Abstract
Three-dimensional printing technology has significant clinical implications for the management of congenital heart disease. Computed tomography and magnetic resonance imaging have been established as imaging tools for the creation of physical three-dimensional models. The potential use of non-invasive bedside imaging techniques such as three-dimensional echocardiography to derive three-dimensional printed models can revolutionize the planning of interventions for complex congenital malformations. The feasibility of deriving three-dimensional printing from ultrasound provides an additional cost-effective and patient-centered option for interventional cardiologists and surgeons for the management and care of congenital heart disease patients.
Keywords: 3D ultrasound, 3D imaging, 3D segmentation, 3D reconstruction, Cardiac imaging, Clinical application
Background
The recent advancements in creating accurate anatomic cardiovascular models using three-dimensional (3D) printing technology has significant clinical implications for cardiologists and surgeons in treating structural heart disease. In 1988, Laschinger et al. were the first to create virtual preoperative three-dimensional models using data from magnetic resonance imaging (MRI) of patients with congenital heart disease [1]. Due to low-resolution images and lack of computer processing power, the technique was not readily adopted for clinical use. Recent advances in technology including high-resolution imaging and 3D printing has enabled clinically relevant visualization and reconstruction of complex cardiac morphology. Utilization of non-invasive imaging to create 3D printed models of structural heart disease is useful to cardiologists and surgeons to help plan the interventional approach and make an informed selection of appropriate catheter course or device. The insight provided by 3D modeling may help to increase procedural efficiency, decrease radiation exposure and procedural complications, and improve surgical outcomes. Currently, 3D printing is produced from volumetric images derived from computed tomography (CT) and magnetic resonance imaging (MRI) [1–4].
Three-dimensional echocardiography is another imaging modality with strong linear correlation with CT and cardiac MRI [5, 6]. As a bedside tool, it is safe for severely ill patients who do not have to be transported to and positioned in a CT or MRI scanner, and eliminates the need for sedation or general anesthesia [6]. Our aim was to demonstrate the feasibility of creating 3D printed cardiovascular models from 3D echocardiography data, which is safe, effective, and patient-centered. This will offer cardiologists and surgeons a better three-dimensional anatomical understanding when planning interventions in patients with congenital heart malformations or other disorders.
Methods
Acquisition of Data
