Abstract
Brain-implanted devices are no longer a futuristic idea. Traditionally, therapies for most neurological disorders are adjusted based on changes in clinical symptoms and diagnostic measures observed over time. These therapies are commonly pharmacological or surgical, requiring continuous or irreversible treatment regimens that cannot respond rapidly to fluctuations of symptoms or isolated episodes of dysfunction. In contrast, closed-loop systems provide intervention only when needed by detecting abnormal neurological signals and modulating them with instantaneous feedback. Closed-loop systems have been applied to several neurological conditions (most notably epilepsy and movement disorders), but widespread use is limited by conceptual and technical challenges. Herein, we discuss how advances in experimental closed-loop systems hold promise for improved clinical benefit in patients with neurological disorders.
Closed-loop systems incorporate feedback between output and input signals to effectively exert control over the system. The benefits of closed-loop feedback are most significant when symptoms fluctuate rapidly depending on external or internal conditions or when symptoms occur in time-limited episodes with periods of intervening normal function. In these cases, the development of automated or programmable closed-loop systems that operate in real time is advantageous. Clinical efficacy for such systems has already been demonstrated for several categories of neurological disease.1 However, effective closed-loop systems for clinical applications require the following key components:(1) salient pathologic signals to serve as inputs; (2) appropriate sensors to capture these signals; (3) real-time, computationally manageable algorithms to process inputs; and (4) appropriate effectors and actuators to deliver interventions with the correct parameters to the target.
A diverse combination of physiological signals and intervention approaches can be used in a closed-loop fashion. For instance, patient motion captured by an accelerometer has been used to guide the display of virtual visual stimuli to improve gait impairment in patients with Parkinson disease.2 Furthermore, drug dosing based on monitored physiological parameters in certain patient populations could be seen as on-demand treatment, and such an approach is effectively used in anesthesiology to ensure maintenance of stable, appropriate levels of anesthesia during operative procedures.3,4 However, most well-established closed-loop devices for neurological applications have used intervention strategies that provide greater temporal precision, typically in the form of on-demand electrical stimulation of the central nervous system, and we focus our discussion on such strategies herein.
Perhaps the earliest closed-loop experiment in an animal model was performed by Delgado and colleagues5 on a chimpanzee named Paddy. Paddy was fitted with a telemetric device (“stimoceiver”), and when spindle patterns were detected from presumed amygdala circuits, feedback stimulation was delivered to the central gray area. The aversive effects of the stimulation reduced the occurrence of spindles, and Paddy became quieter, less attentive, and less motivated during behavior altesting.5 Delgado et al6 envisioned that such closed-loop feedback stimulation could be used to reduce seizures, panic attacks, or other neurological symptoms and explored the feasibility of such potential therapy in humans. Many technical hurdles prevented the practicality of the closed-loop method in clinical use at that time, but neurostimulation is now an established therapy for several neurological disorders. Patients with intractable neuropathic pain experience improved pain relief and quality of life when body position information acquired by an accelerometer is used to adjust the parameters of spinal cord stimulation (RestoreSensor; Medtronic, Inc7). Progress has also been made in automated triggering of deep brain stimulation (DBS) based on local field potentials recorded from the basal ganglia in patients with Parkinson disease8 (Activa PC + S; Medtronic, Inc [clinicaltrials.gov identifiers NCT02115802, NCT01934296, NCT01990313, and NCT02235792]). Two closed-loop options are currently available as adjunctive therapy for patients with refractory epilepsy. Closed-loop therapy in epilepsy requires early seizure detection and effective feedback selectively at the time of seizures. This method is in stark contrast to traditional approaches in which treatment is irreversible (eg, surgical removal of tissue), constantly applied, or applied without regard to the current brain state (Figure). The NeuroPace system (NeuroPace, Inc), which uses abnormal electrocorticography signals to trigger focal cortical stimulation, is approved by the US Food and Drug Administration and has demonstrated clinical safety and efficacy in reduction of seizure frequency in a select patient population.11 Closed-loop vagal nerve stimulation (AspireSR; Cyberonics, Inc [clinicaltrials.gov identifier NCT01325623]) is approved for use in Europe, although the trigger for stimulation is ictal tachycardia rather than brain-derived electrophysiological signals.
