Abstract
Background
Little is known about ageing-related changes in the brain that affect emergence from general anaesthesia. We used young adult and aged Fischer 344 rats to test the hypothesis that ageing delays emergence from general anaesthesia by increasing anaesthetic sensitivity in the brain.
Methods
Time to emergence was determined for isoflurane (1.5 vol% for 45 min) and propofol (8 mg kg−1 i.v.). The dose of isoflurane required to maintain loss of righting (LOR) was established in young adult and aged rats. The efficacy of methylphenidate to reverse LOR from general anaesthesia was tested. Separate young adult and aged rats with implanted electroencephalogram (EEG) electrodes were used to test whether ageing increases sensitivity to anaesthetic-induced burst suppression.
Results
Mean time to emergence from isoflurane anaesthesia was 47 s [95% CI 33, 60; young adult) compared with 243 s (95% CI 185, 308; aged). For propofol, mean time to emergence was 13.1 min (95% CI 11.9, 14.0; young adult) compared with 23.1 min (95% CI 18.8, 27.9; aged). These differences were statistically significant. When methylphenidate was administered after propofol, the mean time to emergence decreased to 6.6 min (95% CI 5.9, 7.1; young adult) and 10.2 min (95% CI 7.9, 12.3; aged). These reductions were statistically significant. Methylphenidate restored righting in all rats during continuous isoflurane anaesthesia. Aged rats had lower EEG power and were more sensitive to anaesthetic-induced burst suppression.
Conclusions
Ageing delays emergence from general anaesthesia. This is due, at least in part, to increased anaesthetic sensitivity in the brain. Further studies are warranted to establish the underlying causes.
Keywords: ageing, delayed emergence from anaesthesia, electroencephalography
Editor's key points.
Ageing increases sensitivity to general anaesthetics, but its impact on emergence from anaesthesia is uncertain.
The return of the righting reflex following isoflurane or propofol anaesthesia was delayed in aged compared with young adult rats.
This effect is pharmacodynamic rather than pharmacokinetic, as the older rats also showed greater anaesthetic-induced suppression of brain activity.
Although the behaviours that define general anaesthesia (unconsciousness, amnesia, analgesia, and akinesia) are reversible, general anaesthesia can cause delirium and persistent cognitive deficits, particularly in the elderly.1–4 Advancing age is a key risk factor for developing postoperative cognitive dysfunction (POCD).5 As the global population ages, more elderly patients are requiring general anaesthesia for surgery and invasive procedures.6 Therefore it is important to better understand how the ageing brain is affected by general anaesthetic drugs.
Ageing decreases the minimum anaesthetic concentration (MAC; the dose of inhaled anaesthetic required to produce immobility in response to pain), and this has been demonstrated in both humans7 and rodents.8 However, inhaled anaesthetics produce immobility by actions in the spinal cord,9,10 and less is known regarding how ageing changes sensitivity to anaesthetic-induced unconsciousness in the brain. It has been reported that ageing delays the recovery of psychomotor functions after general anaesthesia11,12 and that the doses of inhaled anaesthetics at which patients emerge from general anaesthesia decrease with age.13
In this study we tested the hypothesis that ageing delays emergence from general anaesthesia by increasing brain sensitivity to anaesthetic drugs. First, time to emergence from general anaesthesia was determined in young adult and aged rats for both isoflurane and propofol. We then established the minimum steady-state dose of isoflurane required to maintain loss of righting (LOR) in aged and young adult rats and also tested whether methylphenidate induces emergence from isoflurane and propofol anaesthesia. Finally, we studied separate cohorts of young adult and aged rats with implanted electroencephalogram (EEG) electrodes to test whether ageing alters sensitivity to burst suppression induced by isoflurane and propofol.
Methods
Animal care and use
Animal studies were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee (IACUC). Twenty-two male Fischer 344 (F344) rats (National Institute on Ageing, Bethesda, MD, USA) were used for this study (weight 360–466 g). Eleven rats were 6–8 months of age (‘young adult’) and 11 rats were 24–26 months of age (‘aged’) at the time of the study. We chose these two age groups because the typical lifespan of a rat is approximately 2–3 years and body weight is similar for these two age groups.14 For all behavioural experiments we used the same six rats from each group, and each animal was provided with at least 3 days of rest between experiments. Separate cohorts of five young adult and five aged rats underwent surgery for implantation of extradural electrodes for EEG studies. Details regarding our anaesthesia equipment and protocols have been published.15,16 All animals were kept on a standard day–night cycle (lights on at 7 am and off at 7 pm) and fed ad libitum. All experiments were performed during the day.
Preparation and delivery of drugs
Isoflurane, propofol, and methylphenidate hydrochloride were purchased from Henry Schein (Melville, NY, USA), APP Pharmaceuticals (Shaumburg, IL, USA) and Sigma-Aldrich (St. Louis, MO, USA), respectively. Methylphenidate was dissolved in 0.5 ml of normal saline (NS) and sterile filtered immediately prior to administration. Intravenous tubing (volume ∼0.6 ml) was flushed with 2 ml of NS after i.v. drug administration to ensure complete delivery of the drug.
