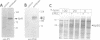Abstract
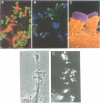
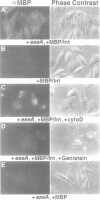
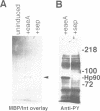
Enteropathogenic E. coli (EPEC) belongs to a group of bacterial pathogens that induce actin accumulation beneath adherent bacteria. We found that EPEC adherence to epithelial cells mediates the formation of fingerlike pseudopods (up to 10 microm) beneath bacteria. These actin-rich structures also contain tyrosine phosphorylated host proteins concentrated at the pseudopod tip beneath adherent EPEC. Intimate bacterial adherence (and pseudopod formation) occurred only after prior bacterial induction of tyrosine phosphorylation of an epithelial membrane protein, Hp90, which then associates directly with an EPEC adhesin, intimin. These interactions lead to cytoskeletal nucleation and pseudopod formation. This is the first example of a bacterial pathogen that triggers signals in epithelial cells which activates receptor binding activity to a specific bacterial ligand and subsequent cytoskeletal rearrangement.
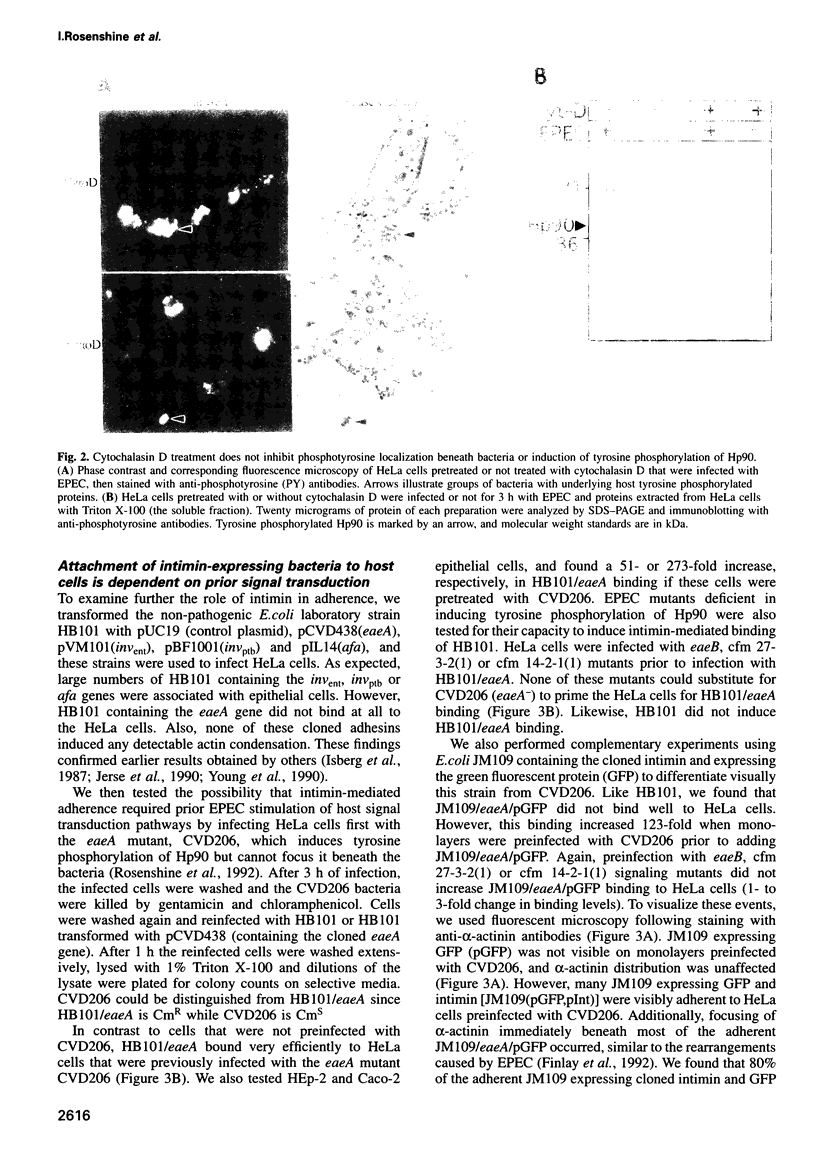
Full text
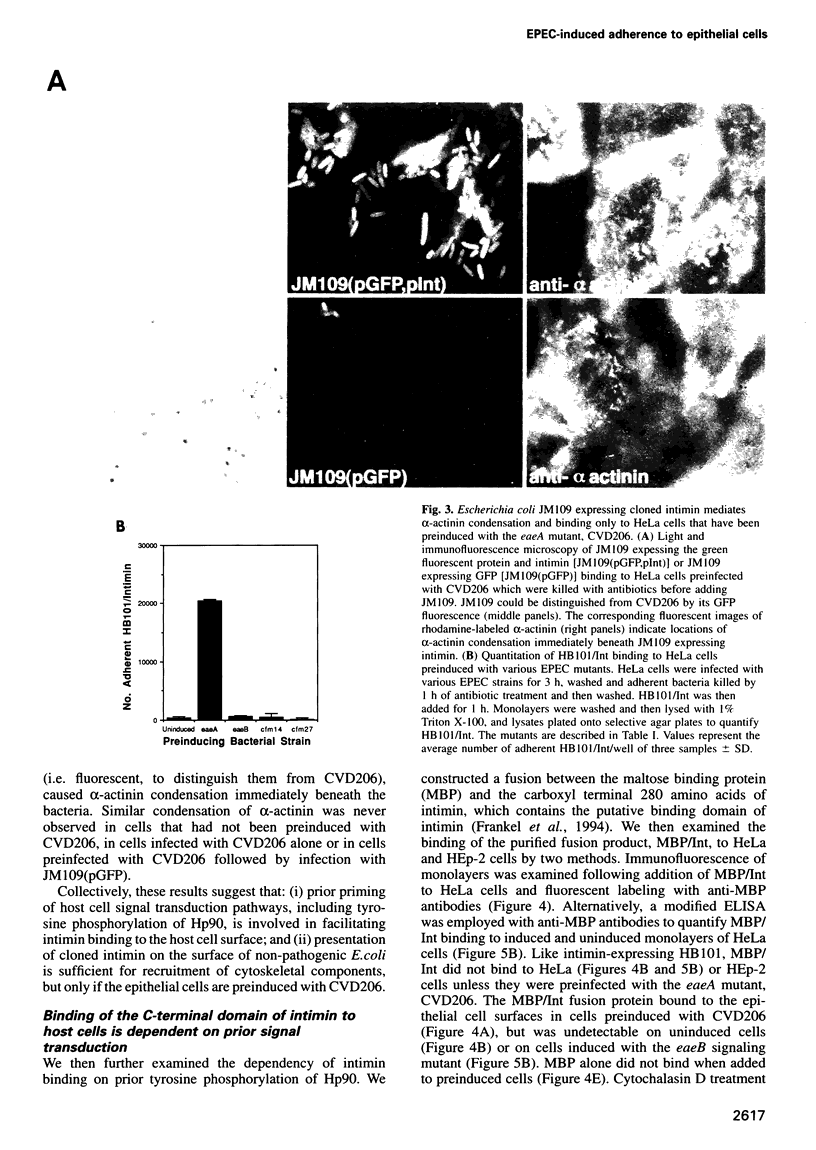
PDF
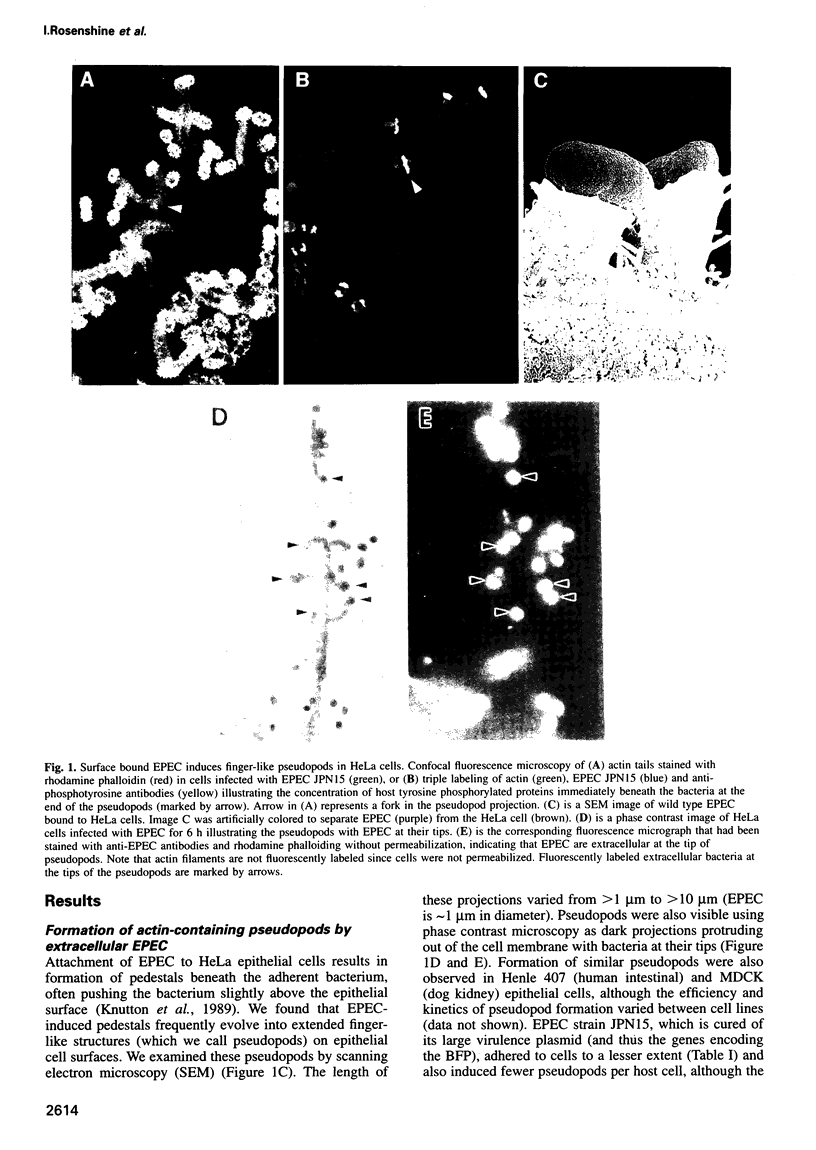
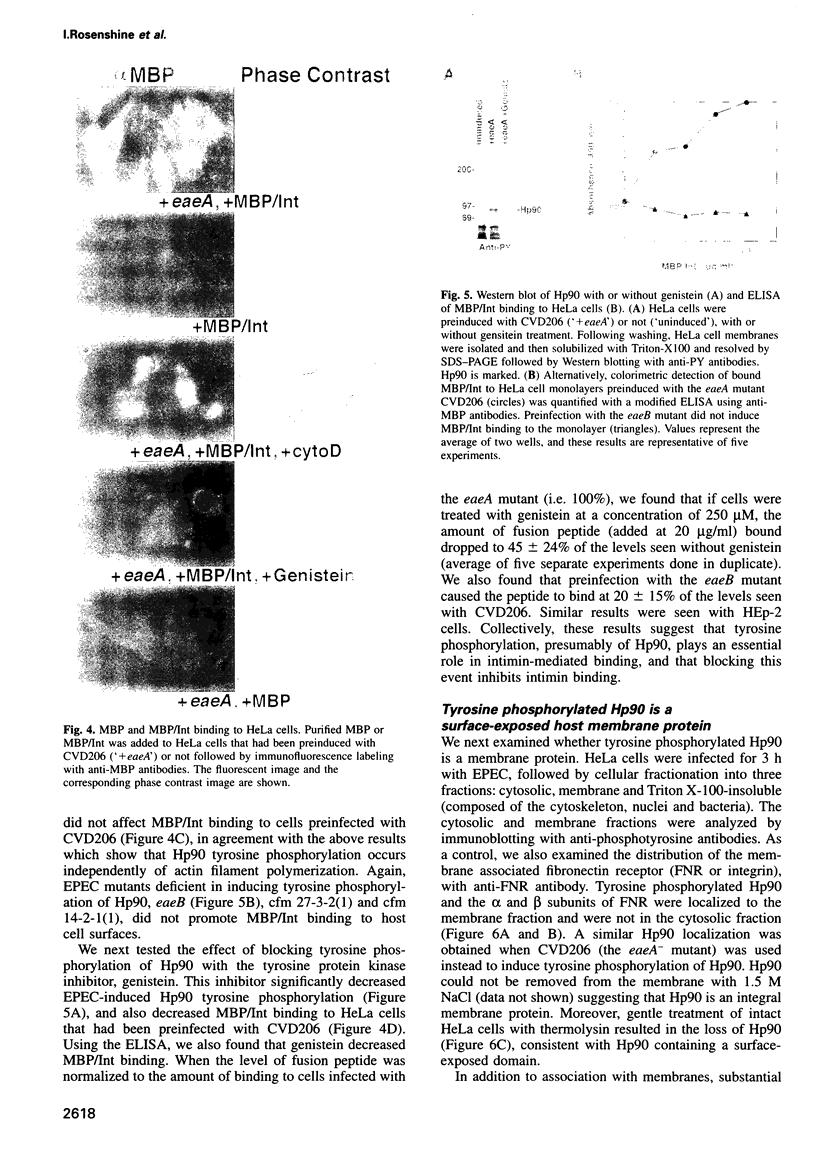





