Abstract
Objective:
To determine whether neuroimaging biomarkers of amyloid-β (Aβ) and neurodegeneration (ND) are associated with greater self-reported subjective cognitive concerns (SCC) in clinically normal older individuals.
Methods:
A total of 257 participants underwent Pittsburgh compound B PET, PET with fluorodeoxyglucose 18F, and structural MRI, as well as a battery of neuropsychological measures including several questionnaires regarding SCC. Individuals were classified into 4 biomarker groups: biomarker negative (Aβ−/ND−), amyloidosis alone (Aβ+/ND−), amyloidosis plus ND (Aβ+/ND+), and ND alone (Aβ−/ND+).
Results:
Both Aβ and ND were independently associated with greater SCC controlling for objective memory performance. By contrast, neither Aβ nor ND was associated with objective memory performance controlling for SCC. Further examination revealed greater SCC in individuals with Aβ or ND positivity compared to biomarker-negative individuals. In addition, greater SCC predicted Aβ positivity when controlling for ND status.
Conclusions:
When individuals were grouped by biomarker status, those who were positive on Aβ or ND had the highest report of SCC compared to biomarker-negative individuals. Findings were consistent when SCC was used to predict Aβ positivity. Taken together, results suggest that both Aβ and ND are associated with SCC, independent of objective memory performance. Enrichment of individuals with SCC may increase likelihood of Aβ and ND markers in potential participants for secondary prevention trials.
Self-report of subjective cognitive concerns (SCC) are common among older individuals, but have often been dismissed as a sign of the worried well, rather than symptoms of an early neurodegenerative process. Accumulating evidence suggests that SCC may herald initial changes in cognitive function that are not detectable by standardized neuropsychological tests, but may be associated with early biomarker evidence of Alzheimer disease (AD) pathology.1,2 Cross-sectional studies in clinically normal older individuals have found a relationship between SCC and increased accumulation of amyloid-β (Aβ) on PET,3,4 as well as biomarkers of neurodegeneration (ND), evidenced by smaller hippocampal/entorhinal volumes5–7 and alterations in glucose metabolism in AD-vulnerable regions.8,9 It remains unclear, however, whether Aβ and ND independently contribute to the likelihood of endorsing SCC.
Guidelines proposed by the National Institute on Aging and the Alzheimer's Association have outlined a biomarker-based staging schema for preclinical AD.10 As individuals advance along the stages (stage 1: amyloidosis; stage 2: amyloidosis and ND; stage 3: amyloidosis, ND, and subtle cognitive decline), risk of progressing to mild cognitive impairment (MCI) and AD dementia increases. Subsequently, an operational approach developed by the Mayo Clinic11,12 uses both Aβ and ND markers to stage individuals, as means of improving predictive accuracy stratified along the preclinical phase. The aim of the current study was to examine SCC across the preclinical phase in a sample of clinically normal older individuals.
METHODS
Participants.
A total of 257 participants (mean age 73.7 years; 57.9% were women) were enrolled in the Harvard Aging Brain Study at the Center for Alzheimer Research and Treatment and the Massachusetts General Hospital over the course of 3 years (2010–2013). Participants were clinically normal, defined by a global Clinical Dementia Rating13 score of 0, an education-adjusted Mini-Mental State Examination (MMSE)14 score of greater than or equal to 25, and a Geriatric Depression Scale (GDS) long-form score of less than 11.15 A detailed review of medical history and functional performance as well as physical and neurologic examinations confirmed their status as clinically normal. None of the participants had a history of alcoholism, drug abuse, head trauma, or current serious medical or psychiatric illness. All study staff who assessed participants clinically were blinded to the biomarker status of the participants. All assessments were conducted within a 6-month window. The original sample was 272 participants, but not all participants underwent or had interpretable imaging across all the modalities, resulting in a total of 257 participants.
All participants underwent structural MRI, PET with Pittsburgh compound B (PiB-PET), and PET with fluorodeoxyglucose 18F (FDG-PET), as well as an extensive battery of neuropsychological measures including several questionnaires regarding SCC. APOE genotype was available for 244 of the 257 participants. Individuals who were APOE 2/4 were excluded, given that the effect of this genotype on risk for AD is unclear (n = 8). This exclusion reduced the sample to 236 individuals for analyses that included APOE.
