Abstract
Full thickness gastrointestinal defects such as perforations, leaks, and fistulae are a relatively common result of many of the endoscopic and surgical procedures performed in modern health care. As the number of these procedures increases, so too will the number of resultant defects. Historically, these were all treated by open surgical means with the associated morbidity and mortality. With the recent advent of advanced endoscopic techniques, these defects can be treated definitively while avoiding an open surgical procedure. Here we explore the various techniques and tools that are currently available for the treatment of gastrointestinal defects including through the scope clips, endoscopic suturing devices, over the scope clips, sealants, endoluminal stents, endoscopic suction devices, and fistula plugs. As fistulae represent the most recalcitrant of defects, we focus this editorial on a multimodal approach of treatment. This includes optimization of nutrition, treatment of infection, ablation of tracts, removal of foreign bodies, and treatment of distal obstructions. We believe that by addressing all of these factors at the time of attempted closure, the patient is optimized and has the best chance at long-term closure. However, even with all of these factors addressed, failure does occur and in those cases, endoscopic therapies may still play a role in that they allow the patient to avoid a definitive surgical therapy for a time while nutrition is optimized, and infections are addressed.
Keywords: Perforation, Fistula, Anastomotic leak, Over the scope clips, Overstitch, Stent, Endoscopic
Core tip: Endoscopic methods are replacing surgical options as the first line therapy for a wide array of gastrointestinal tract defects. Here we will review the available endoscopic modalities, their appropriate applications and their respective success rates. The fusion of standard surgical principles with flexible, intra-luminal modalities is likely to be the key to the successful endoscopic management of these challenging clinical problems.
INTRODUCTION
Whether in the form acute perforations, acute or sub-acute anastomotic leaks or chronic fistulae, full-thickness gastrointestinal (GI) tract defects remain a challenging and highly morbid healthcare problem. According to the Center for Disease Control (CDC) over 6 million abdominal procedures (including upper and lower endoscopies) were performed in the United States in 2010[1]. As the number of abdominal procedures performed annually in the United States increases, the number of full thickness GI defects that occur as a result will also increase. Historically, full-thickness luminal defects mandated surgical exploration (with its associated high rates of morbidity and mortality)[2,3]. Recent advancements in the comprehensive endoscopic management of GI defects have yielded encouraging results.
In centers of expertise, endoscopic methods have begun to replace surgical options as the first line therapy for a wide array of GI tract defects[4-6]. Here we will review the available endoscopic modalities, their appropriate applications and their respective success rates. The fusion of standard surgical principles with flexible, intra-luminal modalities is likely to be the key to the successful endoscopic management of these challenging clinical problems. Much like polyp resection, gastrostomy tube insertion and GI bleeding, we believe that surgery for full-thickness luminal defects will shortly be relegated only to patients who fail endoscopic therapy in the majority of cases[7].
SCOPE OF THE PROBLEM
GI tract defects include perforations, anastomotic leaks and fistulae and occur with numerous disease states as well as following a wide array of endoscopic, surgical and radiologic procedures. They vary greatly in their presentation and in their associated morbidity and mortality; an acute esophageal perforation from an endoscope and a persistent gastro-cutaneous fistula following gastrostomy tube removal are clearly different clinical entities. Yet until recently, these processes were thought of similar when endoscopic modalities were considered. As the volume of cases within which endoscopic closure could be attempted increases, it becomes increasingly clear that a spectrum of endoscopic therapies is necessary.
Technical limitations are not the only hurdle to overcome in the complete endoscopic management of these conditions. Many surgeons are unaware of or unwilling to permit (and/or not able to perform themselves) the application of novel therapies to patients who have perforations, leaks or fistulae. Many endoscopists with the skill and expertise to manage full thickness perforations do not have access to the patients presenting with these problems (unless they are the result of an iatrogenic endoscopic injury). There is therefore a disconnect between those individuals with the knowledge and skill to manage full thickness perforations and those who are evaluating and caring for the patients. The volume of this patient population is not inconsequential. The spectrum of diseases to which endoscopic methods could be applied includes:
Esophageal
The incidence of acute perforations during esophagogastroduodenoscopy (EGD) is approximately 0.03%[8,9]. One series of 217, 507 EGD procedures had a perforation rate of 0.033% with the esophagus being injured most commonly (51%)[9]. That same series showed a mortality rate of 17% despite intervention. The CDC reported that the total number of upper endoscopies performed in the United States in 2010 (including both diagnostic and therapeutic) was 1.1 million[1]. With an average perforation rate of 0.03%, this would equal 330 perforations.
Anastomotic leaks after esophagectomy ranges from 8%-10%[10,11]. Furthermore, patients with esophageal leaks after surgical resection have an increased mortality rate ranging from 18%-35% compared to patients undergoing similar procedures without leaks[10,12-14].
Gastric
Postoperative gastric leaks range from 1.7%-2.5% after Roux-en-Y gastric bypass (RYGB) and 1.5%-7% after sleeve gastrectomy[5,15-17]. The mortality rate for patients who develop leak ranges from 0.6%-14%[18,19]. The American Society for Metabolic and Bariatric Surgery reported that the number of bariatric surgeries performed in the United States is steadily rising with 158000 cases in 2011 and 179000 cases in 2013.
Iatrogenic gastric perforations during upper endoscopy are rare, but it is the site of injury in 3% of all iatrogenic injuries during both diagnostic and therapeutic EGD[9].
Gastrogastric fistulae occur in patients who underwent Roux-en-Y gastric bypass and develop a fistulous connection between the gastric pouch and the native bypassed stomach that is left in-situ. In one series of 1292 patients who underwent Roux-en-Y gastric bypass, 1.2% developed gastrogastric fistulae[20].
Gastrocutaneous (GC) fistulae represent an abnormal connection between the stomach and the skin. GC fistulae can occur at the site of percutaneous endoscopic gastrostomy (PEG) tubes, which are subsequently removed. In the vast majority of cases, these fistulous tracts close spontaneously after the PEG tube is removed. However, in 1.1% of cases these fistulae persist[21,22]. Approximately 216000 PEG tubes are placed each year[21].
Duodenal and small bowel
Worldwide, peptic ulcer disease affects 4 million people annually[23]. Between 2%-14% of those ulcers will perforate with mortality ranging from 10%-40%[24,25]. In the setting of acute perforation during upper endoscopy, the duodenum is the location of perforation in 32% of cases[9].
Enterocutaneous (EC) fistulae, or tracts from the small bowel to the skin, are a devastating complication of abdominal surgery with mortality rates approaching 20%[26]. Patients with EC fistulae suffer from malnutrition, dehydration, skin excoriation, infection and sepsis. Although the largest percentage of EC fistulae are in patients with Crohn’s disease, other inflammatory processes, malignancy, abdominal surgery, trauma, and radiation are all well-known causes[27].
Colon
The incidence of acute colonic perforations during screening colonoscopies ranges from 0.07% to 0.082%[15,28]. These numbers are similar for both screening and therapeutic colonoscopies. In 2010 there were roughly 500000 colonoscopies performed in the United States which would mean that there were approximately 400 perforations[1].
