Abstract
Biological attributions for depression, which are currently ascendant, can lead to prognostic pessimism—the perception that symptoms are relatively immutable and unlikely to abate (Kvaale, Haslam, & Gottdiener, 2013; Lebowitz, Ahn, & Nolen-Hoeksema, 2013). Among symptomatic individuals, this may have important clinical ramifications, as reduced confidence in one’s own ability to overcome depression carries the risk of becoming a self-fulfilling prophecy. Previous research (Lebowitz, Ahn, et al., 2013) has demonstrated that educational interventions teaching symptomatic individuals about how the effects of genetic and neurobiological factors involved in depression are malleable and can be modified by experiences and environmental factors can reduce prognostic pessimism. While previous research demonstrated such effects only in the immediate term, the present research extends these findings by testing whether such benefits persist six weeks after the intervention. Indeed, among individuals who initially considered biological factors to play a major role in influencing their levels of depression, exposure to malleability-focused psychoeducation reduced levels of depression-related prognostic pessimism and stronger belief in their ability to regulate their moods. Critically, this benefit persisted six weeks after the intervention. Clinical implications of the findings are discussed.
Keywords: Depression, Psychoeducation, Biological Explanations, Prognostic Pessimism, Agency
Biological attributions and explanations for mental disorders, including depression, are currently in ascendancy. Large majorities of the American public view neurochemical imbalances and genetic abnormalities as causes of depression (Pescosolido, et al., 2010). In general, biomedical approaches to understanding, studying and treating psychopathology have become predominant (Deacon, 2013; Kemp, Lickel, & Deacon, 2014).
Although biological conceptualizations of psychopathology have been touted for their well-documented power to reduce the blame ascribed to sufferers for their own symptoms, they also have notable negative consequences among members of the general public; for example, they can increase prognostic pessimism—the perception that disorders are unlikely to remit (Kvaale, et al., 2013). This effect may occur because biological explanations can lead to so-called “essentialist” assumptions, in which abnormalities in individuals’ brains or genes come to be seen as deep-seated, fundamental, immutable essences of their symptoms (Dar-Nimrod & Heine, 2011; Haslam, 2011; Medin & Ortony, 1989).
In recent years, several studies have examined the effect of biological attributions specifically among people who display psychiatric symptoms (Lebowitz, 2014). For example, among people with elevated levels of depressive symptomatology, attributing one’s symptoms to neurochemical or genetic causes is associated with pessimistic expectations about the duration of one’s own depression (Lebowitz, Ahn, et al., 2013). Relatedly, individuals who were told that they carried a gene associated with alcoholism rated themselves as less able to avoid drinking alcohol, compared to individuals who were told that they did not carry such a gene (Dar-Nimrod, Zuckerman, & Duberstein, 2013). Biological explanations for generalized anxiety disorder also increased prognostic pessimism among people whose self-report suggested the presence of the condition (Lebowitz, Pyun, & Ahn, 2014). More recently, when individuals with depression were given purported biological test results indicating that their symptoms were caused by a neurochemical imbalance, it increased their pessimism about their own prognoses and decreased their belief in their ability to regulate their own moods (Kemp, et al., 2014).
The clinical implications of the aforementioned findings represent an important cause for concern. Specifically, the prognostic expectancies of people with depression and other disorders are significant predictors of actual clinical outcomes and responsiveness to treatment (Greenberg, Constantino, & Bruce, 2006; Mondloch, Cole, & Frank, 2001; Rutherford, Wager, & Roose, 2010). That is, when people are pessimistic about their own prospects of overcoming a disorder or benefitting from treatment—which may be especially likely if they attribute their symptoms to biological causes—their negative outlooks can become self-fulfilling prophecies.
However, essentialist beliefs about the biology of depression and other mental disorders are inconsistent with current science. The brain maintains the ability to change and adapt to environments and experiences, known as neuroplasticity, well into adulthood (Lozano, 2011), and psychiatric treatments—including non-biomedical psychotherapies—cause observable neurobiological changes in patients (Linden, 2006). The relationship between genes and risk for psychiatric disorders, including depression, can also be moderated by environments and experiences, and in some cases these factors can lead to epigenetic changes that chemically alter gene expression (Lau & Eley, 2010; Rutter, Moffitt, & Caspi, 2006). Clearly, the assumption that biological understandings of psychopathology imply that symptoms are immutable and outside the control of their sufferers—an assumption upon which the common association of biological attributions with prognostic pessimism appears to be based—is misguided.
