Abstract
Depression and substance use, the most common comorbidities with HIV, are both associated with poor treatment outcomes and accelerated HIV disease progression. Though previous research has demonstrated short-term and follow-up success for cognitive behavioral therapy for adherence and depression (CBT-AD) on depression outcomes among patients with HIV in care (Safren et al., 2009) and among patients with HIV in active substance abuse treatment for injection drug use (IDU) (Safren et al., 2012), there is little information regarding possible moderating effects of active use versus abstinence on depression treatment gains. The present study aimed to examine recent substance use at treatment initiation as a moderator of the acute and maintenance effects of CBT-AD on depression. We used data from a two-arm, randomized controlled trial (N= 89) comparing CBT-AD to enhanced treatment as usual (ETAU) in individuals in treatment for IDU (Safren et al., 2012). To test whether depression at time of presentation affected outcomes, repeated-measures ANOVAs were conducted for two time-frames: 1) acute phase (baseline to post-treatment) (acute) and 2) maintenance phase (baseline to 12-month follow-up). To further examine maintenance of gains, we additionally looked at post-treatment to 12-month follow up. Depression scores derived from the Clinical Global Impression (CGI) for severity and the Montgomery-Asberg Depression Rating Scale (MADRS) served as the primary outcome variables. Acute (baseline-post treatment) moderation effects were found for those patients endorsing active drug use at baseline in the CBT-AD condition, who demonstrated the greatest reductions in MADRS scores at post-treatment (F[1,76]=6.78, p=0.01) and follow up (F[1,61]=5.46, p=0.023). Baseline substance use did not moderate differences from post-treatment to 12-month follow-up as depression treatment gains that occurred acutely from baseline to post-treatment were maintained across both patients engaged in substance use and abstainers. We conclude that CBT-AD for triply diagnosed patients (i.e., HIV, depression, substance dependence) is useful for treating depression for both patients with a history of substance use, as well as patients currently engaged in substance use.
Keywords: CBT-AD, HIV, injection drug use, depression
Cognitive behavioral therapy for adherence and depression (CBT-AD) (Safren et al., 2007a,b) has been found to successfully improve adherence and depression in individuals in HIV care (Safren et al., 2009) and reduce depression in patients with injection drug use (IDU) histories undergoing treatment for their substance use disorder (Safren et al., 2012). An important clinical consideration is whether pretreatment substance use would interfere with potential treatment gains in patients in treatment for substance dependence. The purpose of this study was to examine if the acute and maintenance effects of CBT-AD on depression varies as a function of (i.e., moderated by) substance use status. We hypothesized that participants actively engaged in substance use would benefit less from depression treatment, during both the acute and maintenance phases, than participants who abstained.
Method
Subjects and Setting
Individuals (N = 89) between the ages of 18 and 65 who were HIV-seropositive, prescribed antiretroviral therapy for HIV, endorsed a history of IDU, were currently enrolled in opioid treatment for at least one month, and met criteria for a diagnosis of current or sub-syndromal depressive mood disorder were included. Exclusion criteria included evidence of active untreated or unstable major mental illness, inability or unwillingness to provide informed consent, or CBT for depression in the past year.
Design and Procedures
For a more detailed description of the original study's design and procedures, see Safren et al. (2012). Participants were randomly assigned to either CBT-AD or Enhanced Treatment as Usual (ETAU). The present study used data from three major assessment visits: 1) baseline, 2) post-treatment (approximately 3-months after baseline), and 3) 12-months.
Measures
Eligibility included a diagnostic evaluation of Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) diagnoses using the Mini International Neuropsychiatric Interview (MINI)(Sheehan et al., 1998), which was completed by a study therapist. An independent assessor conducted the clinician-administered outcome assessments, which included: 1) the Montgomery-Asberg Depression Rating Scale (MADRS; Montgomery & Asberg, 1979), and 2) a rating of global distress and impairment for depression using the Clinical Global Impression (CGI) for severity (e.g., 1 = not ill to 7 = extremely ill)(National Institute of Mental Health, 1985). Substance use was assessed with the Addictions Severity Index Lite (ASI-Lite; McLellan et al., 1992, 1980), as well as toxicology screens using saliva assays, which utilized gas chromatography-mass spectrometry (GC/MS) analysis (Cone, Presley, Lehrer, Seiter, Smith, Kardos, Fritch, Salamone, & Niedbala, 2002). A composite dichotomous measure indicating substance use was derived from the ASI-Lite and toxicology screens.
