Abstract
Background
Subclinical doses of propofol produce anterograde amnesia, characterized by an early failure of memory consolidation. It is unknown how propofol affects the amygdala-dependent emotional memory system, which modulates consolidation in the hippocampus in response to emotional arousal and neurohumoral stress. We present an event-related functional magnetic resonance imaging study of the effects of propofol on the emotional memory system in human subjects.
Methods
Thirty-five healthy subjects were randomized to receive propofol, at an estimated brain concentration of 0.90 μg ml−1, or placebo. During drug infusion, emotionally arousing and neutral images were presented in a continuous recognition task, while blood-oxygen-level-dependent activation responses were acquired. After a drug-free interval of 2 h, subsequent memory for successfully encoded items was assessed. Imaging analysis was performed using statistical parametric mapping and behavioural analysis using signal detection models.
Results
Propofol had no effect on the stereotypical amygdalar response to emotional arousal, but caused marked suppression of the hippocampal response. Propofol caused memory performance to become uncoupled from amygdalar activation, but it remained correlated with activation in the posterior hippocampus, which decreased in proportion to amnesia.
Conclusions
Propofol is relatively ineffective at suppressing amygdalar activation at sedative doses, but abolishes emotional modulation and causes amnesia via mechanisms that commonly involve hyporesponsiveness of the hippocampus. These findings raise the possibility that amygdala-dependent fear systems may remain intact even when a patient has diminished memory of events. This may be of clinical importance in the perioperative development of fear-based psychopathologies, such as post-traumatic stress disorder.
Clinical trial registration
Keywords: amnesia, chemically induced; amygdala; fMRI; hippocampus; memory, drug effects; propofol, pharmacology
Editor′s key points.
Functional magnetic resonance imaging can detect regional neurophysiological responses to diverse stimuli.
The authors studied the influence of propofol on emotional memory systems in healthy volunteers.
Sedative propofol doses suppressed hippocampal but not amygdalar responses to emotional arousal.
Subsequent amnesia for emotional stimuli was mediated via suppression of hippocampal responses.
At subclinical doses, the γ-aminobutyric acid (GABA)ergic agonist propofol selectively produces anterograde amnesia, but leaves consciousness, working memory, and retrieval processes intact. This amnesia is characterized by a failure of memory consolidation,1–3 the cascade of processes through which an initially fragile memory trace becomes stabilized. Although the consolidation sequence takes hours, days, or longer to unfold fully, the key mechanism of propofol amnesia is likely to involve an extremely early component. Action on later components should lead to a window of retrograde amnesia, as observed when consolidation is interrupted by electroconvulsive shock or protein synthesis inhibitors, but this does not occur.4
Previously, we reported a detailed behavioural and event-related potential study of propofol amnesia using a memory paradigm called the continuous recognition task.1 Here, we investigate the neuroanatomical correlates by adapting that paradigm to an event-related functional magnetic resonance imaging (fMRI) study.
Our hypothesis and study design focus on two medial temporal lobe structures: the hippocampus, which is essential for the normal acquisition and consolidation of declarative memory,5 and the amygdala, which underlies fear learning and memory, and modulates hippocampal consolidation in response to emotion, arousal, and stress via noradrenergic mechanisms.6 Amygdalar hyperactivation is critical to the mechanism of fear memory disorders, including post-traumatic stress disorder and anxiety.7 This is relevant to the domain of the anaesthetist, because the stress response to surgery provides the substrate for an intense activation of amygdalar fear systems. This activation may be gated by local GABAergic circuits,8 but the α1- and α2-subunit GABAA receptors found in the amygdala are distinct from the α5-subunit GABAA receptors found predominantly in the hippocampus.9,10 GABAergic anaesthetics may not suppress amygdalar and hippocampal processes with equal efficacy.11
The effects of propofol and other GABAergic anaesthetics on the emotional memory system in humans are largely unknown. Previous neuroimaging studies with sedative concentrations of propofol have principally focused on cortical processing and encoding processes3,12–15 or resting state connectivity.16,17 A study of sevoflurane reported decreased amygdalohippocampal connectivity, but evaluated only resting networks.18 In this study, we target amygdala-dependent memory modulation by presenting emotionally arousing images, a technique demonstrated in previous fMRI studies.19,20
Methods
Subjects
Forty healthy, right-handed volunteers [29 female, 11 male, age range 18–37 yr, mean 25.5 (4.3) yr] were recruited using the online notice board Craigslist (http://newyork.craigslist.org/vol/, last accessed September 12, 2014). All subjects were non-obese and with no known medical or psychiatric comorbidity. Subjects were excluded if they had dental work likely to compromise imaging. Pregnancy was excluded using urinary β-human chorionic gonadotrophin. All subjects provided written consent and were remunerated. The study was approved and monitored by the Institutional Review Board of Weill Cornell Medical College (New York, NY, USA).
Exclusions and stopping point
Subjects were randomly allocated to either propofol or placebo, with enrolment continuing until a minimum of 18 technically complete studies were included in each group. Four subjects were excluded because their studies were incomplete: two subjects (one placebo, one propofol) because of scanner malfunction; one subject (propofol) because drug delivery malfunctioned; and one subject (placebo) because they fell asleep and had inadequate task performance. One subject (placebo) had a technically complete study, but was excluded during the quality control phase of analysis because of excessive susceptibility-induced field inhomogeneity.
Stimuli
Stimuli were images obtained from the International Affective Picture System (IAPS),21 a widely used collection of colour photographs characterized by normative values for valence (negative ↔ neutral ↔ positive) and arousal (non-arousing ↔ neutral ↔ arousing). Four sets of images were created: (i) a set of 40 negative–arousing images for use as targets; (ii) a set of 40 negative–arousing images for use as foils; (iii) a set of 40 neutral images for use as targets; and (iv) a set of 40 neutral images for use as foils. Further detail on the IAPS images used is provided in the Supplementary material.
To validate the use of the normative IAPS values, subjects rated a diverse set of 40 images for valence and arousal during the orientation session conducted ∼1–2 weeks before the study, using the exact IAPS methodology.21
Stimuli were presented using E-Prime 2 (Psychology Software Tools, Inc., Sharpsburg, PA, USA). Items presented in the scanner were routed using the Integrated Function Imaging System (Invivo Corp., Gainesville, FL, USA) and viewed on a 7.5-inch LCD monitor. Items presented outside the scanner were viewed on a 17-inch laptop monitor.