Fistula formation from the colon to other structures or to the skin is most commonly due to diverticular disease, but may also occur in patients after surgical intervention. In one review from the Cleveland Clinic of all patients treated for diverticular disease from 1960 to 1986, 20.4% had internal fistulae with colovesicular fistulae being the most common (65%)[29]. In one series examining colocutaneous fistulae, 88 of 93 patients (94.6%) were following surgery[30].
The incidence of leaks after colorectal resection and anastomosis ranges from 2.6%-26.2%[31]. Many patients who develop an anastomotic leak and require reoperation ultimately receive a permanent stoma[32,33]. Historically, the majority of these cases were treated surgically with the associated morbidity and difficulty of caring for these patients who are often in extremis. The advent of multiple endoscopic techniques and modalities has provided a safe and effective alternative to open surgical management of these complex problems[34].
Other conditions
There are a myriad of other types and combinations of GI tract leaks that are potentially addressable endoscopically including those related to cancer, radiation therapy, urologic procedures and radiologic interventions. Radiation therapy to the abdomen for other reasons can result in abdominal pathology including perforation and fistulae in up to 5% of patients[35]. In one review of fluoroscopically placed intraperitoneal chemotherapy catheters 6 of 750 patients (0.8%) experienced bowel perforation at the time of catheter placement[36].
When one considers the total volume of patients who present with a full thickness GI tract defect, it becomes clear that endoscopic therapies have the ability to change the way we think about managing a wide array of complex disease states.
AVAILABLE ENDOSCOPIC THERAPIES IN THE ACUTE OR CHRONIC SETTING AND THEIR OUTCOMES
The majority of the published literature describing the success of endoscopic management of GI defects consists of small case series and retrospective reviews. To date, there have been no randomized trials to evaluate the efficacy of endoscopic management versus traditional surgical management. The small reported successes of endoscopic management compared to the increased morbidity and mortality associated with surgical management of these disease processes are pushing the use of endoscopic therapies forward and expanding their scope of application. Larger, randomized trials need to be performed to further establish the following endoscopic therapies as both effective and superior to open surgical techniques.
It should be emphasized that the chronicity of the defect has implications about its etiology. Csendes et al[37] defined defects appearing 1-4 d as acute, 5-9 d as intermediate, and 10 or more days as late. Leaks presenting less than 2 d from the procedure likely represent a technical error such as stapler misfire or tissue injury while leaks presenting 5-7 d after the procedure more often represent ischemia[38].
Acute GI perforations are those that are identified at the time of injury or immediately afterwards by the sequelae that most commonly accompany perforations including fever, tachycardia, elevated white blood cell count, abdominal pain, peritonitis, systemic inflammatory response syndrome, and sepsis[39]. Early diagnosis and treatment of the defect is essential for improved patient outcomes[37].
Chronic defects are evidenced by contained fluid collections, or established fistulae to the skin or other tubular structures. The success of endoscopic therapies in the setting of longstanding leaks and fistulae has been more limited with fistulae being particularly difficult to manage[40-43]. Our experience has been similar to what has previously been reported. Since 2012 we have endoscopically managed 14 patients with GI fistulae and 6 patients with leaks and achieved a long-term closure rate of 64% and 100% respectively[44]. We believe there are multiple factors affecting the outcome in more chronic GI defects that we will explore in more detail later.
Through the scope clips
Endoscopic clips that are passed through the endoscopic working channel and are deployed within the lumen of the GI tract were initially designed for hemostasis and endoluminal marking (Figure 1). They are also referred to as through the scope clips (TTSC), hemoclips and endoclips. In the late 1990’s, reports emerged describing their use as a method to close gastric and colonic perforations[45,46]. Although effective at closing smaller defects, the ability to close larger defects is quite poor due to the small size of the clips, the low grasping force that they generate and the inability to grasp deeper tissues[45]. They are more effective at closing surgically incised tissue with straight regular edges, as opposed to tissue that was bluntly perforated with irregular, striated or gaping edges. Their effectiveness at closing surgically incised mucosal edges has been well documented in the areas of submucosal dissection and POEM (Figure 2)[47-50].
Figure 1.
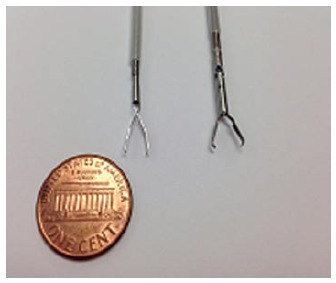
Examples of through the scope clips prior to deployment. Left: QuickClip 2 (Olympus Medical Systems Co., Tokyo, Japan); Right: Resolution Clip (Boston Scientific, Marlborough, MA).
Figure 2.

Endoscopic view of mucosotomy during peroral endoscopic myotomy. A: Esophageal mucosal defect after completion of peroral endoscopic myotomy; B: Defect closed with sequentially placed through the scope clips.
TTSC have been shown to be successful in closing iatrogenic defects in the GI tract with clinical success rates ranging from 59%-83%[51,52]. It is felt that the limitation to their success is their small size, small closing force and mucosa-only tissue apposition, although in the right setting such as small defects that are not gaping, they can be quite effective.
These two factors about endoclip use have introduced bias into the initial clinical experience with acute GI tract perforations. Many acute defects are successfully closed with readily available endoscopic equipment and therefore escape the preview of surgical consultation. Larger defects are more likely to be unsuccessfully managed with TTSC clips and therefore surgeons receive a biased view of the true success rate of the most commonly applied endoscopic therapy.
Endoscopic suturing devices
The endoscopic suturing platform (Overstitch, Apollo Endosurgery, Austin, TX) is a disposable device that is attached to the end of a therapeutic double channel endoscope (Figure 3). It allows for placement of full-thickness absorbable or non-absorbable sutures. The device can be used multiple times without the need to remove the scope from the patient. The sutures can also be applied in a running or interrupted fashion (including simple and figure-of-8 sutures). Since its introduction, it has been successfully used in the closure of GI fistulae, acute perforations and at sites of endoscopic resection[53,54].
Figure 3.

Endoscopic suturing device (Overstitch, Apollo Endosurgery, Austin, TX).
Endoscopic suturing devices have been found to provide safe and effective suturing. In one human in-vivo study, the Overstitch device was found to place sutures consistently at a subserosal depth in the colon without full thickness penetration or injury to adjacent structures[55]. It has been used successfully in the closure of staple-line leaks after sleeve gastrectomy, anchoring stents to help prevent migration, and closing gastrogastric fistulae[6,54,56,57]. However, the long-term success has been mixed with one study of 95 patients with gastrogastric fistulae achieving a 35% long-term closure rate[58].