All participants received an individual single-session intervention on HIV medication adherence (Life-Steps), which involved 11 informational, problem-solving, and cognitive behavioral steps (Safren, Otto, & Worth, 1999) to target common problem areas associated with poor adherence (e.g., getting to appointments, coping with medication effects). Those assigned to the experimental condition received eight individual sessions of CBT-AD delivered by trained masters level or higher therapists.
Statistical Analyses
To test for moderation of the acute effects of treatment on depression, a three-way repeated measures analysis of variance (RMANOVA) was employed with baseline CD4 held constant. Predictor variables were time, treatment condition, and baseline substance use status (use vs. non-use). In two separate models, scores from the CGI and the MADRS served as the repeated measure. A similar RMANOVA was used to test for baseline substance use as a moderator of the maintenance of the treatment effect on depression, first, examining results from baseline to 12-month follow-up, and second, examining results from post-treatment to 12-month follow-up.
Results
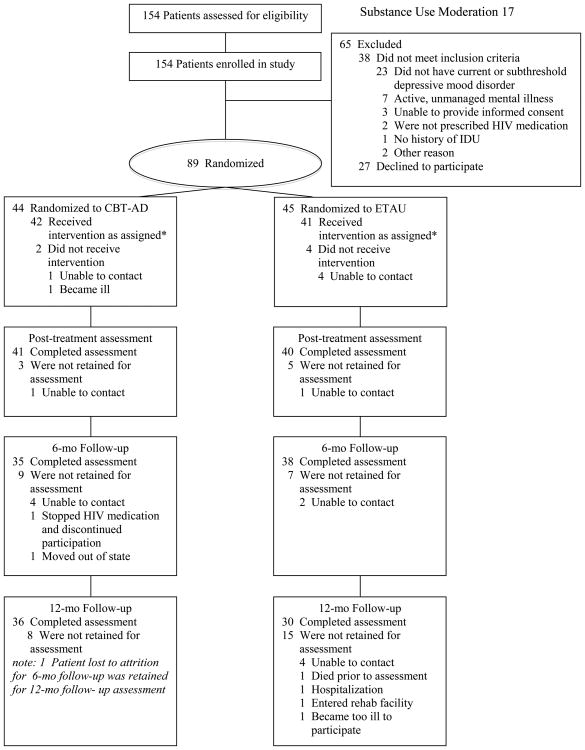
Table 1 presents baseline participant characteristics. At baseline, 56% of the participants reported being on an antidepressant medication. Of the initial 89 randomized participants, 81 patients (91.0%) completed the post-treatment assessment, and 66 patients (74.2%) completed the 12-month assessment. Rate of attrition for the two conditions was not significantly different (Figure 1).
Table 1.
Demographic characteristics of participants (N = 81).
| Mean (SD)/N (%) |
Mean (SD)/N (%) by Gender | ||
|---|---|---|---|
|
| |||
| Male | Female | ||
|
| |||
| Age | 46.8 (7.0) | 47.1 (1.0) | 46.7 (1.3) |
|
| |||
| Gender | |||
| Male | 49 (60.5) | -- | -- |
|
| |||
| Education | |||
| Eighth grade or lower | 13 (16.0) | 6 (7.7) | 7 (9.0) |
| Some high school | 21 (25.9) | 7 (9.0) | 14 (17.9) |
| High school graduate/GED | 20 (24.7) | 15 (19.2) | 4 (5.1) |
| Some college | 21 (25.9) | 16 (20.5) | 5 (6.4) |
| College graduate | 3 (3.7) | 2 (2.6) | 1 (1.3) |
|
| |||
| Race | |||
| African American | 24 (29.6) | 14 (17.3) | 10 (12.3) |
| Caucasian | 41 (50.6) | 23 (28.3) | 18 (22.2) |
| Other | 16 (19.8) | 12 (14.8) | 4 (4.9) |
|
| |||
| Ethnicity | |||
| Hispanic/Latino | 21 (25.9) | 13 (16.0) | 8 (9.9) |
|
| |||
| Relationship Status | |||
| Married | 27 (33.3) | 15 (19.0) | 12 (15.2) |
| Single | 32 (39.5) | 18 (22.8) | 14 (17.7) |
| Other | 20 (24.6) | 15 (19.0) | 5 (6.3) |
|
| |||
| Depression | |||
| CGI (Depression) | 4.3 (1.4) | 4.1 (0.2) | 4.8 (0.2) |
| MADRS | 25.0 (10.1) | 23.4 (1.5) | 27.6 (1.6) |
|
| |||
| Substance Use | |||
| Any Illicit Use | 59 (72.8) | 33 (41.7) | 25 (31.6) |
| Any Alcohol Use | 18 (22.2) | 13 (16.3) | 4 (5.0) |
| Alcohol Use to Intoxication | 5 (6.2) | 4 (5.0) | 1 (1.2) |
| Heroin Use | 20 (24.7) | 14 (17.5) | 6 (7.5) |
| Other Opiate Use | 19 (23.5) | 10 (12.5) | 8 (10.0) |
| Sedative/Tranquilizer Use | 32 (40.0) | 17 (21.5) | 15 (19.0) |
| Cocaine Use | 21 (25.9) | 13 (16.3) | 8 (10.0) |
