Abstract
The aims of this study were to evaluate the effectiveness of six disinfection methods and the influence of these methods on the adaptation of maxillary dentures. Acrylic resin specimens contaminated with fungi were exposed to the following disinfection treatments: 1. microwave oven (900 W) at full power for 5 min (with soaking the specimen in 250 ml water), 2. microwave oven at medium power for 5 min (with soaking the specimen in 250 ml water), 3. sodium hypochlorite 5.25 % for 5 min, 4. diluted sodium hypochlorite 1:420 for 5 h, 5. Chlorhexidine gluconate for 5 h, 6. effervescent tablets for 15 min, 7. soaking in 250 ml tap water for 15 min. Colony forming units (CFUs) of remaining cells were counted and compared with t test (p ≤ 0.05). Dimensional stability was evaluated using aluminum die simulating the maxillary edentulous arch. Posterior palatal gaps were measured. Data were analyzed using one-way ANOVA test and t test (p ≤ 0.05). Microwave irradiation (at full or medium power) and sodium hypochlorite 5.25 % for 5 min were able to reduce the CFUs of fungi by more than 4 log10 whereas diluted sodium hypochlorite, chlorhexidine gluconate, and effervescent tablets did not achieve a reduction of >2.8, 2.68 and 1.66, respectively. For dimensional stability test, t test revealed significant difference between control group and the microwave at full power group (p = 0.000). Within the limits of this study, microwave oven at medium power and sodium hypochlorite (5.25 %) are effective and safe methods of disinfecting removable dentures.
Keywords: Chemical disinfection, Microwave, Sodium hypochlorite, Fungi, Base adaptation, Dimensional stability
Introduction
Denture stomatitis (DS) is a common recurring problem of the denture wearers. The aetiology of the disease includes infection, trauma and probably a defect in the host defense mechanism [1]. Fungal species of Candida have high affinity for adhering to and colonizing acrylic surfaces [2] which is considered the first step in the pathogenesis of DS. Therefore, the presence of Candida species on dentures is considered a major factor in the development of this infection [3].
The effective removal of denture plaque by brushing requires a certain degree of manual dexterity which is commonly compromised in the elderly. In addition, the irregularities and porosities present on the acrylic resin surface may also contribute to penetration of micro-organisms into dentures, making it difficult to clean them by brushing [4]. Microwave irradiation and immersion in chemical solutions have been recommended for denture disinfection. However, the effect of these procedures on the surface characteristics of denture base and reline resins has not been completely evaluated [5]. Since it was first introduced to sterilize dental instruments in 1985 [6], microwave irradiation has been studied to detect its effectiveness and its influence on physical properties of complete denture materials.
Some studies have demonstrated the effectiveness of microwave irradiation as an alternative method for disinfection of denture base acrylic resins [7–11]. In addition, denture microwave disinfection was as effective as topical antifungal therapy for treating DS [12, 13].
The influence of microwave irradiation on physical properties of denture materials was investigated by research [14–21]. Some studies found that microwave had deleterious effect on the base adaptation while others found it safe. Some researchers evaluated occlusion vertical dimension of complete dentures after microwave disinfection when the maxillary complete dentures submitted to microwave disinfection (650 W/3 min), three times a week, for 4 weeks [22].
The aims of this study were to evaluate the effectiveness of six disinfection methods towards fungi usually found on acrylic resin dentures and the influence of these methods on the adaptation of maxillary denture bases.
Materials and Methods
Disinfection Test
Twelve patients wearing maxillary complete dentures were recruited to this study. The ethics committee in the Faculty of Dentistry/Damascus University/approved of this study. The patients were healthy, did not currently have any medication and had worn their dentures for 2–8 years. The principle of the experiment was to contaminate sterile acrylic resin specimens with fungi usually found on acrylic resin dentures, then to expose these specimens to one of six disinfection methods to determine any reduction in count of viable fungi cells by disinfection.