Drugs and drug delivery
In the propofol group, the targeted effect site (brain) concentration was 0.90 μg ml−1, based on the Schneider pharmacokinetic model,22 as used in the earlier behavioural/neurophysiology study.1 In the placebo group, propofol was replaced with normal saline. Subjects were blinded to their assignment. Drug administration was controlled using STANPUMP pharmacokinetic software (freely available from the WorldSIVA Open TCI Initiative; http://opentci.org/doku.php, last accessed September 12, 2014), installed on a laptop computer and driving a Harvard 22 syringe pump (Harvard Apparatus, Inc., Holliston, MA, USA).
Experimental protocol
After positioning in the scanner, blood pressure, oxygen saturation, and exhaled carbon dioxide monitors were placed, and oxygen was administered via nasal cannula at 2 litres min−1. The button-press device was placed in the right hand.
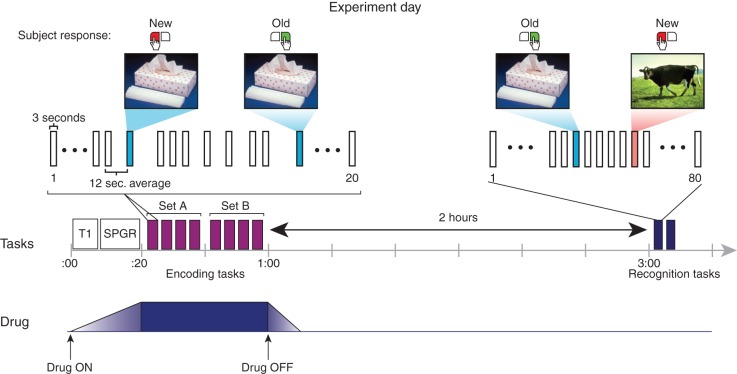
The experimental sequence is shown in Figure 1. The drug infusion was commenced; then, during the 20 min required for the equilibrium concentration to be achieved, the required structural scans were performed.
Fig 1.
Experimental paradigm. The middle line of the figure depicts the experimental sequence, and the bottom line the timing of the drug infusion. The top section shows segments of the experimental tasks in fine detail. The encoding task was performed in the scanner during the drug infusion. A sequence of 160 IAPS pictures was presented in 8 blocks of 20 items (purple bars), with 40 negative—arousing and 40 neutral images each shown twice as part of an old/new continuous recognition task. The recognition task was performed 120 min later outside of the scanner. Here, the 80 images previously seen in the encoding task and 80 novel images were shown in a random sequence across two blocks (blue bars).
The encoding task was performed in the scanner. Subjects viewed a pseudorandom sequence of 160 images, made up of the 40 negative–arousing targets and 40 neutral targets, each presented twice (mean interval between first and second presentations 9.1 images). The task was to indicate with the button-press device whether the image they were seeing was being presented for the first time (‘new’) or whether it had been presented earlier in the sequence (‘old’). The sequence was divided into eight blocks of 20 images, with a short break in between blocks. The interstimulus interval was jittered to an average of 12 s (range 6–18 s). Images were presented for 3000 ms and separated by a white fixation cross. For counterbalancing, four versions of the sequence were randomly assigned. The entire encoding task took ∼35 min, after which the drug was stopped and the subject removed from the scanner.
The recognition task was performed outside the scanner, 120 min after the conclusion of the encoding task. Subjects viewed a pseudorandom sequence of 160 images, made up of the 80 targets seen earlier in the scanner, interspersed with 40 negative–arousing and 40 neutral foils. The task was to indicate whether the image they were seeing was being presented for the first time (‘new’) or whether it had been presented earlier in the scanner (‘old’). The interstimulus interval was 5200 ms. Images were presented for 3000 ms and separated by a white fixation cross. The sequence was divided into two blocks.
Anxiety assessment
Given that anxiety modifies the amygdalohippocampal response to emotional dynamics,23,24 subjects completed the Spielberger State-Trait Anxiety Inventory.25 Trait (phenotypic) anxiety was assessed at the orientation session 1–2 weeks before the study and state anxiety assessed immediately before the experiment.
Behavioural analysis procedures
The signal detection sensitivity measure d′ was calculated as z(hits)−z(false alarms), where z(p), p∈[0,1] is the inverse of the cumulative Gaussian distribution. The value of d′ provides a measure of sensitivity immune to changes in response bias. False alarms were images incorrectly designated as ‘old’, when they were in fact being presented for the first time (i.e. ‘new’). Response bias was assessed by the criterion location (c), calculated as −0.5[z(hits)+z(false alarms)]. Changes in c represent shifts in the decision criteria used to nominate an item as ‘old’ or ‘new’.
Behavioural statistical analysis
All behavioural statistical calculations were performed using SYSTAT 13 (Systat Software, Inc., Chicago, IL, USA). Tests with a normal distribution were conducted using Student's paired or unpaired t tests or repeated-measures anova with Holm–Sidak post hoc t testing and are reported with the t or F statistic, degrees of freedom, and mean (sem). Tests with a non-normal distribution were conducted using the Mann–Whitney U test and are reported with the U statistic and median [quartile, quartile].
Imaging and analysis procedures
Imaging and analysis theory and procedures are fully detailed in the Supplementary material.
Briefly, images were acquired using a 3 T GE Signa magnetic resonance imaging scanner (General Electric Company, Waukesha, WI, USA). Functional scans were acquired using a gradient-echo echo-planar imaging sequence. For co-registration, T1-weighted whole-brain anatomical images were acquired using a spoiled gradient recalled acquisition sequence. Functional image processing was conducted using a modified version26 of SPM99 software (base program freely available from the Wellcome Institute of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm/software/, last accessed September 12, 2014). Images were stereotactically normalized to the Montreal Neurologic Institute version of Talairach space.
