Abstract
Background
Step length asymmetry (SLA) is a common hallmark of gait post-stroke. Though conventionally viewed as a spatial deficit, SLA can result from differences in where the feet are placed relative to the body (spatial strategy), the timing between foot-strikes (step time strategy), or the velocity of the body relative to the feet (step velocity strategy).
Objective
The goal of this study was to characterize the relative contributions of each of these strategies to SLA.
Methods
We developed an analytical model that parses SLA into independent step position, step time, and step velocity contributions. This model was validated by reproducing SLA values for twenty-five healthy participants when their natural symmetric gait was perturbed on a split-belt treadmill moving at either a 2:1 or 3:1 belt-speed ratio. We then applied the validated model to quantify step position, step time, and step velocity contributions to SLA in fifteen stroke survivors while walking at their self-selected speed.
Results
SLA was predicted precisely by summing the derived contributions, regardless of the belt-speed ratio. Although the contributions to SLA varied considerably across our sample of stroke survivors, the step position contribution tended to oppose the other two – possibly as an attempt to minimize the overall SLA.
Conclusions
Our results suggest that changes in where the feet are placed or changes in interlimb timing could be used as compensatory strategies to reduce overall SLA in stroke survivors. These results may allow clinicians and researchers to identify patient-specific gait abnormalities and personalize their therapeutic approaches accordingly.
Keywords: Locomotion, stroke, motor coordination, biomechanics, rehabilitation
Introduction
Stroke survivors often walk with unequal step lengths. This asymmetry can take two forms: individuals either take a smaller step with their paretic leg if they have trouble advancing it, or take a smaller step with the non-paretic leg if they have trouble standing on the paretic leg1–4. While reducing this asymmetry is a common goal following stroke5–7, current rehabilitation efforts have limited success correcting step length asymmetry8,9. For example, body-weight supported treadmill training can improve speed and single-limb stance time, but is largely unable to change step length asymmetry10. Similarly, balance training can lead to improved symmetry during standing, and a faster walking speed, but it does not improve step length asymmetry11. One possible explanation for the limited capability to reduce step length asymmetry is that multiple factors can contribute to an observed asymmetry, and effective interventions may need to identify and target these factors.
Step length asymmetry is determined not only by how far the feet are placed in front of the body, but also by the relative timing between foot-strikes. In other words, step length asymmetry has distinct contributions from control of foot placement (spatial strategy) and control of inter-limb timing (temporal strategy)12. Consequently, understanding the relative contributions of spatial and temporal strategies to step length asymmetry may help clinicians to customize their treatment based on whether individuals have more difficulty with temporal or spatial control of their limbs.
Split-belt treadmill training has the potential to correct step length asymmetry and target spatial and temporal asymmetries separately13. When individuals are initially exposed to walking with the belts moving at different speeds, step length asymmetry is magnified, and asymmetries are observed in both the spatial and temporal domains12,14–16. This initial increase in asymmetry provides a stimulus driving individuals to recalibrate their motor commands in a feed-forward manner to reduce asymmetry. When the belts are driven at the same speeds following a period of adaptation, the resulting after-effect can leave hemiparetic individuals with more symmetric step lengths17,18, and repeated exposure adaptation can cause lasting reductions in step length asymmetry19,20. Previous work has shown that the spatial strategy used to alleviate step length asymmetry can be adapted independently from the temporal strategy when individuals are provided with feedback of foot-strike location13. Though spatial and temporal aspects of walking can be dissociated during split-belt adaptation in patients with stroke21, the relative contribution of each strategy to changes in overall symmetry remains unknown.
The goal of this study is to characterize the relative contributions of spatial and temporal control to step length asymmetry. Here, spatial control refers to the location where the feet are placed, and temporal control refers to timing between foot strikes. To this end, we developed an analytical model that parses step length symmetry into independent spatial and temporal contributions. This model is used to quantify changes in spatial and temporal contributions to correct step length asymmetry during locomotor adaptation on a split-belt treadmill. Finally, we applied the model to dissociate the spatial and temporal contributions to natural step length asymmetry in hemiparetic individuals. Our results demonstrate that step length asymmetry post-stroke can result from deficits in spatial and/or temporal limb control, and how seemingly symmetric step lengths in stroke survivors can arise from opposing asymmetries in space and time.
Methods
Experimental Paradigm
Twenty-five healthy individuals (13 Female, age: 25 ±4 years) and fifteen stroke survivors (4 Female) participated in this study. Our sample of stroke survivors was recruited through physician referrals at Johns Hopkins Hospital. Individuals were included in the study if they had a diagnosis of stroke and could walk on the treadmill continuously for a minimum of five minutes. Exclusion criteria included any neurological disorders or orthopedic conditions that prevented them from walking on the treadmill. Clinical characteristics for the stroke survivors are presented in Table 1. The data from healthy individuals (previously published12,22) were reanalyzed using the framework presented in this paper. All subjects provided written informed consent before testing. The experimental protocol was approved by the Johns Hopkins University Institutional Review Board, and the study was conducted according to the principles expressed in the Declaration of Helsinki.
Table 1.