Such misunderstandings may be a promising target for intervention. In previous research, we developed a psychoeducation intervention, delivered in the form of a brief audiovisual presentation, which focused on the malleability of biological factors involved in depression, including information about neuroplasticity and how gene effects can be altered through epigenetics and gene-by-environment interactions (Lebowitz, Ahn, et al., 2013). Among people with and without elevated depressive symptomatology, this intervention significantly decreased prognostic pessimism, increased feelings of agency regarding the ability to positively influence one’s own mood, and reduced generalized feelings of hopelessness.
However, these effects were observed immediately following administration of the intervention, leaving the question of whether such an intervention might have longer-term effects unresolved. A demonstration of long-term benefits would be critical to determining whether this kind of intervention might have useful real-world clinical applications.
Thus, in the present study, we tested whether the beneficial effects of a similar psychoeducation intervention would remain at follow-up after six weeks. We also measured participants’ beliefs about the extent to which biological factors influenced their moods and levels of depression. Because the intervention was targeted at changing their views about the role of biology in depression, we hypothesized that it might be more effective among individuals who more strongly believed that biological factors (e.g., genes, neurochemistry) determine their moods and levels of depression.
Method
Participants and Recruitment
Participants were recruited using the online Mechanical Turk system from Amazon.com, which allows users to complete short tasks in exchange for monetary compensation (Buhrmester, Kwang, & Gosling, 2011). The initial sample consisted of 454 U.S. adults (55.3% female, 43.4% male, 1.3% unknown gender; 83.9% White/Caucasian) ranging in age from 18 to 70 years (M=33.27, SD=10.62).
Six weeks after their initial (“T1”) participation, participants were contacted and asked to provide follow-up (“T2”) data (see Procedures). Mechanical Turk user IDs were used to match T2 responses to the T1 responses of the same participant. The follow-up sample (n=255) was demographically comparable to the full initial sample. Specifically, T2 respondents were 52.5% female, 46.3% male, 1.2% unknown gender, and 86.7% White/Caucasian; they ranged in age from 18 to 70 years (M=34.63, SD=11.14).
Procedures
Study procedures were administered using Qualtrics.com online survey software. Upon initially providing informed consent at the beginning of T1, participants were randomly assigned to one of two conditions: intervention (n=227) or control (n=227). All participants then completed the Beck Depression Inventory-II (BDI-II), a well-validated and widely used measure of depression symptomatology (Dozois, 2010). (We omitted one BDI-II item, “Suicidal Thoughts or Wishes,” because our online procedures precluded appropriate responses to reports of suicidality.) We collected this data to ensure that there was no systematic difference in depressive symptomatology between the two conditions. Indeed, there were no significant differences in BDI-II scores between participants in the intervention (M=13.22, SD=11.36) and control (M=13.52, SD=11.35) conditions, t(452)=.28, p=.78.
Next, participants were presented with a list of six factors that could plausibly lead to depressive symptoms (e.g., “Stress”) and asked to “indicate the extent to which you believe each of the following factors is involved in determining your mood (for example, how much each factor affects whether or not you feel sad or depressed).” These ratings were made on a 7-point scale (1 = “Not at all” 7 = “Very much”). Two of the factors were biological: “Genetics” and “Neurobiology (e.g. brain chemistry).” The remaining items were fillers to disguise the true reason for these ratings (e.g. “Your childhood or the way you were raised,” “Your environment or events that take place in your life”).