For every patient, denture biofilm sample was taken from the fitting surface of every maxillary denture with a sterile cotton swab. The swab was placed in test tube containing Sabouraud liquid medium (Scharlau, Barcelona, EU) with seven sterile acrylic resin specimens measuring 10 × 10 × 2.5 mm (sterilized by autoclave 15 min., 121 °C, 15 psi). The total size number of this test was 84 acrylic resin specimens. Test tubes were incubated at 27 °C for 72 h (PIF 600-England) that allows the fungi found on the swab to grow and contaminate the acrylic resin specimens. Following incubation, the specimens were exposed to following treatments: 1. microwave oven (Sharp R-750B, 900 W, Sharp Corporation) at full power (100 % potency) for 5 min (the specimen was immersed in a beaker containing 250 ml water), 2. microwave oven (Sharp R-750B) at medium power (50 % potency) for 5 min (the specimen was immersed in a beaker containing 250 ml water), 3. sodium hypochlorite 5.25 % (Clorox, Abudawood and partners for industry Co. Jeddah, Saudi Arabia) for 5 min, 4. diluted sodium hypochlorite (Clorox) 1:420 giving 125 ppm for 5 h, 5. Chlorhexidine gluconate 0.12 % (Zak, Maleh Chemical Products, Syria) for 5 h, 6. effervescent tablets (Bony Plus, Bonyf AG, Liechtenstein) for 15 min, 7. soaking in 250 ml tap water for 15 min (as a control group). Then, the specimens were dried by placing them covered by sterile gauze in an incubator (PIF 600-England) at 37 °C for 30 min. Every specimen was placed in a new test tube containing 1 ml sterile saline (0.9 % sodium chloride solution). Test tubes were manually shacked for 3 min to allow the remaining fungi to move to the surrounding solution. 10 µl of the saline were streaked on Sabouraud agar plates (Sabouraud Dextrose Agar, BBL Becton Dickenson & Company, USA). Sabouraud agar plates were incubated at 27 °C for 72 h. Colony forming units (CFUs) were counted (Fig. 1). Samples of growing colonies were colored with methylene blue and microscopically examined. The mean remaining viable cells for the various experiments were compared by a two-tailed, two-sample t test with the assumption of unequal variances at a significance level p ≤ 0.05. Logarithmic reduction of every disinfection method was calculated.
Fig. 1.

Colony forming units on Sabouraud agar plates were counted for every disinfection method
Evaluating Dimensional Stability
Dimensional stability was evaluated using aluminum die simulating the maxillary edentulous arch (Fig. 2). Four notches were cut along the border of the cast to serve as indices for re-positioning the acrylic resin dentures. Stone casts were prepared by duplicating the metal master die. Similarity in denture base thickness and tooth position was ensured using silicone moulds used to fabricate identical acrylic resin dentures with 2.6 mm base thickness (n = 35). Dentures were fabricated using heat-cure acrylic resin (Triplex Hot, Ivoclar, Liechtenstein) and the conventional compression technique. The flasks were opened after bench cooling. Dentures were trimmed of excess resin flash but were not polished and a new stone cast was made for every denture by pouring dental stone inside the fitting surface of every denture. Then, the dentures had been stored in water for 24 h. Thirty disinfection cycles (with 6 h intervals) started after the 24 h water storage. Dimensional stability test groups were similar to those of disinfection test. Any dimensional change due to disinfection would appear as a palatal gap along the posterior palatal border of the denture base. This gap was measured using light microscope connected to computer (Leica, LEITZ DMRXE, Germaney) (Fig. 3) at seven locations: 1. buccal vestibules (right and left), 2. points at the junction of the horizontal and vertical slopes of the palate (right and left), 3. midline. 4. points halfway between points at “2” and midline (right and left). The average of the seven gap measurements indicated the misfit of every denture. This gap is mainly caused by the applied disinfection method. Data were analyzed using one-way ANOVA test to detect any significant difference between groups. Student t test was applied to identify the significance between control group and the other groups. The significance level was set at p = 0.05.
Fig. 2.

