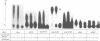Abstract
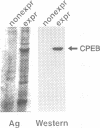
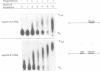
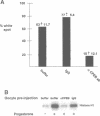
Cytoplasmic polyadenylation is a key mechanism controlling maternal mRNA translation in early development. In most cases, mRNAs that undergo poly(A) elongation are translationally activated; those that undergo poly(A) shortening are deactivated. Poly(A) elongation is regulated by two cis-acting sequences in the 3'-untranslated region (UTR) of responding mRNAs, the polyadenylation hexanucleotide AAUAAA and the U-rich cytoplasmic polyadenylation element (CPE). Previously, we cloned and characterized the Xenopus oocyte CPE binding protein (CPEB), showing that it was essential for the cytoplasmic polyadenylation of B4 RNA. Here, we show that CPEB also binds the CPEs of G10, c-mos, cdk2, cyclins A1, B1 and B2 mRNAs. We find that CPEB is necessary for polyadenylation of these RNAs in egg extracts, suggesting that this protein is required for polyadenylation of most RNAs during oocyte maturation. Our data demonstrate that the complex timing and extent of polyadenylation are partially controlled by CPEB binding to multiple target sites in the 3' UTRs of responsive mRNAs. Finally, injection of CPEB antibody into oocytes not only inhibits polyadenylation in vivo, but also blocks progesterone-induced maturation. This is due to inhibition of polyadenylation and translation of c-mos mRNA, suggesting that CPEB is critical for early development.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahringer J., Kimble J. Control of the sperm-oocyte switch in Caenorhabditis elegans hermaphrodites by the fem-3 3' untranslated region. Nature. 1991 Jan 24;349(6307):346–348. doi: 10.1038/349346a0. [DOI] [PubMed] [Google Scholar]
- Ballantyne S., Bilger A., Astrom J., Virtanen A., Wickens M. Poly (A) polymerases in the nucleus and cytoplasm of frog oocytes: dynamic changes during oocyte maturation and early development. RNA. 1995 Mar;1(1):64–78. [PMC free article] [PubMed] [Google Scholar]
- Bienroth S., Wahle E., Suter-Crazzolara C., Keller W. Purification of the cleavage and polyadenylation factor involved in the 3'-processing of messenger RNA precursors. J Biol Chem. 1991 Oct 15;266(29):19768–19776. [PubMed] [Google Scholar]
- Bilger A., Fox C. A., Wahle E., Wickens M. Nuclear polyadenylation factors recognize cytoplasmic polyadenylation elements. Genes Dev. 1994 May 1;8(9):1106–1116. doi: 10.1101/gad.8.9.1106. [DOI] [PubMed] [Google Scholar]
- Christerson L. B., McKearin D. M. orb is required for anteroposterior and dorsoventral patterning during Drosophila oogenesis. Genes Dev. 1994 Mar 1;8(5):614–628. doi: 10.1101/gad.8.5.614. [DOI] [PubMed] [Google Scholar]
- Curtis D., Lehmann R., Zamore P. D. Translational regulation in development. Cell. 1995 Apr 21;81(2):171–178. doi: 10.1016/0092-8674(95)90325-9. [DOI] [PubMed] [Google Scholar]
- Dworkin M. B., Dworkin-Rastl E. Functions of maternal mRNA in early development. Mol Reprod Dev. 1990 Jul;26(3):261–297. doi: 10.1002/mrd.1080260310. [DOI] [PubMed] [Google Scholar]
- Fox C. A., Sheets M. D., Wahle E., Wickens M. Polyadenylation of maternal mRNA during oocyte maturation: poly(A) addition in vitro requires a regulated RNA binding activity and a poly(A) polymerase. EMBO J. 1992 Dec;11(13):5021–5032. doi: 10.1002/j.1460-2075.1992.tb05609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. A., Sheets M. D., Wickens M. P. Poly(A) addition during maturation of frog oocytes: distinct nuclear and cytoplasmic activities and regulation by the sequence UUUUUAU. Genes Dev. 1989 Dec;3(12B):2151–2162. doi: 10.1101/gad.3.12b.2151. [DOI] [PubMed] [Google Scholar]
