Abstract
The physiological functions of neurotransmitter:sodium symporters (NSS) in reuptake of neurotransmitters from the synapse into the presynaptic nerve have been shown to be complemented by their involvement, together with non-plasma membrane neurotransmitter transporters, in the reverse transport of substrate (efflux) in response to psychostimulants. Recent experimental evidence implicates highly anionic phosphatidylinositol 4,5-biphosphate (PIP2) lipids in such functions of the serotonin (SERT) and dopamine (DAT) transporters. Thus, for both SERT and DAT, neurotransmitter efflux has been shown to be strongly regulated by the presence of PIP2 lipids in the plasma membrane, and the electrostatic interaction of the N-terminal region of DAT with the negatively charged PIP2 lipids. We examine the experimentally established phenotypes in a structural context obtained from computational modeling based on recent crystallographic data. The results are shown to set the stage for a mechanistic understanding of physiological actions of neurotransmitter transporters in the NSS family of membrane proteins.
Keywords: Cell signalling, phosphorylation sites, psychostimulant drugs of abuse, reverse transport of substrate, amphetamine-induced efflux, membrane composition and PIP2 lipids, lipid segregation in the membrane, electrostatic interactions, molecular dynamics simulations, continuum mean-field theory
1. Introduction
In the past decade the mechanistic evaluation of the structural basis for the function of the neurotransmitter:sodium symporters (NSS) proteins has been enriched greatly by the availability of crystallographically determined molecular models, starting with very first X-ray structure of Leucine transporter (LeuT) [1], the bacterial homolog of the neurotransmitter transporters [2] that rapidly became a prototype for extensive structure-function studies. The initial structure of LeuT revealed a transmembrane (TM) bundle composed of 12 helical segments that incorporated a centrally located primary substrate binding site (S1) and two Na+ binding sites, Na1 and Na2. These sites were occluded from both intracellular (IC) and extracellular (EC) vestibules exposed to the aqueous environment. Subsequent crystallographic studies [3-9] captured LeuT and its mutant constructs (mostly dictated by crystallization requirements, or an engineered Cl− site [10]), in various other conformations (including outward- and inward-facing), and in complex with distinct ligands in the S1 site, as well as with tricyclic antidepressants in the EC vestibule. A variety of yet additional states that can be accessible to the proteins that share LeuT-like architecture, were inferred from forms of LeuT in the presence and absence of ligands and ions [11], and crystallographic models of other bacterial transporters with similar architecture (the “LeuT fold”) [12-21].
The availability of such a rich repertoire of structures has prompted various formulation of mechanistic models of the transport cycle in NSS proteins directly from structure [22-25], consistent with the alternating access mechanism [23]. Not surprisingly, however, experimental and computational studies of the dynamic properties of the LeuT prototype under various conditions showed that these direct approximations from structure were incomplete (see, for example, Refs. [26-28]). Indeed, ensemble and single-molecule level spectroscopic measurements on LeuT [28-31] and on Mhp1 [26], as well as enlightening atomistic scale computational simulations of LeuT [9, 27-39] and of homology models of mammalian dopamine (DAT) [40-44] and serotonin (SERT) [45, 46] NSS transporters, provided valuable mechanistic insights into ion- and ligand-dependent conformational dynamics in NSS proteins. Together, these findings suggest that the alternating access is achieved through concerted dynamic rearrangements in specific structural motifs of the protein [47]. Indeed, extensive computational modelling and simulations of the system [27, 32-38] focused on the detailed manner in which binding of substrate and ions from the extracellular side of the transporter induces conformational changes in the protein leading to the translocation of substrate and ions towards the intracellular vestibule. Furthermore, ligand binding and uptake measurements in LeuT together with computational modelling [32, 48, 49] have suggested a mechanistic model of allosteric Na+-coupled symport in which intracellular release of the S1-bound substrate is triggered by the binding of a second substrate molecule in the extracellular vestibule located ~11Å above the S1 site, termed the S2 site.
Recently, the very first X-ray structure of eukaryotic NSS protein, DAT from Drosophila melanogaster (dDAT) in complex with tricyclic antidepressant nortriptyline, has been reported [50]. In this structure, dDAT is in outward-open state, similar to LeuT model stabilized by an inhibitor tryptophan [4], providing novel insights into relationships between the bacterial prototype LeuT, and the eukaryotic neurotransmitter transporters.
Members of the NSS family, in addition to DAT and SERT, include the transporters of the neurotransmitters γ-aminobutyric acid, glycine, and norepinephrine (respectively known as GAT, GlyT, and NET), which are responsible for the clearance of the neurotransmitters from the synaptic cleft and their translocation back into the presynaptic nerve terminal [51-56]. In the function of these NSS, neurotransmitter reuptake into the presynaptic cell is powered by coupling to the transmembrane sodium gradients. Their functions in neuronal signalling have made these transporters primary targets for medications [57], and they have been implicated in the mechanisms of action of abused psychostimulants, such as cocaine and amphetamine (AMPH), and in various psychiatric and neurological disorders that include drug addiction, schizophrenia, and Parkinson's disease [58].
Interestingly, while there have been several reports regarding a role for the membrane in which the NSS are embedded, in their functional mechanisms (e.g., see [59-61]), until recently there had been no experimental evidence to suggest a role for plasma membrane components in specific modes of regulation of the NSS proteins. This was changed by in vitro and in vivo studies on DAT [62] and SERT [63] that have unambiguously established a direct involvement of PIP2 (phosphatidylinositol 4,5-biphosphate) lipids in the transport mechanisms of these NSS proteins.
PIP2 lipids are strongly anionic (with a net charge of −4e at neutral pH) and represent only a minor fraction of the phospholipid composition of the cytoplasmic leaflet of plasma membranes [64, 65]. Nevertheless, they regulate many cellular processes by affecting function and organization of both peripheral and integral membrane proteins. For example, PIP2 lipids anchor various proteins to the cell membrane through interactions with specific sensor domains, such as the pleckstrin homology (PH) domain [66] and have been shown to regulate the function of various channels and enzymes (reviewed in Ref. [67]). The evidence implicating PIP2 lipids, for the first time, in the function of the NSS is thus much more recent [62, 63].
Here we review the recent findings regarding NSS/PIP2 lipid interactions and discuss their significance to the physiology of the NSS transporters. The evaluation of the mechanisms at the molecular-level that has emerged from combined experimental and computational studies of the phenotypes, are shown to produce novel mechanistic insights regarding the role of the long N- and C-terminal regions of the mammalian NSS members in functional mechanisms regulated by PIP2 lipids. As the mechanisms of regulation involve direct association of these terminal domains with PIP2 lipids, we provide a structural context, from computational modelling, for the dynamics of the interactions between PIP2 lipids and the N-terminus of the NSS proteins and the manner in which functional properties of specific physiological actions of the NSS are affected.
2. Terminal loops as functional units of neurotransmitter transporters
While the crystallographic data shows that the TM bundle of LeuT and dDAT share remarkable similarity, as would be expected for closely related members of the LeuT-fold protein family [1], one of the major structural differences between the bacterial transporters and the neurotransmitter transporters (NT-s) is the presence in NT proteins of long intracellular N-terminal (N-term) and C-terminal (C-term) segments. This difference is expected to be of significant mechanistic importance as intracellular loop regions of membrane proteins are often identified as functional domains. For example, as substrates for kinases in EGFR, and connections to the signalosome of GPCRs (e.g., see [68, 69]). In the structures of the NT-s, the N-term and C-term segments contain numerous putative phosphorylation sites (Figure 1), and various protein kinases have been implicated in the regulation of the function of the transporter proteins on this basis [70-73]. For DAT, specifically, the phosphorylation of the Ser residues distal (farthest from the TM bundle) in the N-terminal segment (Fig. 1) has been shown to be carried out by Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) and to require binding of CaMKII to the C-term of the DAT [74]. Notably, this phosphorylation is involved in the interesting phenotype of amphetamine-dependent efflux whereby the substrate, e.g., DA, is transported via the transporter (DAT) in the reverse direction, out of the cell [71, 75-78]. The process of efflux initiated under physiological conditions by the action of psychostimulants like AMPH, was shown to require the presence of various juxtamembrane proteins such as the lipid raft protein Flotillin-1 [79, 80], as well as association of the DAT N-term with the SNARE protein Syntaxin-1A (STX) [81]. Functional interactions of STX with N-terminal regions of other NSS proteins, such as SERT, NET, and GAT have also been reported [82-86].
Figure 1.
Sequences of the N-terminal regions of the human dopamine transporter (hDAT), serotonin transporter (hSERT), norepinephrine transporter (hNET), and vesicular monoamine transporter 2 (hVMAT2); positively charged Arg/Lys residues are indicated in red, Ser/Thr residues that are potentially targeted for phosphorylation, are in green.