| Cannabis Use | 11 (13.6) | 4 (5.0) | 7 (8.8) |
| Hallucinogen Use | 1 (1.2) | 1 (1.2) | 0 (0.0) |
| Inhalant Use | 1 (1.2) | 1 (1.2) | 0 (0.0) |
| Polysubstance Use | 25 (30.9) | 15 (18.8) | 10 (12.5) |
| Barbiturate Toxicology Screen | 1 (1.2) | 1 (1.2) | 0 (0.0) |
| Benzodiazepine Toxicology Screen | 7 (8.6) | 5 (6.7) | 2 (2.7) |
| Amphetamine Toxicology Screen | 1 (1.2) | 1 (1.2) | 0 (0.0) |
| Cannabis Toxicology Screen | 4 (4.9) | 2 (2.7) | 2 (2.7) |
| Cocaine Toxicology Screen | 18 (22.2) | 11 (14.7) | 7 (9.3) |
| Opiate Toxicology Screen | 9 (11.1) | 4 (5.4) | 4 (5.4) |
|
| |||
| Log Viral Load | 2.3 (0.9) | 2.3 (0.1) | 2.4 (0.2) |
|
| |||
| CD4 | 450.53 (260.4) | 462.6 (32.6) | 440.2 (58.2) |
Note. Demographic data based on N=81 of 89 randomized participants that completed through post-test. Three participants did not report education level and two participants did not report relationship status, resulting in < 100%.
Figure 1. CONSORT participant flow chart.
Moderation of Acute (Pre-Post Treatment) Effects
A significant three-way interaction between time, treatment condition, and baseline substance use status when depression was assessed with the MADRS was found (F[1,76]=6.78, p=0.01, Figure 2), indicating that change in depression was moderated by treatment condition and use status. Post-hoc analyses found that substance-using CBT-AD patients experienced a greater reduction in MADRS score from baseline to 3-month follow-up (t = -9.77, p = 0.003) than substance-using ETAU patients. There were no other significant group differences.
Figure 2.
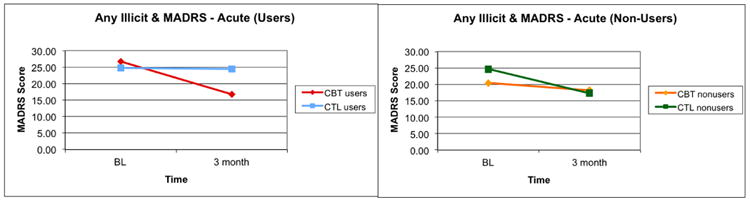
Moderation of acute depression treatment effects as assessed by the MADRS.
A RMANOVA with CGI score as the dependent variable resulted in a trend towards a significant three-way interaction (F[1,76]=2.82, p=0.097); however, there was a significant time by condition interaction (F[1,76]=8.74, p=0.004). Post-hoc analyses demonstrated that CBT-AD patients had a greater reduction in CGI score from baseline to 3-month follow-up. (t = -3.84, p< 0.001) than ETAU patients.
Moderation of Maintenance Effects
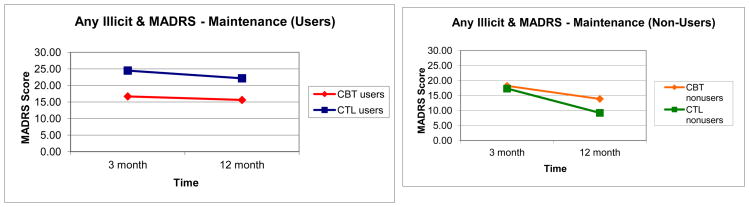
A RMANOVA for baseline to 12-month with MADRS scores as the dependent variable resulted in a significant three-way interaction between time, condition, and baseline substance use status (F[1,61]=5.46, p=0.023; Figure 3), indicating that change in depression scores was moderated by treatment condition and substance use status. Post-hoc analyses revealed a trend towards a significant group difference, with substance-using CBT-AD individuals experiencing a greater reduction in MADRS scores from baseline to 12-month follow-up than substance-using ETAU individuals (t = -8.12, p = 0.08). There were no other significant group differences. Findings were similar when the CGI score was used as the dependent variable (F[1,61]=5.99, p=0.021). Follow-up analyses found that substance-using CBT-AD participants experienced a greater reduction in depression from baseline to 12-month follow-up compared to substance-using ETAU participants (t=-1.27, p=0.016).