Clinical characteristics of stroke survivors
| Gender | Age | Paretic Limb |
Fugl-Meyer (Lower Limb) |
Self-selected Speed (m/s) |
|
|---|---|---|---|---|---|
| S1 | F | 62 | R | 21 | 0.37 |
| S2 | M | 65 | R | 18 | 0.71 |
| S3 | M | 55 | R | 18 | 0.54 |
| S4 | M | 42 | L | 22 | 0.96 |
| S5 | F | 56 | R | 33 | 0.57 |
| S6 | F | 68 | R | 23 | 0.26 |
| S7 | M | 57 | R | 17 | 0.61 |
| S8 | M | 52 | L | 32 | 1.02 |
| S9 | M | 67 | L | 27 | 0.33 |
| S10 | M | 53 | R | 19 | 0.17 |
| S11 | M | 54 | L | 31 | 0.35 |
| S12 | F | 29 | L | 16 | 0.14 |
| S13 | M | 70 | R | 16 | 0.06 |
| S14 | M | 60 | L | 33 | 0.28 |
| S15 | M | 78 | L | 26 | 0.10 |
| 4 F | 58±14 | 7L | 23±2 | 0.43±0.07 | |
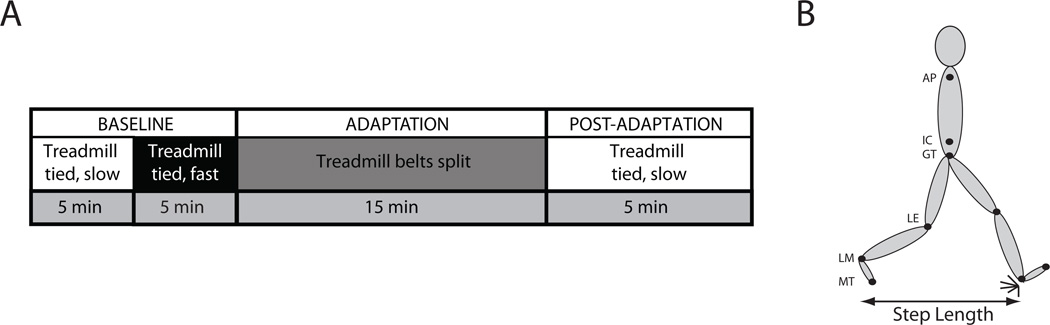
Healthy participants adapted their walking pattern on a custom split-belt treadmill with two belts moving independently (Woodway USA, Waukesha, WI). The experimental paradigm is illustrated in Figure 1a. All participants began the experiment with two 5-minute baseline trials when the belts moved at 0.75 m/s and 1.5 m/s, respectively. During the adaptation period, participants walked for 15 minutes in one of two conditions. For the 2:1 group, fourteen individuals walked on the treadmill while the left and right belts were driven at 0.75 m/s and 1.5 m/s respectively22. For the 3:1 group, eleven individuals walked on the treadmill while the left and right belts were driven at 0.5 m/s and 1.5 m/s, respectively12. During the post-adaptation period, participants walked at 0.75 m/s (2:1 group) or 0.5 m/s (3:1 group) for 5 minutes to washout the effects of adaptation.
Figure 1.
Experimental paradigm. a) Phases of the split-belt treadmill adaptation studies. Participants began with two baseline periods where both belts moved at the same speeds. This was followed by an adaptation period where the belts moved at a 2:1 or 3:1 ratio. Each study concluded with a post-adaptation period where both belts moved at the slow speed. b) Marker locations and illustration of step length. Step length was calculated as the anterior-posterior distance between the ankle markers at foot-strike.
Stroke survivors walked at a constant speed on a split-belt treadmill with both belts moving at the same speed. We found each participant’s self-selected speed by gradually increasing the speed of the treadmill until they acknowledged that the current speed was comparable to what they would use when walking outside at a casual pace. Each stroke survivor then walked for 5-minutes at this self-selected walking speed.
Kinematics
Kinematic data for all subjects were acquired with a digital camera system (Optotrak Certus, Northern Digital Inc., Waterloo, ON) at 100 Hz. This motion analysis system was used to record the position of the following anatomical landmarks bilaterally (Figure 1b): the acromion process (shoulder), iliac crest (pelvis), greater trochanter (hip), lateral femoral epicondyle (knee), lateral malleolus (ankle), and fifth metatarsal (toe). Heel-strikes and toe-offs were determined from peak anterior and posterior excursions of the toe and ankle markers using the coordinate-based algorithm developed by Zeni et al.23 which has been validated for use in both healthy individuals and stroke survivors23.
Data Analysis
We characterized the learning process using the difference in step lengths, which has been shown to characterize the adaptation of gait symmetry during split-belt walking14. Here step length is defined to be the anterior-posterior distance between the ankle markers at heel-strikes. We define step length asymmetry (SLasym) as
| (1) |
where SLfast is the step length at heel strike on the fast belt and SLslow is the step length at heel strike on the slow belt. For the stroke survivors, step length asymmetry was the non-paretic step length minus the paretic step length.
Derivation of Spatial and Temporal Contributions to Symmetry
We hypothesized that step length and step length asymmetry can be influenced by independent spatial and temporal changes in the walking pattern. In this section, we derive a mathematical expression for step length asymmetry parsing it into independent spatial and temporal contributions. This derivation is only meant to apply to step lengths, which are discrete events, and not to the entire gait cycle.