Aluminum die simulating the maxillary edentulous arch
Fig. 3.

Light microscope connected to computer used for measuring the posterior gaps
Results
Microscopic examination of Sabouraud agar colonies revealed that they were of Candida Species (Fig. 4).
Fig. 4.
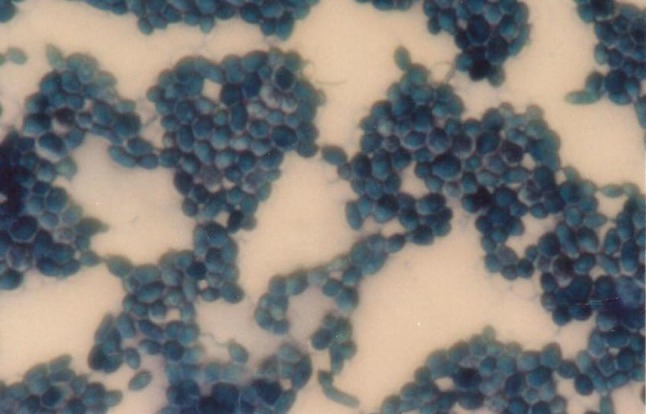
Microscopic examination showing Candida species
Table 1 shows mean and standard deviation of CFUs found in 10 µl of serum (or broth) whereas, the percent reduction and logarithmic reduction of the number of CFUs with tested different disinfection methods are shown in Table 2. Microwave irradiation (at full power or medium power) and sodium hypochlorite 5.25 % for 5 min were able to reduce the CFUs of fungi by more than 4 log10 whereas diluted sodium hypochlorite, chlorhexidine gluconate, and effervescent tablets did not achieve a reduction of >2.8, 2.68 and 1.66, respectively. CFUs of diluted sodium hypochlorite was comparable to chlorhexidine gluconate 0.12 % (p = 0.74). Effervescent tablet was the weakest method of killing fungi (log reduction was 1.66 log10). CFUs of effervescent tablet was statistically different of diluted sodium hypochlorite (p = 0.000) and chlorhexidine gluconate (p = 0.000).
Table 1.
Mean and standard deviation of CFUs found in 10 µl of serum (or broth) for test groups
| Mean | Standard deviation | |
|---|---|---|
| Broth | 10,400,000 | 6,581,931 |
| Control (tap water) | 1,316 | 787 |
| Microwave 100 % | 0 | 0 |
| Microwave 50 % | 0 | 0 |
| Sodium hypochlorite | 0 | 0 |
| Diluted sodium hypochlorite | 2.1 | 5 |
| Chlorhexidine gluconate | 2.8 | 4.8 |
| Effervescent tablets | 28.5 | 12.3 |
Table 2.
Percent reduction and logarithmic reduction of the number of CFUs with tested different disinfection methods
| Percent reduction | Logarithmic reduction | |
|---|---|---|
| Microwave 100 % | 100 | >4 |
| Microwave 50 % | 100 | >4 |
| Sodium hypochlorite | 100 | >4 |
| Diluted sodium hypochlorite | 99.84 | 2.8 |
| Chlorhexidine gluconate | 99.787 | 2.68 |
| Effervescent tablets | 97.834 | 1.66 |
Table 3 shows the mean values and standard deviations of posterior palatal gaps of test groups. One way ANOVA revealed a significant difference between groups (p = 0.000). Student t test revealed significant difference between control group and the microwave at full power group (p = 0.000), whereas, the differences between all other groups and control group were insignificant (p > 0.05).
Table 3.
Mean values and standard deviations of the posterior palatal gaps (μm)
| Mean | Standard deviation | |
|---|---|---|
| Control (tap water) | 207.85 | 36.99 |
| Microwave 100 % | 1,013.45 | 94.59 |
| Microwave 50 % | 240 | 59.04 |
| Sodium hypochlorite | 183.33 | 31.53 |
| Diluted sodium hypochlorite | 179.65 | 29.78 |
| Chlorhexidine gluconate | 283.95 | 114.35 |
| Effervescent tablets | 183.83 | 36.63 |
Discussion
Denture disinfection is a very important step to treat denture stomatitis, prevent cross-contamination between patients, and to remove bad odor and stains of acrylic resin dentures. This procedure should not cause harmful changes to denture base materials.