Participants in the intervention condition were then presented with an onscreen audiovisual psychoeducation intervention in the form of a YouTube video, approximately seven minutes in length. Based on a similar intervention developed for earlier research (Lebowitz, Ahn, et al., 2013), it focused on the malleability of biological factors involved in depression, including a primer on how genes can be “turned on and off” through epigenetic mechanisms and how brain chemistry and activity can be modulated through experience, including psychotherapy. Participants who watched the video were then instructed, before proceeding, to write a short letter to a depressed individual, using information from the video they watched, to persuade the person to see depression “in a new light.” This approach took advantage of the “saying-is-believing” effect, a tendency for people to internalize viewpoints they have advocated (Aronson, Fried, & Good, 2002; Higgins, 1999; Lebowitz, Ahn, et al., 2013; Walton & Cohen, 2011). Participants in the control condition did not view any video or receive any other intervention and did not complete this “saying-is-believing” procedure.
All participants then completed the two T1 dependent measures. The first, which gauged participants’ perceptions of their own agency in responding to possible future episodes of depression, was a version of the Negative Mood Regulation (NMR) scale (Catanzaro & Mearns, 1990; Kemp, et al., 2014) that was modified for the present research. The original measure consists of a single stem (“When I’m upset, I believe that…”) and asks respondents to rate each of 30 possible sentence-completing items (e.g., “…I can do something to feel better”) on a five-point scale. To create our modified version, we removed some items (e.g., “Doing something nice for someone else will cheer me up”) deemed irrelevant to the contents of our intervention, and altered others to increase their relevance (e.g., from “I can find a way to relax” to “Reducing my stress will help cheer me up”). That is, the intervention video did not discuss doing nice things for others as a helpful approach against depression, but it did discuss managing stress as such a beneficial strategy. We sought a measure in which all items would be directly relevant to the contents of the intervention. We also changed the stem to “If I become depressed in the future, I believe that…” and converted all items to future tense (e.g. from “Wallowing in it is all I can do” to “Wallowing in it is all I will be able to do”). The modified NMR scale included a total of 17 items (Cronbach alpha=.92). Responses to all items were averaged to compute a single NMR score for each participant, ranging from 1 to 5, with higher scores indicating greater confidence in one’s own agency in responding to depression.
The other dependent measure gauged prognostic pessimism by asking participants to imagine if they were to become depressed in the future and then rate how permanent this depression would be, on a scale from 1 (“Not at all permanent”) to 7 (“Very permanent”). At the end of the T1 study procedures, participants were asked optional demographic questions. They were then redirected to a separate online form into which they were asked to enter their e-mail addresses so that they could subsequently be invited to complete T2 measures. This yielded 403 e-mail addresses, which were stored separately from (and could not be linked to) T1 data in order to preserve the anonymous nature of participants’ responses.
Six weeks later, a message was sent to all of the e-mail addresses including a link allowing recipients to enroll in T2 via Mechanical Turk. Participants were given a maximum of one week to complete the T2 measures. Of the 255 T2 respondents, 125 had been assigned to the control condition at T1, while the other 130 had received the intervention at T1. The T2 procedure was the same for both conditions, and consisted only of the dependent measures completed at T1 (the modified NMR scale and the prognostic pessimism item) without any reminder of the contents of the intervention for those in the intervention condition.
Results
Unsurprisingly, BDI-II scores had a significant negative correlation with NMR scores (T1: r=−.53, p<.001; T2: r=−.56, p<.001) and a significant positive correlation with prognostic pessimism ratings (T1: r=.55, p<.001; T2: r=.58, p<.001). (See Appendix for further analyses involving BDI-II scores).
Ratings of the two biological factors as determinants of a participant’s mood were significantly correlated (r=.53, p<.001) and were thus averaged to compute an index of the extent to which participants attributed their moods to biological causes. Among participants in the control condition, biological attributions were negatively correlated with NMR scores (r=−.19, p<.01) and positively correlated with prognostic pessimism scores (r=.19, p<.01). This replicated previous research demonstrating that absent any intervention, the more people attribute their mood states to biological factors, the more prognostic pessimism they tend to exhibit (Lebowitz, Ahn, et al., 2013).
The mean length of letters to a hypothetical depressed person written by the 227 participants who completed T1 in the intervention condition as part of the “saying-is-believing” procedure was 673.70 characters (SD=334.82); all but two participants wrote at least 100 characters. Interestingly, the length of these letters had a significant positive correlation with NMR scores (r=.15, p=.03) and significant negative correlation with prognostic pessimism ratings (r=−.19, p<.01). This finding is consistent with the notion that the saying-is-believing effect successfully increased the strength of the intervention.