Stents
The use of stents as a diversion method in full thickness GI defects is a non-FDA approved use that has been widely accepted by surgeons and endoscopists alike as a method for defect management. Stent deployment at the site of the defect helps by allowing diversion of enteric contents away from the defect. Multiple types of stent have been studied including metallic (partially or completely covered), plastic (covered, expandable), and biodegradable (Figure 4). Stent placement often permits continued enteral nutrition and can be used in cases of larger defect (> 1.5 cm)[59-61]. Although stents have been successful at treating GI defects, they are prone to migration in as much as 20%-30% of cases and require frequent observation with radiographic monitoring[61,62]. This has been addressed with techniques using TTSC and endoscopic suturing devices to anchor the stent in place. Stents also do not create a complete seal within the GI tract and, although variable in its amount, leak around stents is a near universal finding. Percutaneous placement of enteric stents have also been effective in patients with high-output EC fistulas by decreasing the output of the fistula, improving wound care, TPN requirements, and oral diet tolerance[63].
Figure 4.

Examples of endoscopic stents. From Left: Fully covered plastic stent, fully covered metal stent, partially covered metal stent, larger diameter partially covered metal stent.
There is a large body of evidence supporting the use of stents in the treatment of GI defects. A recent meta-analysis of 7 studies of stent placement for acute leak after bariatric surgery showed a radiographically confirmed closure rate after stent removal of 87.8% (95%CI: 79.4%-94.2%)[64]. That same analysis showed a migration rate of 16.9% and only 9% of patients undergoing reoperation. Some authors advocate for clip placement to anchor the stents to help prevent migration. One study used 2 to 4 endoscopic clips to anchor the stent in 23 of 44 consecutive patients and found that stent migration occurred in 13% of patients with clips and 34% of patients without[65].
Sealants
Tissue adhesives and hemostatic agents, including fibrin sealant, have been used with varying degrees of success in the management of GI track defects. Fibrin sealant is composed of fibrinogen and thrombin, which are combined to make an acellular clot at the site of application. In one report fibrin glue was injected into the submucosa of a tracheoesophageal fistula causing a wheal and subsequent occlusion of the fistula in a pediatric patient[66]. In another series of 15 patients with persistent fistulae after conservative treatment, fibrin glue was used to occlude the fistula opening and resulted in long-term closure in 86.6% of patients after a mean of 2.5 sessions[67]. Tissue adhesives and sealants will likely be utilized primarily as an adjunct therapy to the definitive closure of leaks with an alternative method (such as a clip or suturing device).
Fistula plugs
SurgiSIS AFP plugs (Cook Biotech, West Lafayette, IN) were developed for the use in anal fistulae and have been used successfully in the treatment of GC fistulae after bariatric surgery[68]. Porcine small intestinal submucosa (SIS) is a bioprosthetic collagen material used in many settings including hernia repair, dressings for venous stasis ulcers, and anal fistulae. One group used SurgiSIS strips to endoscopically occlude GI fistulae in 25 patients with an 80% long-term closure rate[69].
Vacuum-assisted devices
Vacuum-assisted sponge closure has been used in the setting of esophageal and colorectal defects. Porous sponge foam is cut to be just smaller than the defect and sutured to the end of a nasogastric feeding tube (Figure 5). This is then grasped with endoscopic graspers and introduced into the defect. The nasogastric tube is then placed on continuous external suction. This suction minimizes secretions escaping through the defect while increasing blood flow to the area. Furthermore, the sponge induces granulation of the surrounding tissue and promotes healing[70]. Sponges need to be changed every 2-3 d. Small defects with adjacent fluid collections that aren’t septated are more amenable to this therapy.
Figure 5.

Vacuum-assisted closure device constructed of porous sponge and sutured to a nasogastric feeding tube.
Vacuum-assisted sponge devices have been used successfully in small esophageal defects. In one series of five patients with fluid collections related to a leak at an esophageal anastomosis, all 5 patients resolved their leak with vacuum-assisted sponge therapy. The median length of therapy was 28 d with 9 sponge changes. Two of the patients developed stenosis at the anastomosis and one suffered from a fatal hemorrhage after a dilation procedure revealed an aortoanastomotic fistula[70].
Managing leaks with endoscopically placed tubes
Other strategies for managing leaks from the GI tract without repairing the defect include using the hole for other therapeutic modalities. Such “tube ostomy” formation is a standard surgical maneuver for difficult perforations in retroperitoneal organs like the colon and duodenum. In patients who presented with an acutely dislodged PEG tube and a leaking gastrotomy, the defect can be used to enter the abdominal cavity endoscopically, and replace the tube correctly, a so called “PEG rescue”[71]. We recently published a similar technique in a patient with a dislodged esophagostomy tube. By passing a wire from the cutaneous opening at the skin, securing this wire in the esophagus endoscopically, and drawing the wire out through the patients mouth, a new esophagostomy tube could be placed without any further surgical intervention[72]. Both of these examples illustrate the ability of the endoscopist to use established techniques to endoscopically manage what would traditionally be managed surgically.
Over the scope clips
Over the scope clips (OTSC) (Ovesco Endoscopy, Tubingen, Germany and Padlock, Aponos Medical, Kingston, NH) have gained popularity for the closure of GI track defects. Their ease of use, large capacity caps and short learning curve are the factors responsible for their surge in use.
Ovesco OTSC are made of elastic, biocompatible nitinol and are capable of full thickness closure of defects measuring 2 cm in diameter[73] (Figure 6). Two devices are available to use in conjunction with the OTSC to aid in apposition of the tissues prior to firing: a twin-grasper and a 3-pronged tissue anchor. Either device can be passed through the working channel and is used to secure the edges of the defect and draw them up into the cap prior to deployment of the OTSC. Because of the larger size of OTSC compared to TTSC they are able to close larger defects and take full-thickness bites of the tissue. They also provide a larger closure force due to their design. The Padlock device consists of a nitinol ring and a clear applicator cap that is placed on the end of the endoscope (Figure 7). Once deployed, the ring provides 360-degree tissue compression and approximation.
Figure 6.

Examples of the over the scope clips (Ovesco Endoscopy, Tubingen, Germany).
Figure 7.

Example of the Padlock over the scope clips (Aponos Medical, Kingston, NH).
OTSC has been reported in many case series to be successful in closing acute perforations, leaks, and fistulae with long-term success rates ranging from 71%-100%[74-78]. A recent multi-centered international review examined 188 patients with acute perforations, leaks, and fistulae who were treated with OTSC and found that long-term closure rates were achieved in 90%, 73.3% and 42.9% respectively[79]. Since 2012 we have endoscopically treated 20 patients with the OTSC (6 with leaks and 14 with fistulae) resulting in a 100% and 64% closure rate respectively[44].
FACTORS LEADING TO SUCCESSFUL OUTCOMES
There are multiple factors that influence the ultimate closure rate in any endoscopic therapy, but common themes emerge in the literature in regards to closure rates. Defect size, that is the size of the luminal defect, not the length of the leak or fistula outside the GI tract, seems to play a role with smaller defects being easier to close than larger ones[58]. This is likely due to the technically difficult closure that larger defects present. Also, even though OTSC has been shown to close larger defects measuring up to 3 cm, in ex-vivo studies the bursting pressures have been much lower in repairs of larger defects compared to smaller ones[80]. Using the right tool for the type and location of the defect is crucial. Time from perforation to attempted closure certainly plays a role, with longer times being less successful[40-43]. Accurately measuring and appreciated the size of the defect and ensuring closure fluoroscopically at the time of attempted closure also play a role. Furthermore, the type of defect remains important, with acute perforations being more successfully closed than leaks or more chronic fistulae[41].