Importantly, the AMPH-induced efflux is measurable also for these other NT proteins, and in all cases reported thus far the efflux process has been linked to the phosphorylation of the N-term of the NT-s. For example, in SERT [87], AMPH-induced serotonin (5-HT) efflux has been suggested to be related to phosphorylation of the highly conserved T81 residue in the N-term (residue numbering according to human SERT, hSERT, see Fig. 1) by protein kinase C (PKC). Interestingly, phosphorylation of the residue analogous to hSERT T81 in the N-term of DAT (i.e., S53 in human DAT, hDAT, see Fig. 1), has also been implicated in AMPH-induced DA efflux [88]. Furthermore, efflux of 5-HT through the vesicular monoamine transporter 2 (VMAT2) [89], triggered by methamphetamine (METH), has been linked to phosphorylation of Ser residues in the 16-SRRSRK-20 stretch of the VMAT2 N-term (Fig. 1).
The structure-function relationship between N-term phosphorylation and efflux has been investigated in various NT-s through deletions of specific motifs or by substitutions of specific Ser or Thr residues (i)-with Asp (S/D, T/D), to mimic phosphorylation, or (ii)-with Ala (S/A, T/A), to interfere with phosphorylation (Fig. 1). Thus, expression in cells of hDAT constructs with either simultaneous S/A mutations in all five Ser residues in the distal N-term (Fig. 1), or with the first 22 residues truncated, led to resistance to AMPH-induced efflux [75]. AMPH-induced efflux was restored by S/D mutations in the same five positions. Remarkably, none of the above structural modifications affected DA uptake by hDAT. These results demonstrated, that substrate transport in the inward and reverse directions could be independently regulated in this NT, raising the possibility of interfering selectively with AMPH-induced DA efflux without altering physiological DA uptake. In this respect, it is important to note the recent discovery of several specific de novo mutations in hDAT that are linked to various neurological disorders and affect the efflux phenotype in particular [90-97].
Interestingly, when constructs of rat DAT with mutations of T53 (S53 in hDAT) to either alanine or aspartate (T53A or T53D) were reconstituted in cells, the detected levels of AMPH-induced efflux were negligible [88], similar to the hSERT constructs in which T81 (analogous to T53 in rat DAT) was substituted with either Ala or Asp and did not produce reverse transport when expressed in cells [87]. Importantly, however, these mutants showed as well decreased levels of normal uptake for both DAT and SERT constructs. Similarly, in VMAT2 [89], simultaneous mutations of N-terminal Ser15 and Ser18 residues to either Ala or Asp (Fig. 1) not only substantially reduced substrate efflux but also lowered normal substrate uptake. Puzzlingly, this is in contrast to the phenotypes identified for S/D and S/A mutants in the distal N-term part of the hDAT (i.e., closest to the N-terminal end of the protein) [75]. Indeed, as described above, simultaneous S/A substitutions in Serines at positions 2,4,7,12, and 13 (see Fig. 1) could not sustain AMPH-induced efflux, whereas S/D mutations in the same residues resulted in efflux-competent transporters. Furthermore, neither S/D or S/A substitutions in the distal N-term affected normal substrate uptake.
Together, these experimental studies have established that N-terminal regions of NT proteins actively participate in the functional mechanisms via phosphorylation. But they also have revealed more multifaceted role of the N-term segment in function of NT-s. This has been perhaps demonstrated best in hDAT, as detailed above, by the experimental results suggesting that while the distal 22 N-terminal residues selectively control the efflux process, the proximal end of the N-term that is adjacent to the hDAT TM bundle, appears to regulate both inward and reverse transport of the substrate.
3. Functional interactions of SERT and DAT with PIP2 components of the membrane
The recent demonstrations that the efflux process in DAT [62] and SERT [63] depend on the availability of plasma membrane PIP2 lipid, point to a novel and important cofactor in the surrounding membrane, which can selectively regulate efflux mechanisms in NSS proteins. The first illustration that PIP2 component plays important role in functional mechanisms in NSS proteins comes from the studies on SERT. Thus, the actions of AMPH at SERT were shown to require PIP2 lipids in the plasma membrane [63] when hSERT-mediated efflux and currents were compared in control cells and in cells treated with either PIP2-sequestering peptide (e.g., the PIP2 binding domain of the Kv7.2 channel) or phospholipase C (PLC) activator agent to deplete PIP2 levels in the membrane. Sequestering or depleting PIP2 reduced SERT-mediated 5-HT efflux induced by an AMPH derivative (pCA ; para-chloramphetamine) while leaving physiological 5-HT uptake unchanged.
Evaluation of electrostatic properties of IC loop regions in hSERT suggested several sites on the transporter that could potentially be involved, directly or indirectly, in interactions with PIP2 lipids. Specifically, three basic residues, R144 (in ICL1), K352 (in ICL3), and K460 (in ICL4) were identified and mutated to Ala separately or in combination in order to test their functional importance. While the hSERT constructs bearing the single point mutations did not alter the functionality of the transporter (i.e. both uptake and reverse transport of the substrate were unchanged), the R144A/K352A, R144A/K460A, and K352A/K460A double mutants of hSERT had lower pCA-induced efflux levels than those measured in cells expressing the wild type hSERT. Efflux in these double mutant constructs was not completely abolished, however, and PIP2 lipid depletion performed in the background of these mutations affected pCA-induced efflux only for R144A/K352A and R144A/K460A hSERT but not for the K352A/K460A variant which neither bound PIP2 nor mediated pCA-evoked currents. In contrast, R144A/K352A and R144A/K460A mutants showed pCA-induced currents that were comparable to those through the wild type hSERT.
While these studies have established the requirement for PIP2 lipids in psychostimulant actions at SERT, it remained unclear (1)-whether PIP2 lipids regulate efflux in SERT, or indeed in NSS proteins in general, through direct interactions with the transporter; and, if they do, (2)-what mode of PIP2 binding underlies the functional mechanisms. This became clearer from more recent studies on DAT [62] which unambiguously demonstrated direct interactions of PIP2 with this NSS. Specifically, co-localization of hDAT with PIP2 lipid sensor (PH domain from PLC) was detected using live confocal imaging on cells transfected with hDAT. Furthermore, the association of PIP2 lipids with DAT was revealed by immunoprecipitation assays performed in striatal tissues of mice, demonstrating that PIP2 associates with DAT both in cell cultures as well as ex vivo. In addition, direct interaction between PIP2 and the N-term of DAT in vitro was shown in lipid binding assays by using GST-fused hDAT N-term construct containing first 64 N-terminal amino acids. That the association between hDAT N-term and PIP2 lipids is driven by electrostatic forces is suggested from additional lipid binding experiments performed on hDAT N-term constructs with either K3A/K5A or K3N/K5N double mutations (Fig. 1). These charge-neutralizing substitutions significantly inhibited the ability of hDAT N-term to bind PIP2 lipids [62].
The functional consequences of the association between the hDAT N-term and PIP2 lipids were probed in assays of substrate uptake and efflux. The PIP2 content of the membranes was manipulated either by depleting cellular stores of PIP2 lipids (through activation of the PLC enzyme that stimulates PIP2 hydrolysis), or with PIP2-sequestering peptides (see above). As in SERT, both PIP2 depletion and sequestration inhibited AMPH-induced DA efflux without affecting DAT-mediated DA uptake. In addition, cells expressing mutant hDAT constructs with K3A/K5A or K3N/K5N substitutions were found to have impaired AMPH-induced DA efflux but normal DA uptake. Remarkably, expression of hDAT K3A/K5A in Drosophila dopaminergic neurons did not affect circadian locomotor activity yet diminished AMPH-induced locomotion and neuronal DA efflux [62]. Collectively, these studies support the role of direct electrostatic interactions between specific regions of the DAT N-term and PIP2 lipids, and demonstrate that disruption of DAT/PIP2 interactions selectively impairs reverse transport but not substrate reuptake by DAT.
However, the key questions still remain whether PIP2 lipids directly associate with the N-terminal domains of other NSS proteins, and if such association relates to specific functional mechanisms. The studies in SERT [63] have raised a possibility that the mutations identified in the IC loops of this transporter have only indirect roles in the observed effects of PIP2. For example, since these loop regions are adjacent to the N- and C- terminal segments, and likely interact with them in some manner, it is reasonable to expect that loop residues can be involved in mediating interactions between PIP2 and the terminal regions in SERT, similar to observations in hDAT (see above). This is particularly plausible in light of the suggested role of phosphorylation of the N-term in SERT-mediated efflux process [87]. In this respect, it is interesting to note that recent computational modeling of N- and C-terminal regions of SERT in the context of the full-length transporter model [98] has revealed the flexible nature of these terminal domains and suggested that the SERT N-term, in particular, can have large conformational freedom when the transporter is in the inward-open state, which is the configuration that has been suggested to be important for efflux [77].