Determination of MAC-LOR for isoflurane
General anaesthesia was induced with 2.5% isoflurane and a 24G i.v. catheter was placed in the lateral tail vein. Rats were then placed supine in a custom-made acrylic anaesthetizing chamber with ports for administration of i.v. drugs and gas sampling. A rectal temperature probe was inserted and a syringe pump was used to infuse NS at a rate of 2 ml h−1 to ensure patency. The isoflurane concentration was adjusted every 40 min as described,15 and MAC-LOR was defined as the lowest dose of isoflurane sufficient to maintain loss of righting for 40 min. After 40 min at the final dose of isoflurane, a 2-ml NS bolus was administered to ensure that bolus delivery did not produce arousal, and the rectal temperature probe was removed. None of the rats exhibited purposeful movement after these manoeuvres. Although the Ohmeda 5250 anaesthesia agent analyser (GE Healthcare, Waukesha, WI) is convenient for continuous monitoring of isoflurane concentration, it is only accurate to ±0.2% isoflurane. Therefore a Riken FI-21 gas indicator (Riken Keiki, Tokyo, Japan) was used to determine the final isoflurane concentration in the chamber with precision (accurate to 0.01% isoflurane).
EEG electrode placement and recording
Extradural EEG electrodes were surgically implanted under isoflurane anaesthesia as described.16 Postoperative analgesia was provided and animals were allowed to rest for a minimum of 7 days to fully recover. The EEG signal was recorded using a QP511 Quad AC Amplifier System (Grass Instruments, West Warwick, RI, USA) and a USB-6009 14-bit data acquisition board (National Instruments, Austin, TX, USA). The sampling rate was 500 Hz and data were bandpass filtered between 0.3 and 50 Hz. A line filter was used to eliminate 60 Hz noise.
In order to characterize neurophysiological differences between aged and young adult rats at different doses of isoflurane, general anaesthesia was induced with 3.0% isoflurane, a rectal temperature probe was inserted, and the chamber was placed on a warming pad to ensure normothermia. The inhaled isoflurane concentration was maintained for 40 min to achieve a steady-state concentration in the brain, after which 10 min of EEG data were recorded and analysed. The inhaled isoflurane concentration was then decreased in steps of 0.5% from 3.0 to 1.5%, with 40 min at each dose prior to recording 10 min of EEG data. For each 10-min epoch of EEG recorded at a certain isoflurane concentration, the data were segmented for burst suppression and burst suppression probability (BSP) was calculated as described below.
To characterize neurophysiological differences between aged and young adult rats with propofol anaesthesia, rats with EEG electrodes had an i.v. catheter placed under isoflurane anaesthesia as described.16 After the rat regained the righting reflex, we waited at least 15 min to ensure complete recovery from isoflurane anaesthesia and then started recording the EEG. Propofol (12 mg kg−1 i.v.) was administered and the animal was placed on a warming pad in room air. After the rat regained the righting reflex, the i.v. catheter was removed.
Identification and segmentation of EEG bursts and suppressions
For both the isoflurane and propofol experiments, the recorded EEG was visually inspected for periods of burst suppression, as described.17 Each EEG recording was first bandpass filtered between 0.1 and 35 Hz using a finite impulse response (FIR) filter with a Blackman window to eliminate noise. A Gaussian function with a width of 2 s was created and convoluted with the filtered EEG. This convolution was then subtracted from the filtered EEG to linearly detrend the signal and the energy of the signal was calculated using the non-linear energy operator.18 The energy was convoluted once more with the Gaussian function to smooth the non-linear energy operator and a threshold was used in the energy domain to clearly separate large energy bursts from lower energy suppressions. Both the width of the Gaussian function and the energy-based threshold were manually set.
Calculation of burst suppression probability
Indices of the EEG that were above the threshold were designated as ‘bursts’ and given a value of 0, while indices that fell below the threshold were designated as ‘suppression’ and given a value of 1. A state-space model was then applied to this binary time series to give the instantaneous burst suppression probability (BSP) over time.19 The BSP is similar to the burst suppression ratio in that a BSP value of 1 indicates a state of EEG suppression, while a value of 0 indicates a lack of suppression or burst. However, unlike the burst suppression ratio, the BSP provides a smoothed signal with corresponding CIs.
