farms with low mechanization compared to farms that were highly mechanized [38]. We assumed that individuals raised on farms with low mechanization would have higher activity levels as a child than individuals raised on farms with a higher level of mechanization. Although we found an effect of greater femoral neck bone area among individuals raised on farms with low mechanization vs. high mechanization, this difference was limited to females. Sundberg and coworkers previously reported differences in spine aBMD among rural vs. urban adolescent boys aged 15–16 years, which supports rural vs. urban bone differences existing earlier in life [39]. Our finding of greater radius cross-sectional area in Hutterite and rural men compared to non-rural men across all ages also is consistent with differences existing prior to our lowest age of 20 years.
There are several unique aspects of the current study. First, previous cross-sectional studies reported higher BMC and aBMD in populations living in rural areas compared to urban areas [4,39], but these studies did not consider the lifestyle of the individual. Our population-based study was conducted in eight counties in eastern South Dakota that are all classified as rural non-metro counties. We chose to identify two extremes of rural lifestyle by including individuals who spent either the majority of their life, or none of their life, on an active working farm. Hutterites actively farm and, in terms of daily activities, are more similar to the rural population than the non-rural population. Second, we collected prospective diet and activity data quarterly for the first three years from study participants. Few reports have collected these data throughout the year. Third, we obtained bone measurements using pQCT in addition to DXA, which allowed us to obtain information on changes in bone geometry with aging.
There are several limitations of this study. pQCT measurements were made at the radius only and did not include tibial measures. Originally, we chose the radius as a non-loading site in order to investigate possible genetic differences in bone measures independent of loading. However, many farming activities (i.e., throwing hale bales, shoveling, etc.) involve the use of the upper body and could lead to increased bone loading at the radius. It is possible that tibia results may have differed from those observed at the radius. Another limitation is that the longitudinal statistical models used assume the within-individual change in a bone measure over time is linear. The cross-sectional changes in some bone measures when viewed over different ages have a non-linear parabolic profile, but the non-linearity occurred over a longer time frame than the 90 months of follow-up used in this study. We did evaluate the potential influence of non-linearity on the estimates for rates of change (data not presented). We found that for almost all bone measures the age-dependent pattern for rates of change were similar for estimates based on 36-month versus 90-month data. Only FN BMC had a substantial discrepancy between rate estimates based on 36- versus 90-month data, as judged by complete separation of corresponding 95% confidence intervals. For example, the 95% confidence intervals for annual rate of change in FN BMC at 65 years of age for rural men was −4.7% to −2.3% based on 36-month data but −1.5% to −0.5% based on 90-month data.
In summary, we found that men living a rural lifestyle had larger bones by both DXA and pQCT, and had greater cortical area and bone strength (pSSI) despite lower cortical vBMD, than men living a non-rural lifestyle. We speculate that these differences in bone size may partially explain rural–urban differences in fracture rates that have previously been reported. The rates of change in femoral neck BMC and aBMD and trabecular vBMD indicated greater losses among non-rural men in their 20s and 60s than in both Hutterite and rural men. Percent time in moderate plus vigorous activity measured by recall was not found to be a potential mediator of these population differences. Although calcium intake at baseline, or changes in calcium intake over the study period, were partial mediators of population differences in bone, the mediation accounted for only a small amount of the population association. We speculate that rural–non-rural differences in adult men are a result of lifestyle differences that occur earlier in life or are a result of factors related to a rural lifestyle that have not yet been identified.
Supplementary Material
Acknowledgments
We would like to thank the participants for their time, effort and commitment that they put into the study.
Footnotes
Funding source: Supported in part by the National Institutes of Health Grant #R01-AR47852 and the EA Martin Endowment in Human Nutrition, South Dakota State University. Funding sources had no involvement in the study design, data analysis or interpretation of data.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bone.2015.04.045.
Financial disclosures
None of the authors have any financial disclosures.