More generally, the recent findings [62, 63] discussed above, regarding the role of N-term/PIP2 interactions, highlight the importance of discerning the different molecular mechanisms that determine different modes of NT function (i.e., efflux versus uptake), including the role of phosphorylation at different sites in various segments of these terminal regions.
4. Electrostatic interactions as key factors controlling structural and functional mechanisms of the N-terminus in DAT
The experimental evidence presented above points to the role of the many charged residues adjacent to Ser/Thr amino acids in the N-term segment of NT proteins (Fig. 1) as key determinants of the mode of interaction with PIP2 lipids and the modulation of the electrostatic properties by phosphorylation, and thus suggests that they are key elements in the observed functional phenotypes. This hypothesis was recently tested at a detailed molecular level for the DAT N-term, as described below.
While the recently determined crystal structure of dDAT has provided detailed information about the TM bundle of the transporter, the X-ray model could not elucidate one of the key aspects of eukaryotic NSS proteins that distinguishes them from their bacterial counterparts, i.e., the long IC N- and C-terminal domains. Indeed, the dDAT X-ray model contains only a small part of the C-term, while the rest of it (its more distal part) as well as the entire N-terminal region have being excised from the experimental construct in order to enhance crystallization. A structural prediction of the DAT N-term region was therefore undertaken [99] using the Rosetta ab initio structure prediction algorithm [100]. The computational evaluation of three-dimensional (3D) folds of the N-terminal fragments from the hDAT (residues 1-59) and VMAT2 (residues 1-20), described in full detail in Ref. [99], was based on a computational protocol that included fold-prediction of the N-term peptides with Rosetta and clustering the predicting structures (1000 per each N-term construct) under various residue exclusion criteria. Clusters containing a majority of the structures were identified (top 2-3 clusters), and the selected models were the ones with the lowest scores (from Rosetta energy function) in each cluster.
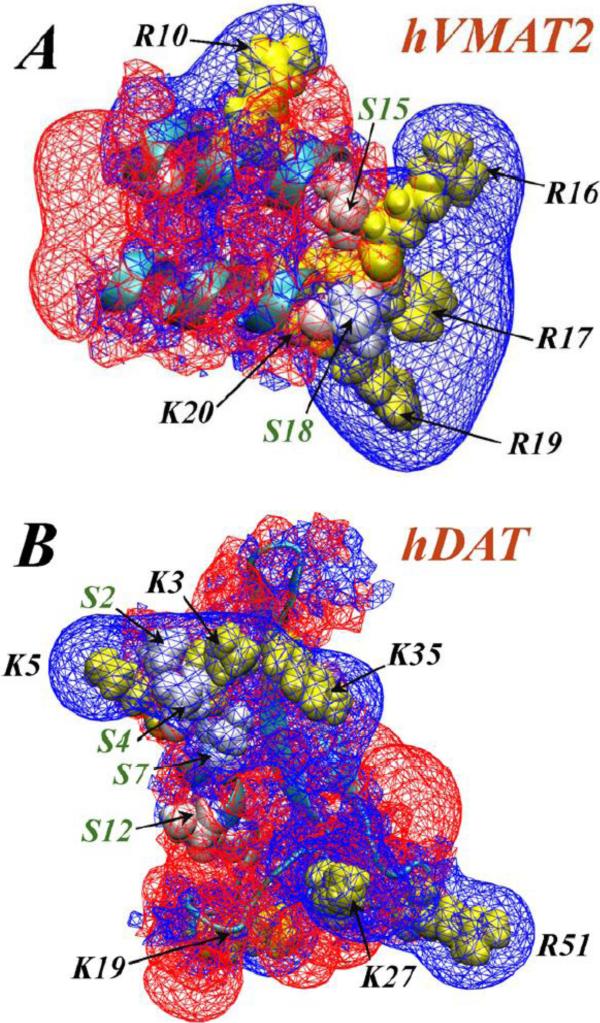
Figure 2 shows the predicted folds obtained in this manner for the hDAT and VMAT2 N-term segments, as well as electrostatic potential isosurfaces (EPI) calculated for these structures (see Fig. 2 captions for the details of methodology). The N-term segments of the two NT-s are characterized by well-defined regions of strong positive EPI (blue wireframe in Fig. 2) expressing the electrostatic effects of the multiple basic residues that are positioned in specific 3D arrangements in these constructs. The spatial properties of the EPI (note the positive EPI in the hDAT N-term emerging from belt-like positioning of Lys/Arg residues) and their effect on interaction with PIP2 have been discussed before [62]; the Lys/Arg residues in the 16-SRRSRK-20 stretch of the N-term in VMAT2 are seen to generate a similarly strongly reinforced positive EPI.
Figure 2.
Electrostatic potential isosurfaces (EPIs) (+1kt/e values shown as blue wireframes and −1kt/e as red wireframes) derived from the predicted structures for: (A) human VMAT2 (hVMAT2, residues 1-20); (B) hDAT N-terminus (residues 1-59). In both panels, the locations of basic residues and Serine residues are indicated by labels and black arrows, and highlighted in yellow and white space-fill representations, respectively. Note the extended EPI in the hDAT N-term, generated by a belt-like arrangement of K/R residues. The electrostatic potential was calculated from the Non-linear Poisson-Boltzmann equation using APBS software [105].
The structural models of the N-term were used in computational studies to evaluate the mechanistic consequences of the well-defined reactivity features. The ability of the hDAT N-term to sequester PIP2 lipids was explored with several levels of computational modeling. First, extensive unbiased atomistic MD simulations (totaling >2.2 μs) were used to compare the conformational dynamics of the N-term segment anchored to PIP2-enriched, and PIP2-depleted lipid membranes. A single palmitoyl tail was attached to the C-terminal end of the 65-residue long hDAT N-terminus to anchor the N-term at the membrane surface (see Ref. [99] for more details).
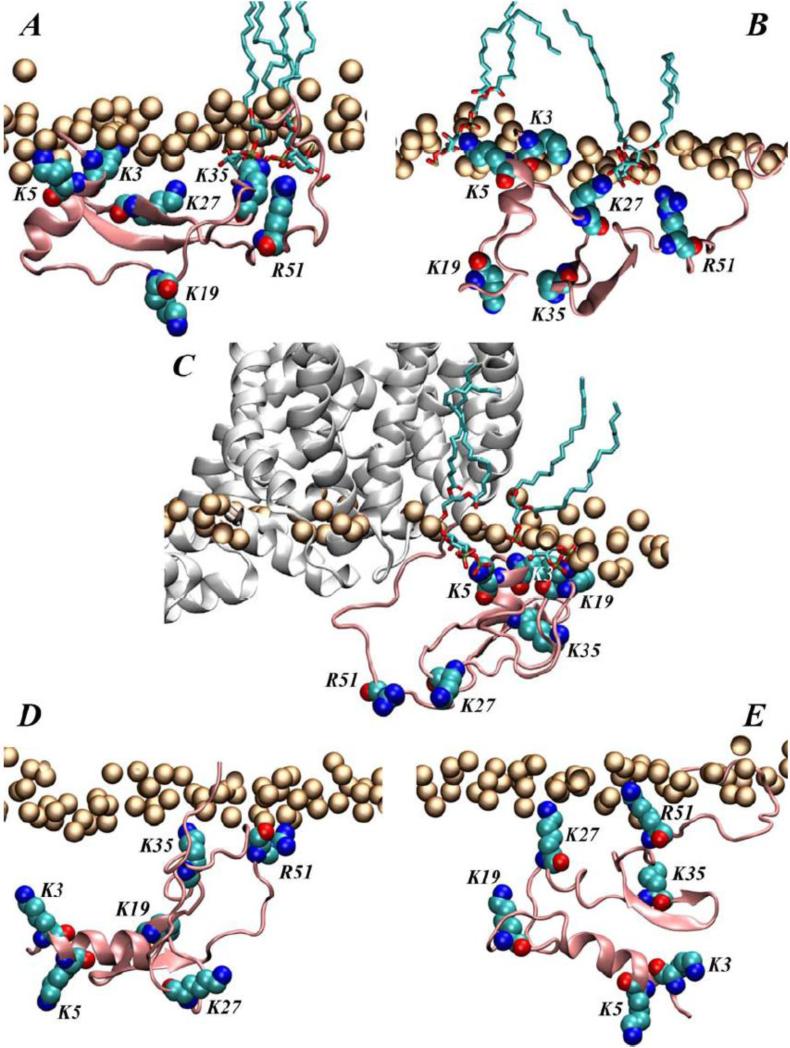
Analysis of the accumulated trajectories revealed that the N-term strongly associates with lipid membranes through interactions with PIP2 lipids (compare panels A-B with D-E on Figure 3) and identified specific regions of the N-term in closest proximity to PIP2. These regions include the distal N-term part harboring residues K3 and K5, and the far end of the N-term, especially residues K27 and R51 (Fig. 3A-B). Moreover, the N-term/PIP2 mode of association involving the residue pair K3/K5 was fully recapitulated in large-scale (>15μs) MD simulations carried out on the full length transporter model in an explicit all atom membrane representation.