Spectral analysis of the EEG
Power spectral density estimations from the baseline (awake) EEG were calculated using multitaper methods.20–22 For the multitaper calculation, 2-s non-overlapping windows were used over approximately 10 min of EEG data in each animal. The half-bandwidth was 1.5 Hz and five tapers were used in its construction. Power spectral density estimates were then pooled across animals to create group estimates of young adult and aged power spectral density. The 95% CIs were calculated from these power spectral density estimations using a percentile bootstrap method.23
Statistical analysis of emergence time and loss of righting
Prism 5.04 (GraphPad Software, San Diego, CA, USA) or MATLAB R2014b (MathWorks, Natick, MA, USA) was used for statistical analysis and, when possible, results were reported in terms of 95% CIs of the mean based on percentile bootstrap analysis. The 95% CIs for the difference of two group means can be used to ascertain significance. If the 95% CIs are >0, then there is a significant increase; if the 95% CIs are <0, then there is a significant decrease; but if the 95% CIs include 0, then there is no significance. This is equivalent to using a significance α of 0.05.
Results
Ageing delays emergence from isoflurane and propofol anaesthesia
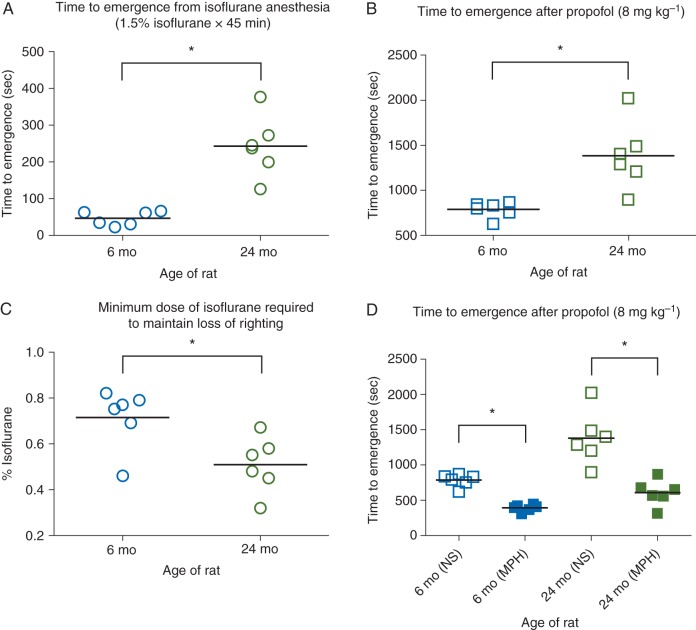
Young adult and aged rats underwent isoflurane anaesthesia at the same dose (1.5% for 45 min), after which they were exposed to room air. As shown in Figure 1a, mean time to emergence was 47 s (95% CI 33, 60; n=6) for young adult rats and 243 s (95% CI 185, 308; n=6) for aged rats. This difference was statistically significant [mean difference 197 s (95% CI 137, 261)]. The mean rectal temperature was 36.9°C (95% CI 36.6, 37.1) for young adult rats and 36.7°C (95% CI 36.5, 36.8) for aged rats. This difference was not statistically significant [mean difference 0.2°C (95% CI −0.1, 0.5)]. As shown in Figure 1b, the mean time to emergence from propofol anaesthesia was 785 s (95% CI 715, 840; n=6) for young adult rats and 1380 s (95% CI 1130, 1670; n=6) for aged rats. This difference was statistically significant [mean difference 598 s (95% CI 338, 893)].
Fig 1.
(a) Scatter plot of time to emergence after isoflurane anaesthesia (1.5% inhaled for 45 min) in young adult (6–8 months old; blue circles) and aged (24–26 months old; green circles) F344 rats. Return of righting was used to define emergence. (b) Scatter plot of time to emergence from propofol anaesthesia (8 mg kg−1 i.v.) in young adult (blue squares) and aged (green squares) F344 rats. (c) Scatter plot of minimum isoflurane dose necessary to maintain loss of righting in young adult (blue circles) and aged (green circles) F344 rats. (d) Scatter plots of time to emergence from propofol anaesthesia (8 mg kg−1 i.v.) in young adult (filled blue squares) and aged (filled green squares) F344 rats that received methylphenidate (MPH; 5 mg kg−1 i.v.). The data from (b) are shown again here to provide direct comparison with controls (NS: normal saline). For each data set, the horizontal line represents the mean. *Significant difference between groups with 95% confidence.
Aged rats have a decreased dose requirement for isoflurane-induced LOR
The mean inhaled concentration of isoflurane required to maintain LOR was 0.71% (95% CI 0.61, 0.79; n=6) for young adult rats and 0.51% (95% CI 0.42, 0.59; n=6) for aged rats (Fig. 1c). This difference was statistically significant [mean difference 0.20% (95% CI 0.07, 0.33)]. The mean rectal temperature was 36.6°C (95% CI 36.5, 36.8) for young adult rats and 36.7°C (95% CI 36.5, 36.9) for aged rats, which was not statistically significant [mean difference 0.1°C (95% CI −0.2, 0.3)].