For disinfection, logarithmic transformation of each CFU count was performed to normalize the data due to the high range of fungi numbers. 4 log1–0 or greater reduction in CFU is considered as a standard for adequate disinfection [23]. Rohrer and Bulard [6] investigated the possibility of using microwave irradiation to sterilize dentures. They designed a three-dimensional rotation device to produce uniform sterilization of the infected denture. They concluded that 8 min of irradiation was sufficient to sterilize dentures. However, this three-dimensional device is not commercially available or practical for use by the average patients. Silva et al. [24] evaluated the effectiveness of microwave irradiation on the disinfection of simulated complete dentures. Dentures were individually immersed in 200 ml of water and submitted to microwave irradiation at 650 W for 6 min. They concluded that microwave irradiation for 6 min at 650 W produced sterilization of complete dentures contaminated with Staphylococcus aureus and Candida albicans and disinfection of those contaminated with Pseudomonas aeruginosa and Bacillus subtilis. Ribeiro et al. [10] showed that microwave irradiation (650 W) for 3 min resulted in sterilization of all dentures evaluated whereas, only a significant decrease in Candida species resulted after microwave irradiation for 2 min. Sanitá et al. [25] found that microwave irradiation for 3 min at 650 W resulted in sterilization of all complete dentures contaminated with all Candida species. Silva et al. [7] inoculated samples of acrylic resin with species of Candida and later immersed in 100 ml of sterile water and irradiated with microwave at 650 W for 3 min. Microwave irradiation was an effective method for disinfection of the acrylic resins inoculated with C. albicans, C. dubliniensis and C. tropicalis. The present study was in agreement with most of the abovementioned studies taking into consideration irradiation time, microwave power and potency, soaking in water and water volume.
Our results agreed with those of Rudd et al. [26] who concluded that using sodium hypochlorite for 5 min sterilized contaminated dentures. However, our results disagreed with Baysan et al. who found that diluted sodium hypochlorite (125 ppm) had killed all the Candida on the contaminated specimens. The difference between the two studies can be attributed to that Baysan et al. [27] washed the contaminated specimens by saline twice before disinfection, in addition, they diluted (1,000 times) the serum which the microorganisms moved to before streaking on agar plates which might hide the few remaining yeast cells. In the present study, no dilution has been applied.
Effervescent tablets (alkaline peroxide) reduced the fungi by 1.66 log1–0. Tamamoto 1985 suggested enzymes for removal fungi because alkaline peroxide does not reduce bacterial plaque level and does not improve inflamed palatal tissues [28]. Lal et al. studied the effect of Peridex oral rinse (0.12 % chlorhexidine gluconate) when used both as mouthrinse and as a denture soak for a period of 24 days. They found that some palatal inflammation was still evident at the end of the treatment. They assumed that these signs were due to surviving yeast on the mucosa or that wound healing repair mechanisms within the tissue were still effective [29]. Our results showed that yeast cells have not been completely eliminated when chlorhexidine gluconate was used as denture disinfectant, which might give another explanation to their observation.
The range of reduction for chlorhexidine gluconate, diluted sodium hypochlorite and- effervescent tablets was well below than 4 log10 which considered the minimum acceptable standard for adequate disinfectant whereas, microwave irradiation at full or medium power and sodium hypochlorite 5.25 % were adequate disinfectants.