Using a median split, we categorized participants whose average biological attribution ratings was at least a 5 (out of 7) as holding strong biological attributions, while others were categorized as holding weak biological attributions. That the median split resulted in a cut-point slightly above the scale midpoint of 4 is perhaps relatively unsurprising, given the prevalence of biological attributions for depression (Pescosolido, et al., 2010). This grouping was used to test whether biological conceptualizations of one’s mood would moderate the intervention’s effects
We first analyzed the T1 data of all participants using 2 (condition: intervention vs. control) × 2 (biological attributions: weak vs. strong) univariate ANOVAs. For NMR scores, there was a significant effect of condition, F(1,450)=24.90, p<.001, η2p=.05. However, this was qualified by a significant two-way interaction, F(1, 450)=6.33, p=.01, η2p=.01 (see Figure 1). Further analysis revealed that the intervention yielded significantly higher T1 NMR scores, compared to the control condition, among participants with weak biological attributions, t(260)=2.05, p=.04, d=.25, and among those with strong biological attributions, t(190)=4.53, p<.001, d=.66. This difference in mean T1 NMR scores between the two conditions was greater among participants with strong biological attributions (Ms = 3.78 vs. 3.28) than among those with weak biological attributions (Ms = 3.74 vs. 3.57). For prognostic pessimism at T1 (see Table 1, top half), ratings were lower overall among participants who received the intervention, F(1,449) = 10.49, p=.001, η2p=.02. Although the two-way interaction did not reach significance, F(1, 449)=3.05, p=.08, η2p=.01, further analysis revealed that the intervention yielded significantly lower T1 prognostic pessimism ratings (versus the control condition) among participants with strong biological attributions, t(189)=3.13, p<.01, d=.46, but not among participants with weak biological attributions, t(260)=1.19, p=.24, d=.15.
Figure 1.
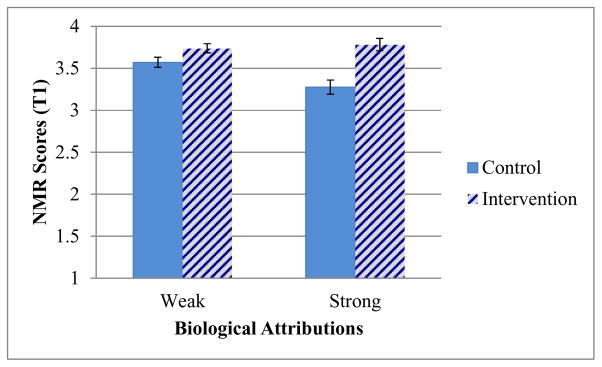
T1 Scores, by condition, on the modified Negative Mood Regulation scale; higher scores on the 7-point scale indicate stronger confidence in one’s ability to regulate one’s own negative moods. Error bars indicate +/− one standard error. N=454.
Table 1.
Prognostic pessimism ratings, by condition, at T1 and T2. Scores indicate how permanent participants felt their condition would be if they were to experience depression in the future, from 1 (Not at All Permanent) to 7 (Very Permanent).
| Time point | Condition | Biological Attributions | Mean | SD | N |
|---|---|---|---|---|---|
| T1 | Control | Weak | 2.70 | 1.57 | 128 |
| Strong | 3.19 | 1.66 | 99 | ||
| Intervention | Weak | 2.49 | 1.28 | 134 | |
| Strong | 2.49 | 1.42 | 92 | ||
| T2 | Control | Weak | 2.61 | 1.58 | 64 |
| Strong | 2.97 | 1.65 | 61 | ||
| Intervention | Weak | 2.77 | 1.52 | 78 | |
| Strong | 2.39 | 1.36 | 51 |
Next, we tested whether the benefit of the intervention persisted over time among participants who completed T2. An initial 2 (condition) × 2 (biological attributions: strong vs. weak) × 2 (time: T1 vs. T2) mixed ANOVA with ‘time’ as a within-subjects variable revealed that the two-way interaction of condition by biological attribution strength was significant overall for both NMR scores, F(1,250)=10.90, p=.001, η2p=.04 and prognostic pessimism ratings, F(1,250)=4.07, p<.05, η2p=.02. These interaction effects were obtained because the intervention effect was stronger among those with strong biological attributions than those with weak biological attributions (see below for details). Most critically, there were no significant three-way interactions involving time for NMR scores or prognostic pessimism ratings (all Fs≤.2, all ps>.65, all η2ps ≤.001), indicating that the two-way interactions of condition by biological attribution strength followed the same pattern across the two time points. To explain this, we report below the interaction effects of condition by biological attribution broken down by time point.