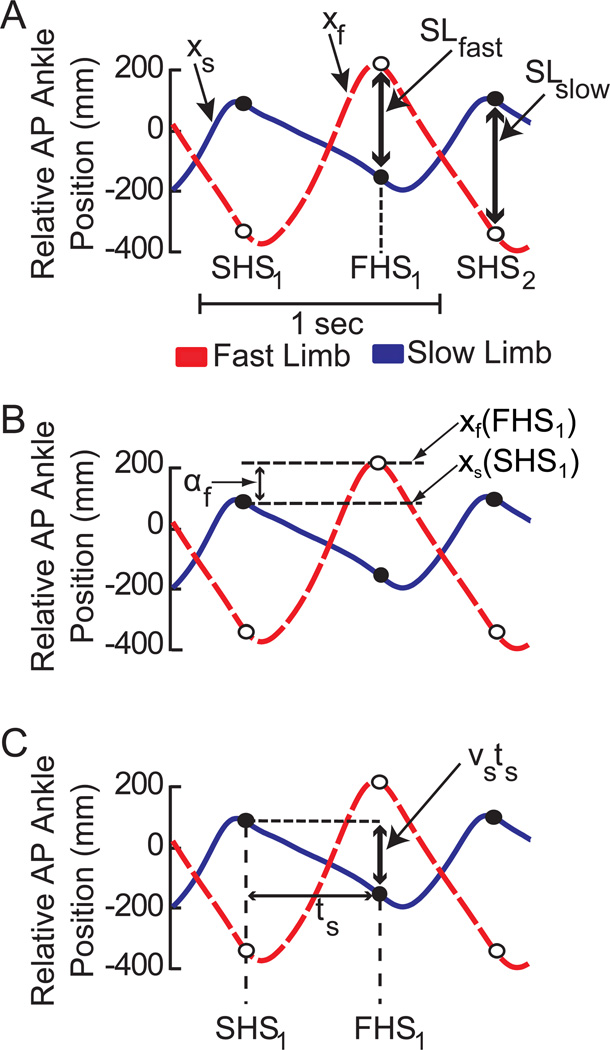
Figure 2a shows, for a single stride, the temporal order of heel strikes to be SHS1 (first slow heel strike), FHS1 (fast heel strike), and SHS2 (second slow heel strike). We define the anterior-posterior position of the ankle markers for the fast and slow foot as xf and xs, (Figure 2a, red and blue traces, respectively). These positions are defined relative to the average of the hip markers so that they are in a body-centered coordinate frame, with positive values indicating that the foot is in front of the hip. Using these coordinates, the step lengths are defined as
| (2) |
| (3) |
as shown in Figure 2a. Here, xf(FHS) and xf(SHS) indicate the ankle position on the fast belt at the fast and the slow heel strikes, FHS and SHS, respectively. Similarly, xs(FHS) and xs(SHS) indicate the ankle position on the slow belt at the fast and the slow heel strikes respectively.
Figure 2.
Illustration of parameters used to derive the spatial and temporal contributions to step length. Each panel contains representative trajectories of anterior-posterior ankle position relative to the hip for the same subject during the early phase of split-belt adaptation. Positive values indicate that the ankle is in front of the hip. Dashed red traces represent the relative ankle position of the fast limb (xf) while solid blue traces represent the ankle position of the slow limb (xs). Open and closed circles represent the locations of the fast and slow markers respectively at heel strike of the leading limb. a) Representation of fast (SLfast) and slow (SLslow) step lengths. SHS1, FHS1, and SHS2 correspond to the initial slow heel strike, the subsequent fast heel strike, and the second slow heel strike respectively. b) Graphical representation of the spatial contribution to the fast step length (af). The spatial contribution is determined by the difference in the leading ankle positions on consecutive heel-strikes. Note that shifts in timing (x-axis) do no affect the amplitude of this component. c) The temporal contribution to step length is determined by the time between heel strikes (t) and the average velocity (v) of the trailing foot relative to the hips during the interval between heel strikes. Spatial shifts in the ankle trajectory (y-axis) do not affect the temporal contribution.
First, we define a spatial variable αf that indicates where the fast foot is placed relative to the previous slow foot placement (Figure 2b). This parameter describes the contribution of leading limb flexion to step length asymmetry. The example in Figure 2b shows that the foot at fast heel strike is placed farther forward from the hip than the foot at the previous slow heel strike
| (4) |
We then derive a temporal contribution that characterizes asymmetries due to the distance that the trailing limb extends beyond the hips. Here, we first define the slow step time, ts, as the time between SHS1 and FHS1 (Figure 2c). Similarly, we define the fast step time, tf as the time between FHS1 and SHS2. The average speed of each foot relative to the hips while on the moving belt can be written as νs (Equation 5) for the slow belt and νf (Equation 6) for the fast belt.
| (5) |
| (6) |
Using this notation, the location of the slow, trailing foot at fast heel strike xs(FHS) can be written as
| (7) |
as shown in Figure 2c. In other words, the slow foot is placed at xs(SHS1) at the first heel strike and then moves backward at a rate of νs relative to the hips for time ts. If we then solve for xf(FHS1) in (4) and xs(FHS1) in (7), we can substitute the results into (2) to obtain
| (8) |
Here, αf is a spatial term that indicates where the fast foot is placed relative to the previous slow foot placement as shown in Figure 2b and νsts is a temporal term that indicates how far the slow foot moved back relative to the hips during the slow step time as shown in Figure 2c. In other words, the location of the trailing (slow) foot relative to the pelvis at heel strike depends on the slow foot’s velocity relative to the pelvis and the amount of time between heel strikes. Similarly, the slow step length can be written as
| (9) |
| (10) |
We can now rewrite eq. (1) by substituting (6) and (7) into (1) as
| (11) |
By rearranging terms, we get the following final equation for step length asymmetry
| (12) |
Here, the first term is referred to as the step position contribution since it only depends on spatial variables. The step time term quantifies step length asymmetry due to differences in step times, and the step velocity component quantifies the step length asymmetry due to differences in step velocity (i.e., velocity of the foot relative to the body during stance). These latter terms are not independent as both have a dependency on step times and step velocities. However, it is important to separate these contributions to explicitly represent whether asymmetries in step time or step velocity add to or reduce step length asymmetry. It should be noted that, reducing asymmetries in step velocity may not necessarily affect the step time contribution if the average step velocity is held constant. Similarly, reducing step time asymmetry will not affect the step velocity contribution if the average step time is held constant. In sum, shifts in foot placement only affect the step position contribution to step length asymmetry, while shifts in inter-limb timing exclusively affect the temporal (step time and step velocity) contributions to step length asymmetry.