Stimuli for which no response was recorded were removed from analysis. At the individual subject level, a voxel-by-voxel whole-brain multiple linear regression model was applied.27 Contrast-effect images were then progressed into group-level models. A random-effects model was used for whole-brain group analyses. Three anatomical regions for which there was an a priori hypothesis were assessed using standard small volume correction: (i) bilateral amygdalae; (ii) bilateral hippocampi; and (iii) bilateral parahippocampi. A priori and non-hypothesized regions were reported as significant if the initial single-tailed uncorrected voxel-wise P value was <0.001, and then the P value was <0.05 after family-wise error correction (Pcorr) for multiple comparisons across the small volume or whole brain. Planned analyses were as follows: (i) whole-brain analysis; (ii) analysis of regional response to emotional dynamics; (iii) analysis of regional response to encoding opportunities; (iv) correlation of regional activity with d′; and (v) subsequent memory effect analysis. Additionally, we planned an assessment of the integrity of the blood-oxygen-level-dependent (BOLD) analysis assumptions.
Results
Subject characteristics
There was no significant difference between the treatment groups in age, sex, body mass index, education, or state/trait anxiety. Full details are provided in the Supplementary material.
Validation of International Affective Picture System normative values
On the representative sample of 40 IAPS images, there was a strong correlation between the cohort rating and the normative values for both valence (r=0.949, P<0.001) and arousal (r=0.705, P<0.001). The mean difference between the cohort rating and the normative values was −0.16 (sem 0.09, t34=−1.67, P=0.105) for valence and 0.19 (sem 0.21, t34=0.928, P=0.360) for arousal. This validated the use of the IAPS normative values in characterizing the emotional content of the images used in the study.
Behavioural results
Memory effects
Behavioural results are comprehensively shown in Table 1. The median response rate during the scanner encoding task was 98%, with no difference between the groups, indicating that the low dose of propofol did not significantly impair the ability of subjects to remain attentive and perform the experimental tasks. The median false-alarm rate was 4% and did not differ between the groups. Propofol caused a modest decrease in correct recognition of the second image presentation (P=0.019).
Table 1.
Behavioural results. Values shown in bold describe the results for the entire image set (i.e. all negative–arousing and neutral images combined). The test statistics and exact P-values shown relate to these comparisons. Tests with a normal distribution are reported with the t statistic and mean (sem). Tests with a non-normal distribution are reported with the Mann–Whitney U statistic and median [quartile, quartile]. Shown below each main result are the results for each emotional subtype. Here, statistical testing was conducted using a Holm–Sidak post hoc t test comparison in a repeated-measures anova, reported as mean (sem), and indicated in the table as follows: *P<0.05, **P<0.01, ***P<0.001. d′=z(hits)−z(false alarms). c=−0.5[z(hits)+z(false alarms)]
| Placebo (n=17) | Propofol (n=18) | Test statistic | P-value | |
|---|---|---|---|---|
| Encoding (scanner) task | ||||
| Response rate | 0.99 [0.95,1.00] | 0.95 [0.87, 0.99] | U=109.50 | 0.151 |
| Hit rate | 0.91 [0.86, 0.96] | 0.83 [0.76, 0.90] | U=74.00 | 0.009 |
| Negative–arousing | 0.92 (0.02)*** | 0.86 (0.02)*** | ||
| Neutral | 0.87 (0.02)*** | 0.79 (0.03)*** | ||
| False-alarm rate | 0.04 [0.01, 0.06] | 0.05 [0.02, 0.12] | U=107.50 | 0.137 |
| Negative–arousing | 0.04 (0.01) | 0.07 (0.02) | ||
| Neutral | 0.04 (0.01) | 0.06 (0.01) | ||
| d′ | 3.21 (0.19) | 2.60 (0.16) | t33=2.47 | 0.019 |
| Negative–arousing | 3.35 (0.17)* | 2.74 (0.17) | ||
| Neutral | 3.02 (0.17)* | 2.43 (0.14) | ||
| Criterion location (c) | 0.24 (0.05) | 0.32 (0.06) | t33=−1.15 | 0.257 |
| Negative–arousing | 0.12 (0.07)* | 0.23 (0.06)* | ||
| Neutral | 0.30 (0.05)* | 0.39 (0.06)* | ||
| Recognition task | ||||
| Response rate | 0.99 [0.98, 1.00] | 0.99 [0.98, 1.00] | U=152.00 | 0.986 |
| Hit rate | 0.78 (0.05) | 0.41 (0.06) | t33=4.97 | <0.001 |
| Negative–arousing | 0.82 (0.04)* | 0.42 (0.06) | ||
| Neutral | 0.73 (0.06)* | 0.39 (0.06) | ||
| False-alarm rate | 0.08 [0.04, 0.12] | 0.09 [0.05, 0.13] | U=139.50 | 0.667 |
| Negative–arousing | 0.15 (0.02)*** | 0.16 (0.03)*** | ||
| Neutral | 0.05 (0.02)*** | 0.05 (0.01)*** | ||
| d′ | 2.26 (0.19) | 1.10 (0.22) | t33=4.08 | <0.001 |
| Negative–arousing | 2.21 (0.20)* | 0.88 (0.22)** | ||
| Neutral | 2.54 (0.22)* | 1.30 (0.19)** | ||
| Criterion location (c) | 0.27 (0.09) | 0.82 (0.09) | t33=−4.36 | <0.001 |
| Negative–arousing | 0.05 (0.08)*** | 0.67 (0.11)*** | ||
| Neutral | 0.53 (0.11)*** | 1.06 (0.10)*** | ||
In the recognition task, the propofol group had a marked and expected decrease in memory for the items presented 120 min earlier (P<0.001). Propofol caused a significant shift in the criterion location, indicating a generalized bias away from nominating items as having been seen previously (P<0.001). The median false-alarm rate was 8%, double that observed in the encoding task. Notably, the false-alarm rate for negative–arousing images was three times that for neutral images in both groups (P<0.001). Hence, on the d′ measure, which corrects for false alarms, performance for negative–arousing images was markedly inferior to that for neutral images in the propofol group (P=0.002) and marginally inferior in the placebo group (P=0.046). The criterion location showed that subjects in both groups were biased such that they were more likely to endorse negative–arousing images than neutral images.