Standard protocol approvals, registrations, and patient consents.
All protocols and informed consent procedures for this study were approved by the Partners Human Research Committee. All participants provided written informed consent.
SCC questionnaires.
Participants were administered 3 different questionnaires that measured SCC: (1) the self-report version of the Everyday Cognition (E-Cog) scale,16 which contains 6 domain-specific factors that include Everyday Memory, Language, Visuospatial Abilities, Planning, Organization, and Divided Attention; (2) the Memory Functioning Questionnaire (MFQ),17 which is divided into several subscales that include the General Frequency of Forgetting, Seriousness of Forgetting, Retrospective Functioning, and Mnemonics Usage; and (3) participants were administered a set of 7 questions that were adapted from the Structured Telephone Interview for Dementia Assessment (STIDA).18,19 A composite of the Memory subscale of the E-Cog, the General Frequency of Forgetting subscale of the MFQ, and the 7 STIDA questions was calculated, as previously described (SCC-Memory).3 An adjusted GDS score was calculated that removed 4 overlapping SCC-Memory items with the GDS.
Neuropsychological testing.
Participants underwent an extensive neuropsychological battery that included measures of episodic memory. A memory factor score, derived in a previous study,20 was used to determine the relationship among SCC-Memory, AD biomarkers, and objective memory. Measures that were included in the factor score included the Face-Name Associative Memory Exam,21,22 Six-Trial Selective Reminding Test,23 and Memory Capacity Test.24
PiB-PET acquisition and processing.
Carbon 11-PiB was synthesized using a previously published protocol25 and imaging was performed using a PET system (ECAT EXACT HR+; Siemens, Munich, Germany). Before injection, 10-minute transmission images for attenuation correction were collected. After injection of 8.5–15 mCi of PiB, 60 minutes of dynamic data were acquired in a 3D acquisition mode.
PiB-PET data were processed with statistical parametric mapping (SPM) v8 using a published protocol.20 PiB images were realigned, and the first 8 minutes of data were averaged and used to normalize data to the Montreal Neurological Institute FDG template. Distribution volume ratio images were created with Logan plotting (40- to 60-minute interval, gray matter cerebellum reference region). An aggregate of cortical regions using the Harvard Oxford atlas that typically have elevated PiB burden in patients with AD including frontal, lateral temporal and parietal, and retrosplenial cortices was used to extract a mean PiB value for each subject. Aβ-positive (Aβ+) and Aβ-negative (Aβ−) classification was derived from a previously reported Gaussian mixture modeling approach, revealing a cutoff value of 1.20.26
FDG-PET acquisition and processing.
Before injection, 10-minute transmission images for attenuation correction were collected. IV 5.0–10.0 mCi was injected, and after a 45-minute uptake period, FDG-PET images were acquired for 30 minutes in 3D acquisition mode.26
The FDG-PET data were realigned, summed, and normalized to a template using SPM8. FDG metabolism was extracted from a meta–region of interest (ROI) that included AD-vulnerable regions (lateral parietal, lateral inferior temporal, and posterior cingulate cortices) and was normalized by the mean from the top 50% of voxels from a pons-vermis reference region.27
Structural MRI acquisition and processing.
MRI scanning was on a Siemens TIM Trio 3T System with a 12-channel head coil. Structural T1-weighted volumetric magnetization-prepared rapid acquisition gradient echo scans were collected (repetition time/echo time/inversion time = 6,400/2.8/900 ms, flip angle = 8°, 1 × 1 × 1.2 mm resolution).28
ROI labeling used a software program (FreeSurfer version 5.1). Hippocampal volume (HV) was combined across hemispheres and adjusted for estimated total intracranial volume (eTIV).28
Classification of ND groups.
Classification of ND status is described elsewhere.11,28 Briefly, participants were divided into ND-positive (ND+) and ND-negative (ND−) groups based on cutoffs derived in Alzheimer's Disease Neuroimaging Initiative participants with AD dementia of 1.249 for meta-ROI-FDG and 6,723 mm3 for adjusted HV. Individuals in this study were considered ND+ if they were below either cutoff value.
Classification of biomarker groups.