Figure 3.
Moderation of maintenance of depression treatment effects as assessed by the MADRS (baseline to 12-month).
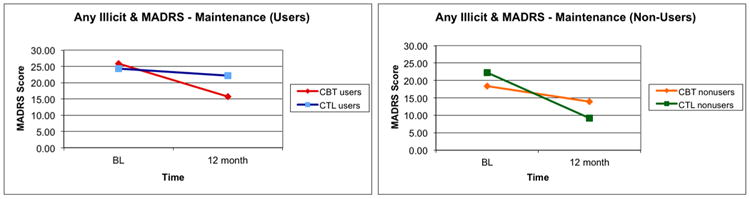
A RMANOVA with MADRS scores as the dependent variable for post-treatment to 12-months resulted in a non-significant three-way interaction (F[1,60]=0.028, p=0.867; Figure 4), indicating that change in depression scores was not moderated by treatment condition and substance use status. Findings were similar when the CGI score was used as the dependent variable (F[1,60]=0.059, p=0.810).
Figure 4.
Moderation of maintenance of depression treatment effects as assessed by the MADRS (post-treatment to 12-month).
Discussion
The purpose of our study was to examine substance use as a moderator of the acute and maintenance effects of CBT-AD on depression. We anticipated that substance use would moderate the effect of CBT-AD on depression such that participants engaged in substance use would benefit less from depression treatment. Results from this study found the opposite to be true – that those actively using illicit substances achieved a greater improvement in depression compared to participants who abstained. This may be because substance-using individuals in the CBT-AD condition had higher scores on the depression measures than participants in any of the other groups, resulting in more room for change to occur. Results also indicate that a majority of treatment gains occurred during the active phase of treatment and that treatment gains were maintained at the same rate by all participants during the follow-up phase, suggesting CBT-AD was effective in reducing depression acutely and maintaining this reduction longer-term.
During the acute phase when the MADRS was used as the outcome measure for depression, substance use significantly moderated the effect of condition on depression treatment gains such that participants engaged in substance use in the CBT-AD condition experienced the greatest change. This finding did not hold with the CGI, though there was a trend towards significance. Because the CGI is an indicator of global severity of depression, this measure may be less sensitive to more subtle improvements in depressive symptomatology. Results for the maintenance phase indicated that depression treatment gains were maintained, regardless of baseline substance use status. This finding highlights that those participants who were actively engaged in substance use at baseline in the CBT-AD condition improved their depression and sustained the improvement over time.
This study has some limitations. First, results may not generalize to HIV patients in different geographical regions, with another type of substance use disorder, or opioid dependent persons not receiving substance abuse treatment. Second, findings may be limited to those patients who are more likely to present for depression treatment, or participate in clinical research, than those who are not. Third, a majority of the participants in this study had an active substance use disorder and, therefore, analyses may have been underpowered to detect depression treatment effects among abstainers. However, this does not nullify the substance use moderation effects on depression treatment found for participants with an active substance use disorder in the CBT-AD condition. Fourth, we did not assess for lifetime CBT and did not involve one's family in the treatment. Fifth, there is evidence suggesting that substance use is associated with long-term cognitive deficits (Gould, 2010) which can affect treatment engagement and outcomes (Aharonovich, Hasin, Brooks, Liu, Bisaga, & Nunes, 2006; Kulik, Nich, & Carroll, 2011), however there is ample evidence demonstrating the effectiveness of CBT for individuals with substance use disorders (Dutra, Stathopoulou, Basden, Leyro, Powers, & Otto, 2008; Magill & Ray, 2009; McHugh, Hearon, & Otto, 2010). Last, substance use categorization was based on endorsement of any substance or a positive toxicology screen at baseline. Results may have been influenced by changes in substance use during treatment and/or the follow-up period that were not accounted for.
Results of this study suggest that CBT-AD for triply diagnosed patients is useful for treating depression in opiate dependent persons who either have a history of substance dependence or actively engaging in substance use. Based on these data, clinicians working with this patient population should not necessarily exclude individuals with active substance use from CBT depression treatment. In fact, results from this study indicate that active substance use does not appear to interfere with potential gains that could occur from receiving CBT-AD. Given its utility, efforts should focus on disseminating and employing this treatment intervention in substance use clinics.