Methylphenidate induces reanimation from general anaesthesia in aged rats
Methylphenidate induces return of the righting reflex from general anaesthesia,15,16 most likely by activating a dopaminergic arousal system.24,25 Methylphenidate (5 mg kg−1 i.v.) was administered after establishing the steady-state dose of isoflurane required for maintaining LOR. As reported previously,15 animals began to show signs of behavioural arousal within seconds, such as eye opening, kicking, and clawing, despite continuous inhalation of the same dose of isoflurane. The righting reflex was restored in all young adult (n=6) and aged (n=6) rats after methylphenidate administration. The mean time to righting after methylphenidate was 50 s (95% CI 36, 64; n=6) for young adult rats and 45 s (95% CI 30, 60; n=6) for aged rats. This difference was not statistically significant [mean difference 5 s (95% CI −16, 26)].
To test the efficacy of methylphenidate in accelerating recovery from propofol anaesthesia, propofol (8 mg kg−1 i.v.) was administered, followed by methylphenidate (5 mg kg−1 i.v.). Methylphenidate decreased the mean time to emergence from 785 s (95% CI 715, 840; n=6) to 394 s (95% CI 357, 426; n=6) for young adult rats and from 1383 s (95% CI 1130, 1674; n=6) to 611 s (95% CI 478, 738; n=6) for aged rats (Fig. 1d). The reductions for both young adult rats [mean difference 391 s (95% CI 313, 460)] and aged rats [mean difference 773 s (95% CI 488, 1094)] were statistically significant.
EEG power is decreased in aged rats
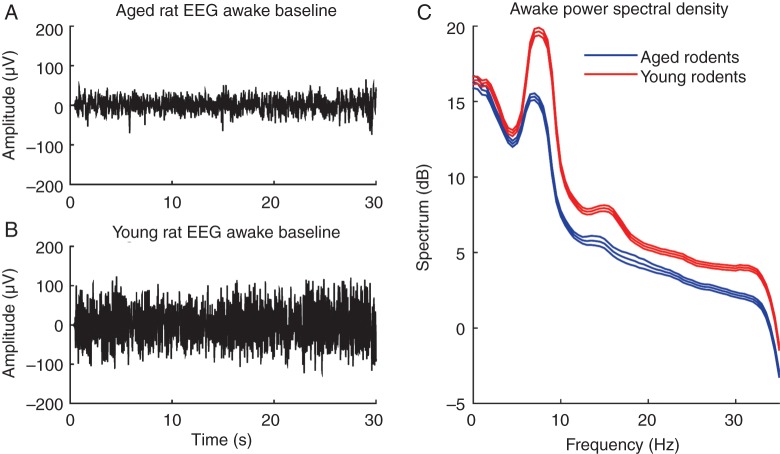
A typical 30-s EEG trace from an aged rat during the awake state is shown in Figure 2a and a similar baseline recording from a young adult rat is shown in Figure 2b. In these examples, peak-to-peak amplitude of the EEG is approximately two-fold greater in the young adult rat than the aged rat. Pooled power spectral densities of baseline recordings from young adult (n=4) and aged rats (n=5) are shown in Figure 2c. The middle line represents the group mean, while the upper and lower lines represent 95% CIs. The CIs do not overlap, indicating that the group power spectral density for young adult rats was significantly higher than for aged rats.
Fig 2.
(a) Representative 30-s EEG recording from an aged rat in the awake state. (b) Representative 30-s EEG recording from a young adult rat in the awake state. (c) Group power spectral density computed from baseline EEG recordings of aged (n=5) and young adult (n=4) rats. Each individual baseline EEG was approximately 10 min in duration. Upper and lower lines represent 95% CIs. Non-overlapping CIs indicate that the young adult group has significantly higher EEG power compared with the aged group.
Lower doses of isoflurane induce burst suppression in aged rats
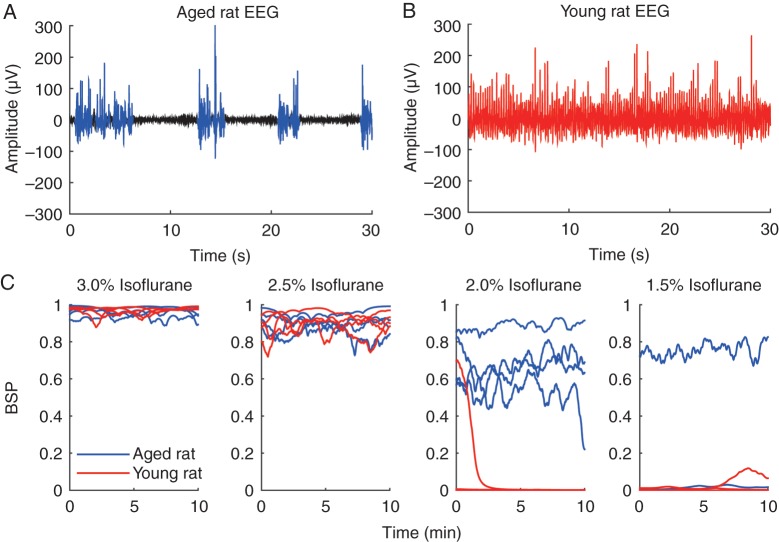
EEG recordings were taken during a continuous isoflurane ramp-down experiment in young adult (n=4) and aged (n=4) rats. Figure 3a shows a typical 30-s EEG trace from an aged rat inhaling 2.0% isoflurane. Bursts are shown in blue and suppressions are shown in black. Figure 3b shows a typical 30-s EEG trace from a young adult rat, also inhaling 2.0% isoflurane. Despite the same dose of isoflurane, the aged rat was in burst suppression while the young adult rat was not. Figure 3c shows the calculated BSP across all concentrations of isoflurane from each of the four aged and four young adult animals. At 3.0 and 2.5% isoflurane the BSP was 0.8–0.9, indicating that all animals were in deep burst suppression. At 2.0% isoflurane, the BSP for the aged animals was in the range of 0.6–0.8, while the BSP for the young adult animals was at or close to 0, indicating that they were not in burst suppression. At 1.5% isoflurane, one aged rat remained in burst suppression, while the rest of the rats were no longer in burst suppression.