Classification of biomarker groups is as follows: stage 0 = Aβ−/ND−; stage 1 = Aβ+/ND−; stage 2 = Aβ+/ND+. Individuals who were Aβ−/ND+ were classified as having suspected non-AD pathophysiology (SNAP).11 Stage 3 of preclinical AD was not included in the current analysis, as subtle cognitive decline may be closely related to SCC, which was the outcome measure.
Statistical methods.
All assumptions of linear modeling were met in the reported analyses. The primary analysis was a standard multiple regression relating Aβ group and ND group and their interaction with SCC-Memory as the dependent variable. This analysis was theoretically driven, as we hypothesized that biomarkers lead to manifestation of SCC. Age and education were used as covariates. Secondary analyses included separate standard regression models that included APOE4 carrier status, as well as the adjusted GDS score. A separate model that controlled for objective memory performance was conducted to determine the relationship between SCC-Memory and biomarkers. The converse model was also employed, in which SCC-Memory was used to predict objective memory performance controlling for covariates.
A logistic regression analysis was performed with SCC-Memory as the predictor variable and Aβ group as the dependent variable, controlling for ND group, age, and education to determine whether SCC-Memory is useful in predicting Aβ status.
SCC-Memory was also compared across biomarker stages (stage 0, stage 1, stage 2, and SNAP), controlling for age and education to determine if level of SCC was related to advancing preclinical stages of AD.
RESULTS
Demographics.
Older age (r = 0.76, p < 0.001) and lower education (r = −0.14, p = 0.03) were associated with higher SCC-Memory. There was no effect of sex on SCC. Adjusted GDS score (despite being at subsyndromal levels) was significantly associated with greater SCC-Memory (r = 0.35, p < 0.01). The memory factor score was significantly correlated with SCC-Memory (r = −0.21, p = 0.001). Mean performance on SCC-Memory was 0.00319 (range −1.25 to 2.86). Skewness was less than 1 and Cronbach α across the 3 subscales (E-Cog, MFQ, and STIDA) was 0.746, supporting the combination of these items as a composite score.
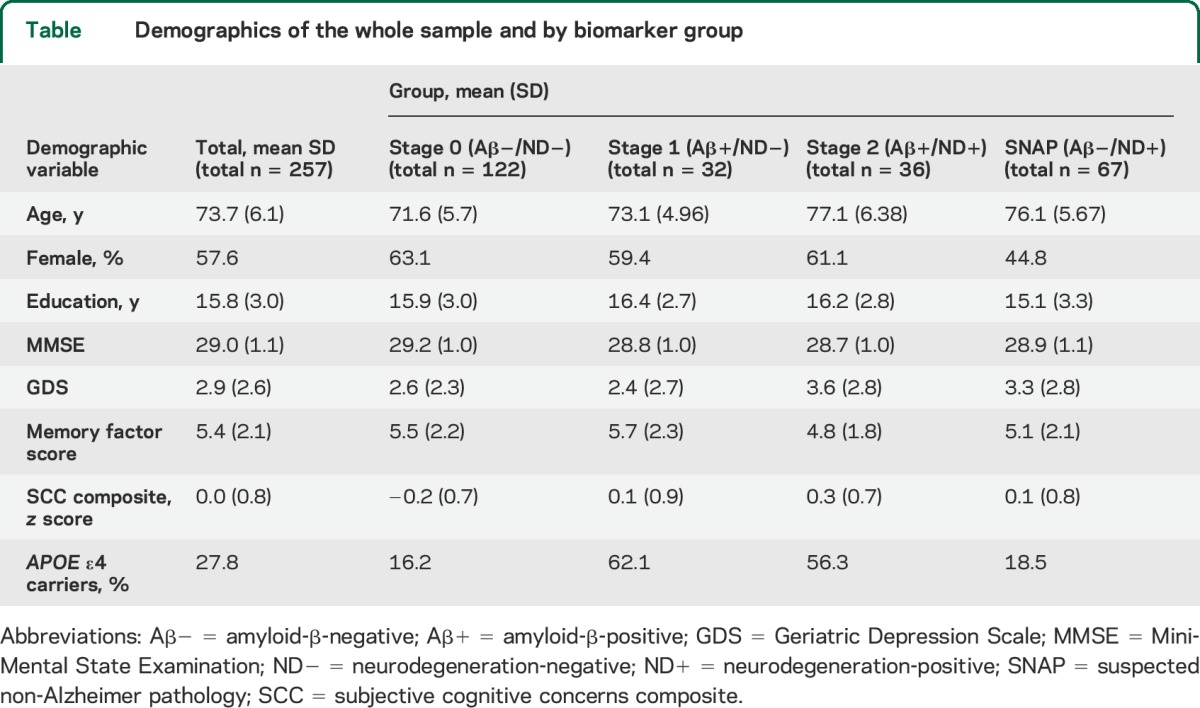
When comparing demographic variables across biomarker groups, stage 2 and SNAP participants were older than stage 0 and stage 1 participants (table). There were no significant differences in sex across biomarker groups. There were differences between APOE4 carrier status, with stage 1 and stage 2 having a higher proportion of APOE4 carriers than stage 0 and SNAP. Stage 0 participants scored higher on the MMSE compared to stage 2 participants. There were no statistical differences in years of education or GDS scores between groups.
