Abstract
Background
Integrating antenatal care (ANC) and HIV care may improve uptake and retention in services along the prevention of mother-to-child transmission (PMTCT) cascade. The current study aimed to determine if integration of HIV services into ANC settings improves PMTCT service utilization outcomes.
Methods
ANC clinics in rural Kenya were randomized to integrated (6 clinics, 569 women) or non-integrated (6 clinics, 603 women) services. Intervention clinics provided all HIV services, including highly active antiretroviral therapy (HAART), while control clinics provided PMTCT services but referred women to HIV care clinics within the same facility. PMTCT utilization outcomes among HIV-infected women (maternal HIV care enrollment, HAART initiation, and 3-month infant HIV testing uptake) were compared using generalized estimating equations and Cox regression.
Results
HIV care enrollment was higher in intervention compared to control clinics (69% versus 36%, Odds Ratio (OR)=3.94, 95% Confidence Interval (CI): 1.14–13.63). Median time to enrollment was significantly shorter among intervention arm women (0 versus 8 days, Hazard Ratio (HR)=2.20, 95% CI: 1.62–3.01). Eligible women in the intervention arm were more likely to initiate HAART (40% versus 17%, OR=3.22, 95% CI: 1.81–5.72). Infant testing was more common in the intervention arm (25% versus 18%), however not statistically different. No significant differences were detected in postnatal service uptake or maternal retention.
Conclusions
Service integration increased maternal HIV care enrollment and HAART uptake. However, PMTCT utilization outcomes were still suboptimal, and postnatal service utilization remained poor in both study arms. Further improvements in the PMTCT cascade will require additional research and interventions.
Keywords: HIV/AIDS, prevention of mother-to-child transmission, service integration, cluster randomized controlled trial, Africa
Introduction
Effective prevention of mother-to-child transmission (PMTCT) programs can reduce MTCT from 15–40% to 1%.1–3 However, achieving this rate has been challenging in sub-Saharan Africa (SSA),4–7 and PMTCT service coverage remains below 50%.6,8 Low coverage may be partially explained by the initial introduction of PMTCT programs as stand-alone vertical programs.9–11 In addition, PMTCT interventions create significant additional work for staff in healthcare systems already suffering from insufficient human, financial, and structural resources.12 Thus, PMTCT implementation is suboptimal and has not produced the expected reduction in MTCT rates.10,13
In addition to health system challenges, PMTCT programs in SSA experience high rates of maternal and infant loss-to-follow-up (LTFU) at each step of the service delivery cascade; i.e., from HIV testing and counseling (HTC), initiation of antiretrovirals (ARVs) for PMTCT prophylaxis or maternal health, and linkage to maternal and pediatric HIV care services for follow-up.8,14,15 Recently, high levels of antenatal HTC and ARV prophylaxis coverage have been achieved in many countries,16,17 and the largest drop-offs in the PMTCT cascade occur with linkage to and retention in HIV care services.18–21
In Kenya, almost all pregnant women attend an antenatal care (ANC) clinic at least once during their pregnancy (94%)22 and receive routine opt-out HTC. However, 2009 national statistics showed that only 79% of HIV+ pregnant women received ARV prophylaxis and only 35% of HIV-exposed infants received early infant diagnostic testing at 6 weeks.23 Furthermore, PMTCT services referred only 56% of HIV+ pregnant women to HIV treatment programs.24 Consequently the national MTCT rate has been estimated to be as high as 10.7%.25
PMTCT service integration with other health services targeting women and children is recommended as a key strategy to improve maternal and child health in low-resource settings with high HIV burdens.8,26,2,27 Integration is posited to 1) improve uptake of and retention in services, 2) reduce the stigma experienced by HIV+ women, and 3) reduce duplication of services and competition for scarce resources.28,29 However, integrating HIV and ANC services could also overburden already weak health systems by increasing work load, leading to poorer quality of care,30 and even higher attrition rates along the PMTCT cascade.31
Systematic reviews report a lack of robust evidence on the impact of integration on PMTCT service uptake and outcomes compared to non-integrated or partially integrated services.32,33 The aim of the Study of HIV and Antenatal care Integration in Pregnancy (SHAIP) cluster-randomized controlled trial was to determine if a comprehensive integrated approach to PMTCT and HIV treatment provision within ANC clinics improved service utilization and health outcomes in Nyanza Province, Kenya. In this manuscript we assess the effects of service integration on women’s and infants’ uptake of and retention in services along the PMTCT cascade.
Methods
Ethics
The SHAIP Trial was approved by the Institutional Review Boards of the University of California, San Francisco (UCSF) and the Kenya Medical Research Institute (KEMRI), as well as by the Associate Director for Science at the Centers for Disease Control and Prevention. All study participants gave written informed consent for the use of their de-identified data. This trial is registered at clinicaltrials.gov NCT00931216.
Setting
The study took place in southern Nyanza Province (see Figure 1) during the period 2009–2011. HIV prevalence in Nyanza Province was estimated to be 16% of reproductive aged women in 2008, more than double the national average.22 All sites were government health facilities receiving PEPFAR support through Family AIDS Care and Education Services (FACES) for both PMTCT and HIV treatment services.34
Figure 1. Map showing distribution of study sites in Southern Nyanza.
Study Design
We conducted a prospective cluster randomized trial comparing service utilization and health outcomes for HIV-infected women and their infants attending integrated ANC clinics with those attending non-integrated ANC clinics. Integrated clinics provided PMTCT and HIV care and treatment services (including highly active antiretroviral therapy [HAART]) within existing ANC services. The same clinic provided all prenatal and postpartum services until a definitive pediatric HIV diagnosis was obtained or the child reached 18 months of age. At this time the woman and infant, if HIV infected, would be referred for long-term care to the facility’s HIV clinic. Non-integrated ANC clinics provided routine PMTCT services and referred HIV+ pregnant women to a separate HIV clinic at the same facility. Additional site details and study methods have been published previously.35
Randomization
Study sites were health facilities that provided ANC, PMTCT, and HIV services with ≥ 20 new ANC clients per month. Facilities (n=12) were stratified as “Health Center/Dispensary” (smaller facilities, N = 8) or “Hospital” (larger sub-district/district hospitals, N = 4) and randomly allocated 1: 1 within strata to provide integrated or non-integrated services using ACluster software36 by the study’s biostatistician who was not involved in fieldwork. Clinics, healthcare providers, patients, and researchers involved in implementing the study were not blinded to the allocation.