Reaction time
For the placebo group, the mean reaction time in the encoding task was 1329.0 (sem 44.6) ms for the first presentation and 1257.5 (49.3) ms for the second presentation. For the propofol group, the mean reaction times were 1553.4 (56.4) and 1509.2 (59.9) ms. In a two-way repeated-measures anova, the reaction time for old items was significantly shorter than for new items (F1,33=10.56, P=0.003), and there was a significant effect of propofol in prolonging overall reaction time (F1,33=10.58, P=0.003). No significant effects were identified in the recognition task.
Functional magnetic resonance imaging results
Blood-oxygen-level-dependent analysis assumptions
To evaluate whether propofol caused significant effects on the BOLD signal as a result of something other than the vascular response to activation, we analysed the raw signal at the peak activation voxel in primary visual sensory cortex identified in the placebo group (x=−12, y=−90, z=−6, Pcorr<0.001). This location was chosen because it serves the maximal degree of automated processing, upstream from drug-induced changes in associative and modulatory function. The group average BOLD signal across all trials over all subjects within each group, as calculated by statistical parametric mapping, was identical (see Supplementary material). We interpreted this as demonstration that BOLD analysis could proceed with the normal assumptions.
Whole-brain analysis
A whole-brain analysis of all stimulus items combined was performed to assess for a main effect of propofol. As such analyses do not have an a priori hypothesis, we adopt a conservative threshold for reporting significance (see Methods above and Supplementary material Methods). Using these strict criteria, no regions were reported as significant.
Two exploratory whole-brain analyses using less conservative criteria were also performed (Fig. 2). At a threshold of uncorrected P<0.001 (Fig. 2a), propofol appeared to decrease activity in the left and right putamen and left parahippocampus and to cause a relative increase in activity in the right inferior frontal operculum, right rectus, left precentral gyrus, and right postcentral gyrus. Figure 2b shows the overall pattern of BOLD activation at a liberal threshold of uncorrected P<0.05 and should be regarded as purely exploratory.
Fig 2.
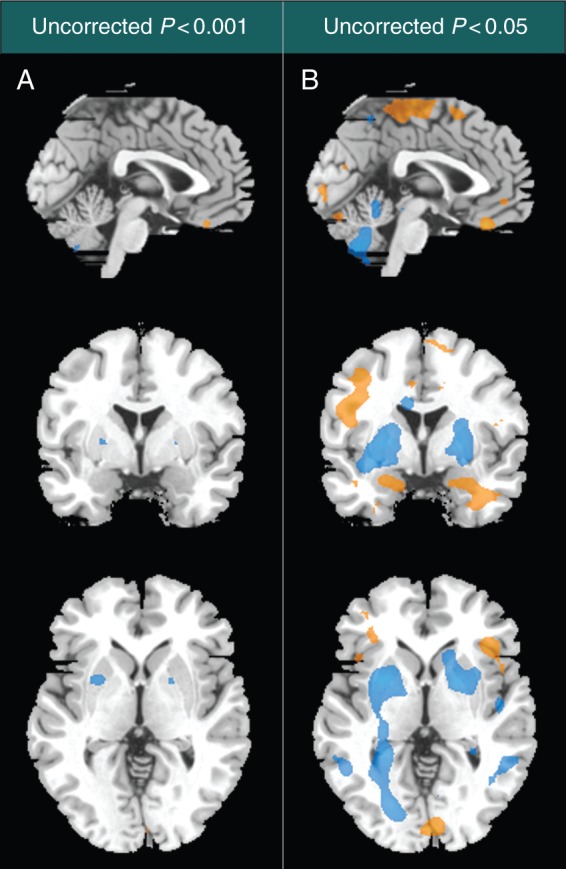
Exploratory assessment of whole-brain changes in the pattern of blood-oxygen-level-dependent (BOLD) activation. Whole-brain contrasts are shown at the exploratory visualization thresholds of uncorrected P<0.001 (column A), and uncorrected P<0.05 (column B). The contrast includes all stimuli and is undifferentiated with regard to the cognitive task. Regions in which propofol has a relative increase in BOLD signal are shown in orange, while regions with a relative decrease are shown in blue. The sagittal, coronal, and axial images represent plane views at Montreal Neurologic Institute co-ordinates x=0, y=0, and z=0.
Response to emotional dynamics
Detailed results are shown in Table 2. In the placebo group, activation on exposure to negative–arousing items was greater than that for neutral items in both the right (P=0.002) and left amygdala (P=0.020) and in both the right (P=0.018) and left hippocampus (P=0.001). In the propofol group, activation for negative–arousing items was greater than that for neutral items in the right (P=0.001) and left amygdala (P=0.008), but not in the hippocampi (Fig. 3). In a between-group contrast to assess whether this effect of emotional valence was less in the propofol than in the placebo group, activation of the right hippocampal–parahippocampal junction was significantly less in propofol-treated subjects than in placebo-treated subjects (P=0.038).
Table 2.
Blood-oxygen-level-dependent response to emotional dynamics. Clusters shown are those containing voxel-wise maxima with Pcorr<0.05 after small volume correction using bilateral automated anatomical labelling masks. Coordinates are quoted using the Montreal Neurological Institute spatial reference
| Drug condition | Region | x | y | z | Zpeak | Voxel-wise Pcorr | Cluster (mm3) |
|---|---|---|---|---|---|---|---|
| Emotional exposure: negative–arousing vs neutral | |||||||
| Placebo | Negative–arousing > neutral | ||||||
| Right amygdala | 24 | 0 | −15 | 4.14 | 0.002 | 513 | |
| Left amygdala | −18 | 0 | −15 | 3.43 | 0.020 | 108 | |
| Left hippocampus | −12 | −36 | 0 | 4.86 | 0.001 | 486 | |
| Right hippocampus | 21 | −33 | −3 | 3.88 | 0.018 | 351 | |
| Propofol | Negative–arousing > neutral | ||||||
| Right amygdala | 27 | −3 | −12 | 4.41 | 0.001 | 972 | |
| Left amygdala | −24 | 0 | −15 | 3.80 | 0.008 | 297 | |
| Propofol vs Placebo | Propofol < placebo | ||||||
| Right hippocampal–parahippocampal junction | 18 | −36 | −6 | −3.59 | 0.038 | 108 | |
Fig 3.