Table.
Demographics of the whole sample and by biomarker group
Association of SCC-Memory with AD biomarkers.
In a multiple regression model, with Aβ group and ND group as simultaneous predictors of SCC-Memory, both the Aβ and ND groups were independently associated with SCC (Aβ group: partial r = 0.162, p = 0.009; ND group: partial r = 0.160, p = 0.01). The interaction between Aβ group and ND group was not significant (p = 0.88), suggesting that each biomarker provides an independent and additive association with SCC-Memory.
Association of SCC-Memory and episodic memory with AD biomarkers.
When episodic memory performance was added as a covariate, Aβ group (partial r = 0.158, p = 0.010) and ND group (partial r = 0.150, p = 0.016) remained significant predictors of SCC-Memory, suggesting that the presence of both Aβ and ND biomarkers predict greater SCC-Memory above and beyond the contribution of objective memory performance. When SCC-Memory, Aβ group, and ND group predicted objective memory performance, SCC-Memory was a significant predictor (partial r = −0.210, p = 0.001), but Aβ group (partial r = 0.010, p = 0.871) and ND group (partial r = −0.067, p = 0.290) were not significant predictors.
Association of SCC-Memory and APOE status with AD biomarkers.
Since APOE4 is related to amyloid status, we sought to determine whether APOE4 genotype was associated with SCC. We found that when APOE4 carrier status was included in the regression model with Aβ and ND groups, APOE4 genotype was not a statistically significant predictor of SCC-Memory (partial r = 0.076, p = 0.204). Aβ group was no longer significant (partial r = 0.119, p = 0.066), while the ND group remained a significant predictor of SCC (partial r = 0.152, p = 0.018). When the interaction term of APOE4 carrier status and Aβ was added to the model, the interaction was not significant (p = 0.473). This finding suggests that APOE4 carrier status does not appear to modify the relationship between SCC-Memory and Aβ, meaning that individuals with high Aβ tend to have higher SCC-Memory compared to those with lower Aβ, regardless of APOE4 carrier status.
Association of SCC-Memory and depression with AD biomarkers.
To explore the relation of depression and SCC with AD biomarkers in the model, the adjusted GDS predicted SCC-Memory (partial r = 0.329, p < 0.001). Aβ group remained a significant predictor of SCC-Memory (partial r = 0.178, p = 0.004), while the ND group was no longer significant (partial r = 0.102, p = 0.103). These findings suggest that Aβ status contributes to SCC-Memory despite controlling for depression.
Association of SCC-Memory with biomarker stages of preclinical AD.
A similar pattern of results emerged when biomarker groups were used to predict SCC-Memory (figure). In this analysis, a main effect of biomarker group was found (F3,256 = 4.46, p < 0.01), controlling for age and education. Post hoc contrasts across biomarker groups revealed statistically significance differences between stage 0 and stage 1 (p = 0.045), between stage 0 and stage 2 (p < 0.001), and between stage 0 and SNAP (p = 0.021). Differences between stage 1 and stage 2 (p = 0.19), between stage 2 and SNAP (p = 0.084), and between stage 1 and SNAP (p = 0.866) were not significant.