Participants
The study population included all pregnant HIV+ women ≥ 18 years who were not enrolled in HIV care at baseline, as well as infants born to study participants.
Outcome Measures
The SHAIP Trial was powered to detect differences in the primary trial outcomes of MTCT and changes in maternal CD4 counts,35 which are reported elsewhere. Secondary PMTCT service utilization outcomes reported in this manuscript are presented below, with primary outcomes for the current analyses indicated in bold:
- MTCT service provision (as documented in medical records)
- maternal ARV prophylaxis dispensed at baseline visit (Y/N)
- baseline CD4 count obtained (within 3 months of study enrollment) (Y/N)
- baseline WHO staging performed (Y/N)
- infant dried blood spot (DBS) HIV testing by 3 and 9 months of age (Y/N)
- time from date birth to first infant HIV test (days)
- Maternal utilization of HIV services (as documented in medical records)
- enrollment in facility’s HIV care and treatment program within 12 months of study enrollment (all HIV+ pregnant women were recommended to enroll in the program, regardless of HAART eligibility) (Y/N)
- time from baseline visit to enrollment in the HIV care and treatment program (days)
- HAART initiation among eligible women (CD4≤350 and/or WHO Stage III or IV) within 12 months of study enrollment (Y/N)
- retention in HIV care (≥2 visits within 6 months of enrollment in care) (Y/N)
- Adherence to PMTCT interventions during pregnancy, labor and delivery (L&D) and postpartum (postpartum maternal self-report)
- maternal ARV use during pregnancy (Y/N)
- delivery at a health facility (Y/N)
- ARV use during labor and delivery (Y/N)
- use of maternal and infant ARVs after the birth (Y/N)
- currently exclusively breastfeeding (Y/N)
Procedures
Healthcare providers at all study clinics received an initial week-long and periodic refresher trainings on high quality ANC, PMTCT, and HIV care service delivery provision as well as instruction on clinic logistics to improve service utilization for both models. ANC nurses at intervention sites received training on appropriate prescription and management of patients on HAART.
At all twelve ANC clinics, protocols mandated that all HIV-positive women should receive ARVs for PMTCT, WHO clinical staging and CD4 testing to determine HAART eligibility, as per national guidelines. Women received AZT/NVP/3TC to take at labor onset, 3TC/AZT post-delivery, and, if eligible, AZT.37 At intervention clinics, HIV+ women were offered enrollment and receipt of all HIV services at the ANC clinic, including HAART if eligible. ANC providers enrolled these women in longitudinal HIV care and managed their HIV care services and paperwork in the ANC clinic. At control clinics, ANC study staff encouraged all women to co-enroll in the facility’s separate HIV clinic and provided a referral form to facilitate enrollment. Women presented separately at these clinics, and HIV clinic providers handled HIV enrollment, paperwork, and care services.
The provision of PMTCT and HIV services followed Kenyan national guidelines in all sites for the duration of the study, with the only difference being the location of HIV services for pregnant and postpartum women (ANC clinic versus the HIV clinic). Guidelines recommended HAART initiation for HIV+ pregnant women at WHO clinical stage III or IV regardless of CD4 count, or WHO clinical stage I or II with CD4 count ≤ 350/mm3 throughout the study period. In August 2010 all sites simultaneously implemented updated PMTCT national guidelines, recommending earlier ARV prophylaxis for pregnant women (at 14 weeks of pregnancy) and postpartum maternal/infant prophylaxis.
Data Collection Methods
Health facility staff members enter all patient socio-demographic and clinical data on paper forms and registers as part of routine clinic care services. Form data are then entered into an electronic database, which employs the Open Medical Record System (OpenMRS®) platform, by program data clerks. Routine programmatic paperwork at all study sites included a “Positive in Pregnancy” form completed at the first ANC visit or at HIV diagnosis, an HIV care enrollment form, HIV care follow-up visit forms, and two postnatal forms: a “Motherhood” form for HIV+ postpartum women and an “HIV-Exposed Infant (HEI) Tracing Form” for infants who did not return to the clinic for HIV testing. Form data are linked using the pregnant woman’s ANC ID. Internal monitoring of data quality was conducted on a monthly basis by the study team, and the trial was also externally monitored.
Study staff abstracted data from this database for each SHAIP participant until 12 months after study enrollment. Completed postnatal forms were available for few study participants (173 [30%] intervention and 152 [25%] control) due to a variety of factors including women not returning to the clinic following delivery, incorrect contact addresses limiting tracing efforts, and incomplete reporting by busy clinicians.
Statistical Analysis
Statistical analysis was conducted using an intention-to-treat approach with all analyses adjusted for clustering of data within clinics. We compared participant baseline characteristics in the two study arms using chi-square and t-tests. Generalized Estimating Equations (GEE), which employed a logit transformation and assumed a binomial distribution, were used to compare dichotomous outcomes, with study arm (intervention or control) as the independent variable. GEE methods were used to account for repeated measures and clustering of data by site. We compared time-to-event outcomes across study arms using proportional hazards Cox regression. Multivariable analyses were not performed because there were no baseline variables that were significantly out of balance in the two arms, and exploratory analyses indicated that potential confounders (such as initial CD4 count) did not confound the relationships between study arms and our key outcomes.
Missing data due to LTFU relative to the measurement of specific outcomes was a significant issue in this study that relied on routine health facility visits and program data. This especially affected analysis of postnatal PMTCT utilization data captured on the two postpartum forms (responses were available for only 325 [28%] of the original 1172 study participants). The amount of missing data varied by site, ranging from 37% to 90%. Thus, data on early uptake of infant testing (before 3 months) were only available for 720 (61%) of mother-infant pairs and the specific timing of early infant testing were only available for 664 (57%) mother-infant pairs.
We used two methods to deal with missing data due to LTFU. First, we employed inverse probability of censoring weighting (IPCW) to account for potential bias associated with missing outcome data. We modeled the probability of remaining in the sample (P(c)) based on calendar time and baseline maternal characteristics (characteristics included in Table 1), then weighted the data (1/(P(c)) so that the distribution of maternal characteristics was similar to the observed baseline data. Second, we used estimates for cases with missing data for infant date of birth (DOB) or infant date of death. We estimated infant’s DOB using the woman’s estimated date of delivery (EDD). For infant death, we estimated the date of death at 1 month following the infant’s DOB (or the EDD if DOB was not available) based on the fact that the majority of infant deaths (60%) occur during the first month of life in Kenya.22 Sensitivity analyses for maternal HIV care enrollment and infant testing uptake were also conducted by excluding maternal and (or) infant deaths and LTFU.