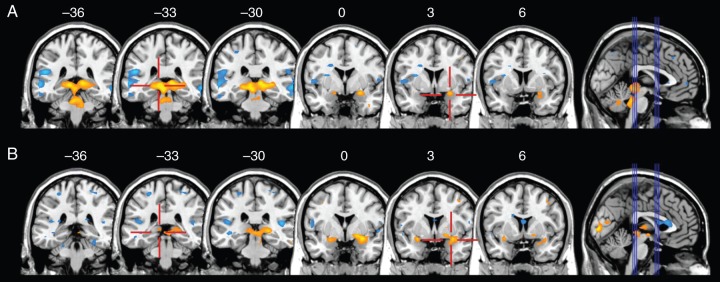
Response to emotional dynamics. Contrast between exposure to negative—arousing items and neutral items. Three coronal slices through the hippocampus (y-co-ordinates −36, −33, −30) and amygdala (0, 3, 6) are selected. (a) is placebo, and (b) propofol. Propofol had no effect on the amygdala response to emotional arousal, but markedly attenuated the hippocampal response. The scale shows color rendering of the groupwise t values, with yellows positive and blues negative. The threshold for color rendering is |t| ≥3.37.
Response to encoding opportunities
At the first presentation, there was no statistically significant difference between propofol and placebo in medial temporal lobe activation; there was a trend towards lower activation of the right hippocampus in the propofol group [Z value at peak voxel (Zpeak)=−3.32, Pcorr=0.076]. At the second presentation, there was significantly lower activation of the left hippocampus in the propofol group than in the placebo group (P=0.047). When both first and second presentations were combined, there was no significant difference between propofol and placebo groups in medial temporal lobe activation.
Correlation of activity with d′
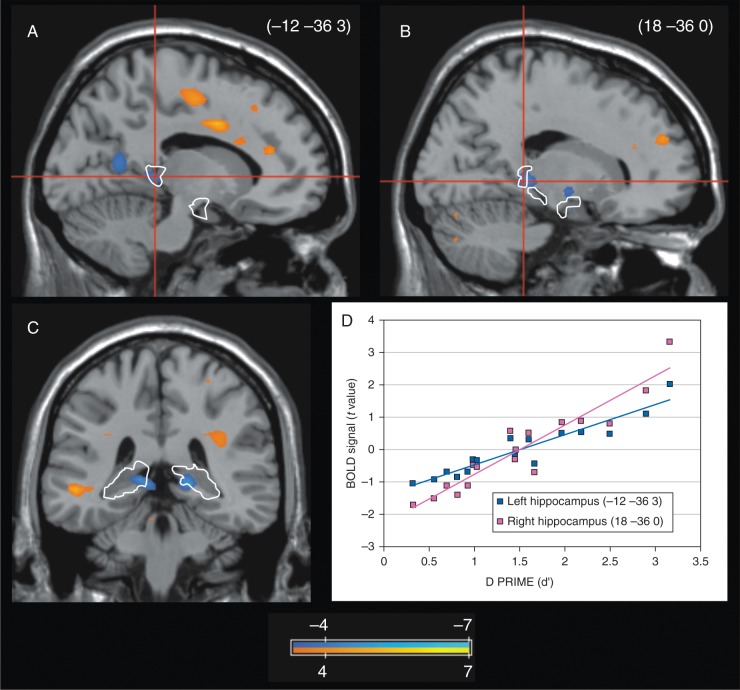
In a regression contrast, the degree of subsequent amnesia caused by propofol, as measured by d′, was correlated with activation of the right hippocampus at the time of the first presentation (P=0.027) and with activation of the left hippocampus at the time of the second presentation (P=0.035). When both first and second presentations were combined, d′ was correlated with decreased activation in both the left (P=0.045) and right hippocampus (P=0.035; Fig. 4).
Fig 4.
Hippocampal predictors of propofol amnesia. Rendering of a regression model correlating d′ with activation in the propofol condition. The analysis combines first and second image presentations. (a) Left parasagittal slice in the plane x=−12; (b) Right parasagittal slice in the plane x=18; (c) Coronal slice in the plane y=−36; (d) Relationship between d′ and the blood-oxygen-level-dependent (BOLD) signal extracted from the peak voxel in the left (blue) and right (red) hippocampus. Scale shows color rendering of the groupwise t values. The threshold for color rendering is |t| ≥3.37. Automated Anatomical Labeling masks for the hippocampus are indicated by the white line.
Subsequent memory effect
All trials in the encoding task were sorted on the basis of whether the item was later reported as remembered or forgotten in the recognition task. Assessment for a subsequent memory effect was conducted by contrasting items later reported as remembered with those later reported as forgotten. Detailed results are shown in Table 3.
Table 3.
Blood-oxygen-level-dependent response and memory. Clusters shown are those containing voxel-wise maxima with Pcorr<0.05 after small volume correction using bilateral automated anatomical labelling masks. Coordinates are quoted using the Montreal Neurological Institute spatial reference
| Drug condition | Region | x | y | z | Zpeak | Voxel-wise Pcorr | Cluster (mm3) |
|---|---|---|---|---|---|---|---|
| Response to encoding opportunities | |||||||
| Propofol vs placebo | Propofol < placebo | ||||||
| Second presentation | Left hippocampus | −27 | −30 | −6 | −3.49 | 0.047 | 54 |
| Correlation of activity with d′ | |||||||
| Propofol | Positive correlation with d′ | ||||||
| First presentation | Right hippocampus | 18 | −36 | 0 | 3.80 | 0.027 | 54 |
| Second presentation | Left hippocampus | −12 | −36 | 3 | 3.70 | 0.035 | 135 |
| Combined presentations | Left hippocampus | −12 | −36 | 3 | 3.62 | 0.045 | 108 |
| Right hippocampus | 18 | −36 | 0 | 3.71 | 0.035 | 54 | |
| Subsequent memory effect | |||||||
| Placebo | Items later remembered > items later forgotten | ||||||
| First presentation | Left hippocampus | −21 | −33 | 3 | 3.89 | 0.016 | 270 |
| Second presentation | Right hippocampus | 24 | −39 | −3 | 3.73 | 0.028 | 162 |
| Combined presentations | Left amygdala | −27 | −3 | −12 | 3.24 | 0.032 | 27 |
| Left hippocampus | −18 | −36 | 0 | 4.12 | 0.008 | 405 | |
In placebo-treated subjects, a subsequent memory effect was identified in the left hippocampus at the time of the first presentation (P=0.016) and in the right hippocampus at the time of the second presentation (P=0.028). When both presentations were combined, a subsequent memory effect was identified in the left amygdala (P=0.034) and in the posterior left hippocampus (P=0.008).