Figure. Comparison of subjective cognitive concerns across biomarker stages defined by amyloid-β and neurodegeneration.
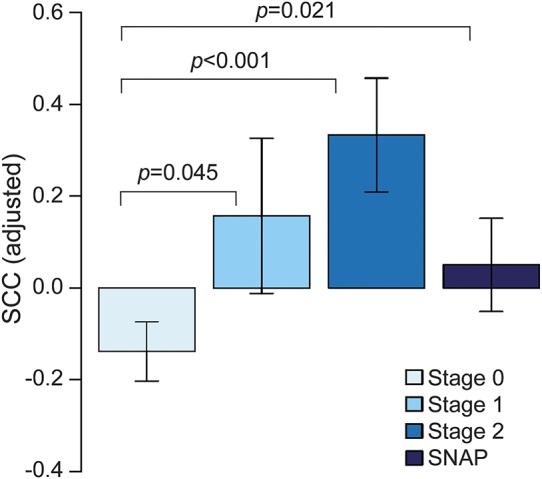
Stage 0: amyloid-β-negative/neurodegeneration-negative; stage 1: amyloid-β-positive/neurodegeneration-negative; stage 2: amyloid-β-positive/neurodegeneration-positive; suspected non-Alzheimer pathology (SNAP): amyloid-β-negative/neurodegeneration-positive. Stage 2 is associated with the greatest subjective cognitive concerns (SCC) compared to stage 0. A difference between stage 1 and stage 0, as well as SNAP and stage 0, is found. Analysis is controlled for age and education.
Association of SCC-Memory with amyloid group.
Logistic regression analysis revealed that SCC predicted Aβ group, such that individuals with higher SCC-Memory were more likely to be Aβ-positive (p = 0.008), controlling for ND group, age, and education.
DISCUSSION
Both Aβ and ND predicted greater self-reported memory concerns in clinically normal older individuals. When individuals were grouped by biomarker status, those who were positive on one or more AD biomarkers (stage 1, stage 2, SNAP) had a statistically significant higher report of SCC compared to biomarker-negative individuals (stage 0). Individuals who were biomarker-positive on both Aβ and ND (stage 2) had the highest SCC compared to individuals who were biomarker-positive on either Aβ or ND in isolation (stage 1 or SNAP), although this difference did not reach statistical significance. When controlling for objective memory performance, Aβ and ND remained significant predictors of SCC.
These findings are consistent with previous reports demonstrating a relationship between greater SCC and putative AD biomarkers in clinically normal older individuals.3–9,29 In particular, we found that both Aβ and ND were independently associated with greater SCC. Studies that have looked at both Aβ and ND in individuals with SCC have reported similar relationships; for example, Aβ and global gray matter atrophy was not found in normal controls or those with MCI,30 but was for SCC, suggesting a convergence between biomarkers before individuals move toward clinical impairment. In addition, a longitudinal study found that stage 1 and stage 2, as defined by cerebrospinal biomarkers, were both associated with greater cognitive decline compared to biomarker-negative individuals in those with SCC.31 A recent pathology study found that neuritic plaques were associated with SCC, although neurofibrillary tangles were not.32 Taken together, future studies will help to elucidate the exact role of Aβ and ND as it relates to SCC along the early AD trajectory, as it is likely to be a dynamic relationship that changes as individuals move toward clinical impairment.
When APOE4 carrier status was added as a covariate, the relationship between Aβ and SCC was no longer significant and APOE4 carrier status was not a significant independent predictor of SCC. This suggests that, despite a well-known association between Aβ and the APOE4 allele, Aβ offers unique information that is not entirely accounted for by APOE4 carrier status with respect to SCC.26 This finding is in contrast to another study that found the strongest relationship between Aβ and SCC was in individuals who were APOE4 carriers, compared to APOE4 noncarriers.29 Possible reasons for this discrepancy may have been the different methods in measuring SCC compared to our study, as well as a large proportion of APOE4 carriers (43%) compared to our sample (26%). Thus, our current sample may be underpowered to detect an interaction between Aβ and APOE4 in predicting SCC.