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade J. R., Da Veiga V. F., De Santa Rosa M. R., Suassuna I. An endocytic process in HEp-2 cells induced by enteropathogenic Escherichia coli. J Med Microbiol. 1989 Jan;28(1):49–57. doi: 10.1099/00222615-28-1-49. [DOI] [PubMed] [Google Scholar]
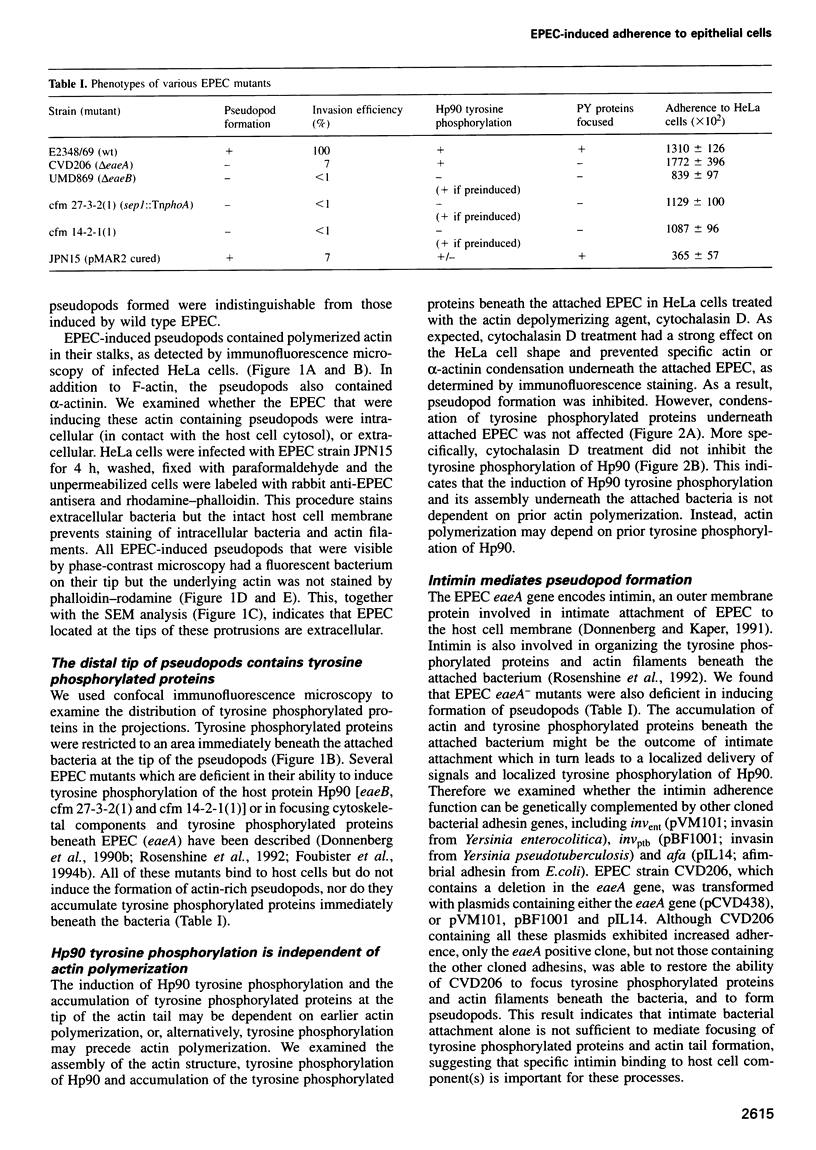
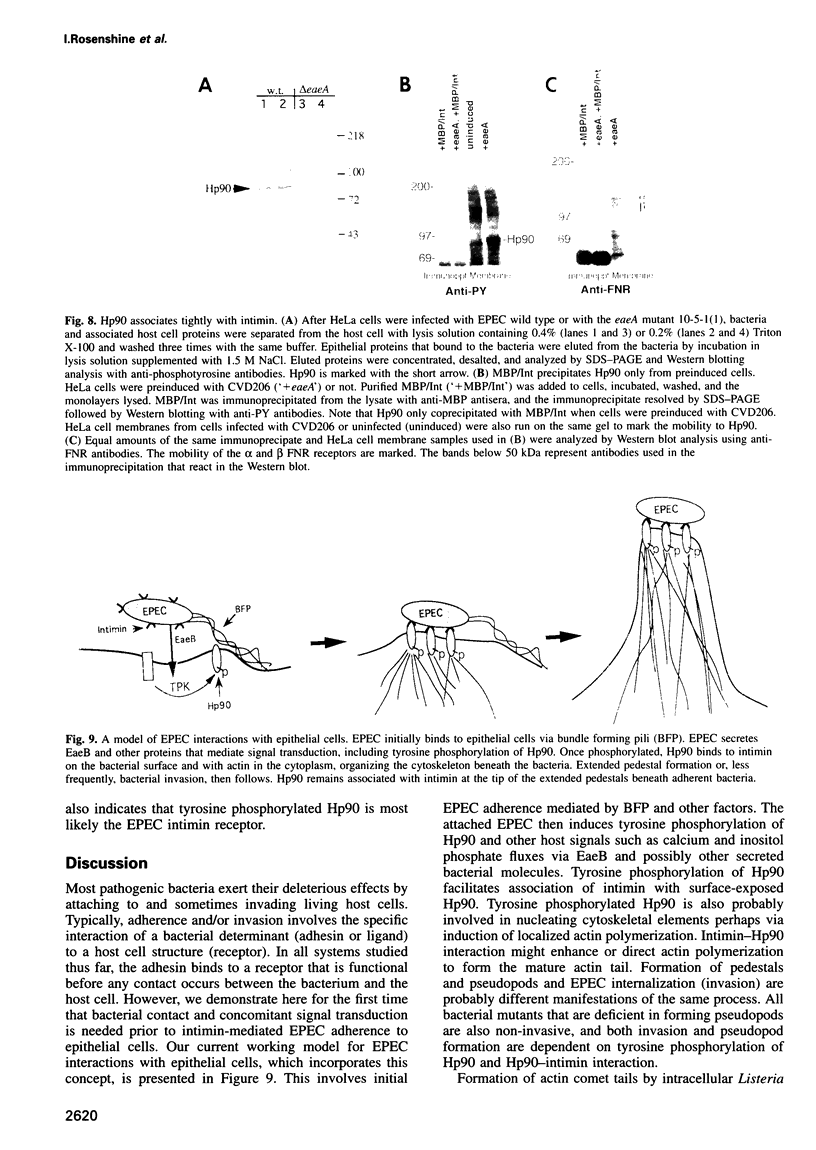
- Cossart P., Kocks C. The actin-based motility of the facultative intracellular pathogen Listeria monocytogenes. Mol Microbiol. 1994 Aug;13(3):395–402. doi: 10.1111/j.1365-2958.1994.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Donnenberg M. S., Calderwood S. B., Donohue-Rolfe A., Keusch G. T., Kaper J. B. Construction and analysis of TnphoA mutants of enteropathogenic Escherichia coli unable to invade HEp-2 cells. Infect Immun. 1990 Jun;58(6):1565–1571. doi: 10.1128/iai.58.6.1565-1571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg M. S., Donohue-Rolfe A., Keusch G. T. A comparison of HEp-2 cell invasion by enteropathogenic and enteroinvasive Escherichia coli. FEMS Microbiol Lett. 1990 May;57(1-2):83–86. doi: 10.1016/0378-1097(90)90417-o. [DOI] [PubMed] [Google Scholar]
- Donnenberg M. S., Girón J. A., Nataro J. P., Kaper J. B. A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localized adherence. Mol Microbiol. 1992 Nov;6(22):3427–3437. doi: 10.1111/j.1365-2958.1992.tb02210.x. [DOI] [PubMed] [Google Scholar]