Figure 3.
Illustration of modes of interaction of the hDAT N-term with PIP2 lipids in the membrane in selected snapshots from long atomistic MD simulation (A-B), and from the simulations of the chimera construct composed of the hDAT N-term and the dDAT TM bundle (C) (see Ref. [99] for more details). (D-E) Snapshots from various time-points in the atomistic MD simulation with a PIP2-depleted membrane. The hDAT N-terminus peptide (in pink) is next to the lipid membrane (orange spheres trace the positions of the lipid head group phosphate atoms). In all panels, lipid head group phosphate atoms are shown as orange spheres, the hDAT N-term (residues 1-65) is depicted in pink cartoon, and residues K3, K5, K27, K19, K35, and R51 are shown in space fill and are labeled. Panels (A-C) also illustrate PIP2 lipids within 3Å of the hDAT N-term (rendered in licorice representation). In panel C the dDAT TM bundle is shown in white cartoon.
To evaluate the N-term/PIP2 interactions in an integrated model of the transporter, the TM bundle of the recently determined dDAT structure was used to construct a chimera in which the terminal regions were from the hDAT sequence, and a full-length hDAT model (Fig. 3C) in which the TM bundle was obtained by homology modeling (see Ref. [99] for more details). The simulations of the full-length hDAT model identified a second site of interactions of the N-term with IC regions of the transporter, which included the ICL4 segment. Extensive simulations of this chimeric construct (Khelashvili et al, in preparation) showed that these modes of interactions involve the R51 residue (in the N-term) associating with the ICL4 region that contains the residue equivalent to K460 in SERT, which has been implicated in efflux mechanisms.
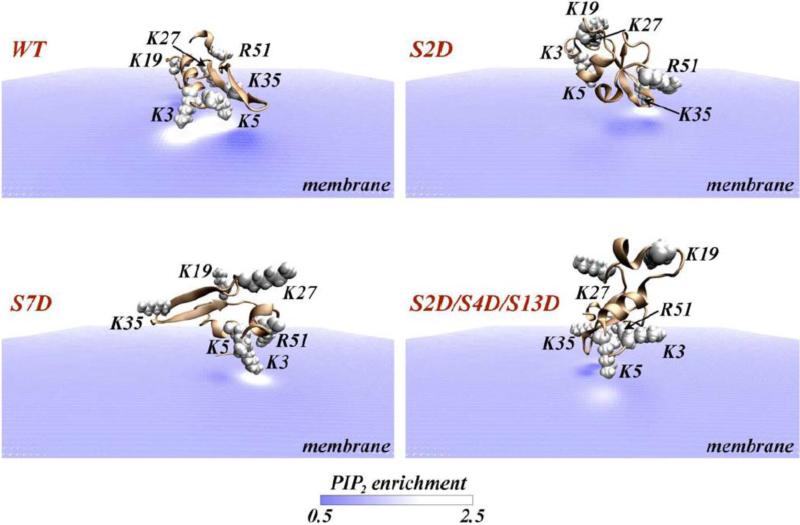
Interestingly, the computational approaches were able to quantify the extent of PIP2 sequestration by the N-term of DAT. This was achieved with an established protocol [62, 99, 101-104], the quantitative continuum-level self-consistent mean-field modeling (SCMFM). Juxtamembrane poses of the N-terminus served as starting configurations for following the dynamics of PIP2 lipids near the N-terminus under the influence of electrostatic forces, taking into account self-consistently the entropic contributions of mixing of lipids in the membrane and moving ions in the surrounding solution (see Refs. [99, 101, 102] for details about the SCMFM approach). The results illustrated in Figure 4 (top panel, WT) show that the configuration of the N-term generating the largest EPI on the bilayer surface in which the K3/K5 pair is facing the membrane produces a strong, ~2.5-fold increase in local PIP2 levels (white shades in Fig. 4 color map) compared to ambient (5%) PIP2 concentration (blue colors in Fig. 4). This level of segregation of the PIP2 lipids out of the random distribution in the membrane, corresponds to the binding of two PIP2 lipid molecules near the K3/K5 pair and is consistent with the atomistic picture obtained from the MD simulations (Fig. 3A-B). The calculations of PIP2 lipid aggregation was carried out in [62] for the hDAT N-term mutants K3A/K5A and K3N/K5N, because these mutations were found to yield an efflux-incompetent transporter when incorporated into the full-length hDAT construct [62]. Consonant with these findings, the mutant N-terms were also found not to bind PIP2-containing liposomes and to exhibit diminished PIP2 segregation. The binding to PIP2-containing lipid membranes was calculated to be unfavorable by ~3kBT [62] (where kB is the Boltzmann constant, and T is the temperature).
Figure 4.
View from the intracellular side, of hDAT N-terminus (cartoon) adsorbing on the lipid membrane. Panels show wild type (WT) and various S/D mutants, as indicated. In each panel, the level of local PIP2 enrichment near the N-terminus is illustrated in color code representing the ratio of local lipid fraction values to the value in the distal bulk regions. Regions of PIP2 aggregation are shaded white (enrichment values > 1), whereas membrane areas depleted of PIP2 are shown in dark blue colors (enrichment values < 1). The positively charged residues are highlighted in space fill and labeled on each panel. Steady state distribution of PIP2 around a given peptide construct was calculated using the SCMFM approach [101, 102], by placing the N-term 2Å away from the lipid surface with average surface charge density of −0.0031e, which corresponds to a lipid mixture with ~5% PIP2. The Non-linear Poisson-Boltzmann equation was then solved numerically using APBS software [105] to obtain reduced electrostatic potential in space, as described previously [101], in a 0.1 M ionic solution of monovalent counterions (corresponding to λ=9.65Å Debye length), and using a dielectric constant of 2 for membrane interior and protein, and 80 for the solution.
4.1. Phospho-mimic mutations impair N-term/PIP2 interactions by altering the electrostatic features of the hDAT N-term
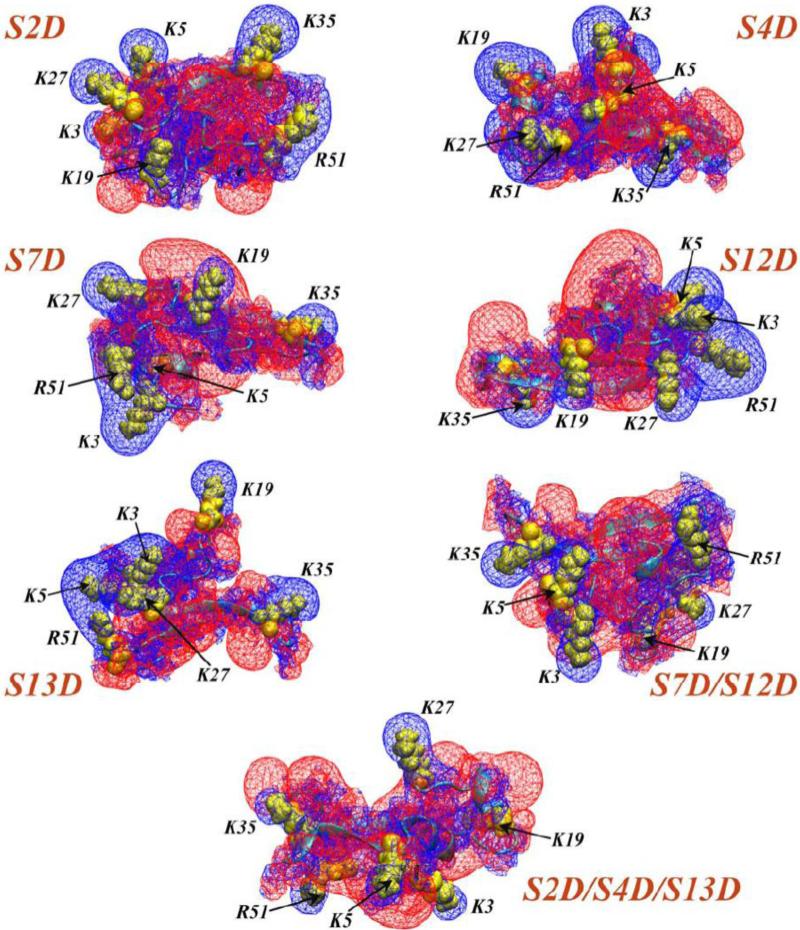
As illustrated in Fig. 2, regions of positive EPI in the N-term models of hDAT and VMAT2 contain Ser residues that have been shown to be phosphorylated in the course of transporter activities. Computational probing of the effect of phosphorylation of these Serines was carried with constructs of the hDAT N-term obtained with the same Rosetta ab initio protocol described above for the prediction of WT structures. The mutant constructs were prepared with various single and multiple phospho-mimic S/D mutations. Figure 5 shows the specific constructs, their predicted conformations and the EPI-s calculated for each of them. Compared to the extended positive EPI in the wild type hDAT N-term (Fig. 2B) the positive EPI is clearly diminished and disrupted in all the S/D mutant peptides, as expected from the introduction of additional negative charges into the hDAT N-term.