- Gebauer F., Richter J. D. Cloning and characterization of a Xenopus poly(A) polymerase. Mol Cell Biol. 1995 Mar;15(3):1422–1430. doi: 10.1128/mcb.15.3.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer F., Xu W., Cooper G. M., Richter J. D. Translational control by cytoplasmic polyadenylation of c-mos mRNA is necessary for oocyte maturation in the mouse. EMBO J. 1994 Dec 1;13(23):5712–5720. doi: 10.1002/j.1460-2075.1994.tb06909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand R. A., Smith L. D. RNA stabilization and continued RNA processing following nuclear dissolution in maturing Xenopus laevis oocytes. Dev Biol. 1983 Oct;99(2):427–436. doi: 10.1016/0012-1606(83)90292-0. [DOI] [PubMed] [Google Scholar]
- Gilmartin G. M., Fleming E. S., Oetjen J., Graveley B. R. CPSF recognition of an HIV-1 mRNA 3'-processing enhancer: multiple sequence contacts involved in poly(A) site definition. Genes Dev. 1995 Jan 1;9(1):72–83. doi: 10.1101/gad.9.1.72. [DOI] [PubMed] [Google Scholar]
- Gilmartin G. M., Nevins J. R. An ordered pathway of assembly of components required for polyadenylation site recognition and processing. Genes Dev. 1989 Dec;3(12B):2180–2190. doi: 10.1101/gad.3.12b.2180. [DOI] [PubMed] [Google Scholar]
- Gotoh Y., Masuyama N., Dell K., Shirakabe K., Nishida E. Initiation of Xenopus oocyte maturation by activation of the mitogen-activated protein kinase cascade. J Biol Chem. 1995 Oct 27;270(43):25898–25904. doi: 10.1074/jbc.270.43.25898. [DOI] [PubMed] [Google Scholar]
- Hake L. E., Richter J. D. CPEB is a specificity factor that mediates cytoplasmic polyadenylation during Xenopus oocyte maturation. Cell. 1994 Nov 18;79(4):617–627. doi: 10.1016/0092-8674(94)90547-9. [DOI] [PubMed] [Google Scholar]
- Huarte J., Belin D., Vassalli A., Strickland S., Vassalli J. D. Meiotic maturation of mouse oocytes triggers the translation and polyadenylation of dormant tissue-type plasminogen activator mRNA. Genes Dev. 1987 Dec;1(10):1201–1211. doi: 10.1101/gad.1.10.1201. [DOI] [PubMed] [Google Scholar]
- Huarte J., Stutz A., O'Connell M. L., Gubler P., Belin D., Darrow A. L., Strickland S., Vassalli J. D. Transient translational silencing by reversible mRNA deadenylation. Cell. 1992 Jun 12;69(6):1021–1030. doi: 10.1016/0092-8674(92)90620-r. [DOI] [PubMed] [Google Scholar]
- Keller W., Bienroth S., Lang K. M., Christofori G. Cleavage and polyadenylation factor CPF specifically interacts with the pre-mRNA 3' processing signal AAUAAA. EMBO J. 1991 Dec;10(13):4241–4249. doi: 10.1002/j.1460-2075.1991.tb05002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P. S., Melton D. A. Translational control of activin in Xenopus laevis embryos. Dev Genet. 1995;17(1):55–64. doi: 10.1002/dvg.1020170107. [DOI] [PubMed] [Google Scholar]
- Kosako H., Gotoh Y., Nishida E. Regulation and function of the MAP kinase cascade in Xenopus oocytes. J Cell Sci Suppl. 1994;18:115–119. doi: 10.1242/jcs.1994.supplement_18.17. [DOI] [PubMed] [Google Scholar]
- Kuge H., Richter J. D. Cytoplasmic 3' poly(A) addition induces 5' cap ribose methylation: implications for translational control of maternal mRNA. EMBO J. 1995 Dec 15;14(24):6301–6310. doi: 10.1002/j.1460-2075.1995.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz V., Chang J. S., Horabin J. I., Bopp D., Schedl P. The Drosophila orb RNA-binding protein is required for the formation of the egg chamber and establishment of polarity. Genes Dev. 1994 Mar 1;8(5):598–613. doi: 10.1101/gad.8.5.598. [DOI] [PubMed] [Google Scholar]
- Manley J. L. A complex protein assembly catalyzes polyadenylation of mRNA precursors. Curr Opin Genet Dev. 1995 Apr;5(2):222–228. doi: 10.1016/0959-437x(95)80012-3. [DOI] [PubMed] [Google Scholar]