RECOMMENDATIONS FOR ENDOSCOPIC CLOSURE
When there is clinical suspicion for acute GI perforation, leak, or fistulae, at an area of the GI tract that is reachable by endoscopic means, we recommend prompt endoscopic evaluation and treatment. The absolute contraindication to endoscopic therapy is evidence of peritonitis on abdominal exam[73]. Prompt endoscopic intervention provides two major benefits: Firstly, the endoscopist is able to provide a direct evaluation of the location and extent of the defect, and secondly, they are able to provide timely therapeutic attempts at closure for those lesions that are appropriate for endoscopic management.
The method of closure in acute full thickness GI defects will be dictated by three factors: the location, the size, and the operator’s proficiency and familiarity with each therapy. Smaller defects may be amenable to TTSC, while larger ones may require one or more deployments of the OTSC. Very proximal perforations may not be amenable to stenting due to the foreign-body sensation that many patients experience with proximal stenting that approaches the upper esophageal sphincter. In many cases of initial failure, multiple attempts with various modalities are often required to ultimately obtain long-term closure[81]. We previously described the use of laparoscopy and endoscopic stent placement for management of leaks following bariatric surgery, but have since moved to definitive endoscopic closure of all leaks with endoscopic suturing or over the scope clips[5]. We now reserve stent use for leaks not amenable to or that have failed previous attempts at definitive closure.
We do not recommend any one type of endoscopic therapy for any specific location in the GI tract. Rather, we recommend that the endoscopist become familiar with all treatment modalities so as to use whichever method he/she deems appropriate based on clinical judgment. We reemphasize that often these defects require multiple attempts with varying modalities to achieve long-term closure, thus familiarity with all types of endoscopic therapies is strongly encouraged.
Because endoscopic closure of fistulae has routinely achieved the lowest long-term success rates, we recommend adopting traditional surgical fistula management techniques jointly with endoscopic attempts at closure[82-85]. Addressing the factors described by the classic acronym FRIENDS (foreign bodies, radiation, infection/inflammation, epithelialization, neoplasm, distal obstruction, and steroids) in the setting of fistula management is imperative to long-term success. It has been our experience that by addressing these issues on a case-by-case basis, we have achieved somewhat higher closure rates in patients with long-term fistulae. Since 2012 we have endoscopically treated 14 patients with GI fistulae with the OTSC resulting in a 64% closure rate[44].
Foreign bodies at the endoluminal opening of any fistula will contribute to its persistence by the foreign body reaction that they perpetuate. We routinely remove any suture, indigestible food matter, or other foreign bodies present within fistulous tract (Figures 8 and 9). Furthermore, once external drains have effectively treated the fluid collection for which they were placed, they should be removed in conjunction with the endoscopic treatment of the fistulous opening.
Figure 8.
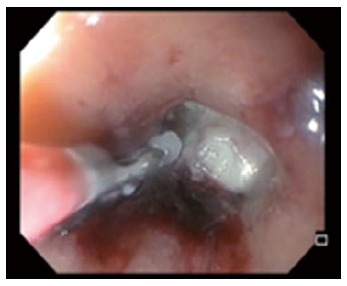
Endoscopic removal of suture foreign body at the opening of a rectal stump fistula.
Figure 9.
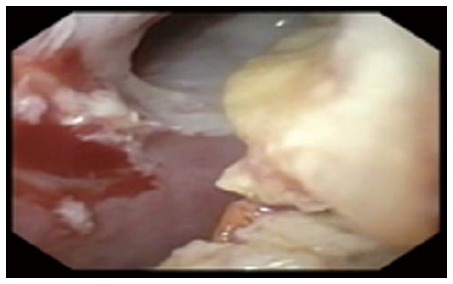
Undigested food within an esophago-cutaneous fistula tract.
Infection must be treated with adequate source control in the form of external drainage for infected fluid collections and organism-specific antibiotic coverage. If the patient displays hemodynamic instability or sepsis due to uncontrolled infection, surgical intervention may be warranted as endoscopic management is typically reserved for the more stable patient.
Inflammation at the site of the fistulous opening is a commonly sited factor for failed closure. It is felt that closure rates are lower due to the difficulty in achieving adequate tissue apposition due to the fibrotic and inflamed edges that are present at the fistula opening[86]. Cauterization of the margins of chronic fistulae has been advocated to facilitate subsequent closure with the OTSC[87]. We routinely ablate the margins prior to clip placement in all chronic fistulae.
Epithelialization of the fistula tract can be addressed by both mechanical and ablative techniques. We frequently use argon plasma coagulation to ablate the epithelialized surface of the fistula tract to help prevent recurrence (Figure 10)[88]. Other authors have described mechanical debridement with biopsy forceps or brushes to disrupt the epithelial lining that may be present with more chronic tracts.
Figure 10.
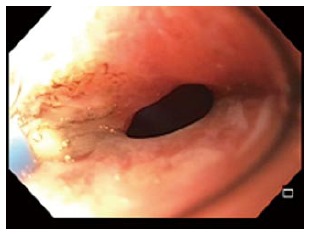
Circumferential argon plasma coagulation catheter ablating epithelialized fistula tract.
Distal obstruction or stenosis may precipitate the failure or breakdown of more proximal anastomoses or staple-lines[89]. When distal stenosis is discovered, this must be addressed concomitantly with any attempt to close proximal fistulae. Stenosis may be adequately treated with serial endoscopic dilations, though more recalcitrant strictures may require stenting (Figure 11)[90].
Figure 11.

Endoscopic balloon-dilation of a stenotic gastrojejunostomy with adjacent jejunoanastomotic fistula.
For the patient who is receiving steroid therapy for unrelated processes, coordination with the provider managing their steroids may provide a window where the steroid load can either be lessened or withheld for a period while endoscopic treatment is attempted. This may not be possible in all cases, but should be addressed on a case-by-case basis.
Maintaining adequate nutrition in these patients is imperative to promote healing. We recommend early enteral feeding in all cases possible. This may be achieved by obtaining feeding tube access distal to the site of the fistula such as a nasojejunal tube, or a percutaneous endoscopic jejunostomy tube. If this is not feasible, total parenteral nutrition may be initiated and continued until enteral feeding is tolerated.
In patients with acute perforations, endoscopic management alone may be sufficient for long-term success. In patients with chronic GI fistulae, all aspects of a patient’s care must be optimized in order to achieve long-term success. Similar to the treatment of a patient with acute GI hemorrhage, multimodality endoscopic therapies may be required for more complex or chronic GI tract perforation. A large gastro-gastric fistula after RYGB, for example, may require suture foreign body removal, argon plasma ablation of the epithelialized track, endoscopic suture closure of the largest portions of the defect, over the scope clip application to smaller portions and endoscopic dilation of a simultaneous gastro-jejunal anastomotic ulcer. Failure to address all of these issues will likely result in short term endoscopic failure.