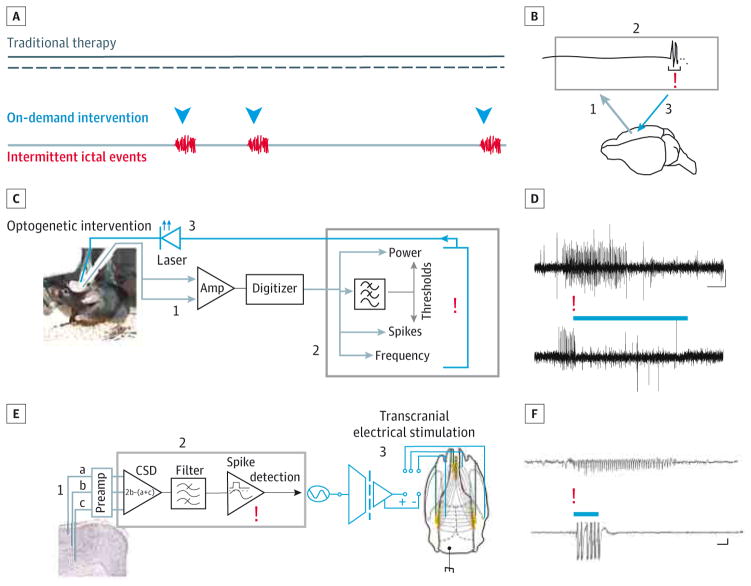
Figure. Closed-Loop Intervention to Inhibit Spontaneous Seizures.
A, In contrast to traditional therapeutic approaches (black lines), which are applied in a constant or scheduled manner without regard to brain states or the actual occurrence of pathologic events such as seizures, on-demand intervention is designed to align intervention (blue arrowheads) with events that require intervention (eg, seizures [red]). B, Such closed-loop approaches use information about ongoing brain activity to determine when to provide intervention. Brain activity is recorded (step 1 [gray]) and processed in real time (step 2 [upper rectangle]) to detect events online (red exclamation point). Detection triggers immediate intervention (step 3 [blue]), altering brain activity and closing the loop. C, Closed-loop optogenetic system used to detect spontaneous temporal lobe seizures based on various properties of the signal, including power, spiking, and frequency components. D, On-demand optogenetic inhibition of excitatory cells reduced seizure duration. The top trace shows a spontaneous seizure without intervention, and the bottom trace shows a spontaneous seizure with light intervention. E, Closed-loop transcranial electrical stimulation intervention system used to inhibit absence seizures in rats (described in detail in the Effectors and Actuators section in the main text). CSD indicates current source density. F, On-demand transcranial electrical stimulation intervention shortens absence seizures. The top trace shows a spontaneous seizure recorded without intervention, and the bottom trace shows a spontaneous seizure detected online (red exclamation point), triggering transcranial electrical stimulation intervention (the period of intervention is denoted by a blue bar [the large amplitude signal is a transcranial electrical stimulation–induced artifact and has been truncated]). Scale bars are 500 ms and 0.5 mV (D) and 5 s and 100 μV (F). C and D are modified with permission and in accord with the Creative Commons license (http://creativecommons.org/licenses/by/3.0/) from Krook-Magnuson et al.9 E and F are modified with permission from Berényi et al.10
Data generated from the use of these devices support the notion that closed-loop therapeutics can offer increased efficacy and clinical benefit of stimulation, as well as decreased stimulation adverse effects, compared with open-loop protocols.7,12 Despite these potential benefits, it remains difficult to develop new closed-loop technologies and refine stimulation parameters in a clinical setting. Herein, we discuss examples of how studies using cutting-edge techniques and analysis methods in a research setting can facilitate improvements in components of a closed-loop therapeutic system.
Input Signals
Determining the appropriate pathologic signals to use as triggers for closed-loop modulation in various disease states remains a major challenge. For example, various features identified in electromyography recordings and electrophysiological signals from basal ganglia or cortical structures are associated with parkinsonism.13,14 Without a sufficient understanding of which signals are critically integrated in the pathologic circuit vs merely correlational, selection of 1 or more of these features for incorporation into a closed-loop paradigm becomes a process of trial and error. By inserting recording electrodes in cortical and basal ganglia structures in the nonhuman primate, Rosin et al12 demonstrated that responsive DBS that was triggered by action potentials in the motor cortex was effective in improving motor function and was associated with a prominent reduction in pallidal oscillatory activity. Conversely, triggering DBS based on action potentials in the pallidum (rather than the motor cortex) increased pallidal oscillatory activity and actually worsened motor activity. These experiments supported the pathologic role of these oscillations and (in concert with other key research and clinical observational studies) helped prompt clinical investigation of closed-loop stimulation in patients with Parkinson disease triggering on oscillatory activity recorded in the basal ganglia.8
Experimentation in animal models offers the advantage of recording signals from individual and populations of neurons in brain regions often inaccessible to clinical recording in humans, as well as the possibility of recording from multiple distributed brain regions simultaneously. Such approaches allow for better characterization of network-level mechanisms at the spatiotemporal scale of neuronal interactions, the significance of which is being increasingly recognized in neurological disorders.15,16 Closed-loop methods have been used successfully to selectively detect and abolish or enhance hippocampal sharp wave ripples by electrical17 or optogenetic18 stimulation and to demonstrate that these oscillations have a causal role in memory trace consolidation. However, the successful translation of animal studies results to clinical applications is dependent on the reliability of the animal model used, emphasizing the importance of an ongoing dialogue among researchers involved in animal and clinical studies of any given disorder.