In propofol-treated subjects, no significant subsequent memory effect was identified. The finding closest to significance was a region in the left hippocampus (x=−18, y=−39, z=6; Zpeak=3.20, Pcorr=0.134) observed in the combined presentations.
We next contrasted the groups to assess whether the subsequent memory effect for propofol was less than that for placebo. There was no statistically significant finding. The finding closest to significance was a region in the left parahippocampus (x=−33, y=−45, z=−9; Zpeak=3.76, Pcorr=0.065).
Discussion
In this event-related fMRI study, we sought to determine the effects of propofol on encoding activity in the medial temporal lobe emotional memory system. The principal finding is that propofol has no discernible effect on the amygdalar response to emotional arousal, but causes marked suppression of the hippocampal response, which corresponds to the degree of amnesia. Together, these findings raise the possibility that amygdala-dependent fear systems may remain intact, even when a patient has diminished or no memory of events.
The region of the amygdala important to the present study is the basolateral complex, which projects to the hippocampus via the entorhinal cortex.28 Activation of the basolateral amygdala in response to neurohumoral stress and emotion modulates consolidation processes in the hippocampus, leading to enhanced learning and memory.29,30 We found that the stereotypic response of the amygdala to emotionally arousing stimuli was unaffected by propofol, whereas the response of the hippocampus was markedly suppressed. In the placebo condition, we demonstrated that memory performance was related to activation of both the amygdala and the hippocampus, replicating previous findings31 and providing strong support for the memory modulation model. However, in the propofol condition, memory performance became unrelated to amygdalar activation, but remained correlated with activation in the hippocampus. Furthermore, the degradation in memory performance caused by propofol was greater for emotional items than it was for neutral ones. A parsimonious explanation is that propofol abolishes emotional modulation and causes amnesia via mechanisms that commonly involve hyporesponsiveness of the hippocampus.
One plausible explanation for the contrasting effects of propofol on amygdalar and hippocampal memory processes points to heterogeneity in the GABAA receptor subtypes found in the two structures. The hippocampus is exclusively dense in α5GABAA receptors,9 and the amnestic effect of other GABAergic anaesthetics are dependent on this subtype.32,33 In contrast, the basolateral amygdala is dominated by α1GABAA receptors,10 and these have been demonstrated to mediate the effect of volatile anaesthetics on amygdala-dependent fear conditioning.34 The potency and efficacy of propofol and other anaesthetics at the GABAA receptor depend on the subtype,35 but are largely uncharacterized. Another observation, at the systems level, is that theta (4–8 Hz) phase locking between the basolateral amygdala and the hippocampus is associated with emotional memory modulation36 and consolidation in fear-based paradigms.37,38 We1 and others39 have previously suggested that the amnestic effects of GABAergic anaesthetics involve interference with synchronized theta activity networks converging on the hippocampus. We do not intend to represent that the present study provides evidence in support of these, or any other, mechanistic possibilities; however, it is important to acknowledge that diversity in the effects of propofol on amygdalar and hippocampal function is not unexpected.
Our study is the first neuroimaging investigation of propofol and the emotional memory system, but can be placed in the context of two previous imaging studies investigating other anaesthetic drugs. Alkire and colleagues18 demonstrated that emotional memory modulation was abolished as the concentration of sevoflurane transitioned from 0.2 to 0.25% and was associated with suppression of amygdalohippocampal effective connectivity. Hayama and colleagues40 investigated the α2A-adrenoceptor agonist dexmedetomidine and demonstrated that both the amygdalar response to emotional arousal and behavioural memory modulation were preserved. This is a critical contrast with the results of our study and those of Alkire and colleagues,18 which found that propofol and sevoflurane preserved the amygdalar response, but blocked downstream memory modulation effects.
Certain aspects of our study design and limitations warrant elaboration. In the continuous recognition task that we applied in the scanner, each image was shown twice. When the subject correctly recognizes the second presentation, it demonstrates that a memory trace has survived beyond immediate working memory and that any amnesia subsequently observed represents the failure of a memory to consolidate, rather than a failure to be acquired. Multiple fMRI studies have demonstrated that hippocampal activity at both presentations is dominated by encoding function,41–43 but it must be acknowledged that the second presentation involves retrieval activity and positive familiarity judgements, and so will not be functionally identical to the first. It is therefore not surprising that we identified lateralization differences between the two presentations. Lateralization of function in the medial temporal lobe memory system is incompletely understood and may depend on nuanced specifics of the experimental paradigm that extend beyond the purpose of this study. We report the results, but caution that they be viewed as exploratory only. Functional organization along the longitudinal axis of the hippocampus is also incompletely understood and sensitive to the paradigm specifics. Our findings were dominantly posterior, in a region previously identified to serve lower-level perceptual processes and true/false judgement.31,44 Again, full interpretation lies beyond the scope of the present study.
To assess the activations associated with memory behaviour, we applied both a subsequent memory analysis and a regression analysis using the sensitivity index d′. A limitation of the subsequent memory analysis is that it assumes that items categorized as remembered are true hits (signal) and does not accommodate for false alarms (noise). In the propofol condition, a low hit rate because of amnesia and a relatively high false-alarm rate combined to diminish the signal-to-noise ratio and compromised our ability to demonstrate a subsequent memory effect with the number of trials available. The sensitivity index d′ compensates for false alarms, and so it is not surprising that it was a more robust assessment in the propofol cohort.
Blood-oxygen-level-dependent signal analysis assumes that the blood flow response is coupled to neuronal activity.45 Functional magnetic resonance imaging studies of anaesthetic drugs must acknowledge the possibility of a direct vascular effect unrelated to neuronal demand. Low concentrations of propofol do not shift the regional cerebral demand–flow relationship at rest,46 but this cannot be extrapolated to exclude coupling variations during activation definitively. As a validation assessment, we examined the haemodynamic response function at the site of maximal automated visual processing and found that BOLD signal at this location was unaffected by propofol. This is reassuring, as is the absence of a substantial main effect of propofol, but we recognize our limitations in comprehensively precluding a drug effect on BOLD signal in all regions.