Although the T1 scores included in this analysis were restricted to those of participants who also provided T2 responses, the two-way (condition by biological attribution strength) interactions for T1 responses were significant for NMR scores, F(1,251)=8.51, p<.01, η2p=.03, and marginally significant for prognostic pessimism, F(1,250)=3.29, p=.07, η2 p=.01. Among participants with weak biological attributions, T1 NMR scores were not significantly different between those who received the intervention and those in the control condition, Ms = 3.70 vs. 3.50, t(111)=1.63, p=.11, though the intervention did yield lower prognostic pessimism ratings (Ms = 2.42 vs. 2.94), t(140)=2.02 p<.05. The significant interaction effect was obtained because as with the full sample, the differences between the two conditions were much greater among participants with strong biological attributions. Among such participants, those who received the intervention had significantly higher T1 NMR scores, Ms = 3.95 vs. 3.19, t(111)=4.95, p<.001, and significantly lower prognostic pessimism ratings (Ms = 2.08 vs. 3.30) compared to those in the control condition, t(110)=4.17, p<.001.
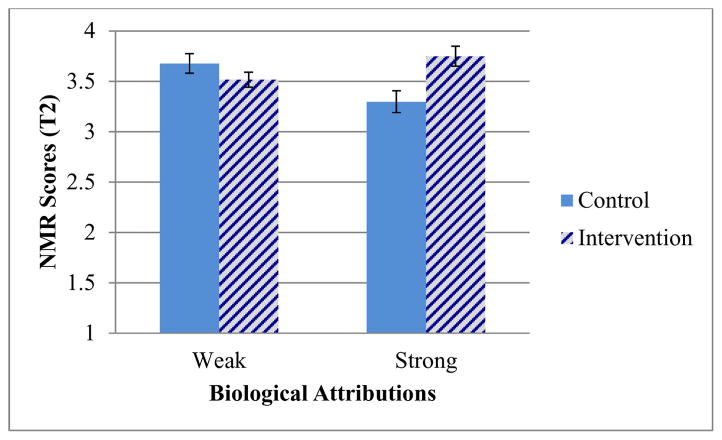
More importantly, the two-way (condition by biological attribution strength) interactions at T2 was still significant for NMR scores, F(1,251)=10.54, p=.001 (see Figure 2), and marginally significant for prognostic pessimism ratings, F(1,251)=3.27, p=.07 (see Table 1, bottom half). Further analysis revealed that among participants with weak biological attributions, the effect of the intervention at T2 was not significant for NMR scores, t(140)=1.35, p=.18, or for prognostic pessimism ratings, t(140)=.61, p=.54. However, among participants with strong biological attributions, those who received the intervention had higher T2 NMR scores, t(111)=3.04, p<.01, and marginally lower prognostic pessimism ratings, t(111)=1.89, p=.06, compared to those in the control condition.
Figure 2.
T2 Scores, by condition, on the modified Negative Mood Regulation scale; higher scores on the 7-point scale indicate stronger confidence in one’s ability to regulate one’s own negative moods. Error bars indicate +/− one standard error. N=255.
Discussion
The present study conceptually replicates earlier research (Lebowitz, Ahn, et al., 2013) demonstrating that a brief psychoeducation intervention focusing on the malleability of biological factors involved in depression can significantly increase people’s confidence in their own ability to recover from future depressive episodes. Most importantly, the present study indicates that the benefits of the intervention are durable rather than limited to the immediate term: significant effects were evident six weeks after administration of the intervention.