Fig 3.
(a) Representative 30-s EEG recording from an aged rat inhaling 1.5% isoflurane, showing a burst suppression pattern. Bursts are highlighted in blue and suppressions are in black. (b) Representative 30-s EEG recording from a young adult rat inhaling 1.5% isoflurane. This animal was not in burst suppression, indicating a lighter depth of anaesthesia. (c) Burst suppression probability (BSP) for 10 min of EEG data at 3.0, 2.5, 2.0, and 1.5% isoflurane. At 2.0% isoflurane, aged rats mainly exhibited a burst suppression pattern, whereas young adult rats did not. One aged rat was still in burst suppression at 1.5% isoflurane.
Aged rats remain in burst suppression longer than young adult rats after a propofol bolus
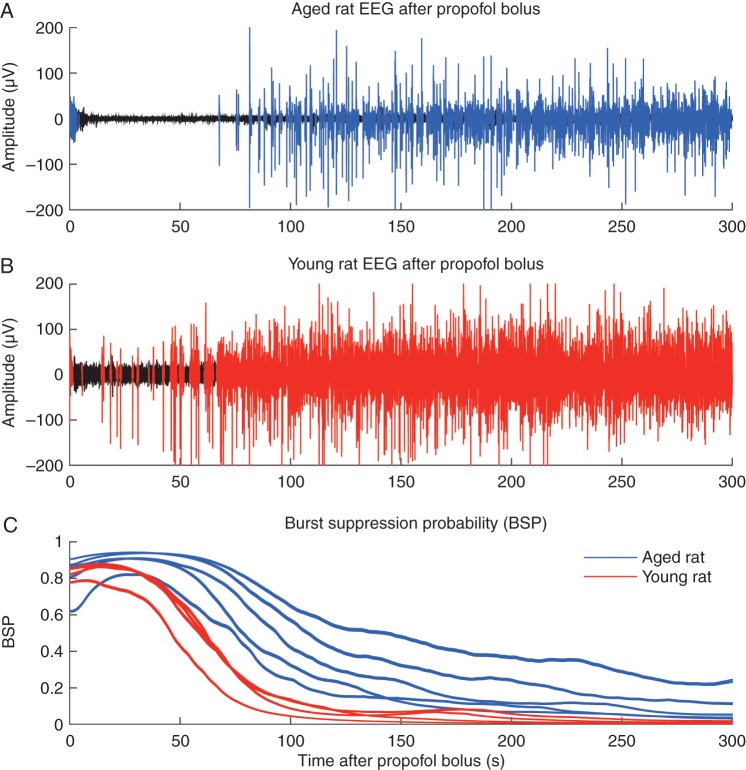
Figure 4a shows a 5-min EEG trace immediately after administration of propofol (12 mg kg−1 i.v.) in an aged rat. Bursts are shown in blue and suppressions are shown in black. Figure 4b shows an analogous trace recorded from a young adult rat, with bursts in red and suppressions in black. At the same propofol dose, the aged rat was in a deeper state of anaesthesia for a longer period of time compared with the young adult rat. Figure 4c shows the calculated BSP for 5 min after propofol injection in five aged (blue) and four young adult (red) rats. The area of the lines represents 95% CIs. Although both groups entered a state of EEG burst suppression, after 50 s the BSP of young adult rats decreased quickly, and was significantly lower than for aged rats.
Fig 4.