Table 1.
Baseline characteristics of HIV-infected pregnant women enrolled in the two study arms (N=1172)
| Intervention (Integrated) (n=569) |
Control (non- integrated) (n=603) |
P value from chi-square or t- test, adjusted for clustering |
Number with missing data |
Percent with missing data |
|
|---|---|---|---|---|---|
| Mean age in years (SE) | 25.0 (0.19) | 24.8 (0.18) | 0.582 | 6 | 0.51 |
| Education, n (%) | 2 | 0.17 | |||
| None or Some Primary | 481 (85%) | 533 (89%) | 0.372 | ||
| Some Secondary or more | 84 (15%) | 68 (11%) | |||
| Marital status, n (%) | 10 | 0.85 | |||
| Married | 472 (84%) | 500 (84%) | 0.986 | ||
| Single/Separated/Divorced | 49 (8%) | 50 (8%) | |||
| Widowed | 43 (8%) | 48 (8%) | |||
| Median Gravidity (IQR) | 3 (2–4) | 3 (2–4) | 0.943 | 19 | 1.62 |
| Median Parity (IQR) | 2 (1–3) | 2 (1–3) | 0.921 | 25 | 2.13 |
| Mean Gestational Age, weeks (SE) | 26 (0.3) | 25.2 (0.3) | 0.100 | 10 | 0.85 |
| WHO stage n (%) | 66 | 5.63 | |||
| WHO Stage 1 | 339 (63%) | 455 (80%) | 0.403 | ||
| WHO Stage 2 | 79 (15%) | 42 (7%) | |||
| WHO Stage 3 or 4 | 31 (6%) | 8 (1%) | |||
| Not Staged | 85 (15%) | 67 (12%) | |||
| Mean Baseline CD4 (SE) | 495 (19.87) | 523 (19.18) | 0.336 | 508 | 43.34 |
| Baseline CD4 category | |||||
| ≤350 | 117 (34%) | 96 (30%) | 0.654 | ||
| 351–499 | 79 (23%) | 83 (26%) | |||
| ≥500 | 146 (43%) | 143(44%) | |||
| Eligible for HAART* | 127 (22%) | 87 (14%) | 0.278 | 0 | 0 |
Participants who were classified as eligible, based on having a CD4 count ≤ 350 and/or WHO Stage 3 or 4.
Statistical analyses were conducted using Stata 12 for baseline, time-to-event, and initial bivariate analyses and SAS 9.3 for IPCW analyses.
Results
Participant baseline characteristics
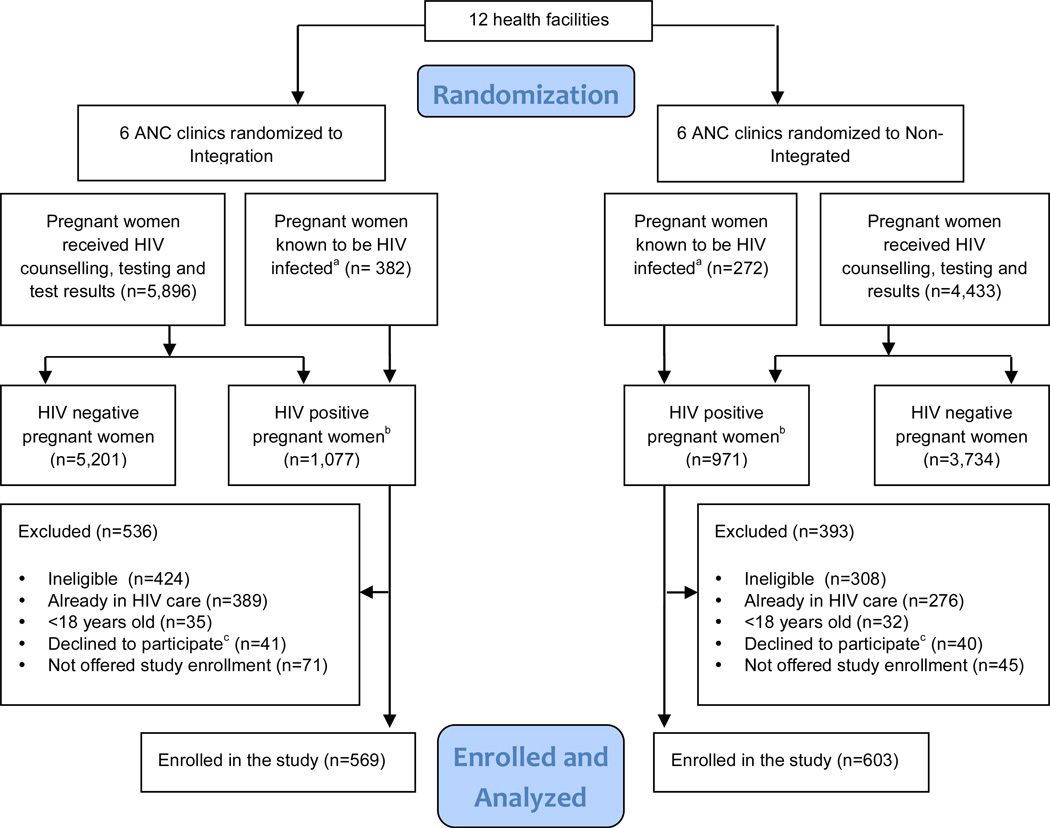
A total of 1,172 HIV+ pregnant women enrolled in the study during June 2009–March 2011 (Figure 2). No significant differences in participant baseline characteristics, i.e., age, education, marital status, gravity, parity, gestational age, mean CD4 count, WHO clinical stage, and HAART eligibility, were detected by study arm (Table 1).
Figure 2. Consort Diagram. The flow diagram of clusters and individuals through the cluster randomized trial.
a The number of pregnant women known to be HIV-positive before the ANC visit may have been underestimated if some women who knew themselves to be HIV-positive did not disclose their status to the health worker and chose to undergo additional HIV testing for confirmation.
b The total number of HIV-positive women (known HIV-positives before the ANC visit plus those who tested HIV-positive during an ANC visit) may be underestimated, as these data were abstracted from routine ANC registers, which may not be complete. The need to rely on these different data sources resulted in lack of agreement for some totals presented in this figure.
c The number of women who declined to participate may be over-estimated, as some women who declined to participate in the study during their first ANC visit may have accepted study enrollment during a subsequent ANC visit.