Quantifying Spatial and Temporal Contributions to Symmetry during Adaptation
The adaptation of step length asymmetry during split-belt walking involves changes to each of the spatial and temporal components in (10). We quantified the magnitude of the perturbation imposed by the difference in belt speeds as the average step velocity contribution during the last 20 strides of adaptation. The step position and step time contributions were also averaged during the last 20 strides of adaptation. These contributions were expressed both in absolute terms with units of mm and in relative terms as a percentage of the perturbation magnitude.
We also quantified the time course of changes in the step position and step time contributions by computing the number of strides required for each parameter to reach a plateau. The plateau was defined as the average value during the last 20 strides, and the threshold for reaching plateau was defined as the point when 10 consecutive strides remained within two standard deviations of the plateau.
Identification of the Spatial and Temporal Contributions to Step length Asymmetry in Stroke Survivors
Data from our group of stroke survivors was used to illustrate how spatial and temporal strategies combine to generate step length asymmetry during hemiparetic gait. For each stride we computed step length asymmetry and the individual contributions to step length asymmetry from the spatial and temporal strategies. The fast and slow limbs in (10) represented the non-paretic and paretic limbs respectively. We expressed each contribution in absolute terms with units of mm, and the relative contributions of each strategy were expressed as a percentage of step length asymmetry.
Statistical Analysis
Statistics were performed using Matlab (Natick, Mass). Significant differences were assessed at p=0.05. A two-way ANOVA was used to look for differences in the contributions of step position and step time for each belt speed ratio. A two-way ANOVA was also used to test whether there was an effect of contribution type (step position and step time) or belt speed ratio on the time to plateau. The Tukey method for post-hoc analyses was used when significant main effects were observed. Finally, multiple linear regression was used to determine if the spatial and temporal contributions scaled linearly with the size of the perturbation during adaptation.
Results
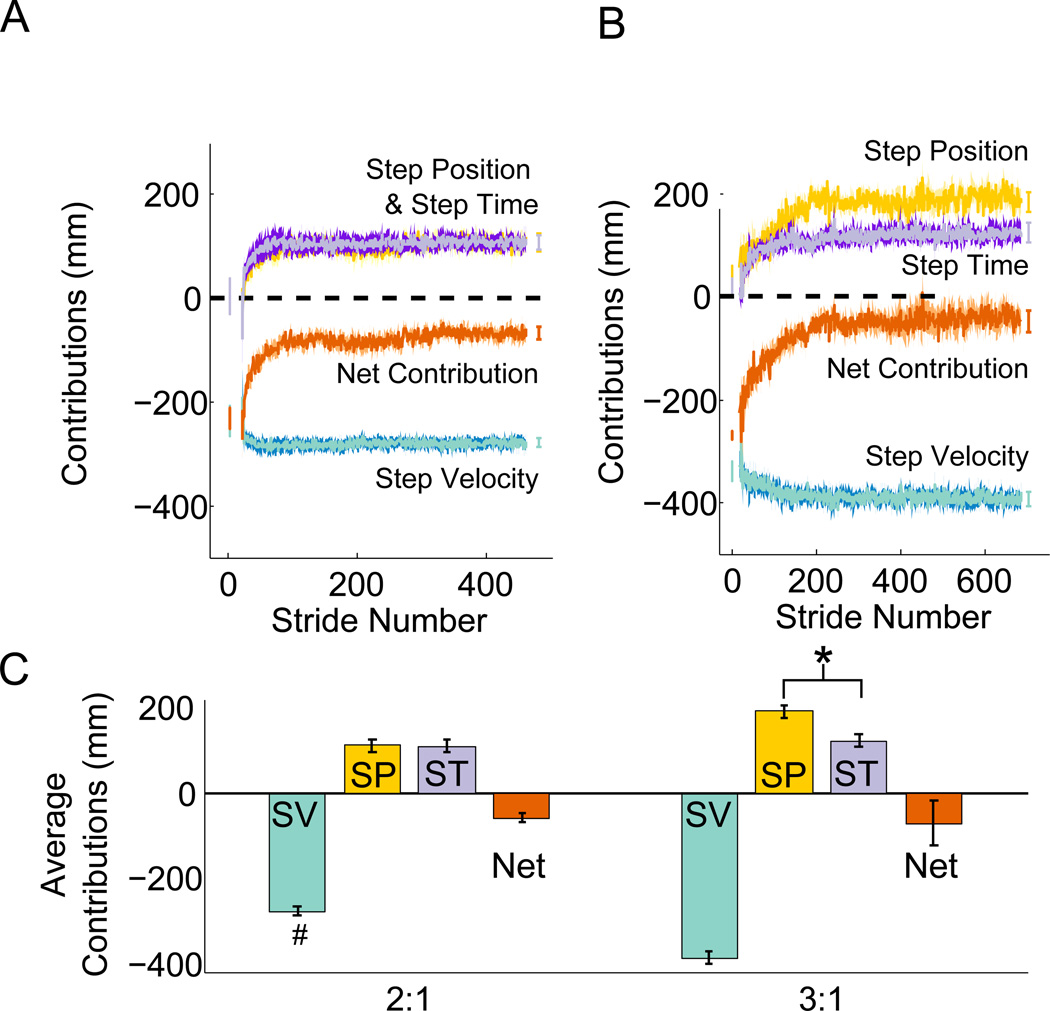
Stride-by-stride values for the individual contributions for the 2:1 and 3:1 groups are illustrated in Figure 3a and 3b. In both groups, the step velocity contribution was relatively constant throughout the entire adaptation process (green trace on Figure 3a and 3b) due to differences in belt speeds. Consequently this contribution was larger for the 3:1 group than the 2:1 group (two-sample t-test, p < 0.001, Figure 3c). While the step velocity contribution (green trace) mirrors the split-belt perturbation, the step position (gold) and step time (purple) contributions increase from initial values near zero to partially offset the step velocity contribution. Because the sum of these contributions is non-zero at the end of adaptation (red), an asymmetry in step length remains. This asymmetry had similar values for the 2:1 and 3:1 groups (2:1: −62 ± 10 mm, 3:1: −36 ± 27 mm, two-sample t-test, p = 0.31). Interestingly, in the 2:1 condition, we observed equal step position (110 ± 15 mm) and step time (109 ± 15 mm) contributions which respectively represented 40 ± 5% and 38 ± 4% of the step velocity contribution. In contrast, in the 3:1 condition, the step position contribution (192 ± 12 