For dimensional stability, Rohrer and Bulard [6] showed that there were no dimensional changes in dentures when exposed to microwave irradiation for up to 16 min at 720 W. Burns et al. [30] examined the effect of microwave irradiation on dimensional stability of acrylic resin rods. They concluded that shrinkage values were clinically insignificant. Another study by Polyzois et al. [31] showed that microwaving rectangular acrylic resin specimens for 3 and 15 min at 500 W produced linear dimensional changes of no clinical importance. However, Burns et al. and Polyzois et al. examined cylindrical and rectangular specimens, respectively, which may make extrapolation to complete denture not valid. Some studies proved that microwave disinfection significantly changes the dimensional stability of denture base resin [16, 18]. Dixon et al. [11] sterilized acrylic resin specimens by microwave irradiation for 5-min while the specimens were immersed in water. They mentioned that 250 ml water started to boil in 2 min and the effect of placing specimens in water had not been reported and should be investigated. The result of the present study proved that full power of 900 W microwave energy caused water to boil in about 80 s and induced about 1 mm posterior palatal gap, and this gap is large enough to cause obvious clinical failure. The other microwave disinfection method (50 % potency for 5 min) was effective and safe alternative disinfection method (water temperature did not exceed 82 °C). In the study of Neppelenbroek et al. [32], water started to boil approximately after 90 s during microwave disinfection. They mentioned that this temperature may cause diffusion of remaining residual monomer molecules into the active sites of the polymer chain. Due to this, there might be further polymerization which leads to increase in linear dimensional change (shrinkage) of denture bases. Craig [33] mentioned that the distortion of acrylic resins occurred when heated from 71 to 90 °C. The results of the present study are in agreement with Senna et al. [15] who concluded that microwave disinfection at 450–630 W for 3 min is safe for poly(methyl methacrylate) PMMA whereas, there was a cumulative effect on linear dimensional stability at 900 W. In another study, no statistically significant differences were observed between control group and the group submitted to microwave disinfection for 3 min at 500 W. However, treatment in microwave oven at 604 W for 10 min produced the greatest discrepancies in the adaptation of maxillary acrylic resin denture bases to the stone casts [21]. Thomas and Webb [34] found that after microwaving dentures for 10 min at 604 W, some measurements showed significant contraction or expansion but reduced exposure (6 min at 331 W) caused much smaller changes. The dimensional changes are inexplicable but at lower radiation are considered to be harmless. Thus, dimensional stability of acrylic resin denture bases after microwave disinfection depends on the specimens form, method of measuring distortion, microwave power and potency, soaking in water, water volume and irradiation time should be considered.
Our results are in agreement with those of Nirale et al. [14] who found that microwave disinfection led to increased shrinkage of denture bases whereas, chemical disinfection with sodium hypochlorite was a safer method of disinfection with regards to physical properties such as changes in dimensional stability. The results in this study are also in agreement with the findings of Sartori et al. [20] who evaluated the effect of disinfection methods (immersion in 100 ppm chloride solution) or microwave disinfection (690 W for 6 min) on the internal adaptation of denture bases and found that bases submitted to microwave disinfection had gradual increase of misfit, while bases immersed in chloride solution did not differ from the control group.
Brondani et al. [35] reviewed papers dealing with microwave therapy for denture cleaning. They found that there was no standardization for microwave use for denture cleaning. Manual cleaning still seemed to be the optimal method for controlling fungal infection and denture stomatitis. However, such a daily routine appeared to be underused, particularly in long-term care facilities.
Senna et al. [36] showed that C. albicans biofilm area influenced disinfection by microwave energy; therefore dentures with larger biofilm areas required longer irradiation exposure to be disinfected which suggest studying on complete denture and not only small contaminated specimens. Senna et al. [37] suggested adding denture cleanser to microwave disinfection regimen to reduce the irradiation time and the exposure of dentures to high temperatures. Physical properties with effective power of microwave irradiation are still needed. Different microwave disinfection protocols should be studied to detect the effective and safe method of denture disinfection.
The strength of this study was that the specimens were closely simulating clinical conditions. Asymmetric distortion might be caused due to the complex design of a denture bases and to relaxation of processing stresses, and this might not be apparent if we only evaluate simple-shaped specimens. The limitation of this study was that this study evaluated the effect on only one denture base material. In addition, only two microwave irradiation protocols were used for disinfecting the samples. Future studies are needed to evaluate the effect of disinfection methods on different denture base materials with different disinfection protocols.