Sensors
Once the relevant signals have been identified, appropriate sensors for acquisition of these signals are required. For electrophysiological signals, sensors should be placed in proximity to the generator of the pathologic signal and be capable of sufficiently high temporal resolution acquisition. For example, changes in neural spiking rates or patterns are linked to the onset of seizures and worsening of parkinsonian symptoms in humans and could potentially be used for triggering intervention in a closed-loopsystem.19,20 However, most clinical electrodes placed on the brain surface (electrocorticography electrodes) or within deep structures (depth electrodes) are too large and often transduce signals too inefficiently to capture high-frequency activity in the form of action potentials. Electrodes or arrays with improved spatiotemporal resolution could substantially enhance the sensitivity and specificity of closed-loop therapeutics. Seizure control is a prime example of the potential benefits of improved triggering methods through improved signal selection and recording methods because it has been demonstrated that early intervention improves the success of seizure termination.21 Evidence from high-density microelectrode arrays (eg, NeuroPort; Cyberkinetics Neurotechnology Systems) inserted into the cortex located in the seizure-onset zone in patients with medically refractory epilepsy revealed microseizures that often preceded detection of seizure activity on the clinical subdural electrodes.22 Multiunit spiking activity acquired by these research electrodes further showed that there was a discrepancy between the onset of the ictal rhythm as detected by conventional electrodes and the time of hypersynchronous neuronal recruitment.16 Therefore, it is possible that the use of refined electrodes capable of acquiring spike resolution data could serve as more effective triggers for closed-loop intervention in epilepsy.
However, the clinical relevance of microelectrode arrays for closed-loop therapy is currently limited by their small spatial coverage (typically 4 × 4 mm), instability in recording unit activity over long periods, and cortical damage associated with array insertion.23,24 These barriers could potentially be overcome by innovations in electrode design. Ongoing mechanical trauma caused by micromotion of implanted electrodes contributes substantially to recording instability and post implantation glial cell activation.24,25 A sinusoidal probe microfabricated from flexible materials has been designed to minimize this motion and has successfully recorded physiological signals for close to 2 years’ duration with decreased neuronal tissue damage in a rabbit model.26 An alternative approach involves detection of spiking activity from the surface of the human brain using conformable, biocompatible, and scalable electrode arrays based on conducting polymers (NeuroGrid27). Further studies are required before such technology could be translated to routine clinical use, but these novel sensors could improve the sensitivity of detection while simultaneously decreasing the potential adverse effects of sensor placement.
Advances in sensors are not limited to electrophysiology. In addition to electrical fields, neurons generate neurochemical signatures in the extracellular space due to neurotransmitter release. Changes in various neuroactive substances are associated with neurological disease. The most well-known is the pallidal dopamine deficiency in Parkinson disease.28 Fast-scan cyclic voltammetry is a method by which changes in the extracellular concentration of electroactive molecules (eg, dopamine, norepinephrine, serotonin, and adenosine) can be measured in real time by oxidation and reduction reactions at a carbon fiber electrode.29 In patients with essential tremor, fast-scan cyclic voltammetry has been incorporated into a wireless, instantaneous, neurochemical concentration sensing system that can quantify changes in adenosine release following DBS electrode insertion.30 Similar sensors could serve as the input component of closed-loop systems that aim to maintain constant extra-cellular concentrations of a targeted neurochemical, such as dopamine in Parkinson disease.31
Detection Algorithms
Fast and reliable pattern detection algorithms with a low incidence of false alarms are needed for making closed-loop stimulation safe and effective in clinical settings. Although such algorithms are required for all closed-loop devices, the inherent difficulties and possibilities for implementation of novel strategies is especially evident for seizure detection in patients with epilepsy and is thus discussed herein.
Seizure detection is a complex issue due to the heterogeneity of seizure characteristics in individual seizures from the same patient and in seizures from different patients. Detection algorithms have been based on features of signals extracted from electroencephalography, electrocorticography, electromyography, electrocardiography, accelerometry, and video recordings,32 but the only closed-loop device currently approved by the Food and Drug Administration (NeuroPace system) uses signals recorded from electrocorticography or implanted depth electrodes.33 This device has 3 detection parameters (bandpass, line length, and area) that can be adjusted by the physician to enhance the sensitivity and specificity for each individual patient’s electrophysiological seizure pattern. Although this detection algorithm is computationally efficient and can detect events within a fraction of a second, adjustment of detection parameters is fine-tuned by trial and error, requiring significant time for the physician and patient. A potential solution to this issue is to use advanced classification methods or learning algorithms, which are increasingly being used in a wide variety of applications from drug discovery to computer vision. Methods that have been tried for seizure detection include support vector machines, artificial neural networks, fuzzy logic models, and Markov modeling.32 Such approaches typically involve a training phase, during which previously acquired data are processed and relevant features indicating seizure onset are extracted. A classifier that can be run in real time is then created and used to detect subsequent seizures. Data from many patients can be used during training, with the goal of creating a generalizable classifier; alternatively, a substantial amount of data from an individual patient can be used to derive a patient-specific classifier.34 A significant challenge to these approaches is to optimize the speed of the real-time classifier to detect seizures within a reasonable latency for closed-loop applications.