Even though none of the participants met criteria for depression, subsyndromal symptoms were correlated with SCC, which is consistent with previous findings.33,34 When an adjusted GDS score was added as a covariate in multiple regression models, Aβ remained a predictor of SCC and ND was no longer significant. Smaller hippocampal volume has been associated with major depression that persists in the remitted state with mechanisms that may be unrelated to AD pathology, such as microvascular disease or glucocorticoid neurotoxicity.35,36 Comparison across ND+ and ND− individuals revealed a significant difference in GDS scores, whereby ND+ individuals reported a greater number of subsyndromal symptoms of depression.37 Thus, a relationship between ND and depression, that may be unrelated to AD, may complicate the picture when investigating SCC.
A significant correlation between greater SCC-Memory and lower education was found in the current study, consistent with a prior study.38 Paradoxically, memory concerns in highly educated individuals have been associated with greater risk of progression to AD than in those with lower education.39 Thus, education may modify the relationship between memory complaints and AD pathology, consistent with the concept of cognitive reserve. Further work is needed to determine the relationship among education, SCC, and AD biomarkers.
Our analyses had several limitations. In order to operationalize Aβ and ND groups, cutoffs had to be created that may have incorrectly classified individuals who were close to the cutoff threshold. In addition, multiple approaches have been used to assess SCC. An SCC composite that assessed a wide range of memory concerns typically reported in older age was created. However, by combining all the questionnaires, we may have obscured our ability to detect relationships across various thematic memory structures that may differentially relate to AD biomarkers.40 Furthermore, it is possible that other nonmemory cognitive concerns may also be important in the earliest stages of AD.
Recent efforts have delineated a diagnostic stage that precedes MCI, called subjective cognitive decline (SCD), that includes features that increase the likelihood of preclinical AD.1 Our results confirm that greater SCC are associated with presence of AD biomarkers and provide further support for the concept of SCD as a useful framework in which to identify individuals who may be at risk for AD.
From a practical standpoint, examination of SCC may be one approach to enrich secondary prevention trials with individuals who may be more likely to exhibit biomarker positivity. Furthermore, assessment of SCC may eventually become one way to define subtle cognitive decline in stage 3 preclinical AD.10 Further work will also be needed to identify which specific SCC items can differentiate among normal age-related changes, preclinical AD, or other pathologies. It will also be important to determine when self-report of SCC becomes inaccurate along the AD trajectory as individuals move toward anosognosia. Ultimately, these findings have potential implications in the clinic setting, where patient report of memory difficulties should not be disregarded, despite an otherwise normal examination.
GLOSSARY
- Aβ
amyloid-β
- AD
Alzheimer disease
- E-Cog
Everyday Cognition scale
- eTIV
estimated total intracranial volume
- FDG-PET
18F fluorodeoxyglucose
- GDS
Geriatric Depression Scale
- HV
hippocampal volume
- MCI
mild cognitive impairment
- MFQ
Memory Functioning Questionnaire
- MMSE
Mini-Mental State Examination
- ND−
neurodegeneration-negative
- ND+
neurodegeneration-positive
- PiB-PET
PET with Pittsburgh compound B
- ROI
region of interest
- SCC
subjective cognitive concerns
- SCD
subjective cognitive decline
- SNAP
suspected non–Alzheimer disease pathophysiology
- SPM
statistical parametric mapping
- STIDA
Structured Telephone Interview for Dementia Assessment
AUTHOR CONTRIBUTIONS
R. Amariglio: design and conduct of the study, collection and interpretation of the data, analysis and interpretation of the data, preparation of the manuscript. E. Mormino: design of the study, analysis and interpretation of the data, review and approval of the manuscript. G. Marshall: collection and interpretation of the data, review and approval of the manuscript. A. Pietras: analysis and interpretation of the data, review and approval of the manuscript. P. Vannini: interpretation of the data, review and approval of the manuscript. K. Johnson: design of the study, interpretation of the data, review and approval of the manuscript. R. Sperling: design of the study, interpretation of the data, review and approval of the manuscript. D. Rentz: design of the study, interpretation of the data, review and approval of the manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