mm) was significantly larger than the step time contribution (123 ± 12 mm), and represented a larger percentage of the step velocity contribution (step position: 50 ± 4%, step velocity: 31 ± 3%). This is revealed by a significant interaction between belt speed ratio and contribution type (step time or step position) (F(1,45) = 9.8, p = 0.033) (Figure 3c). There were no significant effects of contribution type (F(1,45) = 1.46, p = 0.234) or belt speed ratio (F(1,45) = 3.04, p = 0.0881) on the number of strides to plateau for the individual contributions to symmetry. On average, the step position and step time contributions plateaued in 116 ± 25 strides and 81 ± 18 strides respectively.
Figure 3.
Spatial and temporal contributions to step length asymmetry during adaptation to walking on a split-belt treadmill. Group averages of the stride-by-stride values for the step velocity (green), step position (gold), step time (purple), and net (red) contributions for the a) 2:1 group and b) 3:1 group. The horizontal dashed line indicates a contribution of zero. Error bars represent the average of each contribution during the first five steps of adaptation and the last 20 steps of adaptation. Shaded areas surrounding each line represent standard errors across subjects. The difference in belt speeds causes a large asymmetry in the step velocity contribution (green) and this is gradually offset by changes in the step position (gold) and step time contributions (purple). The sum of each contribution gives the actual difference in step lengths (red). c) Average contributions for each belt speed ratio. #: significant differences across speed ratios. *: significant differences in contribution size.
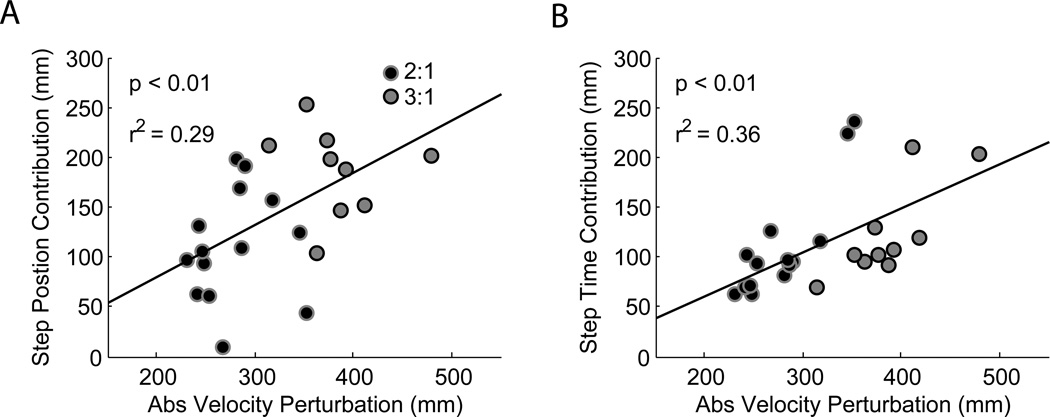
Although there was no group effect of belt speed ratio on the step time contribution, (Figure 3c), we found that both the step position and step time contributions scaled with the magnitude of the velocity perturbation (Figure 4a and 4b). 65% of the variance in the velocity perturbation was explained by the step position and step time contributions. Regression coefficients were 0.58 ± 0.27 and 0.83 ± 0.35 for the step position and step time contributions respectively. Note that there are two outliers in the 2:1 group whose contributions are greater than 200 mm (Figure 4b). These individuals are likely responsible for the absence of an effect of belt speed ratio on the step time contribution for the 2:1 group (Figure 3c).
Figure 4.
Step position and step time contributions as a function of velocity perturbation magnitude. In each plot, black dots with gray outlines represent data from participants who walked at a 2:1 belt speed ratio and gray circles with black outlines represent data from participants who walked at a 3:1 ratio. Both the a) step position and b) step time contributions increased proportionally with the magnitude of the velocity perturbation.
Spatial and Temporal Contributions to Step Length Asymmetry in Hemiparetic Gait
Our model quantified the spatial and temporal contributions to step length asymmetry in a group of post-stroke survivors while walking at their preferred speed on the treadmill. Because the belts are moving at equal speeds, the step velocity contribution is not influenced by the speed of the belts as it was during split-belt walking. During hemiparetic gait, the step velocity contribution results from differences in the velocity of the pelvis relative to the trailing foot. This could result from differences in propulsion during push-off, and would be expected in hemiparetic individuals who often present with plantar flexor weakness24.