The current demonstration of the long-term effects of the intervention has several important empirical and clinical implications. First, it shows that the previously demonstrated effects of this type of intervention (Lebowitz, Ahn, et al., 2013) were unlikely to have been due to demand characteristics, in which participants who received the intervention provided responses that were consistent with the videos they watched moments before. Even without any reminder of the intervention’s content, our participants still showed reduced prognostic pessimism and increased feelings of agency and control over their moods six weeks after they watched the video. This suggests that the intervention is likely to have truly produced longstanding changed in beliefs about biology’s role in mood.
Clinically, the fact that the effects of our audiovisual presentation appeared to last at least six weeks suggests the possibility that such an intervention might be effectively utilized as a part of treatment programs, even if it takes the form of a brief psychoeducation module administered a single time, without subsequent reminders. Nevertheless, future studies could test whether the effects of such an intervention would last over even longer periods of time. Of particular relevance might be research testing whether the beneficial effects would last for the duration of treatment for depression (which can last months or years), or over stressful periods (e.g., major life transitions, etc.). Any findings that suggest limits to the durability of the intervention’s effects could be informative in decisions regarding the optimal intervals at which to administer reminders of its content, if such reminders are found to be necessary.
On the other hand, it is also possible that the effects of an intervention like the one used in the present research may last longer than six weeks. Previous research that served as an inspiration for the present study (Walton & Cohen, 2011) showed that a brief intervention among college freshman framing social difficulties as common and temporary had a lasting beneficial effect: over a three-year observation period, it improved the grades, self-reported health, and wellbeing of African-American students, a socially disadvantaged group in academic contexts. One reason why such interventions can have such long-lasting benefits is that they may alter how individuals perceive their experience, creating a positive feedback loop in which initial benefits reinforce the contents of the intervention, leading to further benefits over time (Walton & Cohen, 2011). In the case of the present intervention, its immediate effects—such as initial inductions of positive mood and increased feelings of empowerment—might lead people to feel less vulnerable to negative moods and depressive episodes, leading to further increases over time in feelings of agency regarding mood-regulation abilities.
Another important clinical implication of the current findings lies in the fact that the intervention used was a short audiovisual presentation, representing a relatively low-cost, highly scalable and accessible means of countering the potential negative consequences of attributing one’s mood to biological factors. Thus, the intervention can be publically available online or readily used in relevant contexts, such as high school health education or freshmen orientations in college to dispel misconceptions about depression.
Our findings suggest that such an intervention is likely to be particularly helpful among individuals who perceive biological factors as having a major role in influencing their moods. Thus, it may be beneficial for clinicians to ascertain whether patients see their symptoms as stemming from biological causes. For those with strong biological attributions, malleability-focused psychoeducation could be helpful, especially since positive outcome expectancies are an important determinant of actual prognosis and responsiveness to treatment (Greenberg, et al., 2006; Mondloch, et al., 2001; Rutherford, et al., 2010). This is especially noteworthy considering that such individuals, are as previous research demonstrates (Lebowitz, Ahn, et al., 2013), especially vulnerable to doubting their own prospects of recovering from depression.
Several methodological aspects of the present study merit discussion. First, the online administration of our study procedures entailed both benefits and limitations. Its advantages over recruitment from a clinical or laboratory setting removed some selection biases (e.g., geographic restrictions, special characteristics of people who seek mental-health treatment). However, administering the study online probably accounted, in large part, for the relatively high attrition rate from T1 to T2. While the demographic background of the sample who returned to T2 was similar to our original sample, a more aggressive follow-up procedure could improve the design of future studies.
Additionally, as with most intervention programs, our intervention was not effective for every participant, while effective enough to show statistically significant effects. Future research could further examine what individual differences might explain resistance to such an intervention. Possible reasons might be relatively minor (e.g., inattentiveness during intervention, skepticism about psychology experiments) or major (e.g., deep-seated misconceptions about the role of biology in depression). Future investigation could shed new light on ways to more effectively mitigate the negative consequences of biological attributions for depression.
Biological understandings of depression are increasingly dominant.
These can lead to pessimistic beliefs about prognosis.
We tested an educational video about the malleability of depression’s biology.
Results suggest that the intervention had both immediate and durable effects.
This could have important clinical implications.
Acknowledgments
This work was supported by the National Institutes of Health [grant number R01-HG007653].