Effectors and Actuators
Ideally, effective stimulation parameters for a closed-loop system should revert pathologic neural activity patterns to physiological patterns. Some disorders could benefit from large volume and relatively diffuse stimulation or suppression of cortex-wide activity, whereas others need focal stimulation. In practice, these parameters can be difficult to establish because the effects of electrical stimulation on populations of neurons are often unknown, and various cell types in the brain may be influenced. For example, it may be theoretically advantageous to suppress the firing of pyramidal (excitatory) cells but not interneurons (inhibitory) at the onset of a seizure. Testing the effect of cell type–specific modulation on pathologic states was not efficiently possible until the application of optogenetic techniques,35 described in detail below.
Optogenetics rests on selective expression of light-sensitive proteins.36 These engineered proteins (called opsins) are not natively expressed. Therefore, the use of optogenetic techniques has so far been limited to animal research. Through restricted expression of the light-sensitive proteins, selective control of specific neuronal populations is possible. In addition, different light-sensitive proteins exist, including excitatory channels (to increase the firing rate of expressing neurons) and inhibitory pumps and channels (to inhibit the firing of expressing neurons). Therefore, on-demand optogenetics provides not only the temporal specificity of electrical stimulation but also cell-type specificity and direct control over the direction of modulation (ie, excitation or inhibition). For example, it is now possible to selectively inhibit a subclass of neurons in a particular brain region at a particular time, at least in animal studies.37 The great specificity of intervention achievable with on-demand optogenetics already makes it an extremely powerful experimental tool, and discoveries made can have implications in the clinical setting.
Optogenetic techniques have been applied in several epilepsy models,38 and on-demand optogenetics specifically has shown success in rodent models of thalamocortical39 and temporal lobe9 epilepsy. One recent study39 reported that experimental cortical stroke could induce thalamocortical epilepsy and examined the potential for on-demand optogenetics targeting the thalamus to stop these cortical stroke–induced seizures. To inhibit thalamic output, an inhibitory opsin (halorhodopsin) was expressed in excitatory cells in the ventrobasal thalamus ipsilateral to the site of induced cortical stroke.39 Seizures were detected online using line-length threshold crossing, rapidly triggering automatic light delivery. This on-demand optogenetic approach successfully truncated thalamocortical seizures.
Several distinct on-demand optogenetic approaches for temporal lobe seizures have been tested.40 First, an inhibitory light-activated chloride pump (halorhodopsin) was expressed in excitatory cells.9 On-demand light delivery to the hippocampus inhibited these cells and dramatically truncated seizures (Figure, C and D). A second approach tested an excitatory light-activated cation channel (channelrhodopsin) expressed in a subpopulation of inhibitory neurons. These neurons comprise less than 5% of the neuronal population.41,42 On-demand light delivery to the hippocampus excited these inhibitory cells (which in turn inhibited excitatory hippocampal neurons).9 Despite directly targeting such a limited population of inhibitory interneurons, this approach also effectively inhibited temporal lobe seizures. These findings indicate not only that a temporally precise on-demand intervention can provide seizure control, but also that a cell-type restricted intervention can work. Recently, it was shown that an on-demand optogenetic approach targeting the cerebellum could inhibit temporal lobe seizures, with optogenetic excitation of the midline cerebellum producing a unique long-lasting inhibition of seizure initiation.43 These studies pave the way for future uses of on-demand optogenetics to study the cell types and networks critically involved in seizures.
Similarly, optogenetics has been used to elucidate cell type–specific output targets in Parkinson disease. Optogenetic activation of direct pathway medium spiny neurons in the striatum of mice with 6-hydroxydopamine–induced parkinsonism improved bradykinesia to prelesion levels, whereas activation of indirect pathway medium spiny neurons generated parkinsonism in mice with previously normal motor behavior.44 These results provide causal support for the role of these pathways in basal ganglia function and establish that pathway-specific stimulation is sufficient to ameliorate parkinsonian symptoms. A different optogenetic approach revealed that inhibition of subthalamic nucleus firing (the presumed mechanism by which DBS operates) was insufficient to improve parkinsonian symptoms in hemiparkinsonian rats.35 These authors were then able to photostimulate only afferent fibers arriving from the cortex rather than subthalamic neurons using a transgenic mouse expressing channelrhodopsin under the Thy1 promoter. This specific stimulation was able to replicate the beneficial effects of DBS in these rats, suggesting a new anatomical target for stimulation in this disorder.