(a) Representative 300-s EEG trace from an aged rat after a propofol bolus (12 mg kg−1 i.v.). Periods of suppression are shown in black and bursts are in blue. (b) A representative 300-s EEG trace from a young adult rat after administration of a propofol bolus (12 mg kg−1i.v.). Periods of suppression are shown in black and bursts are in red. (c) The individual burst suppression probabilities (BSPs) for aged (n=5) and young adult (n=4) rats for 300 s after propofol administration. For the BSP, a value of 1 indicates complete suppression while a value of 0 indicates no suppression. Shaded areas around the BSP indicate 95% CIs. Aged rats remained in burst suppression significantly longer than younger rats.
Discussion
Our results show that ageing causes delayed emergence from general anaesthesia in F344 rats. Ageing prolongs emergence after both inhaled isoflurane and i.v. propofol, demonstrating that delayed emergence is not a unique feature of a particular general anaesthetic or route of delivery. However, time to emergence from general anaesthesia can be greatly affected by both pharmacokinetic and pharmacodynamic factors. To test specifically for pharmacodynamic differences, we determined the steady-state dose of isoflurane necessary to maintain LOR and found that aged rats had a 32% decrease in dose requirement, showing a significant pharmacodynamic contribution to the delayed emergence observed with ageing.
Because factors outside the brain (such as muscle atrophy with ageing) might affect a rat's ability to achieve a behavioural endpoint such as righting, additional studies were performed using rats with implanted EEG electrodes. This made it possible to monitor neurophysiological activity at different doses of general anaesthetics without this confounding factor. In the awake state, aged rats had markedly lower EEG power compared with young adult rats. Furthermore, aged rats were more susceptible to EEG burst suppression induced by isoflurane or propofol, providing evidence that the aged brain is more sensitive to anaesthetic drugs. The present findings are consistent with human studies that reported lower dose requirements for burst suppression in elderly patients with isoflurane26 and propofol.27
Stijnen and colleagues28 reported that in BN/BiRij rats, ageing did not appreciably alter the pharmacokinetics of phenobarbital, but the threshold cerebrospinal fluid concentration for LOR was significantly lower in aged rats. The authors concluded that altered pharmacodynamics are responsible for the increased sensitivity to phenobarbital in aged rats, which is consistent with the results of the present study using F344 rats with isoflurane and propofol. Neither the blood–gas partition coefficient29 nor most tissue–gas partition coefficients30 change significantly for isoflurane with ageing, providing further evidence that pharmacodynamics likely play the dominant role.
Studies on anaesthetic sensitivity and ageing have focused on the endpoint of immobility, or lack of movement in response to noxious stimulation, which is used to determine MAC.31 Loss and colleagues8 reported that aged (24-month-old) F344 rats had a 17% reduction in halothane MAC compared with young adult (5-month-old) controls, suggesting that F344 rats are a suitable animal model to study ageing-related changes in MAC. However, the spinal cord is the major mediator of anaesthetic-induced immobility,9,10 whereas the righting reflex is mediated by midbrain structures,32 which are more closely related to the structures underlying arousal and consciousness. Because the brain and spinal cord might be differentially affected by the process of ageing, it is important to study ageing-related changes in both areas of the central nervous system.
Postoperative delirium occurs in 20–40% of patients >60 yr of age, and recent studies suggest that early postoperative delirium might be a harbinger of persistent postoperative cognitive dysfunction (POCD).1,2 In two large studies, 10–12% of patients >60 yr of age had persistent POCD 3 months after non-cardiac surgery.3,4 Because elderly patients are more sensitive to general anaesthetics, they might be more susceptible to unnecessary overdosing, which in turn could exacerbate cognitive side effects.33,34 Because anaesthetic sensitivity varies among individuals, utilizing neurophysiological monitoring to tailor the anaesthetic dose to each patient could avoid unnecessary overdosing.
The biological mechanisms by which ageing makes the brain more sensitive to anaesthesia are just beginning to be understood,35 and increased anaesthetic sensitivity with age could be due to numerous factors. Decreases in cholinergic tone with ageing might lead to delayed emergence from the deepest stages of anaesthesia.36 GABAergic neurones might increase in relative proportion to other cell types, particularly in cognitively affected subjects.37 Ageing alters the sensitivity of the GABA carrier to propofol and etomidate.38 Finally, there is growing evidence that multiple arousal systems undergo degenerative changes with ageing,39 which could affect anaesthetic sensitivity. More work is needed to understand the causes of increased anaesthetic sensitivity in the ageing brain.
The dose of inhaled anaesthetic required to induce general anaesthesia has been reported to be greater than the dose required for emergence (‘neural inertia’).40 In this study we established the time to emergence from general anaesthesia, as well as the lowest steady-state dose of isoflurane required to maintain LOR. However, we did not determine the dose of isoflurane required to induce LOR, and therefore it is unclear whether the process of ageing affects neural inertia.