References
- 1.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 2.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353:878–82. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 3.Melton LJ, III, Crowson CS, O'Fallon WM. Fracture incidence in Olmsted County, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporos Int. 1999;9:29–37. doi: 10.1007/s001980050113. [DOI] [PubMed] [Google Scholar]
- 4.Gardsell P, Johnell O, Nilsson BE, Sernbo I. Bone mass in an urban and a rural population: a comparative, population-based study in southern Sweden. J Bone Miner Res. 1991;6:67–75. doi: 10.1002/jbmr.5650060112. [DOI] [PubMed] [Google Scholar]
- 5.Sanders KM, Nicholson GC, Ugoni AM, Seeman E, Pasco JA, Kotowicz MA. Fracture rates lower in rural than urban communities: the Geelong Osteoporosis Study. J Epidemiol Community Health. 2002;56:466–70. doi: 10.1136/jech.56.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mannius S, Mellstroem D, Oden A, Rundgren A, Zetterberg C. Incidence of hip fracture in Western Sweden 1974–1982. Acta Orthop Scand. 1987;58:38–42. doi: 10.3109/17453678709146340. [DOI] [PubMed] [Google Scholar]
- 7.Chevalley T, Herrmann FR, Delmi M, Stern R, Hoffmeyer P, Rapin CH, et al. Evaluation of the age-adjusted incidence of hip fractures between urban and rural areas: the difference is not related to the prevalence of institutions for the elderly. Osteoporos Int. 2002;13:113–8. doi: 10.1007/s001980200002. [DOI] [PubMed] [Google Scholar]
- 8.Meyer HE, Berntsen GKR, Sogaard AJ, Langhammer A, Schei B, Fonnebo V, et al. Higher bone mineral density in rural compared with urban dwellers: the NOREPOS study. Am J Epidemiol. 2004;160:1039–46. doi: 10.1093/aje/kwh337. [DOI] [PubMed] [Google Scholar]
- 9.Omsland TK, Gjesdal CG, Emaus N, Tell GS, Meyer HE. Regional differences in hip bone mineral density levels in Norway: the NOREPOS study. Osteoporos Int. 2009;20:631–8. doi: 10.1007/s00198-008-0699-7. [DOI] [PubMed] [Google Scholar]
- 10.Specker B, Binkley T, Fahrenwald N. Rural vs. non-rural differences in BMC, volumetric BMD, and bone size: a population based cross-sectional study. Bone. 2004;35:1389–98. doi: 10.1016/j.bone.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Streeten EA, McBride DJ, Lodge AL, Pollin TI, Stinchcomb DG, Agarwala R, et al. Reduced incidence of hip fracture in the Old Order Amish. J Bone Miner Res. 2004;19:308–13. doi: 10.1359/JBMR.0301223. [DOI] [PubMed] [Google Scholar]
- 12.Melton LJ, III, Khosla S, Atkinson EJ, O'Connor MK, O'Fallon WM, Riggs BL. Cross-sectional versus longitudinal evaluation of bone loss in men and women. Osteoporos Int. 2000;11:592–9. doi: 10.1007/s001980070080. [DOI] [PubMed] [Google Scholar]
- 13.Lauretani F, Bandinelli S, Griswold ME, Maggio M, Semba R, Guralnik JM, et al. Longitudinal changes in BMD and bone geometry in a population-based study. J Bone Miner Res. 2008;23:400–8. doi: 10.1359/JBMR.071103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orwoll ES, Oviatt SK, Biddle JA. Precision of dual-energy x-ray absorptiometry: development of quality control rules and their application in longitudinal studies. J Bone Miner Res. 1993;8:693–9. doi: 10.1002/jbmr.5650080607. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa Y, Schneider P, Reiners C. Age, sex, and grip strength determine architectural bone parameters assessed by peripheral quantitative computed tomography (pQCT) at the human radius. J Biomech. 2001;34:497–503. doi: 10.1016/s0021-9290(00)00211-6. [DOI] [PubMed] [Google Scholar]
- 16.Paffenbarger RS, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–75. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 17.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–6. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 18.Osowski J, Beare T, Specker B. Validation of a food frequency questionnaire for assessment of dietary calcium intake in rural populations. J Am Diet Assoc. 2007;107:1349–55. doi: 10.1016/j.jada.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Wiley-Interscience; Hoboken, N.J.: 2004. [Google Scholar]
- 20.Wey HE, Binkley T, Beare T, Wey CL, Specker B. Cross-sectional versus longitudinal associations of lean and fat mass with pQCT bone outcomes in children. J Clin Endocrinol Metab. 2011;96:106–14. doi: 10.1210/jc.2010-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taes YEC, Lapauw B, Vanbillemont G, Bogaert V, De Bacquer D, Zmierczak H, et al. Fat mass in negatively associated with cortical bone size in young healthy male siblings. J Clin Endocrinol Metabol. 2009;94:2325–31. doi: 10.1210/jc.2008-2501. [DOI] [PubMed] [Google Scholar]