Figure 5.
Electrostatic potential isosurfaces (EPIs) (+1kt/e shown as blue wireframes and −1kt/e as red wireframes) derived from the predicted structures for various S/D mutants of the hDAT N-term, as indicated. Locations of basic residues are highlighted in yellow space-fill representations, and are designated by corresponding labels and black arrows. The electrostatic potential was calculated with the Non-linear Poisson-Boltzmann equation using APBS software [105].
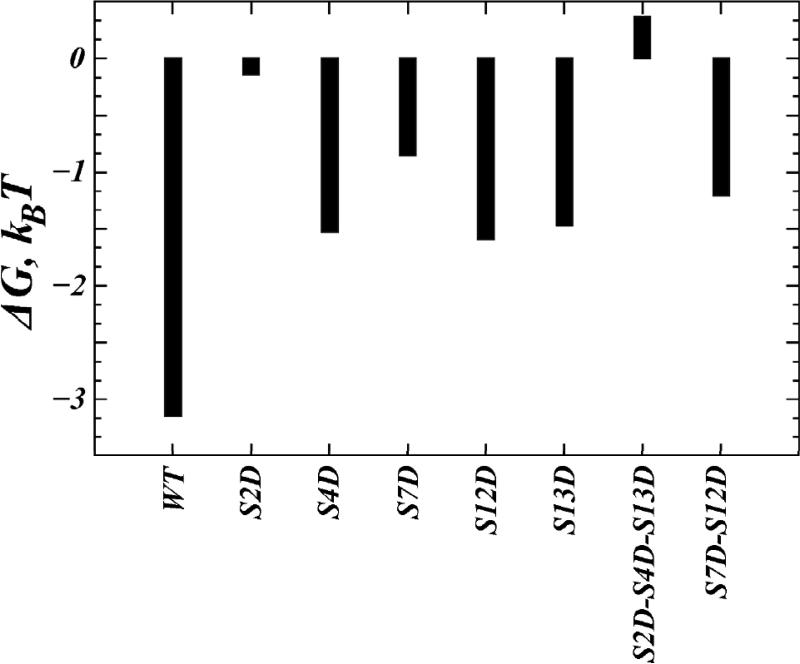
The consequences of the observed changes in electrostatic properties produced by the phospho-mimic mutations for the ability of the N-term constructs to interact with PIP2 lipids, were evaluated with calculations using the SCMFM approach described above. PIP2 lipid aggregation around the S/D N-term variants (see Fig. 4) is seen to be lower for the S/D mutants than for the wild type N-term (compare the extent of white shades). Correspondingly, the adsorption free energies (ΔG, Figure 6) of the single S/D mutants are less favorable than for the wild type N-term. Among the single S/D mutants considered, ΔG was affected strongest by the S2D and S7D variants, whereas overall the triple mutation (S2D/S4D/S13D) showed the largest change (> 3kBT) in the adsorption free energy compared to the wild type construct. The prediction based on these findings is that S/D mutations will reduce N-term/PIP2 association.
Figure 6.
Steady-state adsorption free energies of hDAT N-term wild type, and phospho-mimic constructs. Units are kBT.
4.2. A mechanistic model for the role of N-term/PIP2 association in the function of neurotransmitter transporter proteins
Inferences from the experimental and modeling studies suggest ways in which PIP2 lipids may be involved in the efflux phenotype. One is that they may place the N-terminal segment next to the lipid membrane in a position suitable for the required phosphorylation of the distal serines (e.g., S7, S12) to be accomplished [62]. Another is that they can regulate the involvement of various other segments of the N-terminal domain in intra-protein interactions before, and after, the phosphorylation step (this is suggested by the experimental observations in SERT [63] regarding interactions with the IC loop regions of the transporter).
The results from computational studies on the hDAT N-term discussed above indicate how PIP2 is involved in anchoring the N-terminal domain to lipid membranes, and suggest that disruption of electrostatic interactions between the N-term and the membrane either by PIP2 depletion or charge neutralizing mutations in the distal K3/K5 residues result in N-term/membrane interactions that are energetically less favorable compared to those under native conditions (i.e. wild type N-term and PIP2-enriched membrane). It is reasonable to suspect that when the N-term is no longer bound to the lipid membrane (see Fig. 3), it could engage with the TM bundle of the transporter to establish new interactions with different IC segments of the NT protein that are usually enriched in positively charged residues. Notably, the modes of interaction with the TM bundle are likely to depend on whether the K3/K5 residues are neutralized and phosphorylation is impaired, so that the modes of engagement with the TM domain may be quite different from the ones established by the N-term when it carries additional negative charge(s) due to phosphorylation. Therefore, the N-term phosphorylated in the distal region might support a conformation favoring efflux, but the conformations explored by the N-term under conditions of PIP2 depletion or charge-neutralizing mutations in K3/K5 positions, which will not support phosphorylation, can interfere with the efflux phenotype. These alternatives establish specific mechanistic hypotheses for the experimentally observed phenotypes that involve the electrostatic interactions of specific residues and segments of the N-term with PIP2 and/or the rest of the transporter. The discrete molecular level of detail in which these hypotheses are expressed can inform and guide the experimental elucidation of the efflux process and shed important new light on the determinant involvement of the membrane and its components.
5. Concluding Remarks
The discovery that the strongly anionic PIP2 lipids known to reside in the cytoplasmic leaflet of neuronal cell membranes, are essential for the regulation of neurotransmitter transporter protein functions, underscores the mechanistic role of the cell membrane in cell signaling. As illustrated previously [59], computational modeling is especially well suited for the exploration of the many ways in which protein-membrane interactions regulate structural and dynamic properties of membrane proteins. Indeed, much progress has emerged from computational studies of the full-length hSERT [98] and hDAT [99] which involved robust structure prediction algorithms combined with multi-scale modeling of protein/lipid interactions (ranging from extensive unbiased atomistic MD simulations to continuum mean-field approaches). The need for reliable 3D structural models of the transporter systems is slowly being addressed by remarkable breakthroughs in crystallographic determinations of such structures [22, 50] and carefully designed and evaluated molecular models obtained from homology modeling and refinements [2, 10, 41-46]. An essential complement in the explorations of the interactions of such models with the membrane environment is the identification, from computational modeling, of reasonably stable (during the course of long time-scale MD simulations [99]), and well-structured elements in the N-terminal domain. This has been achieved recently for DAT and other NT proteins (SERT [98], VMAT2). With this progress, and taking advantage of the wealth of information about functional and mechanistic properties gleaned from extensive studies of the structurally known LeuT prototype [10, 22, 28-32, 48], the studies characterized by integrated experimental/computational approaches will continue to guide the design of new ways of exploring and understanding transporter function and its multifaceted involvement in cell signaling.
Highlights.
-
1)
Neurotransmitter transporter (NT) proteins play important role in cellular signalling
-
2)
Terminal loops are functional units of NTs
-
3)
Functional mechanisms of NTs are regulated by lipid-protein interactions at their termini
-
4)
Computations provide important structural insights into functional mechanisms in NTs.