In previous studies with Sprague–Dawley rats, we found that methylphenidate induces return of the righting reflex from general anaesthesia with isoflurane15 and propofol.16 A similar effect can be induced by a D1 dopamine receptor agonist24 and electrical stimulation of the ventral tegmental area,25 a key dopamine nucleus in the midbrain, suggesting that methylphenidate acts by stimulating a dopaminergic arousal circuit projecting from this area. We found that methylphenidate induces emergence from isoflurane anaesthesia with similar efficacy in aged and young adult F344 rats, and also reduces time to emergence from propofol anaesthesia. These findings suggest that monoaminergic arousal circuits stimulated by methylphenidate remain largely intact in aged rats.
In summary, we show that aged rats have delayed emergence from general anaesthesia with isoflurane and propofol, and that altered pharmacodynamic factors likely play the dominant role over pharmacokinetic factors. These findings suggest that aged F344 rats are a suitable animal model for further in vivo research on the causes of ageing-related changes in anaesthetic sensitivity. Because elderly patients are susceptible to anaesthetic overdosing that might lead to long-term cognitive dysfunction, the present findings suggest that neurophysiological monitoring of elderly patients under general anaesthesia is highly desirable. Further research is necessary to elucidate the mechanisms underlying increased anaesthetic sensitivity in the ageing brain.
Authors' contributions
J.J.C.: collected data, analysed data, and critically revised the manuscript. J.D.K.: collected data, analysed data, and critically revised the manuscript. O.O.: collected data, analysed data, and critically revised the manuscript. N.E.T.: analysed data and critically revised the manuscript. E.Y.K.: analysed data and critically revised the manuscript. P.L.P.: analysed data and critically revised the manuscript. E.N.B.: conceived project, interpreted data, and critically revised the manuscript. K.S.: conceived project, collected data, analysed data, interpreted data, and wrote the manuscript.
Declaration of interest
None declared.
Funding
This work was supported by National Institutes of Health grant numbers TR01-GM104948 (E.N.B., K.S.), DP1-OD003646 (E.N.B.), K08-GM094394 (K.S.), and T32-NS048005 (E.K.).
References
- 1.Bickel H, Gradinger R, Kochs E, Forstl H. High risk of cognitive and functional decline after postoperative delirium. A three-year prospective study. Dement Geriatr Cogn Disord 2008; 26: 26–31 [DOI] [PubMed] [Google Scholar]
- 2.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med 2012; 367: 30–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 1998; 351: 857–61 [DOI] [PubMed] [Google Scholar]
- 4.Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 2008; 108: 18–30 [DOI] [PubMed] [Google Scholar]
- 5.Hudson AE, Hemmings HC., Jr Are anaesthetics toxic to the brain? Br J Anaesth 2011; 107: 30–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown EN, Purdon PL. The aging brain and anesthesia. Curr Opin Anaesthesiol 2013; 26: 414–9 [DOI] [PubMed] [Google Scholar]
- 7.Eger EI., II Age, minimum alveolar anesthetic concentration, and minimum alveolar anesthetic concentration-awake. Anesth Analg 2001; 93: 947–53 [DOI] [PubMed] [Google Scholar]
- 8.Loss GE, Jr, Seifen E, Kennedy RH, Seifen AB. Aging: effects on minimum alveolar concentration (MAC) for halothane in Fischer-344 rats. Anesth Analg 1989; 68: 359–62 [PubMed] [Google Scholar]
- 9.Antognini JF, Schwartz K. Exaggerated anesthetic requirements in the preferentially anesthetized brain. Anesthesiology 1993; 79: 1244–9 [DOI] [PubMed] [Google Scholar]
- 10.Rampil IJ. Anesthetic potency is not altered after hypothermic spinal cord transection in rats. Anesthesiology 1994; 80: 606–10 [DOI] [PubMed] [Google Scholar]
- 11.Keita H, Peytavin G, Giraud O, et al. Aging prolongs recovery of psychomotor functions at emergence from propofol-alfentanil anaesthesia. Can J Anaesth 1998; 45: 1211–4 [DOI] [PubMed] [Google Scholar]
- 12.Shinozaki M, Usui Y, Yamaguchi S, Okuda Y, Kitajima T. Recovery of psychomotor function after propofol sedation is prolonged in the elderly. Can J Anaesth 2002; 49: 927–31 [DOI] [PubMed] [Google Scholar]
- 13.Katoh T, Suguro Y, Ikeda T, Kazama T, Ikeda K. Influence of age on awakening concentrations of sevoflurane and isoflurane. Anesth Analg 1993; 76: 348–52 [PubMed] [Google Scholar]
- 14.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci 1999; 54: B492–501 [DOI] [PubMed] [Google Scholar]