- 22.Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 23.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Section 4.5: mediation. Regression methods in biostatistics: linear, logistic, survival, and repeated measures models. 2nd ed. Springer; New York: 2012. pp. 94–9. [Google Scholar]
- 24.Fairchild AJ, MacKinnon DP. A general model for testing mediation and moderation effects. Prev Sci. 2009;10(2):87–99. doi: 10.1007/s11121-008-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nordstrom P, Neovius M, Nordstrom A. Early and rapid bone mineral density loss of the proximal femur in men. J Clin Endocrinol Metab. 2007;92:1902–8. doi: 10.1210/jc.2006-2613. [DOI] [PubMed] [Google Scholar]
- 26.Ohlsson C, Darlid A, Nilsson M, Melin J, Mellstroem D, Lorentzon M. Cortical consolidation due to increased mineralization and endosteal contraction in young adult men: a five-year longitudinal study. J Clin Endocrinol Metab. 2011;96:2262–9. doi: 10.1210/jc.2010-2751. [DOI] [PubMed] [Google Scholar]
- 27.Emaus N, Berntsen GKR, Joakimsen RM, Fonnebo V. Longitudinal changes in forearm bone mineral density in women and men aged 25–44 years. Am J Epidemiol. 2005;162:633–43. doi: 10.1093/aje/kwi258. [DOI] [PubMed] [Google Scholar]
- 28.Khosla S, Melton J, III, Atkinson EJ, O'Fallon WM. Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metabol. 2001;86:3555–61. doi: 10.1210/jcem.86.8.7736. [DOI] [PubMed] [Google Scholar]
- 29.Orwoll ES. Toward an expanded understanding of the role of the periosteum in skeletal health. J Bone Miner Res. 2003;18:949–54. doi: 10.1359/jbmr.2003.18.6.949. [DOI] [PubMed] [Google Scholar]
- 30.Frost H. Mechanical usage, bone mass, bone fragility: a brief overview. In: Kleerekoper M, Krane S, editors. Clinical disorders of bone and mineral metabolism. Liebert; New York: 1989. pp. 15–40. [Google Scholar]
- 31.Bakker I, Twisk JWR, Van Mechelen W, Kemper HCG. Fat-free body mass is the most important body composition determinant of 10-yr longitudinal development of lumbar bone in adult men and women. J Clin Endocrinol Metab. 2003;88:2607–13. doi: 10.1210/jc.2002-021538. [DOI] [PubMed] [Google Scholar]
- 32.Riggs BL, Melton LJ, III, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, et al. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008;23:205–14. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S. Age- and gender-related differences in the geometric properties and biomechanical significance of intra-cortical porosity in the distal radius and tibia. J Bone Miner Res. 2010;25:983–93. doi: 10.1359/jbmr.091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicks KM, Amin S, Atkinson EJ, Riggs BL, Melton LJ, III, Khosla S. Relationship of age to bone microstructure independent of areal bone mineral density. J Bone Miner Res. 2012;27:637–44. doi: 10.1002/jbmr.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Havill L, Mahaney M, Binkley TL, Specker BL. Effects of genes, gender, age and activity on BMC, bone size, and areal and volumetric BMD. J Bone Miner Res. 2007;22:737–46. doi: 10.1359/jbmr.070213. [DOI] [PubMed] [Google Scholar]
- 36.Bass S, Pearce G, Bradney M, Hendrich E, Delmas PD, Harding A, et al. Exercise before puberty may confer residual benefits in bone density in adulthood: studies in active prepubertal and retired female gymnasts. J Bone Miner Res. 1998;13:500–7. doi: 10.1359/jbmr.1998.13.3.500. [DOI] [PubMed] [Google Scholar]
- 37.Lorentzon M, Mellstroem D, Ohlsson C. Association of amount of physical activity with cortical bone size and trabecular volumetric BMD in young adult men: the GOOD Study. J Bone Miner Res. 2005;20:1936–43. doi: 10.1359/JBMR.050709. [DOI] [PubMed] [Google Scholar]
- 38.McCormack LA, Binkley TL, Specker BL. Effect of level of farm mechanization early in life on bone later in life. J Musculoskelet Neuronal Interact. 2012;12:7–15. [PMC free article] [PubMed] [Google Scholar]
- 39.Sundberg M, Duppe H, Gardsell P, Johnell O, Ornstein E, Sernbo I. Bone mineral density in adolescents. Higher values in a rural area—a population-based study of 246 subjects in southern Sweden. Acta Orthop Scand. 1997;68:456–60. doi: 10.3109/17453679708996262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.