- McGrew L. L., Dworkin-Rastl E., Dworkin M. B., Richter J. D. Poly(A) elongation during Xenopus oocyte maturation is required for translational recruitment and is mediated by a short sequence element. Genes Dev. 1989 Jun;3(6):803–815. doi: 10.1101/gad.3.6.803. [DOI] [PubMed] [Google Scholar]
- McGrew L. L., Richter J. D. Translational control by cytoplasmic polyadenylation during Xenopus oocyte maturation: characterization of cis and trans elements and regulation by cyclin/MPF. EMBO J. 1990 Nov;9(11):3743–3751. doi: 10.1002/j.1460-2075.1990.tb07587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Blow J. J., Hunt T. Translation of cyclin mRNA is necessary for extracts of activated xenopus eggs to enter mitosis. Cell. 1989 Mar 24;56(6):947–956. doi: 10.1016/0092-8674(89)90628-4. [DOI] [PubMed] [Google Scholar]
- Murthy K. G., Manley J. L. Characterization of the multisubunit cleavage-polyadenylation specificity factor from calf thymus. J Biol Chem. 1992 Jul 25;267(21):14804–14811. [PubMed] [Google Scholar]
- Murthy K. G., Manley J. L. The 160-kD subunit of human cleavage-polyadenylation specificity factor coordinates pre-mRNA 3'-end formation. Genes Dev. 1995 Nov 1;9(21):2672–2683. doi: 10.1101/gad.9.21.2672. [DOI] [PubMed] [Google Scholar]
- Nebreda A. R., Hunt T. The c-mos proto-oncogene protein kinase turns on and maintains the activity of MAP kinase, but not MPF, in cell-free extracts of Xenopus oocytes and eggs. EMBO J. 1993 May;12(5):1979–1986. doi: 10.1002/j.1460-2075.1993.tb05847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J., Kirschner M. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell. 1982 Oct;30(3):675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Newport J., Kirschner M. A major developmental transition in early Xenopus embryos: II. Control of the onset of transcription. Cell. 1982 Oct;30(3):687–696. doi: 10.1016/0092-8674(82)90273-2. [DOI] [PubMed] [Google Scholar]
- O'Keefe S. J., Wolfes H., Kiessling A. A., Cooper G. M. Microinjection of antisense c-mos oligonucleotides prevents meiosis II in the maturing mouse egg. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7038–7042. doi: 10.1073/pnas.86.18.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J., Philippe M. Poly(A) metabolism and polysomal recruitment of maternal mRNAs during early Xenopus development. Dev Biol. 1990 Jul;140(1):221–224. doi: 10.1016/0012-1606(90)90070-y. [DOI] [PubMed] [Google Scholar]
- Paris J., Richter J. D. Maturation-specific polyadenylation and translational control: diversity of cytoplasmic polyadenylation elements, influence of poly(A) tail size, and formation of stable polyadenylation complexes. Mol Cell Biol. 1990 Nov;10(11):5634–5645. doi: 10.1128/mcb.10.11.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J., Swenson K., Piwnica-Worms H., Richter J. D. Maturation-specific polyadenylation: in vitro activation by p34cdc2 and phosphorylation of a 58-kD CPE-binding protein. Genes Dev. 1991 Sep;5(9):1697–1708. doi: 10.1101/gad.5.9.1697. [DOI] [PubMed] [Google Scholar]
- Pascal E., Tjian R. Different activation domains of Sp1 govern formation of multimers and mediate transcriptional synergism. Genes Dev. 1991 Sep;5(9):1646–1656. doi: 10.1101/gad.5.9.1646. [DOI] [PubMed] [Google Scholar]
- Paynton B. V., Bachvarova R. Polyadenylation and deadenylation of maternal mRNAs during oocyte growth and maturation in the mouse. Mol Reprod Dev. 1994 Feb;37(2):172–180. doi: 10.1002/mrd.1080370208. [DOI] [PubMed] [Google Scholar]