Conclusions
Within the limits of this study, the following conclusion can be drawn:
Microwave oven (900 W, at full or medium power, using a beaker containing 250 ml water) and sodium hypochlorite (5.25 %) for 5 min are effective methods against Candida species usually found on removable dentures whereas, diluted sodium hypochlorite, chlorhexidine gluconate 0.12 % and effervescent tablets do not achieve the minimal standard of disinfection.
Complete dentures deform when exposed to microwave irradiation at full power whereas, other methods of disinfecting do not cause any significant deformation in complete dentures.
When using or studying microwave oven for disinfection, factors as microwave power and potency, soaking in water, water volume and irradiation time should be considered.
Acknowledgments
The author would like to thank Assoc. Prof. Abir Al-Kafri, Faculty of Medicine, for her assistance in disinfection test and Prof. Dr. Rafi Jabra, Higher Institution for Applied Science and Technology, for his help in dimensional stability test. Special acknowledgment is directed to Damascus University for the financial support of this study.
Conflict of interest
The author has no known conflicts of interest associated with the products used in this study and there has been no financial support for this work that could have influenced its outcome.
References
- 1.Jeganathan S, Lin C. Denture stomatitis: a review of the aetiology, diagnosis and management. Aust Dent J. 1992;37:107–114. doi: 10.1111/j.1834-7819.1992.tb03046.x. [DOI] [PubMed] [Google Scholar]
- 2.McCourtie J, Douglas L. Extracellular polymer of Candida albicans: isolation, analysis and role in adhesion. J Gen Microbiol. 1985;131:495–503. doi: 10.1099/00221287-131-3-495. [DOI] [PubMed] [Google Scholar]
- 3.Theilade E, Budtz-Jorgensen E. Predominant cultivable microflora of plaque on removable dentures in patients with denture induced stomatitis. Oral Microbiol Immunol. 1988;3:8–13. doi: 10.1111/j.1399-302X.1988.tb00597.x. [DOI] [PubMed] [Google Scholar]
- 4.Kulak Y, Arikan A, Kazazoglu E. Existence of Candida albicans and microorganisms in denture stomatitis patients. J Oral Rehabil. 1997;24:788–790. doi: 10.1046/j.1365-2842.1997.00550.x. [DOI] [PubMed] [Google Scholar]
- 5.Machado A, Breeding L, Vergani C, da Cruz Perez L. Hardness and surface roughness of reline and denture base acrylic resins after repeated disinfection procedures. J Prosthet Dent. 2009;102:115–122. doi: 10.1016/S0022-3913(09)60120-7. [DOI] [PubMed] [Google Scholar]
- 6.Rohrer M, Bulard R. Microwave sterilization. J Am Dent Assoc. 1985;110:194–198. doi: 10.14219/jada.archive.1985.0250. [DOI] [PubMed] [Google Scholar]
- 7.Silva M, Consani R, Sardi J, Mesquita M, Macêdo A, Takahashi J. Microwave irradiation as an alternative method for disinfection of denture base acrylic resins. Minerva Stomatol. 2013;62:23–29. [PubMed] [Google Scholar]
- 8.Altieri K, Sanitá P, Machado A, Giampaolo E, Pavarina A, Vergani C. Effectiveness of two disinfectant solutions and microwave irradiation in disinfecting complete dentures contaminated with methicillin-resistant Staphylococcus aureus. J Am Dent Assoc. 2012;143:270–277. doi: 10.14219/jada.archive.2012.0152. [DOI] [PubMed] [Google Scholar]