Optogenetic techniques are not currently available for humans, in part due to safety concerns arising from the need for gene therapy approaches to achieve opsin expression (because these proteins are not natively expressed).45,46 However, safety has been demonstrated for a variety of viral vectors for gene therapy for Parkinson disease,47 and orphan drug status has been given to a viral vector–based channelrhodopsin (RetroSense Therapeutics) as part of the process to potentially establish clinical trials for optogenetic restoration of vision in patients with retinitis pigmentosa. As such, it is conceivable that, in the future, optogenetic techniques may transition from a method to refine output parameters to an actuator in closed-loop therapeutic devices.
At present, most actuators in closed-loop neurological systems involve modification of electromagnetic fields in the brain via focal electrical stimulation. Current is injected via depth leads or subdural strips (NeuroPace system) or via a depth electrode with 4 cylindrical contacts (DBS Therapy; Medtronic, Inc). However, there is substantial clinical interest in the development of noninvasive closed-loop actuators, which would likely decrease cost and device adverse effects. Transcranial electrical stimulation (TES) may be an effective noninvasive actuator for paroxysmal neurological disorders due to its great temporal precision. Furthermore, TES does not require the bulky and more expensive equipment needed for trans-cranial magnetic stimulation. The excitement for the potential of TES is evident in the range of applications recently tested in humans, from addiction to stroke rehabilitation, spatial tactile acuity, and reading performance.48,49 Transcranial electrical stimulation can be applied in a variety of manners, including alternating current or direct current schemes. Transcranial electrical stimulation is reported to be safe, with only limited and minor adverse effects, including itching, tingling, and fatigue.50 Most important, effects of TES are reported to be brain state dependent.10,48,51,52 In this regard, combining TES with a closed-loop system may not only reduce unnecessary intervention but also actually improve symptom control.10
Indeed, a study10 examining the use of TES in a rodent model of absence epilepsy (also referred to as thalamocortical epilepsy) indicated that on-demand intervention may provide superior seizure control. Long-Evans rats with spontaneously recurring spike-and-wave episodes (which are characteristic of absence seizures) were implanted with electrodes to record neuronal activity and these seizure events. Three recording electrodes were placed at equally increasing depths in the neocortex (a, b, and c in Figure, E). The recorded signal was first amplified and filtered, and a current source density (CSD) trace was generated using the following equation: CSD = 2b − (a + c). Then, spike-and-wave episodes were rapidly detected online as voltage crossings of a preset threshold level of the CSD trace, and TES was delivered via transcranial stimulating electrodes implanted on the skull (Figure, E). Effects of constant (not on-demand) 1-Hz sinusoidal stimulation and on-demand stimulation (50-millisecond gaussian waveforms triggered by online seizure detection) were examined. While sinusoidal stimulation was able to entrain the firing of neurons (recorded as multiunit firing) and modulate the amplitude of spike-and-wave episodes, it was unable to reduce the duration of spike-and-wave episodes. In contrast, on-demand TES significantly inhibited seizure duration and thereby reduced the amount of time the animal spent seizing (Figure, F). These findings demonstrate 2 critical points. First, TES can effectively inhibit absence seizures. Second, seizure control can be improved by using an on-demand approach.
A similar approach has been piloted for tremor reduction in patients with Parkinson disease.53 In this case, TES was applied focally to the motor cortex at the frequency of the patient’s tremor, as recorded by an accelerometer on the wrist. Closed-loop stimulation that produced a specific phase alignment of TES and motor tremor was more effective than open-loop stimulation in reduction of tremor amplitude. Although the efficacy of this method has only been demonstrated acutely, it illustrates the potential efficacy of noninvasive technologies when used in a closed-loop fashion.
Despite the success of these TES-based closed-loop paradigms, the mechanisms by which TES affects neural activity acutely and chronically in specific brain regions remain obscure. Therefore, in parallel with the development of TES devices, carefully designed studies should be undertaken to clarify the underlying physiological basis for observed effects. Such studies10,54 in animal models, where it is possible to couple invasive neural recordings with TES, have already begun to make progress in understanding the modulations of local field potential and spiking activity that occur in behaving animals during TES stimulation.