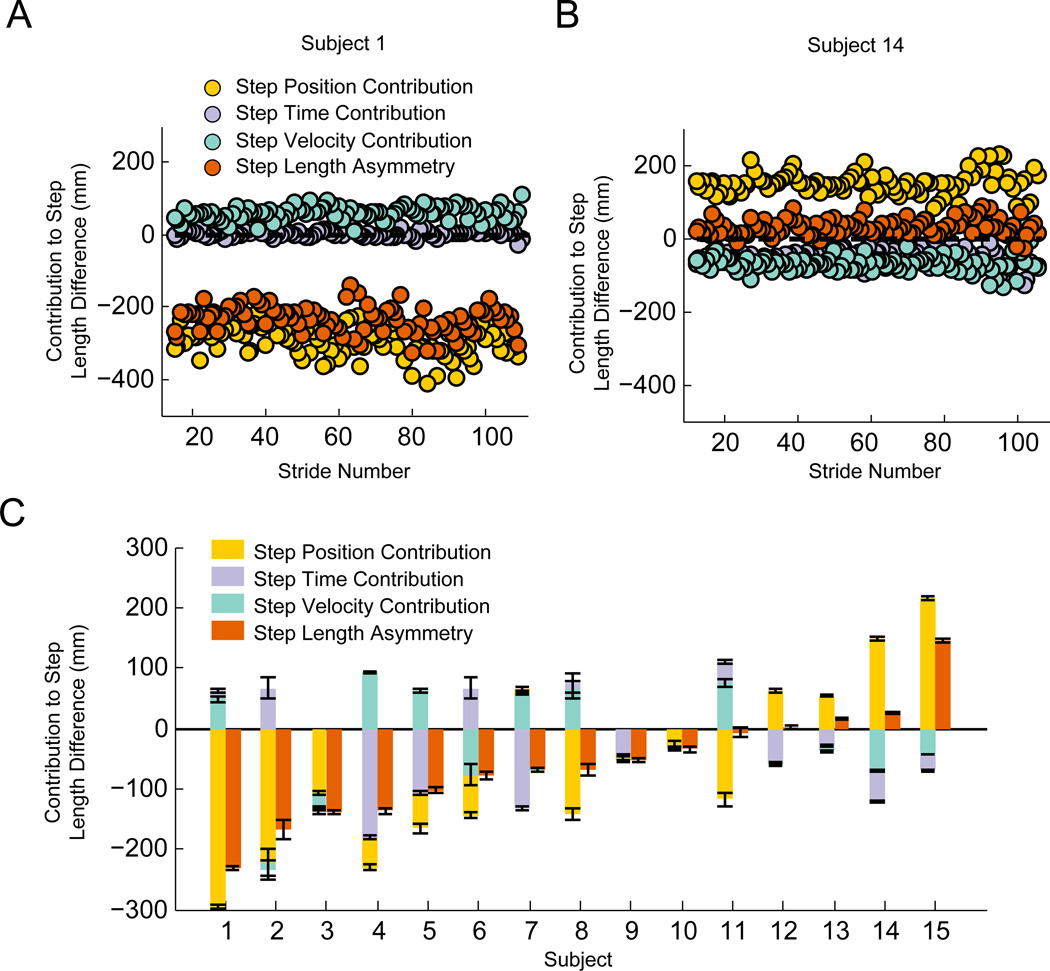
We found that the absolute step length asymmetry and each of the factors contributing to it varied considerably between patients (Figure 5). For example, subject 1 (Figure 5a) had a large step length asymmetry (−232 ± 4 mm), and this was almost exclusively due to a step position asymmetry (−294± 4 mm). Asymmetries in the temporal domain were much smaller (step time: 14 ± 4 mm; step velocity: 48 ± 4 mm) and did little to reduce the step length asymmetry. In contrast, subject 14 (Figure 5b) had relatively symmetric step lengths (SLdiff: 26 ± 2 mm). Surprisingly, this subject had a considerable step position asymmetry (148 ± 2 mm) that opposed by asymmetries in the two temporal contributions (step time: −53 ± 2 mm; step velocity: −69 ± 2 mm). This difference between participants demonstrates that asymmetries in the spatial and temporal domain post-stroke can be combined in ways that either reduce or increase overall step length asymmetry.
Figure 5.
Spatial and temporal contributions to step length asymmetry post-stroke. Examples of stride-to-stride values for each contribution for two representative subjects (a) & (b). Positive contributions correspond to larger values for the non-paretic limb and negative contributions correspond to larger values for the paretic limb. c) Average step position, step time, and step velocity contributions for each subject in the stroke group. Error bars represent standard errors computed during the first 140 strides of walking for each person. The participants are ordered from left to right by the amplitude of step length asymmetry. For each participant, the contribution with the largest amplitude is plotted adjacent to the abscissa, and the remaining contributions are stacked in order of descending amplitude.
Across the group of participants with stroke, we observed a heterogeneous distribution in the direction of step length asymmetry and the relative contribution of each strategy (Figure 5c). Most participants (11 of 15) walked with larger paretic steps than non-paretic steps, similar to a previous report25. Across all participants, the step position contribution tended to increase asymmetry as it was in the same direction as step length difference for 14 of 15 participants. In contrast, both temporal contributions typically opposed the step position contribution. The step time contribution opposed the step position contribution in 11 of 15 subjects and the step velocity contribution opposed it in 10 of 15 participants. Surprisingly, these results indicate that asymmetries in timing are generally beneficial for reducing overall step length asymmetry in stroke survivors. These examples demonstrate how our model can provide further insight into the characteristics of asymmetry in hemiparetic individuals, and this could potentially be used to help tailor therapeutic approaches for reducing step length asymmetry.