Our results raise an important clinical question. If propofol is ineffective at suppressing the amygdalar response to neurohumoral stress, is it possible that neural processes with psychopathological potential could still occur under propofol anaesthesia, despite a patient reporting amnesia?47,48 Recent data suggest that as many as 15% of patients may screen positive for symptoms of post-traumatic stress disorder after surgery.49 Functional magnetic resonance imaging studies clearly demonstrate that fear learning can be produced in the absence of awareness,50,51 and there is evidence suggesting that surgical stress facilitates priming, a non-emotional form of unconscious memory, in patients receiving propofol.52 We stress that our results cannot be extrapolated to the concentrations of propofol used in general anaesthesia, but they should raise concern. A thorough characterization should also address whether the sedative effects of the drug block protective top-down cognitive controls and whether the co-administration of other agents is protective. Answering these questions is methodologically challenging, but should be attempted as the specialty addresses the long-term cognitive sequelae of anaesthesia and perioperative stress.
Supplementary material
Supplementary material is available at British Journal of Anaesthesia online.
Authors' contributions
K.O.P., J.C.R., E.S., H.P., and D.A.S. contributed to the study design; K.O.P. and M.M. contributed to the acquisition of data; K.O.P., J.C.R., M.M., E.S., H.P., R.A.S., and D.A.S. contributed to data analysis and interpretation; K.O.P. and J.C.R. wrote the manuscript.
Declaration of interest
K.O.P. is an member of the Associate Editorial Board of the BJA.
Funding
Foundation for Anesthesia Education and Research (Research Starter Grant to K.O.P.); National Institutes of Health (Bethesda, MD, USA; K08 GM083213 to K.O.P.); Department of Anesthesiology, Weill Cornell Medical College (New York, NY, USA).
Supplementary Material
Acknowledgements
The authors wish to thank Daniel Feiler, BA, Meg Kane, BA, Jackie Bogan, BA, and Anne Blackstock-Bernstein, BA (Research Assistants, Department of Anesthesiology, Weill Cornell Medical College, New York, NY, USA).
References
- 1.Pryor KO, Reinsel RA, Mehta M, Li Y, Wixted JT, Veselis RA. Visual P2-N2 complex and arousal at the time of encoding predict the time domain characteristics of amnesia for multiple intravenous anesthetic drugs in humans. Anesthesiology 2010; 113: 313–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veselis RA, Pryor KO, Reinsel RA, Li Y, Mehta M, Johnson R., Jr Propofol and midazolam inhibit conscious memory processes very soon after encoding: an event-related potential study of familiarity and recollection in volunteers. Anesthesiology 2009; 110: 295–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veselis RA, Pryor KO, Reinsel RA, Mehta M, Pan H, Johnson R., Jr Low-dose propofol-induced amnesia is not due to a failure of encoding: left inferior prefrontal cortex is still active. Anesthesiology 2008; 109: 213–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pryor KO, Proekt A, Blackstock-Bernstein AS, Wixted JT, Root JC. Administration of propofol after learning improves memory performance in human subjects via loss of competitive consolidation: evidence that propofol amnesia occurs at the induction of consolidation and reconsolidation. Anesthesiology 2012; 117: BOC09 [Google Scholar]
- 5.Eichenbaum H. A cortical–hippocampal system for declarative memory. Nat Rev Neurosci 2000; 1: 41–50 [DOI] [PubMed] [Google Scholar]
- 6.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci 2009; 10: 423–33 [DOI] [PubMed] [Google Scholar]
- 7.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010; 35: 169–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron 2009; 62: 757–71 [DOI] [PubMed] [Google Scholar]
- 9.Wainwright A, Sirinathsinghji DJS, Oliver KR. Expression of GABAA receptor α5 subunit-like immunoreactivity in human hippocampus. Brain Res Mol Brain Res 2000; 80: 228–32 [DOI] [PubMed] [Google Scholar]
- 10.Marowsky A, Fritschy JM, Vogt KE. Functional mapping of GABAA receptor subtypes in the amygdala. Eur J Neurosci 2004; 20: 1281–9 [DOI] [PubMed] [Google Scholar]
- 11.Dutton RC, Maurer AJ, Sonner JM, Fanselow MS, Laster MJ, Eger EI., 2nd The concentration of isoflurane required to suppress learning depends on the type of learning. Anesthesiology 2001; 94: 514–9 [DOI] [PubMed] [Google Scholar]
- 12.Veselis RA, Reinsel RA, Feshchenko VA, Dnistrian AM. A neuroanatomical construct for the amnesic effects of propofol. Anesthesiology 2002; 97: 329–37 [DOI] [PubMed] [Google Scholar]
- 13.Veselis RA, Feshchenko VA, Reinsel RA, Beattie B, Akhurst TJ. Propofol and thiopental do not interfere with regional cerebral blood flow response at sedative concentrations. Anesthesiology 2005; 102: 26–34 [DOI] [PubMed] [Google Scholar]
- 14.Davis MH, Coleman MR, Absalom AR, et al. Dissociating speech perception and comprehension at reduced levels of awareness. Proc Natl Acad Sci USA 2007; 104: 16032–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adapa RM, Davis MH, Stamatakis EA, Absalom AR, Menon DK. Neural correlates of successful semantic processing during propofol sedation. Hum Brain Mapp 2014; 35: 2935–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gili T, Saxena N, Diukova A, Murphy K, Hall JE, Wise RG. The thalamus and brainstem act as key hubs in alterations of human brain network connectivity induced by mild propofol sedation. J Neurosci 2013; 33: 4024–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stamatakis EA, Adapa RM, Absalom AR, Menon DK. Changes in resting neural connectivity during propofol sedation. PLoS One 2010; 5: e14224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alkire MT, Gruver R, Miller J, McReynolds JR, Hahn EL, Cahill L. Neuroimaging analysis of an anesthetic gas that blocks human emotional memory. Proc Natl Acad Sci USA 2008; 105: 1722–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kensinger EA, Schacter DL. Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. J Neurosci 2006; 26: 2564–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Britton JC, Taylor SF, Sudheimer KD, Liberzon I. Facial expressions and complex IAPS pictures: common and differential networks. Neuroimage 2006; 31: 906–19 [DOI] [PubMed] [Google Scholar]