R. Amariglio was supported by Alzheimer's Association NIRG-12-243012 and NIH grant K23AG044431. She is coinvestigator for Eisai, Eli Lilly, and Merck. E. Mormino received funding from NIH grant F32AG044054. A. Pietras reports no disclosures relevant to the manuscript. G. Marshall has served as paid consultant for Halloran Consulting Group and Grifols. He is a site principal investigator coinvestigator for Janssen Alzheimer Immunotherapy, Wyeth/Pfizer Pharmaceuticals, Eisai Inc., Eli Lilly and Company, Avid Radiopharmaceuticals, Bristol-Myers-Squibb, Merck, and Navidea clinical trials. These relationships are not related to the content in the manuscript. G. Marshall receives research support for NIH grant K23 AG033634. P. Vannini received funding from NIH grant 8KL2TR000168-05. K. Johnson has served as paid consultant for Bayer, Biogen Idec, Bristol-Myers Squibb, GE Healthcare, Isis Pharmaceuticals Inc., Janssen Alzheimer's Immunotherapy, Piramal, Siemens Medical Solutions, and Genzyme. He is a site principal investigator or coinvestigator for Lilly/Avid, Biogen Idec, Bristol-Myers Squibb, Eisai, Pfizer, Janssen Alzheimer Immunotherapy, Merck, and Navidea clinical trials. He has spoken at symposia sponsored by Janssen Alzheimer's Immunotherapy, GEHC, Lundbeck, and Pfizer. These relationships are not related to the content in the manuscript. K. Johnson receives research support from NIH grants R01EB014894, R21 AG038994, R01 AG026484, R01 AG034556, P50 AG00513421, U19 AG10483, P01 AG036694, R13 AG042201174210, R01 AG027435, and R01 AG037497. R. Sperling has served as a paid consultant for Bristol-Myers Squibb, Eisai, Janssen Alzheimer Immunotherapy, Pfizer, Merck, and Roche, and as an unpaid consultant to Avid and Eli Lilly. She is a site principal investigator or coinvestigator for Avid, Bristol-Myers Squibb, Pfizer, and Janssen Alzheimer Immunotherapy clinical trials. She has spoken at symposia sponsored by Eli Lilly, Pfizer, and Janssen Alzheimer Immunotherapy. These relationships are not related to the content in the manuscript. R. Sperling receives research support for NIH grants U01 AG032438, U01 AG024904, R01 AG037497, R01 AG034556, K24 AG035007, P50 AG005134, U19 AG010483, R01 AG027435, and P01 AG036694. D. Rentz received research support from the NIH grants P01 AG036694, R01 MH090291, U01 AG024904, R01 AG027435, R01 AG037497, and P50 AG005134, a Fidelity Investigator-Initiated grant, and Alzheimer Association grant SGCOG-13-282201. She is coinvestigator for Eli Lilly. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement 2014;10:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knopman DS. Subjective cognitive impairment: fickle but fateful. Neurology 2012;79:1308–1309. [DOI] [PubMed] [Google Scholar]
- 3.Amariglio RE, Becker JA, Carmasin J, et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 2012;50:2880–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging: a Pittsburgh compound B positron emission tomography study in normal elderly individuals. Arch Neurol 2012;69:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jessen F, Feyen L, Freymann K, et al. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging 2006;27:1751–1756. [DOI] [PubMed] [Google Scholar]
- 6.Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology 2006;67:834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Flier WM, van Buchem MA, Weverling-Rijnsburger AW, et al. Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. J Neurol 2004;251:671–675. [DOI] [PubMed] [Google Scholar]
- 8.Mosconi L, De Santi S, Brys M, et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry 2008;63:609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheef L, Spottke A, Daerr M, et al. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology 2012;79:1332–1339. [DOI] [PubMed] [Google Scholar]
- 10.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jack CR, Jr, Knopman DS, Weigand SD, et al. An operational approach to national institute on aging-Alzheimer's association criteria for preclinical Alzheimer disease. Ann Neurol 2012;71:765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knopman DS, Jack CR, Jr, Wiste HJ, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology 2012;78:1576–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 15.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale. J Psychiatry Res 1982-1983;17:37–49. [DOI] [PubMed] [Google Scholar]