Discussion
Spatial and temporal contributions to split-belt walking adaptation
We formally express the relationship describing the relative contributions of spatial and temporal control to step length asymmetry. The spatial contribution reflects differences in where the feet are placed relative to the body while the temporal contribution reflects when (and thus where) toe-off occurs relative to the leading foot. The contribution of each strategy to reduce step length asymmetry was evaluated in healthy individuals during split-belt walking and in stroke survivors during normal tied-belt walking.
These results extend our knowledge about the spatial and temporal aspects of locomotor learning by identifying the fraction of step length asymmetry that is due to spatial versus temporal control strategies. This is important because step length asymmetry serves as a single parameter that represents the net effect of spatial and temporal asymmetries and has been used extensively to characterize both split-belt treadmill walking13–15,22,26 and hemiparetic gait dysfunction1–4. Our previous work has established that the extent of adaptation of spatial and temporal parameters is differentially modified by distraction12 and these parameters exhibit different degrees of generalization to over-ground walking27. A recent study also demonstrated that adaptation of spatial and temporal parameters can occur independently, and that spatial and temporal errors are resolved through adjustments in the relative foot strike locations and adjustments in the duration between heel strikes respectively13. One of the limitations of our model is that it focuses on sagittal plane kinematics and does not account for potential contributions of foot external rotation to measures of step length. This may be particularly relevant for stroke survivors who exhibit heightened toe-out on the paretic limb.28
Spatial and temporal contributions to asymmetry post-stroke
Step length asymmetry has long been appreciated as a common feature of hemiparetic gait, but it has generally been assumed to be an exclusively spatial deficit. Our decomposition of step length asymmetry into independent spatial and temporal components provides a quantitative assessment of how trailing limb extension, leading limb flexion, and step timing contribute to step length asymmetry. In our model, the spatial contribution to step length asymmetry was due to differences in the position of the leading foot relative to the body on consecutive foot-strikes. This corresponds to differences in foot progression relative to the trunk as has been described in a prior study29. In our framework, positive step position contributions indicate that the non-paretic limb progresses further in front of the trunk than the paretic limb. Likewise, negative step position contributions indicate that the paretic limb progresses further than the non-paretic limb. The majority of our post-stroke participants had more paretic than non-paretic limb progression. Individuals who have more paretic limb progression may prefer to walk with the paretic limb in increased flexion throughout the gait cycle while individuals with more non-paretic flexion may rely more heavily on non-paretic hip flexion for forward progress.
The temporal contributions to step length asymmetry were due to differences in step times and differences in the angular velocities of the trailing limb during forward propulsion. Negative step time contributions were observed in a majority of our participants, and represent shorter stance-phase durations in the paretic versus non-paretic limb. This likely occurs because hemiparetic individuals prefer to limit the time period when the paretic leg has to fully bear the weight of the body. The step velocity and step time contributions were often similar in magnitude and typically smaller than the step position contributions. Negative step velocity contributions, which are indicative of greater angular velocity of the non-paretic limb, were observed in 9 of 15 participants, and this strategy would be expected from individuals with marked weakness of the paretic plantar flexors24,30. It remains to be determined whether each of these contributions reflects specific joint-level impairments affecting step length asymmetry.
Lastly, our model may also be useful for identifying subsets of stroke survivors who may be best suited for split-belt treadmill training. A recent study found that not all stroke survivors reduce their step length asymmetry after 12 sessions of split-belt treadmill training (Reisman et al., 2013). It is possible that split-belt training is not equally effective across patients because this type of training may primarily target spatial versus temporal asymmetries or vice versa. We are currently analyzing the behavior of patients with stroke during and after split-belt walking. This will hopefully allow us to understand if spatial versus temporal deficits are preferentially modified, and better identify those individuals who would most likely benefit from split-belt training.
Acknowledgments
Funding: This work was supported by NIH Grants HD048741 and HD007414.