Unfortunately, there will be patients who ultimately fail endoscopic therapy and will require surgical intervention. However, even in these patients, early endoscopic management can lessen the symptoms of high-output fistulae, enable patients to leave the hospital if even for a brief period, allow time for nutritional status to be improved, infections to be treated, and time for more in-depth operative planning that would otherwise not be available in the emergent setting.
CONCLUSION
There has been a great deal of advancement in the field of endoscopic treatment of full thickness GI defects with high rates of long-term closure. TTSCs, endoscopic suturing devices, stents, sealants, fistula plugs, vacuum-assisted devices, and OTSC have all been shown to be effective modalities. The treatment of acute perforations is generally more effective than the treatment of chronic fistulae. Because of this, we recommend a marriage of endoscopic therapies with classic fistula management to give the patient the best chance at long-term closure. Ultimately, even in the case of failure, endoscopic therapy can “buy time” for patient optimization prior to definitive surgical management.
Footnotes
Conflict-of-interest statement: Winder JS has no financial affiliations to disclose; Pauli EM has received honoraria from Cook Biotech for speaking and consulting.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 29, 2015
First decision: March 6, 2015
Article in press: May 8, 2015
P- Reviewer: Bilir C, Kouraklis G, Syam AF, Tsuji Y S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
References
- 1.Centers for Disease Control and Prevention. National Hospital Discharge Survey: 2010 Table, Procedures by selected patient characteristics accessed 2015 Jan 28] Available from: http://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf.
- 2.Lüning TH, Keemers-Gels ME, Barendregt WB, Tan AC, Rosman C. Colonoscopic perforations: a review of 30,366 patients. Surg Endosc. 2007;21:994–997. doi: 10.1007/s00464-007-9251-7. [DOI] [PubMed] [Google Scholar]
- 3.Svanes C. Trends in perforated peptic ulcer: incidence, etiology, treatment, and prognosis. World J Surg. 2000;24:277–283. doi: 10.1007/s002689910045. [DOI] [PubMed] [Google Scholar]
- 4.Pauli EM, Schomisch SJ, Blatnik JA, Krpata DM, Sanabria JS, Marks JM. A novel over-the-scope deployment method for enteral stent placement. Surg Endosc. 2013;27:1410–1411. doi: 10.1007/s00464-012-2564-1. [DOI] [PubMed] [Google Scholar]
- 5.Juza RM, Haluck RS, Pauli EM, Rogers AM, Won EJ, LynSue JR. Gastric sleeve leak: a single institution’s experience with early combined laparoendoscopic management. Surg Obes Relat Dis. 2015;11:60–64. doi: 10.1016/j.soard.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Pauli EM, Beshir H, Mathew A. Gastrogastric fistulae following gastric bypass surgery-clinical recognition and treatment. Curr Gastroenterol Rep. 2014;16:405. doi: 10.1007/s11894-014-0405-1. [DOI] [PubMed] [Google Scholar]
- 7.Pauli EM, Ponsky JL. A modern history of the surgeon-endoscopist. Techniques in Gastrointestinal Endoscopy. 2013;15:166–172. [Google Scholar]
- 8.Geraci G, Pisello F, Modica G, Li Volsi F, Arnone E, Sciumè C. Complications of elective esophago-gastro-duodenoscopy (EGDS). Personal experience and literature review. G Chir. 2009;30:502–506. [PubMed] [Google Scholar]
- 9.Merchea A, Cullinane DC, Sawyer MD, Iqbal CW, Baron TH, Wigle D, Sarr MG, Zielinski MD. Esophagogastroduodenoscopy-associated gastrointestinal perforations: a single-center experience. Surgery. 2010;148:876–880; discussion 881-882. doi: 10.1016/j.surg.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Rutegård M, Lagergren P, Rouvelas I, Lagergren J. Intrathoracic anastomotic leakage and mortality after esophageal cancer resection: a population-based study. Ann Surg Oncol. 2012;19:99–103. doi: 10.1245/s10434-011-1926-6. [DOI] [PubMed] [Google Scholar]
- 11.Lerut T, Coosemans W, Decker G, De Leyn P, Moons J, Nafteux P, Van Raemdonck D. Surgical techniques. J Surg Oncol. 2005;92:218–229. doi: 10.1002/jso.20363. [DOI] [PubMed] [Google Scholar]
- 12.Alanezi K, Urschel JD. Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg. 2004;10:71–75. [PubMed] [Google Scholar]
- 13.Junemann-Ramirez M, Awan MY, Khan ZM, Rahamim JS. Anastomotic leakage post-esophagogastrectomy for esophageal carcinoma: retrospective analysis of predictive factors, management and influence on longterm survival in a high volume centre. Eur J Cardiothorac Surg. 2005;27:3–7. doi: 10.1016/j.ejcts.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Sauvanet A, Mariette C, Thomas P, Lozac’h P, Segol P, Tiret E, Delpero JR, Collet D, Leborgne J, Pradère B, et al. Mortality and morbidity after resection for adenocarcinoma of the gastroesophageal junction: predictive factors. J Am Coll Surg. 2005;201:253–262. doi: 10.1016/j.jamcollsurg.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Arora G, Mannalithara A, Singh G, Gerson LB, Triadafilopoulos G. Risk of perforation from a colonoscopy in adults: a large population-based study. Gastrointest Endosc. 2009;69:654–664. doi: 10.1016/j.gie.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Morales MP, Miedema BW, Scott JS, de la Torre RA. Management of postsurgical leaks in the bariatric patient. Gastrointest Endosc Clin N Am. 2011;21:295–304. doi: 10.1016/j.giec.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Sakran N, Goitein D, Raziel A, Keidar A, Beglaibter N, Grinbaum R, Matter I, Alfici R, Mahajna A, Waksman I, et al. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27:240–245. doi: 10.1007/s00464-012-2426-x. [DOI] [PubMed] [Google Scholar]
- 18.Almahmeed T, Gonzalez R, Nelson LG, Haines K, Gallagher SF, Murr MM. Morbidity of anastomotic leaks in patients undergoing Roux-en-Y gastric bypass. Arch Surg. 2007;142:954–957. doi: 10.1001/archsurg.142.10.954. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Carmody B, Wolfe L, Demaria E, Kellum JM, Sugerman H, Maher JW. Effect of location and speed of diagnosis on anastomotic leak outcomes in 3828 gastric bypass cases. J Gastrointest Surg. 2007;11:708–713. doi: 10.1007/s11605-007-0085-3. [DOI] [PubMed] [Google Scholar]