Conclusions
Closed-loop devices have been successfully used to treat several neurological disorders in severely affected patients for whom noninvasive therapies are insufficient. However, closed-loop technology may improve therapeutic efficacy and reduce adverse effects of treatment in a broader patient population by limiting intervention to specific times when it is required and responding to an individual patient’s internal brain dynamics. Currently, the invasiveness of such devices and lack of comprehensive understanding of which pathophysiological signals to modify, as well as how to precisely modify them, preclude widespread use. Experimental models and approaches such as those described herein can help improve all aspects of these technologies and provide a unique opportunity to study the underlying mechanisms of physiological and pathologic neural processes.18,55 These studies will be critical as closed-loop paradigms are applied to increasingly diverse clinical disorders, including stroke rehabilitation,56 minimally consciousstate,57 and a host of psychiatric disorders.58,59 Effective closed-loop systems for clinical use require advanced understanding of pathophysiological brain signals combined with sophisticated technology for signal processing and subsequent modulation. Only when advancements in basic science, engineering, and materials science are coupled to clinical need will closed-loop technologies translate efficiently to improvements in therapeutic devices for patients with neurological disorders.
Acknowledgments
Funding/Support: This work was supported by a Citizens United for Research in Epilepsy Taking Flight Award (Dr Krook-Magnuson), grant K99NS087110 from the National Institutes of Health (Dr Krook-Magnuson), a Pediatric Scientist Development Program fellowship funded by the March of Dimes (Dr Gelinas), grant NS74432 from the National Institutes of Health (Dr Soltesz), grants MH54671 and NS074015 from the Human Frontier Science Program, The G. Harold & Leila Y. Mathers Charitable Foundation (Dr Buzsáki), grant NS090583 from the National Institutes of Health Brain Research Through Advancing Innovative Neurotechnologies Initiative (Drs Soltesz and Buzsáki), and Temporal Dynamics of Learning Center grant SBE-0542013 from the National Science Foundation.
Footnotes
Conflict of Interest Disclosures: None reported.
Author Contributions: Dr Buzsáki had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Krook-Magnuson and Gelinas contributed equally to this work.
Study concept and design: All authors.
Acquisition, analysis, or interpretation of data: Buzsáki.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: Krook-Magnuson, Gelinas, Buzsáki.
Obtained funding: Krook-Magnuson, Soltesz, Buzsáki.
Administrative, technical, or material support: Gelinas.
Study supervision: Soltesz, Buzsáki.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Sun FT, Morrell MJ. Closed-loop neurostimulation: the clinical experience. Neurotherapeutics. 2014;11(3):553–563. doi: 10.1007/s13311-014-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Espay AJ, Baram Y, Dwivedi AK, et al. At-home training with closed-loop augmented-reality cueing device for improving gait in patients with Parkinson disease. J Rehabil Res Dev. 2010;47(6):573–581. doi: 10.1682/jrrd.2009.10.0165. [DOI] [PubMed] [Google Scholar]
- 3.Liu N, Chazot T, Genty A, et al. Titration of propofol for anesthetic induction and maintenance guided by the Bispectral Index: closed-loop versus manual control: a prospective, randomized, multicenter study. Anesthesiology. 2006;104(4):686–695. doi: 10.1097/00000542-200604000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Morley A, Derrick J, Mainland P, Lee BB, Short TG. Closed loop control of anaesthesia: an assessment of the bispectral index as the target of control. Anaesthesia. 2000;55(10):953–959. doi: 10.1046/j.1365-2044.2000.01527.x. [DOI] [PubMed] [Google Scholar]
- 5.Delgado JM, Johnston VS, Wallace JD, Bradley RJ. Operant conditioning of EEG in the unrestrained chimpanzee. Electroencephalogr Clin Neurophysiol. 1969;27(7):701–702. doi: 10.1016/0013-4694(69)91347-9. [DOI] [PubMed] [Google Scholar]
- 6.Delgado JM, Mark V, Sweet W, et al. Intracerebral radio stimulation and recording in completely free patients. J Nerv Ment Dis. 1968;147(4):329–340. doi: 10.1097/00005053-196810000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Schultz DM, Webster L, Kosek P, Dar U, Tan Y, Sun M. Sensor-driven position-adaptive spinal cord stimulation for chronic pain. Pain Physician. 2012;15(1):1–12. [PubMed] [Google Scholar]