Three-dimensional echocardiography image acquisition was performed using the Philips iE33 ultrasound system (Philips Medical Systems, Andover, Massachusetts, USA). Dataset was anonymized and exported from the ultrasound quantification software, Philips QLAB (Philips Medical Systems, Andover, Massachusetts, USA), in the Cartesian digital imaging and communications in medicine (DICOM) format. Multiplanar reformatting of the images was then performed to define the accuracy of the dataset and to adjust the gain and resolution setting to delineate the anatomy under review.
Segmentation and Generation of the Model
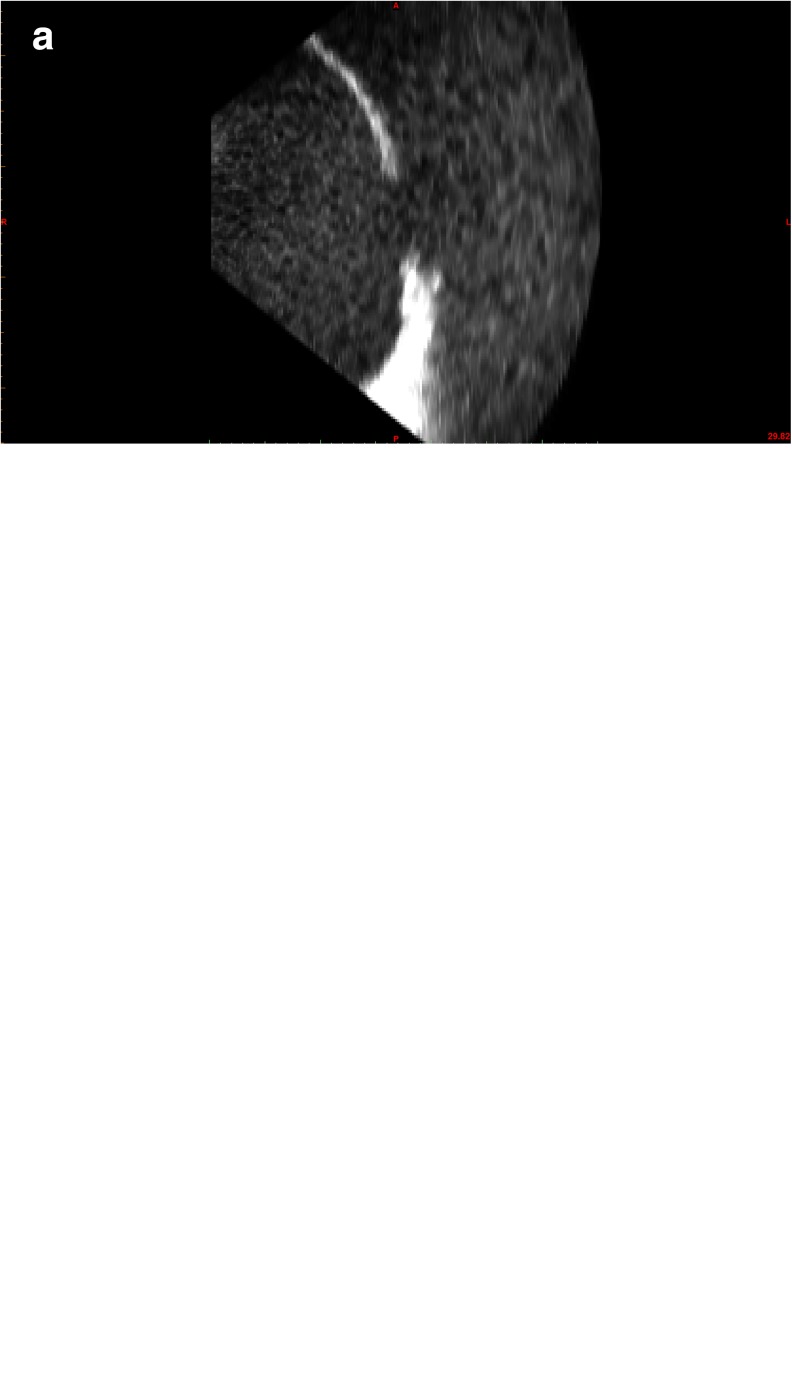
The Cartesian DICOM echocardiography dataset was imported into a dedicated post-processing software (Mimics® Innovation Suite, Materialise NV, Leuven, Belgium). The data was processed using the Mimics® software to reduce imaging noise and isolate the anatomy of interest, which in our case was the atrial septum (Fig. 1a). The atrial septum was segmented from the data via thresholding and interactive editing operations (Fig. 1b). After segmentation was completed, a 3D computer model was rendered for visualization and measurements (Fig. 1c). The Mimics® software was then utilized to smooth the surface of the anatomy and ensure that it was suitable for 3D printing before exporting in the stereolithography (.stl) format. The creation of the physical 3D model was done using a 3D printer with the HeartPrint® flex material (Materialise NV) (Fig. 2).
Fig. 1.
Three-dimensional echocardiography evaluation of atrial septal defect. a Imported image from 3D echocardiography dataset. b Segmented atrial septum. c 3D reconstruction of atrial septum with visible atrial septal defect
Fig. 2.
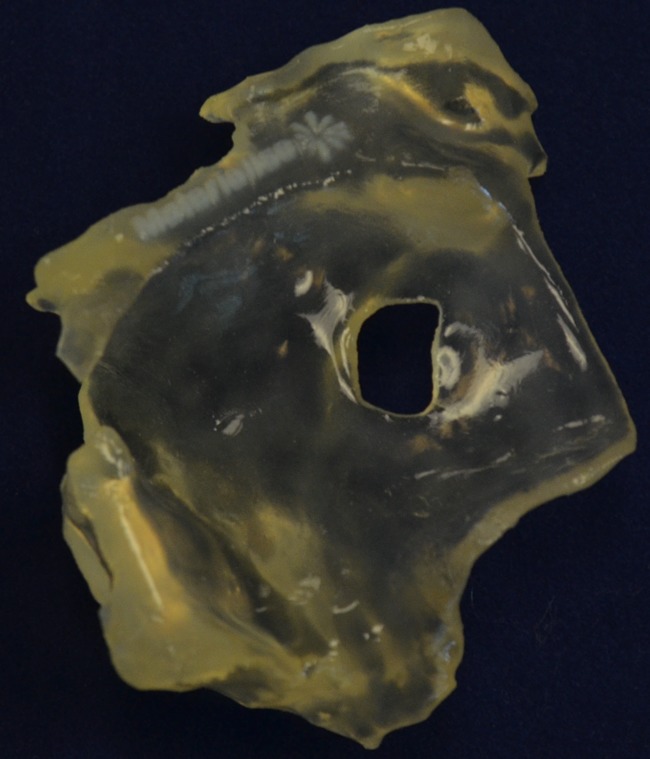
HeartPrint® flex 3D printed model
Results and Discussion
Significant advancement in echocardiography has enabled improved visualization of both morphological and functional information in patients with congenital heart disease. The clinical applications of 3D echocardiography (Table 1) has enhanced preoperative decision-making in congenital heart defects as it provides adequate image resolution with the most integrated display of structure, function, and flow [2, 5]. Unlike CT and MRI, 3D echocardiography is a bedside tool useful in obtaining adequate quality images in most patients without general anesthesia. Images can be easily acquired in the outpatient setting as well as in intubated patients in the preoperative or intensive care settings [6, 7]. Three-dimensional echocardiography does not use radiation and is therefore considered safe. As a result, 3D echocardiography has advantages of being patient-centered and low cost when compared to CT and MRI [6, 8]. Therefore, creating tangible 3D cardiovascular models using 3D echocardiography imaging is desirable.