- 15.Solt K, Cotten JF, Cimenser A, Wong KF, Chemali JJ, Brown EN. Methylphenidate actively induces emergence from general anesthesia. Anesthesiology 2011; 115: 791–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chemali JJ, Van Dort CJ, Brown EN, Solt K. Active emergence from propofol general anesthesia is induced by methylphenidate. Anesthesiology 2012; 116: 998–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenny JD, Westover MB, Ching S, Brown EN, Solt K. Propofol and sevoflurane induce distinct burst suppression patterns in rats. Front Syst Neurosci 2014; 8: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser JF. On a simple algorithm to calculate the energy of a signal. In: 1990 International Conference on Acoustics, Speech, and Signal Processing. ICASSP-1990 New York: IEEE, 1990: 381–4 [Google Scholar]
- 19.Chemali J, Ching S, Purdon PL, Solt K, Brown EN. Burst suppression probability algorithms: state-space methods for tracking EEG burst suppression. J Neural Eng 2013; 10: 056017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babadi B, Brown EN. A review of multitaper spectral analysis. IEEE Trans Biomed Eng 2014; 61: 1555–64 [DOI] [PubMed] [Google Scholar]
- 21.Mitra P, Bokil H. Observed Brain Dynamics. Oxford: Oxford University Press, 2008 [Google Scholar]
- 22.Thomson DJ. Spectrum estimation and harmonic analysis. Proc IEEE 1982; 70: 1055–96 [Google Scholar]
- 23.Efron B, Tibshirani R. An Introduction to the Bootstrap. New York: Chapman & Hall, 1993 [Google Scholar]
- 24.Taylor NE, Chemali JJ, Brown EN, Solt K. Activation of D1 dopamine receptors induces emergence from isoflurane general anesthesia. Anesthesiology 2013; 118: 30–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solt K, Van Dort CJ, Chemali JJ, Taylor NE, Kenny JD, Brown EN. Electrical stimulation of the ventral tegmental area induces reanimation from general anesthesia. Anesthesiology 2014; 121: 311–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz AE, Tuttle RH, Poppers PJ. Electroencephalographic burst suppression in elderly and young patients anesthetized with isoflurane. Anesth Analg 1989; 68: 9–12 [PubMed] [Google Scholar]
- 27.Schultz A, Grouven U, Zander I, Beger FA, Siedenberg M, Schultz B. Age-related effects in the EEG during propofol anaesthesia. Acta Anaesthesiol Scand 2003; 48: 27–34 [DOI] [PubMed] [Google Scholar]
- 28.Stijnen AM, Danhof M, Van Bezooijen CF. Increased sensitivity to the anesthetic effect of phenobarbital in aging BN/BiRij rats. J Pharmacol Exp Ther 1992; 261: 81–7 [PubMed] [Google Scholar]
- 29.Lerman J, Gregory GA, Willis MM, Eger EI., II Age and solubility of volatile anesthetics in blood. Anesthesiology 1984; 61: 139–43 [DOI] [PubMed] [Google Scholar]
- 30.Lerman J, Schmitt-Bantel BI, Gregory GA, Willis MM, Eger EI., II Effect of age on the solubility of volatile anesthetics in human tissues. Anesthesiology 1986; 65: 307–11 [PubMed] [Google Scholar]
- 31.Eger EI, II, Saidman LJ, Brandstater B. Minimum alveolar anesthetic concentration: a standard of anesthetic potency. Anesthesiology 1965; 26: 756–63 [DOI] [PubMed] [Google Scholar]
- 32.Bignall KE. Ontogeny of levels of neural organization: the righting reflex as a model. Exp Neurol 1974; 42: 566–73 [DOI] [PubMed] [Google Scholar]
- 33.Sieber FE, Zakriya KJ, Gottschalk A, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc 2010; 85: 18–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radtke FM, Franck M, Lendner J, Kruger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth 2013; 110(Suppl 1): i98–105 [DOI] [PubMed] [Google Scholar]
- 35.Salat DH, Buckner RL, Snyder AZ, et al. Thinning of the cerebral cortex in aging. Cereb Cortex 2004; 14: 721–30 [DOI] [PubMed] [Google Scholar]
- 36.Smith ML, Booze RM. Cholinergic and GABAergic neurons in the nucleus basalis region of young and aged rats. Neuroscience 1995; 67: 679–88 [DOI] [PubMed] [Google Scholar]
- 37.Banuelos C, LaSarge CL, McQuail JA, et al. Age-related changes in rostral basal forebrain cholinergic and GABAergic projection neurons: relationship with spatial impairment. Neurobiol Aging 2013; 34: 845–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keita H, Lasocki S, Henzel-Rouelle D, Desmonts JM, Mantz J. Aging decreases the sensitivity of the GABA carrier to propofol and etomidate. Br J Anaesth 1998; 81: 249–50 [DOI] [PubMed] [Google Scholar]
- 39.Stern AL, Naidoo N. Wake-active neurons across aging and neurodegeneration: a potential role for sleep disturbances in promoting disease. Springerplus 2015; 4: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedman EB, Sun Y, Moore JT, et al. A conserved behavioral state barrier impedes transitions between anesthetic-induced unconsciousness and wakefulness: evidence for neural inertia. PLoS One 2010; 5: e11903. [DOI] [PMC free article] [PubMed] [Google Scholar]