- 16.Farias ST, Mungas D, Reed BR, et al. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology 2008;22:531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilewski MJ, Zelinski EM, Schaie KW. The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychol Aging 1990;5:482–490. [DOI] [PubMed] [Google Scholar]
- 18.Amariglio RE, Townsend MK, Grodstein F, Sperling RA, Rentz DM. Specific subjective memory complaints in older persons may indicate poor cognitive function. J Am Geriatr Soc 2011;59:1612–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Go RC, Duke LW, Harrell LE, et al. Development and validation of a structured telephone interview for dementia assessment (STIDA): the NIMH Genetics initiative. J Geriatr Psychiatry Neurol 1997;10:161–167. [DOI] [PubMed] [Google Scholar]
- 20.Hedden T, Mormino EC, Amariglio RE, et al. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. J Neurosci 2012;32:16233–16242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amariglio RE, Frishe K, Olson LE, et al. Validation of the face name associative memory exam in cognitively normal older individuals. J Clin Exp Neuropsychol 2012;34:580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rentz DM, Amariglio RE, Becker JA, et al. Face-name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia 2011;49:2776–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masur DM, Fuld PA, Blau AD, Thal LJ, Levin HS, Aronson MK. Distinguishing normal and demented elderly with the selective reminding test. J Clin Exp Neuropsychol 1989;11:615–630. [DOI] [PubMed] [Google Scholar]
- 24.Rentz DM, Locascio JJ, Becker JA, et al. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol 2010;67:353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol 2007;62:229–234. [DOI] [PubMed] [Google Scholar]
- 26.Mormino EC, Betensky RA, Hedden T, et al. Amyloid and APOE epsilon4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology 2014;82:1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landau SM, Harvey D, Madison CM, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging 2011;32:1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mormino EC, Betensky RA, Hedden T, et al. Synergistic effect of beta-amyloid and neurodegeneration on cognitive decline in clinically Normal individuals. JAMA Neurol 2014;71:1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowe CC, Ellis KA, Rimajova M, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging 2010;31:1275–1283. [DOI] [PubMed] [Google Scholar]
- 30.Chetelat G, Villemagne VL, Bourgeat P, et al. Relationship between atrophy and beta-amyloid deposition in Alzheimer disease. Ann Neurol 2010;67:317–324. [DOI] [PubMed] [Google Scholar]
- 31.van Harten AC, Smits LL, Teunissen CE, et al. Preclinical AD predicts decline in memory and executive functions in subjective complaints. Neurology 2013;81:1409–1416. [DOI] [PubMed] [Google Scholar]
- 32.Kryscio RJ, Abner EL, Cooper GE, et al. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology 2014;83:1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Comijs HC, Deeg DJ, Dik MG, Twisk JW, Jonker C. Memory complaints; the association with psycho-affective and health problems and the role of personality characteristics: a 6-year follow-up study. J Affect Disord 2002;72:157–165. [DOI] [PubMed] [Google Scholar]
- 34.Minett TS, Da Silva RV, Ortiz KZ, Bertolucci PH. Subjective memory complaints in an elderly sample: a cross-sectional study. Int J Geriatr Psychiatry 2008;23:49–54. [DOI] [PubMed] [Google Scholar]
- 35.Gerritsen L, Comijs HC, van der Graaf Y, Knoops AJ, Penninx BW, Geerlings MI. Depression, hypothalamic pituitary adrenal axis, and hippocampal and entorhinal cortex volumes: the SMART Medea study. Biol Psychiatry 2011;70:373–380. [DOI] [PubMed] [Google Scholar]
- 36.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 1999;19:5034–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donovan NJ, Hsu DC, Dagley AS, et al. Depressive symptoms and biomarkers of Alzheimer's disease in cognitively normal older adults. J Alzheimers Dis 2015;46:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatric Psychiatry 2000;15:983–991. [DOI] [PubMed] [Google Scholar]
- 39.van Oijen M, de Jong FJ, Hofman A, Koudstaal PJ, Breteler MM. Subjective memory complaints, education, and risk of Alzheimer's disease. Alzheimers Dement 2007;3:92–97. [DOI] [PubMed] [Google Scholar]
- 40.Buckley RF, Saling MM, Irish M, et al. Autobiographical narratives relate to Alzheimer's disease biomarkers in older adults. Int Psychogeriatr 2014;26:1737–1746. [DOI] [PubMed] [Google Scholar]