- 9.Dovigo L, Pavarina A, Ribeiro D, de Oliveira J, Vergani C, Machado A. Microwave disinfection of complete dentures contaminated in vitro with selected bacteria. J Prosthodont. 2009;18:611–617. doi: 10.1111/j.1532-849X.2009.00489.x. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro D, Pavarina A, Dovigo L, Palomari Spolidorio D, Giampaolo E, Vergani C. Denture disinfection by microwave irradiation: a randomized clinical study. J Dent. 2009;37:666–672. doi: 10.1016/j.jdent.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Dixon D, Breeding L, Faler T. Microwave disinfection of denture base materials colonized with Candida albicans. J Prosthet Dent. 1999;81:207–214. doi: 10.1016/S0022-3913(99)70250-7. [DOI] [PubMed] [Google Scholar]
- 12.Silva M, Mima E, Colombo A, Sanitá P, Jorge J, Massucato E, Vergani C. Comparison of denture microwave disinfection and conventional antifungal therapy in the treatment of denture stomatitis: a randomized clinical study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:469–479. doi: 10.1016/j.oooo.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Sanita P, Machado A, Pavarina A, Massucato E, Colombo A, Vergani C. Microwave denture disinfection versus nystatin in treating patients with well-controlled type 2 diabetes and denture stomatitis: a randomized clinical trial. Int J Prosthodont. 2012;25:232–244. [PubMed] [Google Scholar]
- 14.Nirale RM, Thombre R, Kubasad G. Comparative evaluation of sodium hypochlorite and microwave disinfection on dimensional stability of denture bases. J Adv Prosthodont. 2012;4:24–29. doi: 10.4047/jap.2012.4.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Senna P, Da Silva W, Faot F, Del Bel Cury A. Microwave disinfection: cumulative effect of different power levels on physical properties of denture base resins. J Prosthodont. 2011;20:606–612. doi: 10.1111/j.1532-849X.2011.00770.x. [DOI] [PubMed] [Google Scholar]
- 16.Basso M, Giampaolo E, Vergani C, Machado A, Pavarina A, Compagnoni M. Influence of microwave disinfection on the linear dimensional stability of complete dentures: a clinical study. Int J Prosthodont. 2010;23:318–320. [PubMed] [Google Scholar]
- 17.Consani R, Iwasaki R, Mesquita M, Mendes W, Consani S. Effect of repeated simulated disinfections by microwave energy on the complete denture base adaptation. Open Dent J. 2008;2:61–66. doi: 10.2174/1874210600802010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo R, Vergani C, Pavarina A, Compagnoni M, Machado A. Influence of microwave disinfection on the dimensional stability of intact and relined acrylic resin denture bases. J Prosthet Dent. 2007;98:216–223. doi: 10.1016/S0022-3913(07)60058-4. [DOI] [PubMed] [Google Scholar]
- 19.Fleck G, Ferneda F, da Ferreira Silva DF, Mota EG, Shinkai RS. Effect of two microwave disinfection protocols on adaptation of poly (methyl methacrylate) denture bases. Minerva Stomatol. 2007;56:121–127. [PubMed] [Google Scholar]
- 20.Sartori E, Schmidt C, Walber L, Shinkai R. Effect of microwave disinfection on denture base adaptation and resin surface roughness. Braz Dent J. 2006;17:195–200. doi: 10.1590/S0103-64402006000300004. [DOI] [PubMed] [Google Scholar]
- 21.Pavan S, Arioli Filho JN, Dos Santos PH, Mollo Fde A., Jr Effect of microwave treatments on dimensional accuracy of maxillary acrylic resin denture base. Braz Dent J. 2005;16:119–123. doi: 10.1590/S0103-64402005000200006. [DOI] [PubMed] [Google Scholar]