Appendix
Analyses of BDI-II scores as a moderator of the intervention’s effects
An initial set of linear regressions revealed that there were no significant three-way (condition × BDI-II score × biochemical/genetic attribution rating) interactions in predicting NMR scores or prognostic pessimism ratings at T1 or T2 (all bs<.01, all ps>.10).
We tested for two-way (condition × BDI-II score) interactions using Hayes and Matthes’s (2009) MODPROBE approach, creating separate regression models for each T1 dependent variable and each T2 dependent variable. This revealed significant two-way interactions at T1 for both NMR scores (b=.02, SE<.01, t=3.72, p<.001) and prognostic pessimism ratings (b=−.05, SE=.01, t=−4.67, p<.001).
Probing these interactions revealed that the conditional effect the intervention on T1 NMR scores at one standard deviation below the mean BDI-II score (i.e., a subclinical score of 2.03) was not significant, b=.10, SE=.08, t=1.21, p=.23. However, receiving the intervention was associated with significantly higher T1 NMR scores at the mean BDI-II score of 13.37 (just below the “mild depression” cutoff of 14), b=.30, SE=.06, t=5.44, p<.001, as well as at one standard deviation above the mean BDI-II score (i.e., a score of 24.72—in the “moderate depression” range), b=.51, SE=.08, t=6.48, p<.001. Similarly, the conditional effect the intervention on T1 prognostic pessimism ratings at one standard deviation below the mean BDI-II score (i.e., a subclinical score of 2.01) was not significant, b=.13, SE=.16, t=.81, p=.42 (note: this model excludes one participant who did not provide a T1 prognostic pessimism rating, so some BDI-II means and SDs are slightly different). However, receiving the intervention was associated with significantly lower T1 prognostic pessimism ratings at the mean BDI-II score of 13.37 (just below the “mild depression” cutoff of 14), b=−.40, SE=.11, t=−3.53, p<.001, as well as at one standard deviation above the mean BDI-II score (i.e., a score of 24.72—in the “moderate depression” range), b=−.94, SE=.16, t=−5.80, p<.001.
These findings suggest that in the immediate term, our intervention was particularly effective among people with elevated levels of depressive symptomatology, which is perhaps unsurprising. However, there were no significant two-way (condition × BDI-II score) interactions for either dependent variable at T2 (all |b| ≤ .01; all p >.17). This may be because of the decreased sample size at T2, or it may suggest that the long-term effects of the intervention are less contingent on the recipient’s levels of depressive symptomatology than its short-term effects are.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aronson J, Fried CB, Good C. Reducing the effects of stereotype threat on African American college students by shaping theories of intelligence. Journal of Experimental Social Psychology. 2002;38:113–125. [Google Scholar]
- Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk: A New Source of Inexpensive, Yet High-Quality, Data? Perspectives on Psychological Science. 2011;6:3–5. doi: 10.1177/1745691610393980. [DOI] [PubMed] [Google Scholar]
- Catanzaro SJ, Mearns J. Measuring generalized expectancies for negative mood regulation: Initial scale development and implications. Journal of Personality Assessment. 1990;54:546–563. doi: 10.1080/00223891.1990.9674019. [DOI] [PubMed] [Google Scholar]
- Dar-Nimrod I, Heine SJ. Genetic essentialism: On the deceptive determinism of DNA. Psychological Bulletin. 2011;137:800–818. doi: 10.1037/a0021860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar-Nimrod I, Zuckerman M, Duberstein PR. The effects of learning about one’s own genetic susceptibility to alcoholism: A randomized experiment. Genetics in Medicine. 2013;15:132–138. doi: 10.1038/gim.2012.111. [DOI] [PubMed] [Google Scholar]
- Deacon BJ. The biomedical model of mental disorder: A critical analysis of its validity, utility, and effects on psychotherapy research. Clinical Psychology Review. 2013;33:846–861. doi: 10.1016/j.cpr.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Dozois DJA. Beck Depression Inventory-II. In: Weiner IB, Craighead WE, editors. The Corsini Encyclopedia of Psychology. 4. New York, NY: Wiley; 2010. [Google Scholar]