- 20.Carrodeguas L, Szomstein S, Soto F, Whipple O, Simpfendorfer C, Gonzalvo JP, Villares A, Zundel N, Rosenthal R. Management of gastrogastric fistulas after divided Roux-en-Y gastric bypass surgery for morbid obesity: analysis of 1,292 consecutive patients and review of literature. Surg Obes Relat Dis. 2005;1:467–474. doi: 10.1016/j.soard.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 21.McElrath L, Pauli EM, Marks JM. Hernia formation and persistent fistula after percutaneous endoscopy gastrostomy: unusual complications of a common procedure. Am Surg. 2012;78:E200–E201. [PubMed] [Google Scholar]
- 22.Shellito PC, Malt RA. Tube gastrostomy. Techniques and complications. Ann Surg. 1985;201:180–185. doi: 10.1097/00000658-198502000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zelickson MS, Bronder CM, Johnson BL, Camunas JA, Smith DE, Rawlinson D, Von S, Stone HH, Taylor SM. Helicobacter pylori is not the predominant etiology for peptic ulcers requiring operation. Am Surg. 2011;77:1054–1060. [PubMed] [Google Scholar]
- 24.Bertleff MJ, Lange JF. Perforated peptic ulcer disease: a review of history and treatment. Dig Surg. 2010;27:161–169. doi: 10.1159/000264653. [DOI] [PubMed] [Google Scholar]
- 25.Lau JY, Sung J, Hill C, Henderson C, Howden CW, Metz DC. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion. 2011;84:102–113. doi: 10.1159/000323958. [DOI] [PubMed] [Google Scholar]
- 26.Fischer JE. The pathophysiology of enterocutaneous fistulas. World J Surg. 1983;7:446–450. doi: 10.1007/BF01655932. [DOI] [PubMed] [Google Scholar]
- 27.Lundy JB, Fischer JE. Historical perspectives in the care of patients with enterocutaneous fistula. Clin Colon Rectal Surg. 2010;23:133–141. doi: 10.1055/s-0030-1262980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warren JL, Klabunde CN, Mariotto AB, Meekins A, Topor M, Brown ML, Ransohoff DF. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150:849–857, W152. doi: 10.7326/0003-4819-150-12-200906160-00008. [DOI] [PubMed] [Google Scholar]
- 29.Woods RJ, Lavery IC, Fazio VW, Jagelman DG, Weakley FL. Internal fistulas in diverticular disease. Dis Colon Rectum. 1988;31:591–596. doi: 10.1007/BF02556792. [DOI] [PubMed] [Google Scholar]
- 30.Steele M, Deveney C, Burchell M. Diagnosis and management of colovesical fistulas. Dis Colon Rectum. 1979;22:27–30. doi: 10.1007/BF02586752. [DOI] [PubMed] [Google Scholar]
- 31.Tan WS, Tang CL, Shi L, Eu KW. Meta-analysis of defunctioning stomas in low anterior resection for rectal cancer. Br J Surg. 2009;96:462–472. doi: 10.1002/bjs.6594. [DOI] [PubMed] [Google Scholar]
- 32.Francone TD, Saleem A, Read TA, Roberts PL, Marcello PW, Schoetz DJ, Ricciardi R. Ultimate fate of the leaking intestinal anastomosis: does leak mean permanent stoma. J Gastrointest Surg. 2010;14:987–992. doi: 10.1007/s11605-010-1190-2. [DOI] [PubMed] [Google Scholar]
- 33.Khan AA, Wheeler JM, Cunningham C, George B, Kettlewell M, Mortensen NJ. The management and outcome of anastomotic leaks in colorectal surgery. Colorectal Dis. 2008;10:587–592. doi: 10.1111/j.1463-1318.2007.01417.x. [DOI] [PubMed] [Google Scholar]
- 34.Kumta NA, Boumitri C, Kahaleh M. New devices and techniques for handling adverse events: claw, suture, or cover. Gastrointest Endosc Clin N Am. 2015;25:159–168. doi: 10.1016/j.giec.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Donner CS. Pathophysiology and therapy of chronic radiation-induced injury to the colon. Dig Dis. 1998;16:253–261. doi: 10.1159/000016873. [DOI] [PubMed] [Google Scholar]
- 36.Asif A, Byers P, Vieira CF, Merrill D, Gadalean F, Bourgoignie JJ, Leclercq B, Roth D, Gadallah MF. Peritoneoscopic placement of peritoneal dialysis catheter and bowel perforation: experience of an interventional nephrology program. Am J Kidney Dis. 2003;42:1270–1274. doi: 10.1053/j.ajkd.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 37.Csendes A, Burdiles P, Burgos AM, Maluenda F, Diaz JC. Conservative management of anastomotic leaks after 557 open gastric bypasses. Obes Surg. 2005;15:1252–1256. doi: 10.1381/096089205774512410. [DOI] [PubMed] [Google Scholar]
- 38.Baker RS, Foote J, Kemmeter P, Brady R, Vroegop T, Serveld M. The science of stapling and leaks. Obes Surg. 2004;14:1290–1298. doi: 10.1381/0960892042583888. [DOI] [PubMed] [Google Scholar]
- 39.Abou Rached A, Basile M, El Masri H. Gastric leaks post sleeve gastrectomy: review of its prevention and management. World J Gastroenterol. 2014;20:13904–13910. doi: 10.3748/wjg.v20.i38.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albert JG, Friedrich-Rust M, Woeste G, Strey C, Bechstein WO, Zeuzem S, Sarrazin C. Benefit of a clipping device in use in intestinal bleeding and intestinal leakage. Gastrointest Endosc. 2011;74:389–397. doi: 10.1016/j.gie.2011.03.1128. [DOI] [PubMed] [Google Scholar]
- 41.Haito-Chavez Y, Law JK, Kratt T, Arezzo A, Verra M, Morino M, Sharaiha RZ, Poley JW, Kahaleh M, Thompson CC, et al. International multicenter experience with an over-the-scope clipping device for endoscopic management of GI defects (with video) Gastrointest Endosc. 2014;80:610–622. doi: 10.1016/j.gie.2014.03.049. [DOI] [PubMed] [Google Scholar]
- 42.Kim HH, Kye BH, Kim HJ, Cho HM. Prompt management is most important for colonic perforation after colonoscopy. Ann Coloproctol. 2014;30:228–231. doi: 10.3393/ac.2014.30.5.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qadeer MA, Dumot JA, Vargo JJ, Lopez AR, Rice TW. Endoscopic clips for closing esophageal perforations: case report and pooled analysis. Gastrointest Endosc. 2007;66:605–611. doi: 10.1016/j.gie.2007.03.1028. [DOI] [PubMed] [Google Scholar]
- 44.Winder JS, Kulaylat AN, Schubart J, Hal HM, Pauli EM. Management of gastrointestinal defects using the over the scope clip (OTSC): a retrospective review of one institution’s experience (SAGES Annual Surgical Congress; 2015 April 15) Nashville, TN: Hershey Medical Center; 2015. [Google Scholar]
- 45.Binmoeller KF, Grimm H, Soehendra N. Endoscopic closure of a perforation using metallic clips after snare excision of a gastric leiomyoma. Gastrointest Endosc. 1993;39:172–174. doi: 10.1016/s0016-5107(93)70060-7. [DOI] [PubMed] [Google Scholar]