- 22.Basso M, Giampaolo E, Machado A, Pavarina A, Vergani C. Evaluation of the occlusion vertical dimension of complete dentures after microwave disinfection. Gerodontology. 2012;29:e815–e821. doi: 10.1111/j.1741-2358.2011.00567.x. [DOI] [PubMed] [Google Scholar]
- 23.Furukawa K, Niagro F, Runyan D, Cameron S. Effectiveness of chlorine dioxide in disinfection on two soft denture liners. J Prosthet Dent. 1998;80:723–729. doi: 10.1016/S0022-3913(98)70061-7. [DOI] [PubMed] [Google Scholar]
- 24.Silva M, Vergani C, Giampaolo E, Neppelenbroek K, Spolidorio D, Machado A. Effectiveness of microwave irradiation on the disinfection of complete dentures. Int J Prosthodont. 2006;19:288–293. [PubMed] [Google Scholar]
- 25.Sanitá P, Vergani C, Giampaolo E, Pavarina A, Machado A. Growth of Candida species on complete dentures: effect of microwave disinfection. Mycoses. 2009;52:154–160. doi: 10.1111/j.1439-0507.2008.01558.x. [DOI] [PubMed] [Google Scholar]
- 26.Rudd R, Senia E, McCleskey F, Adams ED. Sterilization of complete dentures with sodium hypochlorite. J Prosthet Dent. 1984;51:318–321. doi: 10.1016/0022-3913(84)90212-9. [DOI] [PubMed] [Google Scholar]
- 27.Baysan A, Whiley R, Wright PS. Use of microwave energy to disinfect a long-term soft lining material contaminated with Candida albicans or Staphylococcus aureus. J Prosthet Dent. 1998;79:454–458. doi: 10.1016/S0022-3913(98)70161-1. [DOI] [PubMed] [Google Scholar]
- 28.Tamamoto M, Hamada T, Miyake Y, Suginaka H. Ability of enzymes to remove Candida. J Prosthet Dent. 1985;53:214–216. doi: 10.1016/0022-3913(85)90112-X. [DOI] [PubMed] [Google Scholar]
- 29.Lal K, Santarpia RP, 3rd, Pollock J, Renner R. Assessment of antimicrobial treatment of denture stomatitis using an in vivo replica model system: therapeutic efficacy of an oral rinse. J Prosthet Dent. 1992;67:72–77. doi: 10.1016/0022-3913(92)90053-D. [DOI] [PubMed] [Google Scholar]
- 30.Burns D, Kazanoglu A, Moon P, Gunsolley J. Dimensional stability of acrylic resin materials after microwave sterilization. Int J Prosthodont. 1990;3:489–493. [PubMed] [Google Scholar]
- 31.Polyzois G, Zissis A, Yannikakis S. The effect of glutaraldehyde and microwave disinfection on some properties of acrylic denture resin. Int J Prosthodont. 1995;8:150–154. [PubMed] [Google Scholar]
- 32.Neppelenbroek K, Pavarina A, Spolidorio D, Vergani C, Mima E, Machado A. Effectiveness of microwave sterilization on three hard chairside reline resins. Int J Prosthodont. 2003;16:616–620. [PubMed] [Google Scholar]
- 33.Craig R. Prosthetic applications of polymers in restorative dental materials. 12. St. Louis: Mosby; 1997. pp. 513–547. [Google Scholar]
- 34.Thomas C, Webb B. Microwaving of acrylic resin dentures. Eur J Prosthodont Restor Dent. 1995;3:179–182. [PubMed] [Google Scholar]
- 35.Brondani MA, Samim F, Feng H. A conventional microwave oven for denture cleaning: a critical review. Gerodontology. 2012;29:e6–e15. doi: 10.1111/j.1741-2358.2010.00442.x. [DOI] [PubMed] [Google Scholar]
- 36.Senna P, da Silva W, Cury A. Denture disinfection by microwave energy: influence of Candida albicans biofilm. Gerodontology. 2012;29:e186–e191. doi: 10.1111/j.1741-2358.2010.00439.x. [DOI] [PubMed] [Google Scholar]
- 37.Senna P, Sotto-Maior B, Silva W, Del Bel Cury A. Adding denture cleanser to microwave disinfection regimen to reduce the irradiation time and the exposure of dentures to high temperatures. Gerodontology. 2013;30:26–31. doi: 10.1111/j.1741-2358.2012.00641.x. [DOI] [PubMed] [Google Scholar]