- Donnenberg M. S., Kaper J. B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991 Dec;59(12):4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg M. S., Kaper J. B. Enteropathogenic Escherichia coli. Infect Immun. 1992 Oct;60(10):3953–3961. doi: 10.1128/iai.60.10.3953-3961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg M. S., Tacket C. O., James S. P., Losonsky G., Nataro J. P., Wasserman S. S., Kaper J. B., Levine M. M. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J Clin Invest. 1993 Sep;92(3):1412–1417. doi: 10.1172/JCI116717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg M. S., Yu J., Kaper J. B. A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia coli to epithelial cells. J Bacteriol. 1993 Aug;175(15):4670–4680. doi: 10.1128/jb.175.15.4670-4680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dytoc M., Fedorko L., Sherman P. M. Signal transduction in human epithelial cells infected with attaching and effacing Escherichia coli in vitro. Gastroenterology. 1994 May;106(5):1150–1161. doi: 10.1016/0016-5085(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Rosenshine I., Donnenberg M. S., Kaper J. B. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect Immun. 1992 Jun;60(6):2541–2543. doi: 10.1128/iai.60.6.2541-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Ruschkowski S., Dedhar S. Cytoskeletal rearrangements accompanying salmonella entry into epithelial cells. J Cell Sci. 1991 Jun;99(Pt 2):283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- Foubister V., Rosenshine I., Donnenberg M. S., Finlay B. B. The eaeB gene of enteropathogenic Escherichia coli is necessary for signal transduction in epithelial cells. Infect Immun. 