Acknowledgments
The authors gratefully acknowledge support from the National Institute of Health Grants P01DA012408, R01DA17293, R01DA015170 and U54GM087519. The computational work was enabled by the following gratefully acknowledged resources: XSEDE allocation at the Texas Advanced Computing Center at the University of Texas at Austin (Stampede supercomputer, projects TG-MCB090132, TG-MCB120008); allocation at the National Energy Research Scientific Computing Center (NERSC, repository m1710) supported by the Office of Science of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231; and computational resources of the David A. Cofrin Center for Biomedical Information in the HRH Prince Alwaleed Bin Talal Bin Abdulaziz Alsaud Institute for Computational Biomedicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 2.Beuming T, Shi L, Javitch JA, Weinstein H. A comprehensive structure-based alignment of prokaryotic and eukaryotic neurotransmitter/Na+ symporters (NSS) aids in the use of the LeuT structure to probe NSS structure and function. Mol Pharmacol. 2006;70:1630–1642. doi: 10.1124/mol.106.026120. [DOI] [PubMed] [Google Scholar]
- 3.Singh SK, Yamashita A, Gouaux E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature. 2007;448:952–956. doi: 10.1038/nature06038. [DOI] [PubMed] [Google Scholar]
- 4.Singh SK, Piscitelli CL, Yamashita A, Gouaux E. A competitive inhibitor traps LeuT in an open-to-out conformation. Science. 2008;322:1655–1661. doi: 10.1126/science.1166777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piscitelli CL, Krishnamurthy H, Gouaux E. Neurotransmitter/sodium symporter orthologue LeuT has a single high-affinity substrate site. Nature. 2010;468:1129–1132. doi: 10.1038/nature09581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, Elferich J, Gouaux E. Structures of LeuT in bicelles define conformation and substrate binding in a membrane-like context. Nat Struct Mol Biol. 2012;19:212–219. doi: 10.1038/nsmb.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piscitelli CL, Gouaux E. Insights into transport mechanism from LeuT engineered to transport tryptophan. EMBO J. 2012;31:228–235. doi: 10.1038/emboj.2011.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Gouaux E. Substrate binds in the S1 site of the F253A mutant of LeuT, a neurotransmitter sodium symporter homologue. EMBO Rep. 2012;13:861–866. doi: 10.1038/embor.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quick M, Winther AM, Shi L, Nissen P, Weinstein H, Javitch JA. Binding of an octylglucoside detergent molecule in the second substrate (S2) site of LeuT establishes an inhibitor-bound conformation. Proc Natl Acad Sci U S A. 2009;106:5563–5568. doi: 10.1073/pnas.0811322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantcheva AK, Quick M, Shi L, Winther AM, Stolzenberg S, Weinstein H, Javitch JA, Nissen P. Chloride binding site of neurotransmitter sodium symporters. Proc Natl Acad Sci U S A. 2013;110:8489–8494. doi: 10.1073/pnas.1221279110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnamurthy H, Gouaux E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature. 2012;481:469–474. doi: 10.1038/nature10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons KJ, Jackson SM, Brueckner F, Patching SG, Beckstein O, Ivanova E, Geng T, Weyand S, Drew D, Lanigan J, Sharples DJ, Sansom MS, Iwata S, Fishwick CW, Johnson AP, Cameron AD, Henderson PJ. Molecular mechanism of ligand recognition by membrane transport protein, Mhp1. EMBO J. 2014;33:1831–1844. doi: 10.15252/embj.201387557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimamura T, Weyand S, Beckstein O, Rutherford NG, Hadden JM, Sharples D, Sansom MS, Iwata S, Henderson PJ, Cameron AD. Molecular basis of alternating access membrane transport by the sodium-hydantoin transporter Mhp1. Science. 2010;328:470–473. doi: 10.1126/science.1186303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weyand S, Shimamura T, Yajima S, Suzuki S, Mirza O, Krusong K, Carpenter EP, Rutherford NG, Hadden JM, O'Reilly J, Ma P, Saidijam M, Patching SG, Hope RJ, Norbertczak HT, Roach PC, Iwata S, Henderson PJ, Cameron AD. Structure and molecular mechanism of a nucleobase-cationsymport-1 family transporter. Science. 2008;322:709–713. doi: 10.1126/science.1164440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malinauskaite L, Quick M, Reinhard L, Lyons JA, Yano H, Javitch JA, Nissen P. A mechanism for intracellular release of Na(+) by neurotransmitter/sodium symporters. Nat Struct Mol Biol. 2014;21:1006–1012. doi: 10.1038/nsmb.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao X, Lu F, Zhou L, Dang S, Sun L, Li X, Wang J, Shi Y. Structure and mechanism of an amino acid antiporter. Science. 2009;324:1565–1568. doi: 10.1126/science.1173654. [DOI] [PubMed] [Google Scholar]
- 17.Fang Y, Jayaram H, Shane T, Kolmakova-Partensky L, Wu F, Williams C, Xiong Y, Miller C. Structure of a prokaryotic virtual proton pump at 3.2 A resolution. Nature. 2009;460:1040–1043. doi: 10.1038/nature08201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma D, Lu P, Yan C, Fan C, Yin P, Wang J, Shi Y. Structure and mechanism of a glutamate-GABA antiporter. Nature. 2012;483:632–636. doi: 10.1038/nature10917. [DOI] [PubMed] [Google Scholar]
- 19.Shaffer PL, Goehring A, Shankaranarayanan A, Gouaux E. Structure and mechanism of a Na+-independent amino acid transporter. Science. 2009;325:1010–1014. doi: 10.1126/science.1176088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faham S, Watanabe A, Besserer GM, Cascio D, Specht A, Hirayama BA, Wright EM, Abramson J. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science. 2008;321:810–814. doi: 10.1126/science.1160406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ressl S, Terwisscha van Scheltinga AC, Vonrhein C, Ott V, Ziegler C. Molecular basis of transport and regulation in the Na(+)/betaine symporter BetP. Nature. 2009;458:47–52. doi: 10.1038/nature07819. [DOI] [PubMed] [Google Scholar]
- 22.Penmatsa A, Gouaux E. How LeuT shapes our understanding of the mechanisms of sodium-coupled neurotransmitter transporters. J Physiol. 2013;592:863–869. doi: 10.1113/jphysiol.2013.259051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forrest LR, Zhang YW, Jacobs MT, Gesmonde J, Xie L, Honig BH, Rudnick G. Mechanism for alternating access in neurotransmitter transporters. Proc Natl Acad Sci U S A. 2008;105:10338–10343. doi: 10.1073/pnas.0804659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211:969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- 25.Forrest LR, Rudnick G. The rocking bundle: a mechanism for ion-coupled solute flux by symmetrical transporters. Physiology (Bethesda) 2009;24:377–386. doi: 10.1152/physiol.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazmier K, Sharma S, Islam SM, Roux B, McHaourab HS. Conformational cycle and ion-coupling mechanism of the Na+/hydantoin transporter Mhp1. Proc Natl Acad Sci U S A. 2014;111:14752–14757. doi: 10.1073/pnas.1410431111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao C, Stolzenberg S, Gracia L, Weinstein H, Noskov S, Shi L. Ion-controlled conformational dynamics in the outward-open transition from an occluded state of LeuT. Biophys J. 2012;103:878–888. doi: 10.1016/j.bpj.2012.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kazmier K, Sharma S, Quick M, Islam SM, Roux B, Weinstein H, Javitch JA, McHaourab HS. Conformational dynamics of ligand-dependent alternating access in LeuT. Nat Struct Mol Biol. 2014;21:472–479. doi: 10.1038/nsmb.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y, Terry DS, Shi L, Quick M, Weinstein H, Blanchard SC, Javitch JA. Substrate-modulated gating dynamics in a Na+-coupled neurotransmitter transporter homologue. Nature. 2011;474:109–113. doi: 10.1038/nature09971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y, Terry D, Shi L, Weinstein H, Blanchard SC, Javitch JA. Single-molecule dynamics of gating in a neurotransmitter transporter homologue. Nature. 2010;465:188–193. doi: 10.1038/nature09057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Claxton DP, Quick M, Shi L, de Carvalho FD, Weinstein H, Javitch JA, McHaourab HS. Ion/substrate-dependent conformational dynamics of a bacterial homolog of neurotransmitter:sodium symporters. Nat Struct Mol Biol. 2010;17:822–829. doi: 10.1038/nsmb.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi L, Quick M, Zhao Y, Weinstein H, Javitch JA. The mechanism of a neurotransmitter:sodium symporter--inward release of Na+ and substrate is triggered by substrate in a second binding site. Mol Cell. 2008;30:667–677. doi: 10.1016/j.molcel.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zomot E, Gur M, Bahar I. Microseconds Simulations Reveal a New Sodium-Binding Site and the Mechanism of Sodium-Coupled Substrate Uptake by LeuT. J Biol Chem. 2014 doi: 10.1074/jbc.M114.617555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng MH, Bahar I. Complete mapping of substrate translocation highlights the role of LeuT N-terminal segment in regulating transport cycle. PLoS Comput Biol. 2014;10:e1003879. doi: 10.1371/journal.pcbi.1003879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng MH, Bahar I. Coupled global and local changes direct substrate translocation by neurotransmitter-sodium symporter ortholog LeuT. Biophys J. 2013;105:630–639. doi: 10.1016/j.bpj.2013.