- Preker P. J., Lingner J., Minvielle-Sebastia L., Keller W. The FIP1 gene encodes a component of a yeast pre-mRNA polyadenylation factor that directly interacts with poly(A) polymerase. Cell. 1995 May 5;81(3):379–389. doi: 10.1016/0092-8674(95)90391-7. [DOI] [PubMed] [Google Scholar]
- Robbie E. P., Peterson M., Amaya E., Musci T. J. Temporal regulation of the Xenopus FGF receptor in development: a translation inhibitory element in the 3' untranslated region. Development. 1995 Jun;121(6):1775–1785. doi: 10.1242/dev.121.6.1775. [DOI] [PubMed] [Google Scholar]
- Sagata N., Oskarsson M., Copeland T., Brumbaugh J., Vande Woude G. F. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988 Oct 6;335(6190):519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Sallés F. J., Darrow A. L., O'Connell M. L., Strickland S. Isolation of novel murine maternal mRNAs regulated by cytoplasmic polyadenylation. Genes Dev. 1992 Jul;6(7):1202–1212. doi: 10.1101/gad.6.7.1202. [DOI] [PubMed] [Google Scholar]
- Sallés F. J., Lieberfarb M. E., Wreden C., Gergen J. P., Strickland S. Coordinate initiation of Drosophila development by regulated polyadenylation of maternal messenger RNAs. Science. 1994 Dec 23;266(5193):1996–1999. doi: 10.1126/science.7801127. [DOI] [PubMed] [Google Scholar]
- Sheets M. D., Fox C. A., Hunt T., Vande Woude G., Wickens M. The 3'-untranslated regions of c-mos and cyclin mRNAs stimulate translation by regulating cytoplasmic polyadenylation. Genes Dev. 1994 Apr 15;8(8):926–938. doi: 10.1101/gad.8.8.926. [DOI] [PubMed] [Google Scholar]
- Sheets M. D., Wu M., Wickens M. Polyadenylation of c-mos mRNA as a control point in Xenopus meiotic maturation. Nature. 1995 Apr 6;374(6522):511–516. doi: 10.1038/374511a0. [DOI] [PubMed] [Google Scholar]
- Simon R., Richter J. D. Further analysis of cytoplasmic polyadenylation in Xenopus embryos and identification of embryonic cytoplasmic polyadenylation element-binding proteins. Mol Cell Biol. 1994 Dec;14(12):7867–7875. doi: 10.1128/mcb.14.12.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R., Tassan J. P., Richter J. D. Translational control by poly(A) elongation during Xenopus development: differential repression and enhancement by a novel cytoplasmic polyadenylation element. Genes Dev. 1992 Dec;6(12B):2580–2591. doi: 10.1101/gad.6.12b.2580. [DOI] [PubMed] [Google Scholar]
- Stebbins-Boaz B., Richter J. D. Multiple sequence elements and a maternal mRNA product control cdk2 RNA polyadenylation and translation during early Xenopus development. Mol Cell Biol. 1994 Sep;14(9):5870–5880. doi: 10.1128/mcb.14.9.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Huarte J., Belin D., Gubler P., Vassalli A., O'Connell M. L., Parton L. A., Rickles R. J., Strickland S. Regulated polyadenylation controls mRNA translation during meiotic maturation of mouse oocytes. Genes Dev. 1989 Dec;3(12B):2163–2171. doi: 10.1101/gad.3.12b.2163. [DOI] [PubMed] [Google Scholar]
- Wahle E. 3'-end cleavage and polyadenylation of mRNA precursors. Biochim Biophys Acta. 1995 Apr 4;1261(2):183–194. doi: 10.1016/0167-4781(94)00248-2. [DOI] [PubMed] [Google Scholar]
- Wahle E., Keller W. The biochemistry of 3'-end cleavage and polyadenylation of messenger RNA precursors. Annu Rev Biochem. 1992;61:419–440. doi: 10.1146/annurev.bi.61.070192.002223. [DOI] [PubMed] [Google Scholar]
- Wormington M. Preparation of synthetic mRNAs and analyses of translational efficiency in microinjected Xenopus oocytes. Methods Cell Biol. 1991;36:167–183. doi: 10.1016/s0091-679x(08)60277-0. [DOI] [PubMed] [Google Scholar]
- Yew N., Mellini M. L., Vande Woude G. F. Meiotic initiation by the mos protein in Xenopus. Nature. 1992 Feb 13;355(6361):649–652. doi: 10.1038/355649a0. [DOI] [PubMed] [Google Scholar]