Table 2. Unadjusted mean depression scores across conditions and time.
| Baseline | 3 Month | 12 Month | |
|---|---|---|---|
|
| |||
| MADRS | |||
| CBT-AD | |||
| Users | 26.69 (9.65) | 16.69 (10.74) | 15.62 (9.45) |
| Abstainers | 20.44 (11.19) | 18.22 (10.71) | 13.86 (8.71) |
| ETAU | |||
| Users | 24.73 (9.91) | 24.50 (8.85) | 22.16 (9.97) |
| Abstainers | 24.60 (11.33) | 17.30 (12.41) | 9.20 (10.08) |
|
| |||
| CGI | |||
| CBT-AD | |||
| Users | 4.5 (1.30) | 2.81 (1.53) | 2.62 (1.12) |
| Abstainers | 3.89 (1.36) | 2.56 (1.59) | 2.43 (0.98) |
| ETAU | |||
| Users | 4.3 (1.29) | 4.07 (1.05) | 3.6 (1.29) |
| Abstainers | 4.3 (1.89) | 3.2 (1.62) | 2.2 (1.1) |
Note. Due to attrition, the N per group decreased over time. CBT-AD Users: Baseline N=32; 3 Month N=32; 12 Month N=29. CBT-AD Abstainers: Baseline N=9; 3 Month N=9; 12 Month N=7. ETAU Users: Baseline N=30; 3 Month N=30; 12 Month N=25. ETAU Abstainers: Baseline N=10; 3 Month N=10; 12 Month N=5.
Acknowledgments
Funding for data collection for this project is from R-01 DA018603 (Safren). Some of the investigator time was supported by Grant K24 MH094214 (Safren), and grant NIH/NIAID 5P30AI060354-09 (Labbe; Harvard University Center for AIDS Research Scholar Award).
References
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug and Alcohol Dependence. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Cone EJ, Presley L, Lehrer M, Seiter W, Smith M, Kardos KW, Fritch D, Salamone S, Niedbala RS. Oral fluid testing for drugs of abuse: positive prevalence rates by Intercept immunoassay screening and GC-MS-MS confirmation and suggested cutoff concentrations. Journal of Analytical Toxicology. 2002;26(8):541–546. doi: 10.1093/jat/26.8.541. [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Addiction and cognition. Addiction Science & Clinical Practice. 2010;5(2):4–14. [PMC free article] [PubMed] [Google Scholar]
- Kulik BD, Nich C, Carroll KM. Relationship of cognitive function and the acquisition of coping skills in computer assisted treatment for substance use disorders. Drug and Alcohol Dependence. 2011;114:169–176. doi: 10.1016/j.drugalcdep.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill M, Ray LA. Cognitive-behavioral treatment with adult alcohol and illicit drug users: A meta-analysis of randomized controlled trials. Journal of Studies on Alcohol and Drugs. 2009;70:516–527. doi: 10.15288/jsad.2009.70.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MgHugh RK, Hearon BA, Otto MW. Cognitive behavioral therapy for substance use disorders. Psychiatric Clinics of North America. 2010;33:511–525. doi: 10.1016/j.psc.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abusers: The Addiction Severity Index. Journal of Mental and Nervous Disease. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health. CGI (Clinical Global Impression) Scale - NIMH. Psychopharmacology Bulletin. 1985;21:839–844. [Google Scholar]
- Safren SA, Gonzalez JS, Soroudi N. Coping with Chronic Illness: Cognitive behavioral therapy for adherence and depression in individuals with chronic illness, Client Workbook. New York, NY: Oxford University Press; 2007a. [Google Scholar]
- Safren SA, Gonzalez JS, Soroudi N. Coping with Chronic Illness: Cognitive behavioral therapy for adherence and depression in individuals with chronic illness, Therapist Guide. New York, NY: Oxford University Press; 2007b. [Google Scholar]
- Safren SA, O'Cleirigh C, Bullis JR, Otto MW, Stein MD, Pollack MH. Cognitive behavioral therapy for adherence and depression (CBT-AD-AD) in HIV-infected injection drug users: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2012;80(3):404–415. doi: 10.1037/a0028208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, O'Cleirigh C, Tan JY, Raminani SR, Reilly LC, Otto MW, Mayer KH. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD-AD) in HIV-infected individuals. Health Psychology. 2009;28(1):1–10. doi: 10.1037/a0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, Otto MW, Worth JL. Life-steps: Applying cognitive behavioral therapy to HIV medication adherence. Cognitive and Behavioral Practice. 1999;6:332–341. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Herqueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. 1998 [PubMed] [Google Scholar]