- 46.Yoshikane H, Hidano H, Sakakibara A, Ayakawa T, Mori S, Kawashima H, Goto H, Niwa Y. Endoscopic repair by clipping of iatrogenic colonic perforation. Gastrointest Endosc. 1997;46:464–466. doi: 10.1016/s0016-5107(97)70045-2. [DOI] [PubMed] [Google Scholar]
- 47.Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H, Kudo S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265–271. doi: 10.1055/s-0029-1244080. [DOI] [PubMed] [Google Scholar]
- 48.Orenstein SB, Raigani S, Wu YV, Pauli EM, Phillips MS, Ponsky JL, Marks JM. Peroral endoscopic myotomy (POEM) leads to similar results in patients with and without prior endoscopic or surgical therapy. Surg Endosc. 2015;29:1064–1070. doi: 10.1007/s00464-014-3782-5. [DOI] [PubMed] [Google Scholar]
- 49.Ponsky JL, Marks JM, Pauli EM. How i do it: per-oral endoscopic myotomy (POEM) J Gastrointest Surg. 2012;16:1251–1255. doi: 10.1007/s11605-012-1868-8. [DOI] [PubMed] [Google Scholar]
- 50.Shimizu Y, Kato M, Yamamoto J, Nakagawa S, Komatsu Y, Tsukagoshi H, Fujita M, Hosokawa M, Asaka M. Endoscopic clip application for closure of esophageal perforations caused by EMR. Gastrointest Endosc. 2004;60:636–639. doi: 10.1016/s0016-5107(04)01960-1. [DOI] [PubMed] [Google Scholar]
- 51.Magdeburg R, Collet P, Post S, Kaehler G. Endoclipping of iatrogenic colonic perforation to avoid surgery. Surg Endosc. 2008;22:1500–1504. doi: 10.1007/s00464-007-9682-1. [DOI] [PubMed] [Google Scholar]
- 52.Cho SB, Lee WS, Joo YE, Kim HR, Park SW, Park CH, Kim HS, Choi SK, Rew JS. Therapeutic options for iatrogenic colon perforation: feasibility of endoscopic clip closure and predictors of the need for early surgery. Surg Endosc. 2012;26:473–479. doi: 10.1007/s00464-011-1903-y. [DOI] [PubMed] [Google Scholar]
- 53.Juza RM, Pauli EM, Mathew A. Endoscopic resection of a gastric gastrointestinal stromal cell tumor with full thickness defect flosure using endoscopic suturing device. American College of Surgeons Clinical Congress; October 27. San Fransisco, CA. Nashville, TN: Hershey Medical Center; 2014. p. Moscone Convention Center. [Google Scholar]
- 54.Kantsevoy SV, Thuluvath PJ. Successful closure of a chronic refractory gastrocutaneous fistula with a new endoscopic suturing device (with video) Gastrointest Endosc. 2012;75:688–690. doi: 10.1016/j.gie.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 55.Pauli EM, Delaney CP, Champagne B, Stein S, Marks JM. Safety and effectiveness of an endoscopic suturing device in a human colonic treat-and-resect model. Surg Innov. 2013;20:594–599. doi: 10.1177/1553350613479204. [DOI] [PubMed] [Google Scholar]
- 56.Cai JX, Khashab MA, Okolo PI, Kalloo AN, Kumbhari V. Full-thickness endoscopic suturing of staple-line leaks following laparoscopic sleeve gastrectomy. Endoscopy. 2014;46 Suppl 1 UCTN:E623–E624. doi: 10.1055/s-0034-1390782. [DOI] [PubMed] [Google Scholar]
- 57.Kantsevoy SV, Bitner M, Mitrakov AA, Thuluvath PJ. Endoscopic suturing closure of large mucosal defects after endoscopic submucosal dissection is technically feasible, fast, and eliminates the need for hospitalization (with videos) Gastrointest Endosc. 2014;79:503–507. doi: 10.1016/j.gie.2013.10.051. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez-Esparrach G, Lautz DB, Thompson CC. Endoscopic repair of gastrogastric fistula after Roux-en-Y gastric bypass: a less-invasive approach. Surg Obes Relat Dis. 2010;6:282–288. doi: 10.1016/j.soard.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 59.D’Cunha J, Rueth NM, Groth SS, Maddaus MA, Andrade RS. Esophageal stents for anastomotic leaks and perforations. J Thorac Cardiovasc Surg. 2011;142:39–46.e1. doi: 10.1016/j.jtcvs.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 60.Eroglu A, Turkyilmaz A, Aydin Y, Yekeler E, Karaoglanoglu N. Current management of esophageal perforation: 20 years experience. Dis Esophagus. 2009;22:374–380. doi: 10.1111/j.1442-2050.2008.00918.x. [DOI] [PubMed] [Google Scholar]
- 61.Gelbmann CM, Ratiu NL, Rath HC, Rogler G, Lock G, Schölmerich J, Kullmann F. Use of self-expandable plastic stents for the treatment of esophageal perforations and symptomatic anastomotic leaks. Endoscopy. 2004;36:695–699. doi: 10.1055/s-2004-825656. [DOI] [PubMed] [Google Scholar]
- 62.Dai Y, Chopra SS, Steinbach M, Kneif S, Hünerbein M. Esophageal stents for leaks and perforations. Semin Thorac Cardiovasc Surg. 2011;23:159–162. doi: 10.1053/j.semtcvs.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 63.Orenstein SB, Wu YV, Pauli EM, Tran TT, Haluck RS, Novitsky YW, Hardacre JM, Ammori JB, Sanchez E, Ponsky JL, et al. A Novel Endoscopic Approach to Managing High Output Entero-atmospheric Fistulae. Salt Lake City, UT: SAGES; 2014. [Google Scholar]
- 64.Puli SR, Spofford IS, Thompson CC. Use of self-expandable stents in the treatment of bariatric surgery leaks: a systematic review and meta-analysis. Gastrointest Endosc. 2012;75:287–293. doi: 10.1016/j.gie.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 65.Vanbiervliet G, Filippi J, Karimdjee BS, Venissac N, Iannelli A, Rahili A, Benizri E, Pop D, Staccini P, Tran A, et al. The role of clips in preventing migration of fully covered metallic esophageal stents: a pilot comparative study. Surg Endosc. 2012;26:53–59. doi: 10.1007/s00464-011-1827-6. [DOI] [PubMed] [Google Scholar]
- 66.Farra J, Zhuge Y, Neville HL, Thompson WR, Sola JE. Submucosal fibrin glue injection for closure of recurrent tracheoesophageal fistula. Pediatr Surg Int. 2010;26:237–240. [PubMed] [Google Scholar]
- 67.Rábago LR, Ventosa N, Castro JL, Marco J, Herrera N, Gea F. Endoscopic treatment of postoperative fistulas resistant to conservative management using biological fibrin glue. Endoscopy. 2002;34:632–638. doi: 10.1055/s-2002-33237. [DOI] [PubMed] [Google Scholar]
- 68.Toussaint E, Eisendrath P, Kwan V, Dugardeyn S, Devière J, Le Moine O. Endoscopic treatment of postoperative enterocutaneous fistulas after bariatric surgery with the use of a fistula plug: report of five cases. Endoscopy. 2009;41:560–563. doi: 10.1055/s-0029-1214606. [DOI] [PubMed] [Google Scholar]