Table 1.
Clinical applications of 3D echocardiography
| • Structure and morphology of heart defects |
|---|
| • Deformation imaging |
| • Volumetry: left ventricle, right ventricle, muscle mass |
| • Color 3D and 3D color angiography |
| • Quantifications of regurgitation |
| • Defect sizing |
| • Delineation of functional morphology of values including Ebstein’s anomaly and atrioventricular septal defect |
| • Septation of complex defects |
| • Cardiac catheter interventions (defect sizing, catheter manipulation) |
Reprinted with permission [6]
Three-dimensional cardiovascular models have the potential to improve the understanding of the morphology for cardiologists and surgeons before device placement or surgery. The ability to plan the interventional approach is advantageous in making informed decisions on catheter course, device, and surgery. Patient safety may also be greatly improved by procedural efficiency, decrease in radiation exposure, and procedural outcomes [1–4].
Conclusion
Our experience shows the feasibility and proof of concept of printing 3D cardiovascular models derived from 3D echocardiography images with speculation on how this technology may have advantages in the surgical and interventional setting. An extensive comparative clinical trial is necessary to evaluate 3D printing in more complex anatomy, the use of 3D models on decision-making in surgical or interventional cases as well as its impact on procedure time. The advantages of echocardiography as a safe and low-cost modality when compared to CT and MRI makes it desirable as a means to obtain images to create anatomically accurate 3D cardiovascular models.
Acknowledgments
Special thanks to Jordan Gosnell, Echocardiography sonographer, Helen DeVos Children’s Hospital of Spectrum Health, Grand Rapids, Michigan for acquisition of the 3D echocardiography images.
Conflict of Interest
The authors Samuel and Pinto have nothing to disclose. Pietila is a full-time employee of Materialise NV. Vettukattil has a non-disclosure agreement with Materialise NV.
References
- 1.Laschinger JC, Vannier MW, Gutierrez E, Gronemeyer S, Weldon CS, Spray TL, Cox JL. Preoperative three-dimensional reconstruction of the heart and great vessels in patients with congenital heart disease. Technique and initial results. J Thorac Cardiovasc Surg. 1988;96:464–473. [PubMed] [Google Scholar]
- 2.Sørensen TS, Pedersen EM, Hansen OK, Sørensen K. Visualization of morphological details in congenitally malformed hearts: virtual three-dimensional reconstruction from magnetic resonance imaging. Cardiol Young. 2003;13:1–10. doi: 10.1017/S1047951100012300. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs S, Grunert R, Mohr FW, Falk V. 3D-Imaging of cardiac structures using 3D heart models for planning in heart surgery: a preliminary study. Interact Cardiovasc Thorac Surg. 2008 doi: 10.1510/icvts.2007.156588. [DOI] [PubMed] [Google Scholar]
- 4.Olivieri L, Krieger A, Chen MY, Kim P, Kanter JP. 3D heart model guides complex stent angioplasty of pulmonary venous baffle obstruction in a Mustard repair of D-TGA. Int J Cardiol. 2014 doi: 10.1016/j.ijcard.2013.12.192. [DOI] [PubMed] [Google Scholar]
- 5.Black D, Vettukattil J. Advanced echocardiographic imaging of the congenitally malformed heart. Curr Cardiol Rev. 2013 doi: 10.2174/1573403X11309030008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vettukattil JJ. Three dimensional echocardiography in congenital heart disease. Heart. 2012 doi: 10.1136/heartjnl-2011-300488. [DOI] [PubMed] [Google Scholar]
- 7.Friedberg MK, Su X, Tworetzky W, Soriano BD, Powell AJ, Marx GR. Validation of 3D echocardiography assessment of left ventricular volumes, mass, and ejection fraction in neonates and infants with congenital heart disease: a comparison study with cardiac MRI. Circ Cardiovasc Imaging. 2010 doi: 10.1161/CIRCIMAGING.109.928663. [DOI] [PubMed] [Google Scholar]
- 8.Dwivedi G, Chan KL, Friedrich MG, Beanlands RSB. Cardiovascular imaging: new directions in an evolving landscape. Can J Cardiol. 2013 doi: 10.1016/j.cjca.2013.01.011. [DOI] [PubMed] [Google Scholar]