- 21.Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): affective ratings of pictures and instruction manual. Technical Report A-6 Gainsville, FL: University of Florida, 2005 [Google Scholar]
- 22.Schnider TW, Minto CF, Gambus PL, et al. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology 1998; 88: 1170–82 [DOI] [PubMed] [Google Scholar]
- 23.Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, Rauch SL. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress Anxiety 2008; 25: 496–505 [DOI] [PubMed] [Google Scholar]
- 24.Mujica-Parodi LR, Korgaonkar M, Ravindranath B, et al. Limbic dysregulation is associated with lowered heart rate variability and increased trait anxiety in healthy adults. Hum Brain Mapp 2009; 30: 47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spielberger CD, Lushene RE, Jacobs GA. Manual for the Stait-Trait Anxiety Inventory, STAI (Form Y). Palo Alto, CA: Consulting Psychologists Press, 1983 [Google Scholar]
- 26.Pan H, Epstein J, Silbersweig DA, Stern E. New and emerging imaging techniques for mapping brain circuitry. Brain Res Rev 2011; 67: 226–51 [DOI] [PubMed] [Google Scholar]
- 27.Friston K, Ashburner J, Kiebel S, Nichols T, Penny W, eds. Statistical Parametric Mapping: the Analysis of Functional Brain Images. London: Academic Press, Elsevier, 2007 [Google Scholar]
- 28.Pikkarainen M, Rönkkö S, Savander V, Insausti R, Pitkänen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol 1999; 403: 229–60 [PubMed] [Google Scholar]
- 29.Roozendaal B, McGaugh JL. Memory modulation. Behav Neurosci 2011; 125: 797–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 2004; 27: 1–28 [DOI] [PubMed] [Google Scholar]
- 31.Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron 2004; 42: 855–63 [DOI] [PubMed] [Google Scholar]
- 32.Cheng VY, Martin LJ, Elliott EM, et al. Alpha5GABAA receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidate. J Neurosci 2006; 26: 3713–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin LJ, Oh GH, Orser BA. Etomidate targets α5γ-aminobutyric acid subtype A receptors to regulate synaptic plasticity and memory blockade. Anesthesiology 2009; 111: 1025–35 [DOI] [PubMed] [Google Scholar]
- 34.Sonner JM, Cascio M, Xing Y, et al. α1 subunit-containing GABA type A receptors in forebrain contribute to the effect of inhaled anesthetics on conditioned fear. Mol Pharmacol 2005; 68: 61–8 [DOI] [PubMed] [Google Scholar]
- 35.Olsen RW. Analysis of γ-aminobutyric acid (GABA) type A receptor subtypes using isosteric and allosteric ligands. Neurochem Res 2014; 39: 1924–41 [DOI] [PubMed] [Google Scholar]
- 36.Pelletier JG, Paré D. Role of amygdala oscillations in the consolidation of emotional memories. Biol Psychiatry 2004; 55: 559–62 [DOI] [PubMed] [Google Scholar]
- 37.Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science 2003; 301: 846–50 [DOI] [PubMed] [Google Scholar]
- 38.Lesting J, Narayanan RT, Kluge C, Sangha S, Seidenbecher T, Pape HC. Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PLoS One 2011; 6: e21714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perouansky M, Rau V, Ford T, et al. Slowing of the hippocampal theta rhythm correlates with anesthetic-induced amnesia. Anesthesiology 2010; 113: 1299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayama HR, Drumheller KM, Mastromonaco M, Reist C, Cahill LF, Alkire MT. Event-related functional magnetic resonance imaging of a low dose of dexmedetomidine that impairs long-term memory. Anesthesiology 2012; 117: 981–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duzel E, Habib R, Rotte M, Guderian S, Tulving E, Heinze HJ. Human hippocampal and parahippocampal activity during visual associative recognition memory for spatial and nonspatial stimulus configurations. J Neurosci 2003; 23: 9439–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stark CE, Okado Y. Making memories without trying: medial temporal lobe activity associated with incidental memory formation during recognition. J Neurosci 2003; 23: 6748–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson JD, Muftuler LT, Rugg MD. Multiple repetitions reveal functionally and anatomically distinct patterns of hippocampal activity during continuous recognition memory. Hippocampus 2008; 18: 975–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cabeza R, Rao SM, Wagner AD, Mayer AR, Schacter DL. Can medial temporal lobe regions distinguish true from false? An event-related functional MRI study of veridical and illusory recognition memory. Proc Natl Acad Sci USA 2001; 98: 4805–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ekstrom A. How and when the fMRI BOLD signal relates to underlying neural activity: the danger in dissociation. Brain Res Rev 2010; 62: 233–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaisti KK, Långsjö JW, Aalto S, et al. Effects of sevoflurane, propofol, and adjunct nitrous oxide on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology 2003; 99: 603–13 [DOI] [PubMed] [Google Scholar]
- 47.Pryor KO, Hemmings HC., Jr Increased risk of awareness under anesthesia: an issue of consciousness or of memory? Anesthesiology 2013; 119: 1236–8 [DOI] [PubMed] [Google Scholar]
- 48.Wang M, Messina AG, Russell IF. The topography of awareness: a classification of intra-operative cognitive states. Anaesthesia 2012; 67: 1197–201 [DOI] [PubMed] [Google Scholar]
- 49.Whitlock EL, Rodebaugh TL, Hassett AL, Jacobsohn E, Mashour GA, Avidan M. Psychological sequelae of surgery in a cohort of patients from three intraoperative awareness prevention trials. Anaesth Analg 2015; 120: 87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson AK, Christoff K, Panitz D, De Rosa E, Gabrieli JD. Neural correlates of the automatic processing of threat facial signals. J Neurosci 2003; 23: 5627–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 1998; 18: 411–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deeprose C, Andrade J, Varma S, Edwards N. Unconscious learning during surgery with propofol anaesthesia. Br J Anaesth 2004; 92: 171–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.