Utilization of PMTCT cascade services by study arm
Table 2 shows women’s receipt/utilization of PMTCT cascade services by study arm. Receipt of prenatal PMTCT services, e.g., CD4 count, WHO staging, and ARVs for PMTCT, were similar in the two study arms.
Table 2.
PMTCT Cascade Service Utilization in the Two Study Arms (N=1172)
| Intervention (n=569 women) |
Control (n=603 women) |
N for analysis (intervention /control) |
Odds Ratio or Hazard Ratio# |
95% CI | IPCW^ Odds Ratio# |
95% CI | |
|---|---|---|---|---|---|---|---|
| PMTCT service delivery outcomes | |||||||
| Baseline CD4 count (Y/N), N (%) | 330 (58%) | 324 (54%) | 569/603 | 1.19 | 0.41–3.46 | n/a | n/a |
| Baseline WHO staging (Staged/not staged), N (%) | 449 (79%) | 505 (84%) | 569/603 | 0.73 | 0.27–1.95 | n/a | n/a |
| ARVs dispensed at baseline visit, N (%) | 528 (93%) | 581 (96%) | 569/603 | 0.53 | 0.28–1.02 | n/a | n/a |
| Infant HIV testing (before 3 months), N% | 143 (25%) | 106 (18%) | 569/603 | 1.57* | 0.61–4.07 | n/a | n/a |
| Infant HIV testing (by 9 months of age), N% | 361 (63%) | 326 (54%) | 569/603 | 1.47* | 0.76–2.86 | n/a | n/a |
| Timing of infant testing (days since DOB (IQR)+ | 122 days (51–237) | 189 days (65–366) | 349/315 | 1.39 | 0.89–2.15 | n/a | n/a |
| Maternal HIV care and treatment enrollment | |||||||
| Enrollment in HIV care and treatment by 12 months, N (%) | 393 (69%) | 218 (36%) | 569/603 | 3.94* | 1.14–13.63 | n/a | n/a |
| Time from study enrollment to women’s enrollment in HIV care (median days (IQR))++ | 0 days (0–0) | 8 days (0–72) | 380/214 | 2.20 | 1.62–3.01 | n/a | n/a |
| HAART initiation among eligible women within 12 months, N (%) | 51 (40%) | 15 (17%) | 127/87 | 3.22 | 1.81–5.72 | 3.22 | 1.81–5.72 |
| At least 2 HIV Care follow-up visits in the first 6 months, N (%)+++ | 190 (48%) | 123 (56%) | 393/218 | 0.73 | 0.47–1.14 | 1.40 | 0.74–2.66 |
| Maternal PMTCT compliance during pregnancy, birth, and postpartum (self-report)** | |||||||
| Maternal ARV use during pregnancy, N (%) | 138 (80%) | 75 (49%) | 173/152 | 4.05 | 2.00–8.00 | 4.54 | 1.94–10.48 |
| Delivered in a health facility, N (%) | 61 (41%) | 51 (34%) | 149/148 | 1.83 | 0.71–4.71 | 1.08 | 0.45–2.56 |
| Use of ARVs during labor & delivery, N (%) | 28 (16%) | 84 (55%) | 173/152 | 0.16 | 0.04–0.68 | 0.10 | 0.02–0.56 |
| Use of ARVs postpartum, N (%) | 22 (13%) | 57 (38%) | 173/152 | 0.24 | 0.08–0.70 | 0.18 | 0.12–0.66 |
| Infant’s ARV use, N (%) | 50 (29%) | 106 (70%) | 173/152 | 0.18 | 0.09–0.35 | 0.14 | 0.06–0.33 |
| Exclusive breastfeeding within the first 3 months, N (%) | 70 (58%) | 69 (58%) | 121/119 | 1.10 | 0.61–2.01 | 0.86 | 0.45–1.63 |
Result obtained using Inverse probability of censoring weighting (IPCW)
Statistically significant results are indicated in bold.
This analysis includes only those infants with a non-missing infant HIV test date (N=664)
This analysis includes only those women with a non-missing enrollment in HIV care date (N=594)
This analysis includes only those women who enrolled in HIV care date (N=611)
This analysis assumes that those with no record of testing/enrollment were not tested/enrolled.
Self report from women with postpartum forms (N=325 (173 intervention / 152 control)
HIV care enrollment was higher in intervention sites compared to control sites (69% versus 36%, Odds Ratio (OR)=3.94, 95% Confidence Interval (CI): 1.14–13.63), and the median time to HIV care enrollment was significantly shorter among women in the intervention arm (0 versus 8 days, Hazard Ratio (HR)=2.20, 95% CI: 1.62–3.01). Although the number of HAART-eligible women identified at baseline was relatively small (n=214), women in the intervention arm were significantly more likely to have initiated HAART by study close compared to women in the control arm (40% in intervention compared to 17% in control, OR=3.22, 95% CI: 1.81–5.72). An additional 15 women in the intervention arm and 7 women in the control arm (who were not HAART-eligible at baseline) became eligible and initiated HAART during the follow-up period.