- Greenberg RP, Constantino MJ, Bruce N. Are patient expectations still relevant for psychotherapy process and outcome? Clinical Psychology Review. 2006;26:657–678. doi: 10.1016/j.cpr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Haslam N. Genetic essentialism, neuroessentialism, and stigma: Commentary on Dar-Nimrod and Heine (2011) Psychological Bulletin. 2011;137:819–824. doi: 10.1037/a0022386. [DOI] [PubMed] [Google Scholar]
- Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behavior Research Methods. 2009;41:924–936. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Higgins ET. ‘Saying is believing’ effects: When sharing reality about something biases knowledge and evaluations. In: Levine JM, Messick DM, Thompson LL, editors. Shared cognition in organizations: The management of knowledge. 1. Mahwah, NJ: Lawrence Erlbaum Associates Inc; 1999. pp. 33–49. [Google Scholar]
- Kemp JJ, Lickel JJ, Deacon BJ. Effects of a chemical imbalance causal explanation on individuals’ perceptions of their depressive symptoms. Behaviour Research and Therapy. 2014;56:47–52. doi: 10.1016/j.brat.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Kvaale EP, Haslam N, Gottdiener WH. The ‘side effects’ of medicalization: A meta-analytic review of how biogenetic explanations affect stigma. Clinical psychology review. 2013;33:782–794. doi: 10.1016/j.cpr.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Lau JYF, Eley TC. The Genetics of Mood Disorders. Annual Review of Clinical Psychology. 2010;6:313–337. doi: 10.1146/annurev.clinpsy.121208.131308. [DOI] [PubMed] [Google Scholar]
- Lebowitz MS. Biological Conceptualizations of Mental Disorders Among Affected Individuals: A Review of Correlates and Consequences. Clinical Psychology: Science and Practice. 2014;21:67–83. [Google Scholar]
- Lebowitz MS, Ahn W, Nolen-Hoeksema S. Fixable or fate? Perceptions of the biology of depression. Journal of Consulting and Clinical Psychology. 2013;81:518–527. doi: 10.1037/a0031730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz MS, Pyun JJ, Ahn W. Biological Explanations of Generalized Anxiety Disorder: Effects on Beliefs About Prognosis and Responsibility. Psychiatric Services. 2014;65:498–503. doi: 10.1176/appi.ps.201300011. [DOI] [PubMed] [Google Scholar]
- Linden DEJ. How psychotherapy changes the brain – The contribution of functional neuroimaging. Molecular Psychiatry. 2006;11:528–538. doi: 10.1038/sj.mp.4001816. [DOI] [PubMed] [Google Scholar]
- Lozano AM. Harnessing Plasticity to Reset Dysfunctional Neurons. New England Journal of Medicine. 2011;364:1367–1368. doi: 10.1056/NEJMcibr1100496. [DOI] [PubMed] [Google Scholar]
- Medin DL, Ortony A. Psychological essentialism. In: Vosniadou S, Ortony A, editors. Similarity and Analogical Reasoning. New York, NY: Cambridge University Press; 1989. pp. 179–195. [Google Scholar]
- Mondloch MV, Cole DC, Frank JW. Does how you do depend on how you think you’ll do? A systematic review of the evidence for a relation between patients’ recovery expectations and health outcomes. CMAJ: Canadian Medical Association Journal. 2001;165:174–179. [PMC free article] [PubMed] [Google Scholar]
- Pescosolido BA, Martin JK, Long JS, Medina TR, Phelan JC, Link BG. “A disease like any other”? A decade of change in public reactions to schizophrenia, depression, and alcohol dependence. The American Journal of Psychiatry. 2010;167:1321–1330. doi: 10.1176/appi.ajp.2010.09121743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford BR, Wager TD, Roose SP. Expectancy and the treatment of depression: A review of experimental methodology and effects on patient outcome. Current Psychiatry Reviews. 2010;6:1–10. doi: 10.2174/157340010790596571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene–environment interplay and psychopathology: multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Walton GM, Cohen GL. A brief social-belonging intervention improves academic and health outcomes of minority students. Science. 2011;331:1447–1451. doi: 10.1126/science.1198364. [DOI] [PubMed] [Google Scholar]