- 8.Little S, Pogosyan A, Neal S, et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol. 2013;74(3):449–457. doi: 10.1002/ana.23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat Commun. 2013;4:1376. doi: 10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berényi A, Belluscio M, Mao D, Buzsáki G. Closed-loop control of epilepsy by transcranial electrical stimulation. Science. 2012;337(6095):735–737. doi: 10.1126/science.1223154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heck CN, King-Stephens D, Massey AD, et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. 2014;55(3):432–441. doi: 10.1111/epi.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosin B, Slovik M, Mitelman R, et al. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron. 2011;72(2):370–384. doi: 10.1016/j.neuron.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Rissanen S, Kankaanpää M, Tarvainen MP, et al. Analysis of surface EMG signal morphology in Parkinson’s disease. Physiol Meas. 2007;28(12):1507–1521. doi: 10.1088/0967-3334/28/12/005. [DOI] [PubMed] [Google Scholar]
- 14.Yang AI, Vanegas N, Lungu C, Zaghloul KA. Beta-coupled high-frequency activity and beta-locked neuronal spiking in the subthalamic nucleus of Parkinson’s disease. J Neurosci. 2014;34(38):12816–12827. doi: 10.1523/JNEUROSCI.1895-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moshel S, Shamir RR, Raz A, et al. Subthalamic nucleus long-range synchronization: an independent hallmark of human Parkinson’s disease. Front Syst Neurosci. 2013;7:79. doi: 10.3389/fnsys.2013.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schevon CA, Weiss SA, McKhann G, Jr, et al. Evidence of an inhibitory restraint of seizure activity in humans. Nat Commun. 2012;3:1060. doi: 10.1038/ncomms2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12(10):1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- 18.Stark E, Roux L, Eichler R, Senzai Y, Royer S, Buzsáki G. Pyramidal cell-interneuron interactions underlie hippocampal ripple oscillations. Neuron. 2014;83(2):467–480. doi: 10.1016/j.neuron.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J Neurosci. 2000;20(20):7766–7775. doi: 10.1523/JNEUROSCI.20-20-07766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wyler AR, Ojemann GA, Ward AA., Jr Neurons in human epileptic cortex: correlation between unit and EEG activity. Ann Neurol. 1982;11(3):301–308. doi: 10.1002/ana.410110311. [DOI] [PubMed] [Google Scholar]
- 21.Motamedi GK, Lesser RP, Miglioretti DL, et al. Optimizing parameters for terminating cortical afterdischarges with pulse stimulation [published correction appears in Epilepsia. 2002;43(11):1441] Epilepsia. 2002;43(8):836–846. doi: 10.1046/j.1528-1157.2002.24901.x. [DOI] [PubMed] [Google Scholar]
- 22.Schevon CA, Ng SK, Cappell J, et al. Microphysiology of epileptiform activity in human neocortex. J Clin Neurophysiol. 2008;25(6):321–330. doi: 10.1097/WNP.0b013e31818e8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell PK, Jones KE, Huber RJ, Horch KW, Normann RA. A silicon-based, three-dimensional neural interface: manufacturing processes for an intracortical electrode array. IEEE Trans Biomed Eng. 1991;38(8):758–768. doi: 10.1109/10.83588. [DOI] [PubMed] [Google Scholar]
- 24.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148(1):1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Dickey AS, Suminski A, Amit Y, Hatsopoulos NG. Single-unit stability using chronically implanted multielectrode arrays. J Neurophysiol. 2009;102(2):1331–1339. doi: 10.1152/jn.90920.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sohal HS, Jackson A, Jackson R, et al. The sinusoidal probe: a new approach to improve electrode longevity. Front Neuroeng. 2014;7:10. doi: 10.3389/fneng.2014.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khodagholy D, Gelinas JN, Thesen T, et al. NeuroGrid: recording action potentials from the surface of the brain. Nat Neurosci. 2015;18(2):310–315. doi: 10.1038/nn.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajput AH, Sitte HH, Rajput A, Fenton ME, Pifl C, Hornykiewicz O. Globus pallidus dopamine and Parkinson motor subtypes: clinical and brain biochemical correlation. Neurology. 2008;70(16 pt 2):1403–1410. doi: 10.1212/01.wnl.0000285082.18969.3a. [DOI] [PubMed] [Google Scholar]
- 29.Robinson DL, Venton BJ, Heien ML, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin Chem. 2003;49(10):1763–1773. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- 30.Chang SY, Kim I, Marsh MP, et al. Wireless fast-scan cyclic voltammetry to monitor adenosine in patients with essential tremor during deep brain stimulation. Mayo Clin Proc. 2012;87(8):760–765. doi: 10.1016/j.mayocp.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grahn PJ, Mallory GW, Khurram OU, et al. A neurochemical closed-loop controller for deep brain stimulation: toward individualized smart neuromodulation therapies. Front Neurosci. 2014;8:169. doi: 10.3389/fnins.2014.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramgopal S, Thome-Souza S, Jackson M, et al. Seizure detection, seizure prediction, and closed-loop warning systems in epilepsy. Epilepsy Behav. 2014;37:291–307. doi: 10.1016/j.yebeh.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 33.Morrell MJ. RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 34.Kharbouch A, Shoeb A, Guttag J, Cash SS. An algorithm for seizure onset detection using intracranial EEG. Epilepsy Behav. 2011;22(suppl 1):S29–S35. doi: 10.1016/j.yebeh.