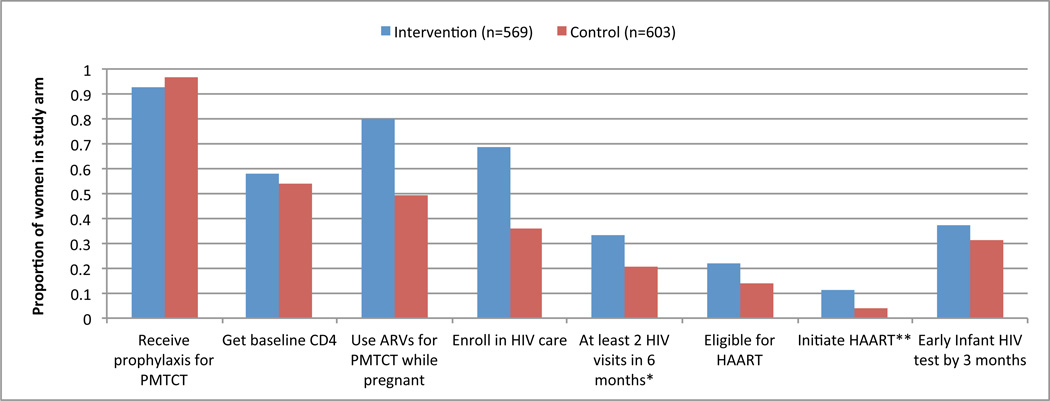
Among the limited number of women with postpartum data (n=325), women in the intervention arm were more likely to report ARV use during pregnancy than those in the control arm (80% in intervention, compared to 49% in control, OR=4.05, 95% CI: 2.00–8.00). However, women in the intervention arm were less likely to report ARV use during L&D (16% intervention versus 55% control; OR=0.16, 95%CI: 0.04–0.68), ARV use following delivery (13% intervention vs. 38% control; OR=0.24, 95%CI: 0.08–0.70), and infant ARV prophylaxis (29% intervention vs. 70% control; OR=0.18, 95%CI: 0.09–0.35) than women in the control arm. There were no statistically significant differences between the study arms in regards to the proportion of women reporting delivery in a health facility or exclusive breastfeeding (Table 2). Although the proportion of infants tested for HIV at 3 and 9 months was slightly higher in the intervention arm, this did not achieve statistical significance. Figure 2 shows the proportion of women completing key cascade steps by study arm.
Sensitivity analyses
For initial analyses of maternal HIV care enrollment, we assumed that women did not enroll in HIV care if there was no HIV care documentation in the medical records. However, study participants might have enrolled elsewhere. As a sensitivity analysis, we repeated this analysis excluding women lacking HIV care documentation in medical records and could not be located at the postpartum tracing visit. This analysis resulted in a lower magnitude OR of 1.94 (95% CI: 0.73–5.15) compared to the initial OR of 3.94 (95% CI: 1.14–13.63) for enrollment in HIV care. Similarly for infant testing uptake, we initially assumed that the absence of an infant HIV test result signified no HIV test. We conducted sensitivity analyses for the infant HIV testing uptake before 3 months of age excluding those LTFU. This resulted in an OR for infant HIV test of 1.31 (95% CI: 0.52 - 3.31), compared to the initial result 1.31 (95% CI: 0.48 - 3.58).
Analyses examining the impact of censoring revealed that adjustment for LTFU did not significantly change our results. Inverse probability of censoring weighting (IPCW) results were of similar direction, magnitude, and statistical significance to the initial results for variables that had substantial missing data (see the last two columns of Table 2).
Discussion
In this first cluster-randomized trial of ANC-HIV treatment integration in SSA, we found that integration had positive effects on some aspects of PMTCT service utilization, but not on others. Despite the convenience and benefits of being offered concurrent enrollment in ANC and HIV care, attrition of women and infants over the course of pregnancy and postpartum remained significant.
Initial services received by HIV+ pregnant women in ANC clinics were similar in both study arms. In both types of clinics, a high percentage of women were clinically staged and received ARVs for PMTCT. Intervention clinics noted improvements in HIV care enrollment, time to enrollment, and HAART initiation among eligible women compared to control clinics. These are clearly important benefits and correspond with previous non-randomized integration study results.38–40 Given the advantages of early HAART initiation for PMTCT and maternal health,41 these important effects should not be taken lightly. However, the proportion of women with baseline CD4 count assessments was low in both arms (58% intervention and 54% control), although higher than in many other settings,8 reflecting the logistical constraints affecting all sites in performing CD4 counts, e.g. stock-outs of blood tubes and/or CD4 reagents. And the 70% HIV care enrollment, not 100%, at intervention sites indicates that women’s hesitations about initiating HIV care, regardless of location, continued to be a barrier for a substantial portion of women.
The self-reported PMTCT adherence data collected from women postpartum had inconsistent results. While there was significantly higher self-reported use of ARVs during pregnancy by women at intervention versus control sites, women from control sites were more likely to report ARV use during delivery and postpartum. However, the small number of study participants with completed postpartum forms (173 of 569 women in the intervention group and 152 of 603 women in the control group) make these results difficult to interpret. Early infant diagnosis was clearly inadequate in both arms of the study, and points to known challenges,42 including inadequate systems to identify HIV-exposed infants in postnatal clinics, insufficient resources to conduct home visits, and continuing stigma associated with HIV.43
In our study women’s retention in HIV care was lower in the intervention arm than in the control arm (although not statistically significant). Similarly, a study in Mozambique found HIV patients attending integrated clinics in primary healthcare services had a higher risk of attrition six months after initiating treatment than HIV patients attending specialized HIV clinics.44 This questions if busy primary healthcare services have sufficient time and attention necessary to follow HIV clients and assure their retention when HIV services are absorbed. In addition, it has been noted that service integration cannot solve existing human resource shortages or problems of inadequate infrastructure.45 Service integration often results in increased provider workload, increased training needs, and even “organizational culture clash.”46 Alternatively women at control sites who followed through and enrolled in HIV care at a separate HIV clinic may have been more motivated than those who were more passively enrolled at the integrated sites.
Despite the benefits of integration seen, it is clear that we still have a long way to go to reduce gaps in the PMTCT cascade in low-resource settings. Other trials in SSA have also concluded that assuring women’s retention in care and adherence to PMTCT recommendations will reduce unacceptably high rates of vertical transmission.47 Integration of clinical services, although important, is probably not sufficient to address all the barriers to utilization of PMTCT services, including risks of stigma, discrimination, and violence.48 An observational study conducted at a sub-set of SHAIP sites revealed that internalized HIV-related stigma was a significant issue for women in both integrated and non-integrated sites.49 A study conducted in Swaziland suggested that clients experienced more unwanted disclosure and risks of stigmatization at integrated versus non-integrated clinics.50
This real-world trial conducted in rural health facilities had a number of limitations. Although use of existing programmatic data as data sources in implementation science has been suggested as an efficient use of resources,51 we found that medical records and health facility registers were often incomplete and/or inconsistent. High rates of maternal and pediatric LTFU, coupled with inadequate completion of postpartum forms resulted in sparse data for some of our PMTCT cascade outcomes. It was also difficult to maintain a consistent level of service quality at the 12 government health facilities throughout the study duration. Insufficient and inconsistent staffing, supplies and systems may have affected fidelity to the site’s randomly assigned service model, the data availability, and outcomes of interest. Changes in national PMTCT guidelines during the study period (although implemented simultaneously at all study sites) also may have affected our results. The relatively small number of sites available for trial inclusion (12) combined with the realities of significant LTFU in the PMTCT cascade resulted in reduced power to detect statistically significant differences between the study arms for some outcomes. Actual intra-cluster correlation coefficients ranged from 0.10 (uptake of infant testing) to 0.28 (maternal HIV care enrollment), compared to the lower estimates that were used in sample size calculations. Lastly, demonstration of cost-effectiveness was not included in the design of this trial, but will be important to investigate as more programs around the world consider implementation of integrated ANC-HIV care services.