1994 Jul;62(7):3038–3040. doi: 10.1128/iai.62.7.3038-3040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foubister V., Rosenshine I., Finlay B. B. A diarrheal pathogen, enteropathogenic Escherichia coli (EPEC), triggers a flux of inositol phosphates in infected epithelial cells. J Exp Med. 1994 Mar 1;179(3):993–998. doi: 10.1084/jem.179.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel G., Candy D. C., Everest P., Dougan G. Characterization of the C-terminal domains of intimin-like proteins of enteropathogenic and enterohemorrhagic Escherichia coli, Citrobacter freundii, and Hafnia alvei. Infect Immun. 1994 May;62(5):1835–1842. doi: 10.1128/iai.62.5.1835-1842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girón J. A., Ho A. S., Schoolnik G. K. Characterization of fimbriae produced by enteropathogenic Escherichia coli. J Bacteriol. 1993 Nov;175(22):7391–7403. doi: 10.1128/jb.175.22.7391-7403.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand J. D., Schaller M. D., Parsons J. T. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxyl terminal domain of focal adhesion kinase. Mol Biol Cell. 1995 Jun;6(6):637–647. doi: 10.1091/mbc.6.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Leong J. M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990 Mar 9;60(5):861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Voorhis D. L., Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987 Aug 28;50(5):769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- Jarvis K. G., Girón J. A., Jerse A. E., McDaniel T. K., Donnenberg M. S., Kaper J. B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse A. E., Yu J., Tall B. D., Kaper J. B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny B., Finlay B. B. Protein secretion by enteropathogenic Escherichia coli is essential for transducing signals to epithelial cells. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7991–7995. doi: 10.1073/pnas.92.17.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Baldwin T., Williams P. H., McNeish A. S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989 Apr;57(4):1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A., Schmidt M. A., Walz W., Falkow S. Genetic organization of the afimbrial adhesin operon and nucleotide sequence from a uropathogenic Escherichia coli gene encoding an afimbrial adhesin. J Bacteriol. 1985 Jun;162(3):1285–1292. doi: 10.1128/jb.162.3.1285-1292.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Edelman R. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol Rev. 1984;6:31–51. doi: 10.1093/oxfordjournals.epirev.a036274. [DOI] [PubMed] [Google Scholar]