- 69.Maluf-Filho F, Hondo F, Halwan B, de Lima MS, Giordano-Nappi JH, Sakai P. Endoscopic treatment of Roux-en-Y gastric bypass-related gastrocutaneous fistulas using a novel biomaterial. Surg Endosc. 2009;23:1541–1545. doi: 10.1007/s00464-009-0440-4. [DOI] [PubMed] [Google Scholar]
- 70.Ahrens M, Schulte T, Egberts J, Schafmayer C, Hampe J, Fritscher-Ravens A, Broering DC, Schniewind B. Drainage of esophageal leakage using endoscopic vacuum therapy: a prospective pilot study. Endoscopy. 2010;42:693–698. doi: 10.1055/s-0030-1255688. [DOI] [PubMed] [Google Scholar]
- 71.Marks JM, Ponsky JL, Pearl JP, McGee MF. PEG “Rescue”: a practical NOTES technique. Surg Endosc. 2007;21:816–819. doi: 10.1007/s00464-007-9361-2. [DOI] [PubMed] [Google Scholar]
- 72.Juza RM, Tran TT, Pauli EM. Endoscopic rescue of dislodged trans-abdominal decompressive esophagostomy tube. In: Baron TH, Raju GS, editors. Gastrointestinal Endoscopy. 2015;81:1001. doi: 10.1016/j.gie.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 73.Al Ghossaini N, Lucidarme D, Bulois P. Endoscopic treatment of iatrogenic gastrointestinal perforations: an overview. Dig Liver Dis. 2014;46:195–203. doi: 10.1016/j.dld.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 74.Lee WC, Ko WJ, Cho JH, Lee TH, Jeon SR, Kim HG, Cho JY. Endoscopic Treatment of Various Gastrointestinal Tract Defects with an Over-the-Scope Clip: Case Series from a Tertiary Referral Hospital. Clin Endosc. 2014;47:178–182. doi: 10.5946/ce.2014.47.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mercky P, Gonzalez JM, Aimore Bonin E, Emungania O, Brunet J, Grimaud JC, Barthet M. Usefulness of over-the-scope clipping system for closing digestive fistulas. Dig Endosc. 2015;27:18–24. doi: 10.1111/den.12295. [DOI] [PubMed] [Google Scholar]
- 76.Kouklakis G, Zezos P, Liratzopoulos N, Gatopoulou A, Oikonomou A, Pitiakoudis M, Efremidou E, Simopoulos C. Endoscopic treatment of a gastrocutaneous fistula using the over-the-scope-clip system: a case report. Diagn Ther Endosc. 2011;2011:384143. doi: 10.1155/2011/384143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manta R, Manno M, Bertani H, Barbera C, Pigò F, Mirante V, Longinotti E, Bassotti G, Conigliaro R. Endoscopic treatment of gastrointestinal fistulas using an over-the-scope clip (OTSC) device: case series from a tertiary referral center. Endoscopy. 2011;43:545–548. doi: 10.1055/s-0030-1256196. [DOI] [PubMed] [Google Scholar]
- 78.Mennigen R, Colombo-Benkmann M, Senninger N, Laukoetter M. Endoscopic closure of postoperative gastrointestinal leakages and fistulas with the Over-the-Scope Clip (OTSC) J Gastrointest Surg. 2013;17:1058–1065. doi: 10.1007/s11605-013-2156-y. [DOI] [PubMed] [Google Scholar]
- 79.Law JK, Stoita A, Wever W, Gleeson FC, Dries AM, Blackford A, Kiswani V, Shin EJ, Khashab MA, Canto MI, et al. Endoscopic ultrasound-guided fine needle aspiration improves the pre-operative diagnostic yield of solid-pseudopapillary neoplasm of the pancreas: an international multicenter case series (with video) Surg Endosc. 2014;28:2592–2598. doi: 10.1007/s00464-014-3508-8. [DOI] [PubMed] [Google Scholar]
- 80.Matthes K, Jung Y, Kato M, Gromski MA, Chuttani R. Efficacy of full-thickness GI perforation closure with a novel over-the-scope clip application device: an animal study. Gastrointest Endosc. 2011;74:1369–1375. doi: 10.1016/j.gie.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 81.Bège T, Emungania O, Vitton V, Ah-Soune P, Nocca D, Noël P, Bradjanian S, Berdah SV, Brunet C, Grimaud JC, et al. An endoscopic strategy for management of anastomotic complications from bariatric surgery: a prospective study. Gastrointest Endosc. 2011;73:238–244. doi: 10.1016/j.gie.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 82.Kirschniak A, Subotova N, Zieker D, Königsrainer A, Kratt T. The Over-The-Scope Clip (OTSC) for the treatment of gastrointestinal bleeding, perforations, and fistulas. Surg Endosc. 2011;25:2901–2905. doi: 10.1007/s00464-011-1640-2. [DOI] [PubMed] [Google Scholar]
- 83.Hagel AF, Naegel A, Lindner AS, Kessler H, Matzel K, Dauth W, Neurath MF, Raithel M. Over-the-scope clip application yields a high rate of closure in gastrointestinal perforations and may reduce emergency surgery. J Gastrointest Surg. 2012;16:2132–2138. doi: 10.1007/s11605-012-1983-6. [DOI] [PubMed] [Google Scholar]
- 84.Dişibeyaz S, Köksal AŞ, Parlak E, Torun S, Şaşmaz N. Endoscopic closure of gastrointestinal defects with an over-the-scope clip device. A case series and review of the literature. Clin Res Hepatol Gastroenterol. 2012;36:614–621. doi: 10.1016/j.clinre.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 85.Baron TH, Song LM, Ross A, Tokar JL, Irani S, Kozarek RA. Use of an over-the-scope clipping device: multicenter retrospective results of the first U.S. experience (with videos) Gastrointest Endosc. 2012;76:202–208. doi: 10.1016/j.gie.2012.03.250. [DOI] [PubMed] [Google Scholar]
- 86.von Renteln D, Denzer UW, Schachschal G, Anders M, Groth S, Rösch T. Endoscopic closure of GI fistulae by using an over-the-scope clip (with videos) Gastrointest Endosc. 2010;72:1289–1296. doi: 10.1016/j.gie.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 87.Iacopini F, Di Lorenzo N, Altorio F, Schurr MO, Scozzarro A. Over-the-scope clip closure of two chronic fistulas after gastric band penetration. World J Gastroenterol. 2010;16:1665–1669. doi: 10.3748/wjg.v16.i13.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zolotarevsky E, Kwon Y, Bains M, Schattner M. Esophagobronchial fistula closure using a novel endoscopic over-the-scope-clip. Ann Thorac Surg. 2012;94:e69–e70. doi: 10.1016/j.athoracsur.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 89.Csendes A, Braghetto I, León P, Burgos AM. Management of leaks after laparoscopic sleeve gastrectomy in patients with obesity. J Gastrointest Surg. 2010;14:1343–1348. doi: 10.1007/s11605-010-1249-0. [DOI] [PubMed] [Google Scholar]
- 90.Eubanks S, Edwards CA, Fearing NM, Ramaswamy A, de la Torre RA, Thaler KJ, Miedema BW, Scott JS. Use of endoscopic stents to treat anastomotic complications after bariatric surgery. J Am Coll Surg. 2008;206:935–938; discussion 938-939. doi: 10.1016/j.jamcollsurg.2008.02.016. [DOI] [PubMed] [Google Scholar]