Based on preliminary results of this trial and other studies, several steps have been undertaken to address PMTCT cascade attrition in Kenya. A variety of interventions—including text message reminders, systems strengthening, community mobilization activities, and efforts to increase male involvement in ANC/PMTCT services—have been implemented to increase engagement of pregnant women in HIV care. Efforts are also underway to develop an integrated point-of-care electronic maternal and child health register, which will allow longitudinal follow-up and timely access to information on women and infants who fall out of care at each point in the PMTCT cascade. Option B+ (universal lifelong HAART for all HIV-positive pregnant women) is currently being scaled up in Kenya and is expected to increase uptake and retention in services.
Conclusions
In this study, we found that integration appears to have important advantages in terms of increasing HIV care enrollment of pregnant HIV+ women and earlier initiation of HAART. However, integration of clinical services may not be enough to retain women in long-term care or ensure infant testing and care. Further research focusing on improving systems and reducing key psycho-social barriers (including stigma) will be necessary in order to improve uptake and retention throughout the entire PMTCT cascade.
Figure 3. Elements of the PMTCT cascade completed for HIV-positive pregnant women attending intervention and control sites (proportions are based on all women enrolled in the study in each study arm).
* Only applicable for women who enrolled in HIV care
** Only applicable for women who were eligible for HAART
ACKNOWLEDGEMENTS
We thank the Kenyan women who participated in the study. We acknowledge the important logistical support of the KEMRI-UCSF Collaborative Group and especially Family AIDS Care and Education Services (FACES). We gratefully acknowledge the Director of KEMRI, the Director of KEMRI’s Centre for Microbiology, and the Kenyan Ministry of Health for their support in conducting this research. We also thank John Oguda, Peter Manwari, George O’chieng, Pheobe Anyango, Cinthia Blat, Jayne Kulzer, Nicole Schmidt, Katie Schwartz, Lisa Dillabaugh, and Benard Otieno for their important contributions to this research. The project described was supported as a Public Health Evaluation by the President’s Emergency Plan for AIDS Relief (PEPFAR)/Centers for Disease Control and Prevention (CDC). The content is solely the responsibility of the authors and does not necessarily represent the official views of PEPFAR or the CDC.
Sources of support:
Funding: The research described was supported as a Public Health Evaluation by the President’s Emergency Plan for AIDS Relief (PEPFAR)/U.S. Centers for Disease Control and Prevention (CDC). The study was funded under the CDC cooperative agreement number 5U2GPS001913-02. The funders had no role in data collection and analysis. The manuscript has received CDC clearance for journal submission. The first author’s time working on this study was partially supported by Award Number K01MH081777 from the U.S. National Institute of Mental Health. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention or the U.S. National Institutes of Health.
Footnotes
- Integration for Impact, Reproductive Health and HIV Services in sub-Saharan Africa, Nairobi, Kenya, September 12–14, 2012.
- 7th IAS Conference on HIV Pathogenesis, Treatment, and Prevention, 30 June – 03 July 2013, Kuala Lumpur, Malaysia.
Competing Interests: The authors have declared that no competing interests exist.
Disclaimer: “The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.”
References
- 1.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic. UNAIDS; 2010. p. 364. [Google Scholar]
- 2.World Health Organisation. [Accessed December 4, 2012];PMTCT Strategic Vision 2010–2015: Preventing mother-to-child transmission of HIV to reach UNGASS and Millennium Development Goals. 2010 Available at: http://www.who.int/hiv/pub/mtct/strategic_vision.pdf.
- 3.Townsend CL, Cortina-Borja M, Peckham CS, de Ruiter A, Lyall H, Tookey PA. Low rates of mother-to-child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000–2006. AIDS. 2008 May 11;22(8):973–981. doi: 10.1097/QAD.0b013e3282f9b67a. [DOI] [PubMed] [Google Scholar]
- 4.Becquet R, Newell ML. Prevention of postnatal HIV infection: infant feeding and antiretroviral interventions. Current opinion in HIV and AIDS. 2007 Sep;2(5):361–366. doi: 10.1097/COH.0b013e3282cecef4. [DOI] [PubMed] [Google Scholar]
- 5.Médecins sans frontières. Responding to the failure of prevention of mother-to-child trans- mission (PMTCT) programmes: what needs to change? Expert roundtable 23. 2008 http://www.msfaccess.org/main/hiv-aids/pmtct-experts-roundtable/.
- 6.Orne-Gliemann J, Becquet R, Ekouevi DK, Leroy V, Perez F, Dabis F. Children and HIV/AIDS: from research to policy and action in resource-limited settings. AIDS. 2008 Apr 23;22(7):797–805. doi: 10.1097/QAD.0b013e3282f4f45a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UNICEF. [Accessed December 4, 2012];Children and AIDS: Second Stocktaking Report. 2008 Available at: http://www.unicef.org/publications/files/ChildrenAIDS_SecondStocktakingReport.pdf.
- 8.WHO/UNAIDS/UNICEF. [Accessed December 3, 2012];Towards Universal Access: Scaling up priority HIV/AIDS interventions in the health sector. 2010 :150. Available at: http://www.who.int/hiv/pub/2010progressreport/report/en/index.html.
- 9.Mazia G, Narayanan I, Warren C, et al. Integrating quality postnatal care into PMTCT in Swaziland. Glob Public Health. 2009;4(3):253–270. doi: 10.1080/17441690902769669. [DOI] [PubMed] [Google Scholar]
- 10.Nkonki LL, Doherty TM, Hill Z, Chopra M, Schaay N, Kendall C. Missed opportunities for participation in prevention of mother to child transmission programmes: simplicity of nevirapine does not necessarily lead to optimal uptake, a qualitative study. AIDS Res Ther. 2007;4:27. doi: 10.1186/1742-6405-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Druce NAK, Campbell White A. [Accessed 4 december 2012];Standing H Strengthening linkages for sexual and reproductive health, HIV and AIDS: progress, barriers and opportunities for scaling up. 2006 http://hdrc.dfid.gov.uk/wp-content/uploads/2012/10/Strenghtening-linkages-for-sexual-and-reproductive-health.pdf.