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Shaikh SA, Enkavi G, Wen PC, Huang Z, Tajkhorshid E. Transient formation of water-conducting states in membrane transporters. Proc Natl Acad Sci U S A. 2013;110:7696–7701. doi: 10.1073/pnas.1218986110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaikh SA, Tajkhorshid E. Modeling and dynamics of the inward-facing state of a Na+/Cl- dependent neurotransmitter transporter homologue. PLoS Comput Biol. 2010;6 doi: 10.1371/journal.pcbi.1000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Tajkhorshid E. Ion-releasing state of a secondary membrane transporter. Biophys J. 2009;97:L29–31. doi: 10.1016/j.bpj.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Celik L, Schiott B, Tajkhorshid E. Substrate binding and formation of an occluded state in the leucine transporter. Biophys J. 2008;94:1600–1612. doi: 10.1529/biophysj.107.117580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shan J, Javitch JA, Shi L, Weinstein H. The substrate-driven transition to an inward-facing conformation in the functional mechanism of the dopamine transporter. PLoS One. 2011;6:e16350. doi: 10.1371/journal.pone.0016350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guptaroy B, Zhang M, Bowton E, Binda F, Shi L, Weinstein H, Galli A, Javitch JA, Neubig RR, Gnegy ME. A juxtamembrane mutation in the N terminus of the dopamine transporter induces preference for an inward-facing conformation. Mol Pharmacol. 2009;75:514–524. doi: 10.1124/mol.108.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K, Newman AH, Javitch JA, Weinstein H, Gether U, Loland CJ. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci. 2008;11:780–789. doi: 10.1038/nn.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kniazeff J, Shi L, Loland CJ, Javitch JA, Weinstein H, Gether U. An intracellular interaction network regulates conformational transitions in the dopamine transporter. J Biol Chem. 2008;283:17691–17701. doi: 10.1074/jbc.M800475200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stockner T, Montgomery TR, Kudlacek O, Weissensteiner R, Ecker GF, Freissmuth M, Sitte HH. Mutational analysis of the high-affinity zinc binding site validates a refined human dopamine transporter homology model. PLoS Comput Biol. 2013;9:e1002909. doi: 10.1371/journal.pcbi.1002909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borre L, Andreassen TF, Shi L, Weinstein H, Gether U. The second sodium site in the dopamine transporter controls cation permeation and is regulated by chloride. J Biol Chem. 2014;289:25764–25773. doi: 10.1074/jbc.M114.574269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koldso H, Autzen HE, Grouleff J, Schiott B. Ligand induced conformational changes of the human serotonin transporter revealed by molecular dynamics simulations. PLoS One. 2013;8:e63635. doi: 10.1371/journal.pone.0063635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LeVine MV, Weinstein H. NbIT--a new information theory-based analysis of allosteric mechanisms reveals residues that underlie function in the leucine transporter LeuT. PLoS Comput Biol. 2014;10:e1003603. doi: 10.1371/journal.pcbi.1003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quick M, Shi L, Zehnpfennig B, Weinstein H, Javitch JA. Experimental conditions can obscure the second high-affinity site in LeuT. Nat Struct Mol Biol. 2012;19:207–211. doi: 10.1038/nsmb.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Lee AS, Bracher S, Jung H, Paz A, Kumar JP, Abramson J, Quick M, Shi L. Identification of a Second Substrate-binding Site in Solute-Sodium Symporters. J Biol Chem. 2014;290:127–141. doi: 10.1074/jbc.M114.584383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Penmatsa A, Wang KH, Gouaux E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature. 2013;503:85–90. doi: 10.1038/nature12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanner BI, Zomot E. Sodium-coupled neurotransmitter transporters. Chem Rev. 2008;108:1654–1668. doi: 10.1021/cr078246a. [DOI] [PubMed] [Google Scholar]
- 52.Focke PJ, Wang X, Larsson HP. Neurotransmitter transporters: structure meets function. Structure. 2013;21:694–705. doi: 10.1016/j.str.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Broer S, Gether U. The solute carrier 6 family of transporters. Br J Pharmacol. 2012;167:256–278. doi: 10.1111/j.1476-5381.2012.01975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rudnick G, Kramer R, Blakely RD, Murphy DL, Verrey F. The SLC6 transporters: perspectives on structure, functions, regulation, and models for transporter dysfunction. Pflugers Arch. 2014;466:25–42. doi: 10.1007/s00424-013-1410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, Stromgaard K, Gether U. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- 56.Pramod AB, Foster J, Carvelli L, Henry LK. SLC6 transporters: structure, function, regulation, disease association and therapeutics. Mol Aspects Med. 2013;34:197–219. doi: 10.1016/j.mam.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gether U, Andersen PH, Larsson OM, Schousboe A. Neurotransmitter transporters: molecular function of important drug targets. Trends Pharmacol Sci. 2006;27:375–383. doi: 10.1016/j.tips.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 58.Sora I, Li B, Fumushima S, Fukui A, Arime Y, Kasahara Y, Tomita H, Ikeda K. Monoamine transporter as a target molecule for psychostimulants. Int Rev Neurobiol. 2009;85:29–33. doi: 10.1016/S0074-7742(09)85003-4. [DOI] [PubMed] [Google Scholar]
- 59.Mondal S, Khelashvili G, Weinstein H. Not Just an Oil Slick: How the Energetics of Protein-Membrane Interactions Impacts the Function and Organization of Transmembrane Proteins. Biophys J. 2014;106:2305–2316. doi: 10.1016/j.bpj.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mondal S, Khelashvili G, Shi L, Weinstein H. The cost of living in the membrane: A case study of hydrophobic mismatch for the multi-segment protein LeuT. Chem Phys Lipids. 2013;169:27–38. doi: 10.1016/j.chemphyslip.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lezon TR, Bahar I. Constraints imposed by the membrane selectively guide the alternating access dynamics of the glutamate transporter GltPh. Biophys J. 2012;102:1331–1340. doi: 10.1016/j.bpj.2012.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamilton PJ, Belovich AN, Khelashvili G, Saunders C, Erreger K, Javitch JA, Sitte HH, Weinstein H, Matthies HJ, Galli A. PIP2 regulates psychostimulant behaviors through its interaction with a membrane protein. Nat Chem Biol. 2014;10:582–589. doi: 10.1038/nchembio.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buchmayer F, Schicker K, Steinkellner T, Geier P, Stubiger G, Hamilton PJ, Jurik A, Stockner T, Yang JW, Montgomery T, Holy M, Hofmaier T, Kudlacek O, Matthies HJ, Ecker GF, Bochkov V, Galli A, Boehm S, Sitte HH. Amphetamine actions at the serotonin transporter rely on the availability of phosphatidylinositol-4,5-bisphosphate. Proc Natl Acad Sci U S A. 2013;110:11642–11647. doi: 10.1073/pnas.1220552110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balla T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev. 2013;93:1019–1137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McLaughlin S, Wang JY, Gambhir A, Murray D. PIP2 AND proteins: Interactions, organization, and information flow. Annual Review of Biophysics and Biomolecular Structure. 2002;31:151–175. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- 66.Lomasney JW, Cheng HF, Wang LP, Kuan Y, Liu S, Fesik SW, King K. Phosphatidylinositol 4,5-bisphosphate binding to the pleckstrin homology domain of phospholipase C-delta1 enhances enzyme activity. J Biol Chem. 1996;271:25316–25326. doi: 10.1074/jbc.271.41.25316. [DOI] [PubMed] [Google Scholar]
- 67.Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys. 2008;37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Endres NF, Engel K, Das R, Kovacs E, Kuriyan J. Regulation of the catalytic activity of the EGF receptor. Curr Opin Struct Biol. 2011;21:777–784. doi: 10.1016/j.sbi.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sensoy O, Weinstein H. A mechanistic role of Helix 8 in GPCRs: Computational modeling of the dopamine D2 receptor interaction with the GIPC1-PDZ-domain. Biochim Biophys Acta. 2015;1848:976–983. doi: 10.1016/j.bbamem.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gnegy ME. The effect of phosphorylation on amphetamine-mediated outward transport. Eur J Pharmacol. 2003;479:83–91. doi: 10.1016/j.ejphar.2003.08.059. [DOI] [PubMed] [Google Scholar]
- 71.Foster JD, Cervinski MA, Gorentla BK, Vaughan RA. Regulation of the dopamine transporter by phosphorylation. Handb Exp Pharmacol. 2006:197–214. doi: 10.1007/3-540-29784-7_10. [DOI] [PubMed] [Google Scholar]