References
- 1.Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch. Phys. Med. Rehabil. 2007;88(1):43–49. doi: 10.1016/j.apmr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Patterson KK, Gage WH, Brooks D, Black SE, McIlroy WE. Evaluation of gait symmetry after stroke: a comparison of current methods and recommendations for standardization. Gait Posture. 2010;31(2):241–246. doi: 10.1016/j.gaitpost.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Allen JL, Kautz SA, Neptune RR. Step length asymmetry is representative of compensatory mechanisms used in post-stroke hemiparetic walking. Gait Posture. 2011;33(4):538–543. doi: 10.1016/j.gaitpost.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall AL, Bowden MG, Kautz SA, Neptune RR. Biomechanical variables related to walking performance 6-months following post-stroke rehabilitation. Clin. Biomech. Bristol Avon. 2012 doi: 10.1016/j.clinbiomech.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wall JC, Turnbull GI. Gait asymmetries in residual hemiplegia. Arch Phys Med Rehabil. 1986;67(8):550–553. [PubMed] [Google Scholar]
- 6.Bohannon RW, Horton MG, Wikholm JB. Importance of four variables of walking to patients with stroke. Int. J. Rehabil. Res. Int. Z. Für Rehabil. Rev. Int. Rech. Réadapt. 1991;14(3):246–250. doi: 10.1097/00004356-199109000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan KJ, Knowlton BJ, Dobkin BH. Step training with body weight support: effect of treadmill speed and practice paradigms on poststroke locomotor recovery. Arch. Phys. Med. Rehabil. 2002;83(5):683–691. doi: 10.1053/apmr.2002.32488. [DOI] [PubMed] [Google Scholar]
- 8.Ada L, Dean CM, Hall JM, Bampton J, Crompton S. A treadmill and overground walking program improves walking in persons residing in the community after stroke: a placebo-controlled, randomized trial. Arch. Phys. Med. Rehabil. 2003;84(10):1486–1491. doi: 10.1016/s0003-9993(03)00349-6. [DOI] [PubMed] [Google Scholar]
- 9.Plummer P, Behrman AL, Duncan PW, et al. Effects of stroke severity and training duration on locomotor recovery after stroke: a pilot study. Neurorehabil. Neural Repair. 2007;21(2):137–151. doi: 10.1177/1545968306295559. [DOI] [PubMed] [Google Scholar]
- 10.Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke J. Cereb. Circ. 2008;39(6):1786–1792. doi: 10.1161/STROKEAHA.107.504779. [DOI] [PubMed] [Google Scholar]
- 11.Winstein CJ, Gardner ER, McNeal DR, Barto PS, Nicholson DE. Standing balance training: effect on balance and locomotion in hemiparetic adults. Arch. Phys. Med. Rehabil. 1989;70(10):755–762. [PubMed] [Google Scholar]
- 12.Malone LA, Bastian AJ. Thinking about walking: Effects of conscious correction versus distraction on locomotor adaptation. J Neurophysiol. 2010;103(4):1954–1962. doi: 10.1152/jn.00832.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malone LA, Bastian AJ, Torres-Oviedo G. How does the motor system correct for errors in time and space during locomotor adaptation? J. Neurophysiol. 2012;108(2):672–683. doi: 10.1152/jn.00391.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol. 2005;94(4):2403–2415. doi: 10.1152/jn.00089.2005. [DOI] [PubMed] [Google Scholar]
- 15.Choi JT, Bastian AJ. Adaptation reveals independent control networks for human walking. Nat Neurosci. 2007;10(8):1055–1062. doi: 10.1038/nn1930. [DOI] [PubMed] [Google Scholar]
- 16.Finley JM, Statton MA, Bastian AJ. A Novel Optic Flow Pattern Speeds Split-belt Locomotor Adaptation. J. Neurophysiol. 2014;111(5):969–976. doi: 10.1152/jn.00513.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain. 2007;130(Pt 7):1861–1872. doi: 10.1093/brain/awm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reisman DS, Wityk R, Silver K, Bastian AJ. Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabil. Neural Repair. 2009;23(7):735–744. doi: 10.1177/1545968309332880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reisman DS, McLean H, Bastian AJ. Split-belt treadmill training poststroke: a case study. J. Neurol. Phys. Ther. JNPT. 2010;34(4):202–207. doi: 10.1097/NPT.0b013e3181fd5eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reisman DS, McLean H, Keller J, Danks KA, Bastian AJ. Repeated Split-Belt Treadmill Training Improves Poststroke Step Length Asymmetry. Neurorehabil. Neural Repair. 2013 doi: 10.1177/1545968312474118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malone LA, Bastian AJ. Spatial and temporal asymmetries in gait predict split-belt adaptation behavior in stroke. Neurorehabil. Neural Repair. 2014;28(3):230–240. doi: 10.1177/1545968313505912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres-Oviedo G, Bastian AJ. Natural error patterns enable transfer of motor learning to novel contexts. J. Neurophysiol. 2012;107(1):346–356. doi: 10.1152/jn.00570.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeni JA, Jr, Richards JG, Higginson JS. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture. 2008;27(4):710–714. doi: 10.1016/j.gaitpost.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadeau S, Gravel D, Arsenault AB, Bourbonnais D. Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. Clin. Biomech. 1999;14(2):125–135. doi: 10.1016/s0268-0033(98)00062-x. [DOI] [PubMed] [Google Scholar]
- 25.Turns LJ, Neptune RR, Kautz SA. Relationships between muscle activity and anteroposterior ground reaction forces in hemiparetic walking. Arch. Phys. Med. Rehabil. 2007;88(9):1127–1135. doi: 10.1016/j.apmr.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finley JM, Bastian AJ, Gottschall JS. Learning to be economical: the energy cost of walking tracks motor adaptation. J. Physiol. 2013;591(Pt 4):1081–1095. doi: 10.1113/jphysiol.2012.245506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torres-Oviedo G, Bastian AJ. Seeing Is Believing: Effects of Visual Contextual Cues on Learning and Transfer of Locomotor Adaptation. J. Neurosci. 2010;30(50):17015–17022. doi: 10.1523/JNEUROSCI.4205-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burdett RG, Borello-France D, Blatchly C, Potter C. Gait comparison of subjects with hemiplegia walking unbraced, with ankle-foot orthosis, and with Air-Stirrup brace. Phys. Ther. 1988;68(8):1197–1203. [PubMed] [Google Scholar]
- 29.Roerdink M, Beek PJ. Understanding inconsistent step-length asymmetries across hemiplegic stroke patients: impairments and compensatory gait. Neurorehabil. Neural Repair. 2011;25(3):253–258. doi: 10.1177/1545968310380687. [DOI] [PubMed] [Google Scholar]
- 30.Hsu A-L, Tang P-F, Jan M-H. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch. Phys. Med. Rehabil. 2003;84(8):1185–1193. doi: 10.1016/s0003-9993(03)00030-3. [DOI] [PubMed] [Google Scholar]