- 12.UNICEF. [Accessed 3 december 2012];Evaluation of United Nations-supported pilot projects for the prevention of mother-to-child transmission of HIV. 2003 http://aidsdatahub.org/dmdocuments/Evaluation_of_United_Nations_Supported_Pilot_Projects_for_the_PMTCT_of_HIV_2003.pdf.pdf.
- 13.Uwimana J, Jackson D, Hausler H, Zarowsky C. Health system barriers to implementation of collaborative TB and HIV activities including prevention of mother to child transmission in South Africa. Trop. Med. Int. Health. 2012 May;17(5):658–665. doi: 10.1111/j.1365-3156.2012.02956.x. [DOI] [PubMed] [Google Scholar]
- 14.Msellati P. Improving mothers' access to PMTCT programs in West Africa: a public health perspective. Soc. Sci. Med. 2009 Sep;69(6):807–812. doi: 10.1016/j.socscimed.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 15.Tugwell P, de Savigny D, Hawker G, Robinson V. Applying clinical epidemiological methods to health equity: the equity effectiveness loop. BMJ. 2006 Feb 11;332(7537):358–361. doi: 10.1136/bmj.332.7537.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byamugisha R, Tumwine JK, Ndeezi G, Karamagi CA, Tylleskar T. Attitudes to routine HIV counselling and testing, and knowledge about prevention of mother to child transmission of HIV in eastern Uganda: a cross-sectional survey among antenatal attendees. J Int AIDS Soc. 2010;13:52. doi: 10.1186/1758-2652-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO, UNAIDS, UNICEF. Global HIV/AIDS Response: Epidemic update and health sector progress towards Universal Access: Progress report 2011. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 18.Stringer EM, Chi BH, Chintu N, et al. Monitoring effectiveness of programmes to prevent mother-to-child HIV transmission in lower-income countries. Bulletin of the World Health Organization. 2008 Jan;86(1):57–62. doi: 10.2471/BLT.07.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manzi M, Zachariah R, Teck R, et al. High acceptability of voluntary counselling and HIV-testing but unacceptable loss to follow up in a prevention of mother-to-child HIV transmission programme in rural Malawi: scaling-up requires a different way of acting. Tropical Medicine and International Health. 2005 Dec;10(12):1242–1250. doi: 10.1111/j.1365-3156.2005.01526.x. [DOI] [PubMed] [Google Scholar]
- 20.Stringer EM, Ekouevi DK, Coetzee D, et al. Coverage of nevirapine-based services to prevent mother-to-child HIV transmission in 4 African countries. JAMA. 2010 Jul 21;304(3):293–302. doi: 10.1001/jama.2010.990. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson L, Grant AD, Watson-Jones D, Kahawita T, Ong'ech JO, DA R. Linking women who test HIV-positive in pregnancy-related services to long-term HIV care and treatment services: a systematic review. Trop. Med. Int. Health. 2012;17:564–580. doi: 10.1111/j.1365-3156.2012.02958.x. [DOI] [PubMed] [Google Scholar]
- 22.Central Bureau of Statistics (CBS) [Kenya], Ministry of Health (MOH) [Kenya], ORC Macro. Kenya Demographic & Health Survey 2008–2009. Calverton, Maryland: KNBS and ICF Macro; 2010. [Google Scholar]
- 23.National AIDS and STI Control programme (NASCOP) TOWARDS THE ELIMINATION OF MOTHER-TO-CHILD TRANSMISSION OF HIV AND KEEPING MOTHERS ALIVE IN KENYA, A STRATEGIC FRAMEWORK, 2012 – 2015. Nairobi, Kenya: Ministry of Health [Kenya]; 2012. [Google Scholar]
- 24.National AIDS / STI Control Programme (NASCOP) Annual Health Sector HIV Report 2009. Nairobi, Kenya: Ministry of Health (Kenya); 2010. [Google Scholar]
- 25.Mwau M, Okoth V, Kadima S, et al. Effectiveness of PMTCT interventions in reducing Mother to Child Transmission of HIV in Kenya. 1st KEMRI Annual Scientific And Health Conference; Feb 9–11, 2011; Nairobi, Kenya. [Google Scholar]
- 26.Ginsburg AS, Hoblitzelle CW, Sripipatana TL, Wilfert CM. Provision of care following prevention of mother-to-child HIV transmission services in resource-limited settings. AIDS. 2007 Nov 30;21(18):2529–2532. doi: 10.1097/QAD.0b013e3282f155f4. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organisation. Technical Consultation on the Integration of HIV Interventions into Maternal, Newborn and Child Health Services: World Health Education. 2006:45.
- 28.IPPF, UNFPA, WHO et al. [Accessed 4 december 2012];Rapid Assessment Tool for Sexual & Reproductive Health and HIV Linkages: A Generic Guide. 2009 http://www.unfpa.org/webdav/site/global/shared/documents/publications/2009/rapid_assesment_2009.pdf. [Google Scholar]
- 29.Israel E, Kroeger M. [Accessed December 4, 2012];Integrating Prevention of Mother-to-Child HIV Transmission into Existing Maternal, Child, and Reproductive Health Programs. Technical Guidance Series. 2003 :22. Available at: http://www2.pathfinder.org/site/DocServer/Technical_Guidance_Series_3_PMTCTweb_01.pdf?docID=242.