- Lo S. H., Chen L. B. Focal adhesion as a signal transduction organelle. Cancer Metastasis Rev. 1994 Mar;13(1):9–24. doi: 10.1007/BF00690415. [DOI] [PubMed] [Google Scholar]
- Manjarrez-Hernandez H. A., Baldwin T. J., Aitken A., Knutton S., Williams P. H. Intestinal epithelial cell protein phosphorylation in enteropathogenic Escherichia coli diarrhoea. Lancet. 1992 Feb 29;339(8792):521–523. doi: 10.1016/0140-6736(92)90340-9. [DOI] [PubMed] [Google Scholar]
- Otey C. A., Burridge K. Patterning of the membrane cytoskeleton by the extracellular matrix. Semin Cell Biol. 1990 Oct;1(5):391–399. [PubMed] [Google Scholar]
- Rosenshine I., Donnenberg M. S., Kaper J. B., Finlay B. B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992 Oct;11(10):3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Magnusson K. E., Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994 Feb 15;13(4):964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sory M. P., Cornelis G. R. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994 Nov;14(3):583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- Young V. B., Miller V. L., Falkow S., Schoolnik G. K. Sequence, localization and function of the invasin protein of Yersinia enterocolitica. Mol Microbiol. 1990 Jul;4(7):1119–1128. doi: 10.1111/j.1365-2958.1990.tb00686.x. [DOI] [PubMed] [Google Scholar]