- 72.Ramamoorthy S, Shippenberg TS, Jayanthi LD. Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacol Ther. 2011;129:220–238. doi: 10.1016/j.pharmthera.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vaughan RA, Foster JD. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol Sci. 2013;34:489–496. doi: 10.1016/j.tips.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fog JU, Khoshbouei H, Holy M, Owens WA, Vaegter CB, Sen N, Nikandrova Y, Bowton E, McMahon DG, Colbran RJ, Daws LC, Sitte HH, Javitch JA, Galli A, Gether U. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51:417–429. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 75.Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, Galli A, Javitch JA. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, Galli A. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc Natl Acad Sci U S A. 2005;102:3495–3500. doi: 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kahlig KM, Javitch JA, Galli A. Amphetamine regulation of dopamine transport. Combined measurements of transporter currents and transporter imaging support the endocytosis of an active carrier. J Biol Chem. 2004;279:8966–8975. doi: 10.1074/jbc.M303976200. [DOI] [PubMed] [Google Scholar]
- 78.Thwar PK, Guptaroy B, Zhang M, Gnegy ME, Burns MA, Linderman JJ. Simple transporter trafficking model for amphetamine-induced dopamine efflux. Synapse. 2007;61:500–514. doi: 10.1002/syn.20390. [DOI] [PubMed] [Google Scholar]
- 79.Cremona ML, Matthies HJ, Pau K, Bowton E, Speed N, Lute BJ, Anderson M, Sen N, Robertson SD, Vaughan RA, Rothman JE, Galli A, Javitch JA, Yamamoto A. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat Neurosci. 2011;14:469–477. doi: 10.1038/nn.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pizzo AB, Karam CS, Zhang Y, Yano H, Freyberg RJ, Karam DS, Freyberg Z, Yamamoto A, McCabe BD, Javitch JA. The membrane raft protein Flotillin-1 is essential in dopamine neurons for amphetamine-induced behavior in Drosophila. Mol Psychiatry. 2013;18:824–833. doi: 10.1038/mp.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Binda F, Dipace C, Bowton E, Robertson SD, Lute BJ, Fog JU, Zhang M, Sen N, Colbran RJ, Gnegy ME, Gether U, Javitch JA, Erreger K, Galli A. Syntaxin 1A interaction with the dopamine transporter promotes amphetamine-induced dopamine efflux. Mol Pharmacol. 2008;74:1101–1108. doi: 10.1124/mol.108.048447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Quick MW. Regulating the conducting states of a mammalian serotonin transporter. Neuron. 2003;40:537–549. doi: 10.1016/s0896-6273(03)00605-6. [DOI] [PubMed] [Google Scholar]
- 83.Ciccone MA, Timmons M, Phillips A, Quick MW. Calcium/calmodulin-dependent kinase II regulates the interaction between the serotonin transporter and syntaxin 1A. Neuropharmacology. 2008;55:763–770. doi: 10.1016/j.neuropharm.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang D, Deken SL, Whitworth TL, Quick MW. Syntaxin 1A inhibits GABA flux, efflux, and exchange mediated by the rat brain GABA transporter GAT1. Mol Pharmacol. 2003;64:905–913. doi: 10.1124/mol.64.4.905. [DOI] [PubMed] [Google Scholar]
- 85.Sung U, Apparsundaram S, Galli A, Kahlig KM, Savchenko V, Schroeter S, Quick MW, Blakely RD. A regulated interaction of syntaxin 1A with the antidepressant-sensitive norepinephrine transporter establishes catecholamine clearance capacity. J Neurosci. 2003;23:1697–1709. doi: 10.1523/JNEUROSCI.23-05-01697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sung U, Blakely RD. Calcium-dependent interactions of the human norepinephrine transporter with syntaxin 1A. Mol Cell Neurosci. 2007;34:251–260. doi: 10.1016/j.mcn.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sucic S, Dallinger S, Zdrazil B, Weissensteiner R, Jorgensen TN, Holy M, Kudlacek O, Seidel S, Cha JH, Gether U, Newman AH, Ecker GF, Freissmuth M, Sitte HH. The N terminus of monoamine transporters is a lever required for the action of amphetamines. J Biol Chem. 2010;285:10924–10938. doi: 10.1074/jbc.M109.083154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Foster JD, Yang JW, Moritz AE, Challasivakanaka S, Smith MA, Holy M, Wilebski K, Sitte HH, Vaughan RA. Dopamine transporter phosphorylation site threonine 53 regulates substrate reuptake and amphetamine-stimulated efflux. J Biol Chem. 2012;287:29702–29712. doi: 10.1074/jbc.M112.367706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Torres B, Ruoho AE. N-terminus regulation of VMAT2 mediates methamphetamine-stimulated efflux. Neuroscience. 2014;259:194–202. doi: 10.1016/j.neuroscience.2013.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hansen FH, Skjorringe T, Yasmeen S, Arends NV, Sahai MA, Erreger K, Andreassen TF, Holy M, Hamilton PJ, Neergheen V, Karlsborg M, Newman AH, Pope S, Heales SJ, Friberg L, Law I, Pinborg LH, Sitte HH, Loland C, Shi L, Weinstein H, Galli A, Hjermind LE, Moller LB, Gether U. Missense dopamine transporter mutations associate with adult parkinsonism and ADHD. J Clin Invest. 2014;124:3107–3120. doi: 10.1172/JCI73778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hamilton PJ, Campbell NG, Sharma S, Erreger K, Herborg Hansen F, Saunders C, Belovich AN, Sahai MA, Cook EH, Gether U, McHaourab HS, Matthies HJ, Sutcliffe JS, Galli A. De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder. Mol Psychiatry. 2013;18:1315–1323. doi: 10.1038/mp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sakrikar D, Mazei-Robison MS, Mergy MA, Richtand NW, Han Q, Hamilton PJ, Bowton E, Galli A, Veenstra-Vanderweele J, Gill M, Blakely RD. Attention deficit/hyperactivity disorder-derived coding variation in the dopamine transporter disrupts microdomain targeting and trafficking regulation. J Neurosci. 2012;32:5385–5397. doi: 10.1523/JNEUROSCI.6033-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bowton E, Saunders C, Erreger K, Sakrikar D, Matthies HJ, Sen N, Jessen T, Colbran RJ, Caron MG, Javitch JA, Blakely RD, Galli A. Dysregulation of dopamine transporters via dopamine D2 autoreceptors triggers anomalous dopamine efflux associated with attention-deficit hyperactivity disorder. J Neurosci. 2010;30:6048–6057. doi: 10.1523/JNEUROSCI.5094-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mazei-Robison MS, Bowton E, Holy M, Schmudermaier M, Freissmuth M, Sitte HH, Galli A, Blakely RD. Anomalous dopamine release associated with a human dopamine transporter coding variant. J Neurosci. 2008;28:7040–7046. doi: 10.1523/JNEUROSCI.0473-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ng J, Zhen J, Meyer E, Erreger K, Li Y, Kakar N, Ahmad J, Thiele H, Kubisch C, Rider NL, Morton DH, Strauss KA, Puffenberger EG, D'Agnano D, Anikster Y, Carducci C, Hyland K, Rotstein M, Leuzzi V, Borck G, Reith ME, Kurian MA. Dopamine transporter deficiency syndrome: phenotypic spectrum from infancy to adulthood. Brain. 2014;137:1107–1119. doi: 10.1093/brain/awu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bowton E, Saunders C, Reddy IA, Campbell NG, Hamilton PJ, Henry LK, Coon H, Sakrikar D, Veenstra-VanderWeele JM, Blakely RD, Sutcliffe J, Matthies HJ, Erreger K, Galli A. SLC6A3 coding variant Ala559Val found in two autism probands alters dopamine transporter function and trafficking. Transl Psychiatry. 2014;4:e464. doi: 10.1038/tp.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mergy MA, Gowrishankar R, Gresch PJ, Gantz SC, Williams J, Davis GL, Wheeler CA, Stanwood GD, Hahn MK, Blakely RD. The rare DAT coding variant Val559 perturbs DA neuron function, changes behavior, and alters in vivo responses to psychostimulants. Proc Natl Acad Sci U S A. 2014;111:E4779–4788. doi: 10.1073/pnas.1417294111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fenollar-Ferrer C, Stockner T, Schwarz TC, Pal A, Gotovina J, Hofmaier T, Jayaraman K, Adhikary S, Kudlacek O, Mehdipour AR, Tavoulari S, Rudnick G, Singh SK, Konrat R, Sitte HH, Forrest LR. Structure and regulatory interactions of the cytoplasmic terminal domains of serotonin transporter. Biochemistry. 2014;53:5444–5460. doi: 10.1021/bi500637f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khelashvili G, Doktorova M, Sahai MA, Johner N, Shi L, Weinstein H. Computational modeling of the N-terminus of the human dopamine transporter and its interaction with PIP2-containing membranes. Proteins: Structure, Function, and Bioinformatics. 2015 doi: 10.1002/prot.24792. doi: 10.1002/prot.24792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Das R, Baker D. Macromolecular modeling with rosetta. Annu Rev Biochem. 2008;77:363–382. doi: 10.1146/annurev.biochem.77.062906.171838. [DOI] [PubMed] [Google Scholar]
- 101.Khelashvili G, Weinstein H, Harries D. Protein diffusion on charged membranes: a dynamic mean-field model describes time evolution and lipid reorganization. Biophys J. 2008;94:2580–2597. doi: 10.1529/biophysj.107.120667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khelashvili G, Harries D, Weinstein H. Modeling membrane deformations and lipid demixing upon protein-membrane interaction: the BAR dimer adsorption. Biophys J. 2009;97:1626–1635. doi: 10.1016/j.bpj.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khelashvili G, Harries D. Modelling signalling processes across cellular membranes using a mesoscopic approach. Annual Reports in Computational Chemistry. 2010;6:236–261. [Google Scholar]
- 104.Khelashvili G, Galli A, Weinstein H. Phosphatidylinositol 4,5-biphosphate (PIP(2)) lipids regulate the phosphorylation of syntaxin N-terminus by modulating both its position and local structure. Biochemistry. 2012;51:7685–7698. doi: 10.1021/bi300833z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]