- 30.Cherutich P, Inwani I, Nduati R, Mbori-Ngacha D. Optimizing paediatric HIV care in Kenya: challenges in early infant diagnosis. Bull. World Health Organ. 2008 Feb;86(2):155–160. doi: 10.2471/BLT.07.040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stinson K, Boulle A, Coetzee D, Abrams EJ, Myer L. Initiation of highly active antiretroviral therapy among pregnant women in Cape Town, South Africa. Trop. Med. Int. Health. 2010 Jul;15(7):825–832. doi: 10.1111/j.1365-3156.2010.02538.x. [DOI] [PubMed] [Google Scholar]
- 32.Both JM, van Roosmalen J. The impact of Prevention of Mother to Child Transmission (PMTCT) programmes on maternal health care in resource-poor settings: looking beyond the PMTCT programme--a systematic review. BJOG. 2010 Nov;117(12):1444–1450. doi: 10.1111/j.1471-0528.2010.02692.x. [DOI] [PubMed] [Google Scholar]
- 33.Tudor Car L, Van Velthoven MHMMT, Brusamento S, et al. Integrating Prevention of Mother-to-Child HIV Transmission Programs to Improve Uptake: A Systematic Review. PLoS ONE. 2012;7(4):e35268. doi: 10.1371/journal.pone.0035268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis Kulzer J, Penner JA, Marima R, et al. Family model of HIV care and treatment: a retrospective study in Kenya. J Int AIDS Soc. 2012;15(1):8. doi: 10.1186/1758-2652-15-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turan JM, Steinfeld RL, Onono M, et al. The study of HIV and antenatal care integration in pregnancy in Kenya: design, methods, and baseline results of a cluster-randomized controlled trial. PLoS One. 2012;7(9):e44181. doi: 10.1371/journal.pone.0044181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ACLUSTER - Software for the Design and Analysis of Cluster Randomized Trials [computer program]. Version 2.1: Update-Software. [Google Scholar]
- 37.Kenya Ministry of Public Health and Sanitation, Kenya Ministry of Medical Services. National Recommendations for Prevention of Mother To Child Transmission of HIV, Infant & Young Child Fedding and Antiretroviral therapy for children, adults and adolescents. 2010 Jul 15; [Google Scholar]
- 38.Killam WP, Tambatamba BC, Chintu N, et al. Antiretroviral therapy in antenatal care to increase treatment initiation in HIV-infected pregnant women: a stepped-wedge evaluation. AIDS. 2010 Jan;24(1):85–91. doi: 10.1097/QAD.0b013e32833298be. [DOI] [PubMed] [Google Scholar]
- 39.Horwood C, Haskins L, Vermaak K, Phakathi S, Subbaye R, Doherty T. Prevention of mother to child transmission of HIV (PMTCT) programme in KwaZulu-Natal, South Africa: an evaluation of PMTCT implementation and integration into routine maternal, child and women's health services. Trop. Med. Int. Health. 2010 Jun 17; doi: 10.1111/j.1365-3156.2010.02576.x. [DOI] [PubMed] [Google Scholar]
- 40.Ong'ech JO, Hoffman HJ, Kose J, et al. Provision of services and care for HIV-exposed infants: a comparison of maternal and child health clinic and HIV comprehensive care clinic models. J. Acquir. Immune Defic. Syndr. 2012 Sep 1;61(1):83–89. doi: 10.1097/QAI.0b013e31825bd842. [DOI] [PubMed] [Google Scholar]
- 41.Chi BH, Adler MR, Bolu O, et al. Progress, challenges, and new opportunities for the prevention of mother-to-child transmission of HIV under the US President's Emergency Plan for AIDS Relief. J. Acquir. Immune Defic. Syndr. 2012 Aug 15;60(Suppl 3):S78–S87. doi: 10.1097/QAI.0b013e31825f3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peltzer K, Mlambo G. Factors determining HIV viral testing of infants in the context of mother-to-child transmission. Acta Paediatr. 2010 Apr;99(4):590–596. doi: 10.1111/j.1651-2227.2009.01670.x. [DOI] [PubMed] [Google Scholar]
- 43.Turan JM, Hatcher AH, Medema-Wijnveen J, et al. The role of HIV-related stigma in utilization of skilled childbirth services in rural Kenya: a prospective mixed-methods study. PLoS Med. 2012;9(8):e1001295. doi: 10.1371/journal.pmed.1001295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lambdin BH, Micek MA, Sherr K, et al. Integration of HIV Care and Treatment in Primary Health Care Centers and Patient Retention in Central Mozambique: A Retrospective Cohort Study. J. Acquir. Immune Defic. Syndr. 2013 Jan 2; doi: 10.1097/QAI.0b013e3182840d4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Topp SM, Chipukuma JM, Chiko MM, Matongo E, Bolton-Moore C, Reid SE. Integrating HIV treatment with primary care outpatient services: opportunities and challenges from a scaled-up model in Zambia. Health Policy Plan. 2013 Jul;28(4):347–357. doi: 10.1093/heapol/czs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freedman LP. Integrating HIV and maternal health services: will organizational culture clash sow the seeds of a new and improved implementation practice? J. Acquir. Immune Defic. Syndr. 2011 Aug;57(Suppl 2):S80–S82. doi: 10.1097/QAI.0b013e31821dba2d. [DOI] [PubMed] [Google Scholar]
- 47.Bisio F, Masini G, Blasi Vacca E, et al. Effectiveness of a project to prevent HIV vertical transmission in the Republic of Congo. J. Antimicrob. Chemother. 2013 May 15; doi: 10.1093/jac/dkt102. [DOI] [PubMed] [Google Scholar]
- 48.Turan JM, Nyblade L. HIV-related Stigma as a Barrier to Achievement of Global PMTCT and Maternal Health Goals: A Review of the Evidence. AIDS Behav. 2013 Mar 9; doi: 10.1007/s10461-013-0446-8. [DOI] [PubMed] [Google Scholar]
- 49.Turan JM, Onono M, Bukusi EA, Cohen CR. HIV-related stigma and enrollment in HIV care in the context of integrated vs. non-integrated antenatal care and HIV services in rural Kenya. Integration for Impact Conference, Reproductive Health and HIV Services in sub-Saharan Africa; Nairobi, Kenya. 2012. [Google Scholar]
- 50.Church K, Wringe A, Fakudze P, Kikuvi J, Simelane D, Mayhew SH. Are integrated HIV services less stigmatizing than stand-alone models of care? A comparative case study from Swaziland. J Int AIDS Soc. 2013;16(1):17981. doi: 10.7448/IAS.16.1.17981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Padian NS, Holmes CB, McCoy SI, Lyerla R, Bouey PD, Goosby EP. Implementation science for the US President's Emergency Plan for AIDS Relief (PEPFAR) J. Acquir. Immune Defic. Syndr. 2011 Mar;56(3):199–203. doi: 10.1097/QAI.0b013e31820bb448. [DOI] [PubMed] [Google Scholar]