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324(5925):354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roux L, Stark E, Sjulson L, Buzsáki G. In vivo optogenetic identification and manipulation of GABAergic interneuron subtypes. Curr Opin Neurobiol. 2014;26:88–95. doi: 10.1016/j.conb.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krook-Magnuson E, Ledri M, Soltesz I, Kokaia M. How might novel technologies such as optogenetics lead to better treatments in epilepsy? Adv Exp Med Biol. 2014;813:319–336. doi: 10.1007/978-94-017-8914-1_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paz JT, Davidson TJ, Frechette ES, et al. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat Neurosci. 2013;16(1):64–70. doi: 10.1038/nn.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Armstrong C, Krook-Magnuson E, Oijala M, Soltesz I. Closed-loop optogenetic intervention in mice. Nat Protoc. 2013;8(8):1475–1493. doi: 10.1038/nprot.2013.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6(4):347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 42.Bezaire MJ, Soltesz I. Quantitative assessment of CA1 local circuits: knowledge base for interneuron–pyramidal cell connectivity. Hippocampus. 2013;23(9):751–785. doi: 10.1002/hipo.22141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M, Soltesz I. Cerebellar directed optogenetic intervention inhibits spontaneous hippocampal seizures in a mouse model of temporal lobe epilepsy. Eneuro. 2014;1(1):e.2014. doi: 10.1523/ENEURO.0005-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kravitz AV, Freeze BS, Parker PR, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466(7306):622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Häusser M. Optogenetics: the age of light. Nat Methods. 2014;11(10):1012–1014. doi: 10.1038/nmeth.3111. [DOI] [PubMed] [Google Scholar]
- 46.Krook-Magnuson E, Soltesz I. Beyond the hammer and the scalpel: selective circuit control for the epilepsies. Nat Neurosci. 2015;18(3):331–338. doi: 10.1038/nn.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartus RT, Weinberg MS, Samulski RJ. Parkinson’s disease gene therapy: success by design meets failure by efficacy. Mol Ther. 2014;22(3):487–497. doi: 10.1038/mt.2013.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dayan E, Censor N, Buch ER, Sandrini M, Cohen LG. Noninvasive brain stimulation: from physiology to network dynamics and back. Nat Neurosci. 2013;16(7):838–844. doi: 10.1038/nn.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turkeltaub PE, Benson J, Hamilton RH, Datta A, Bikson M, Coslett HB. Left lateralizing transcranial direct current stimulation improves reading efficiency. Brain Stimul. 2012;5(3):201–207. doi: 10.1016/j.brs.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kessler SK, Turkeltaub PE, Benson JG, Hamilton RH. Differences in the experience of active and sham transcranial direct current stimulation. Brain Stimul. 2012;5(2):155–162. doi: 10.1016/j.brs.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shahbabaie A, Golesorkhi M, Zamanian B, et al. State dependent effect of transcranial direct current stimulation (tDCS) on methamphetamine craving. Int J Neuropsychopharmacol. 2014;17(10):1591–1598. doi: 10.1017/S1461145714000686. [DOI] [PubMed] [Google Scholar]
- 52.Ozen S, Sirota A, Belluscio MA, et al. Transcranial electric stimulation entrains cortical neuronal populations in rats. J Neurosci. 2010;30(34):11476–11485. doi: 10.1523/JNEUROSCI.5252-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brittain JS, Probert-Smith P, Aziz TZ, Brown P. Tremor suppression by rhythmic transcranial current stimulation. Curr Biol. 2013;23(5):436–440. doi: 10.1016/j.cub.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Márquez-Ruiz J, Leal-Campanario R, Sánchez-Campusano R, et al. Transcranial direct-current stimulation modulates synaptic mechanisms involved in associative learning in behaving rabbits. Proc Natl Acad Sci U S A. 2012;109(17):6710–6715. doi: 10.1073/pnas.1121147109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rolston JD, Gross RE, Potter SM. Closed-loop, open-source electrophysiology. Front Neurosci. 2010;4:4. doi: 10.3389/fnins.2010.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gonzalez Andino SL, Herrera-Rincon C, Panetsos F, Grave de Peralta R. Combining BMI stimulation and mathematical modeling for acute stroke recovery and neural repair. Front Neurosci. 2011;5:87. doi: 10.3389/fnins.2011.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schiff ND, Plum F, Rezai AR. Developing prosthetics to treat cognitive disabilities resulting from acquired brain injuries. Neurol Res. 2002;24(2):116–124. doi: 10.1179/016164102101199576. [DOI] [PubMed] [Google Scholar]
- 58.Neumann WJ, Huebl J, Brücke C, et al. Different patterns of local field potentials from limbic DBS targets in patients with major depressive and obsessive compulsive disorder. Mol Psychiatry. 2014;19(11):1186–1192. doi: 10.1038/mp.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riva-Posse P, Choi KS, Holtzheimer PE, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76(12):963–969. doi: 10.1016/j.biopsych.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]