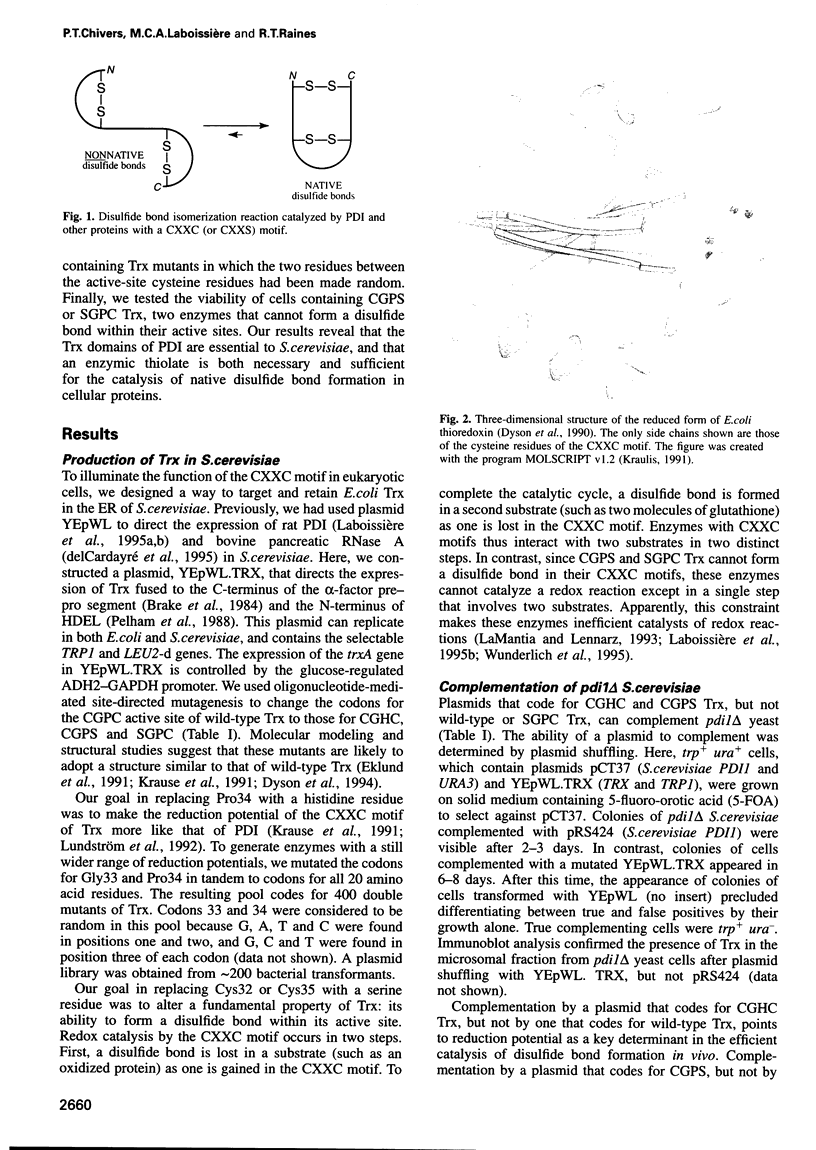
Abstract
The rapid formation of native disulfide bonds in cellular proteins is necessary for the efficient use of cellular resources. This process is catalyzed in vitro by protein disulfide isomerase (PDI), with the PDI1 gene being essential for the viability of Saccharomyces cerevisiae. PDI is a member of the thioredoxin (Trx) family of proteins, which have the active-site motif CXXC. PDI contains two Trx domains as well as two domains unrelated to the Trx family. We find that the gene encoding Escherichia coli Trx is unable to complement PDI1 null mutants of S.cerevisiae. Yet, Trx can replace PDI if it is mutated to have a CXXC motif with a disulfide bond of high reduction potential and a thiol group of low pKa. Thus, an enzymic thiolate is both necessary and sufficient for the formation of native disulfide bonds in the cell.
Full text
PDF
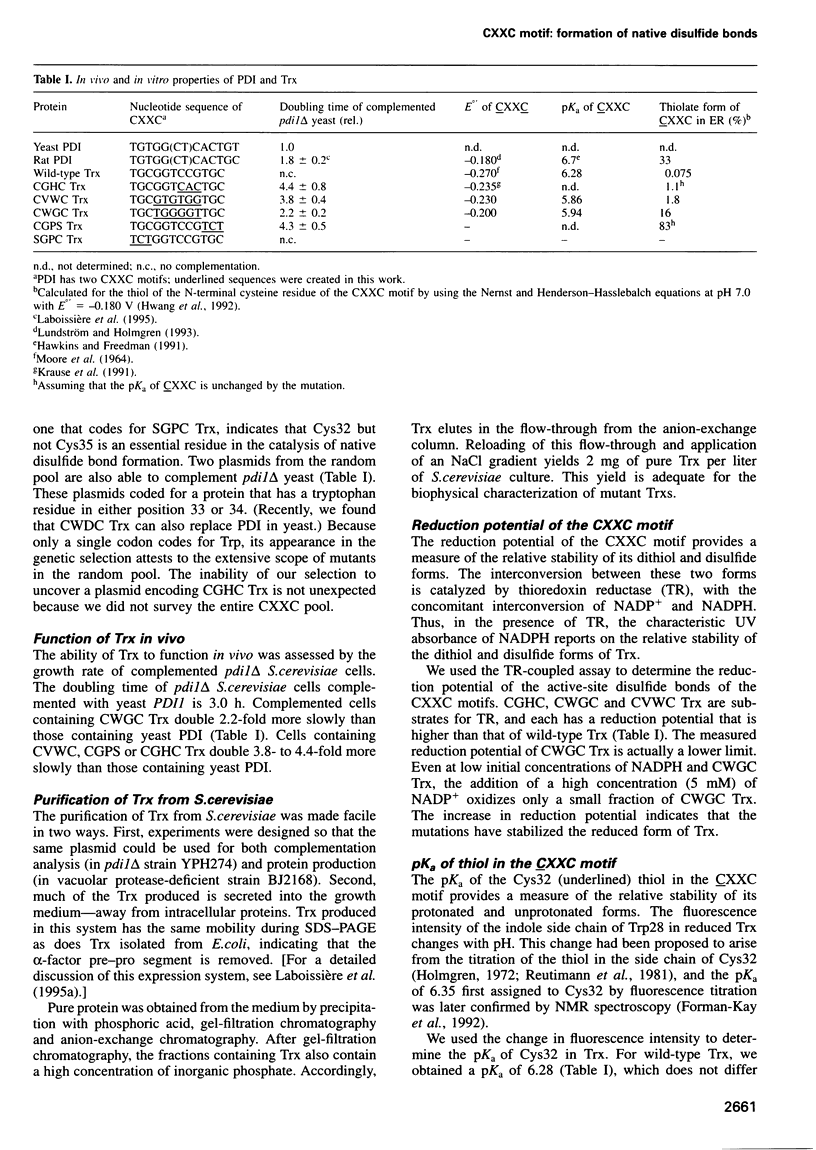

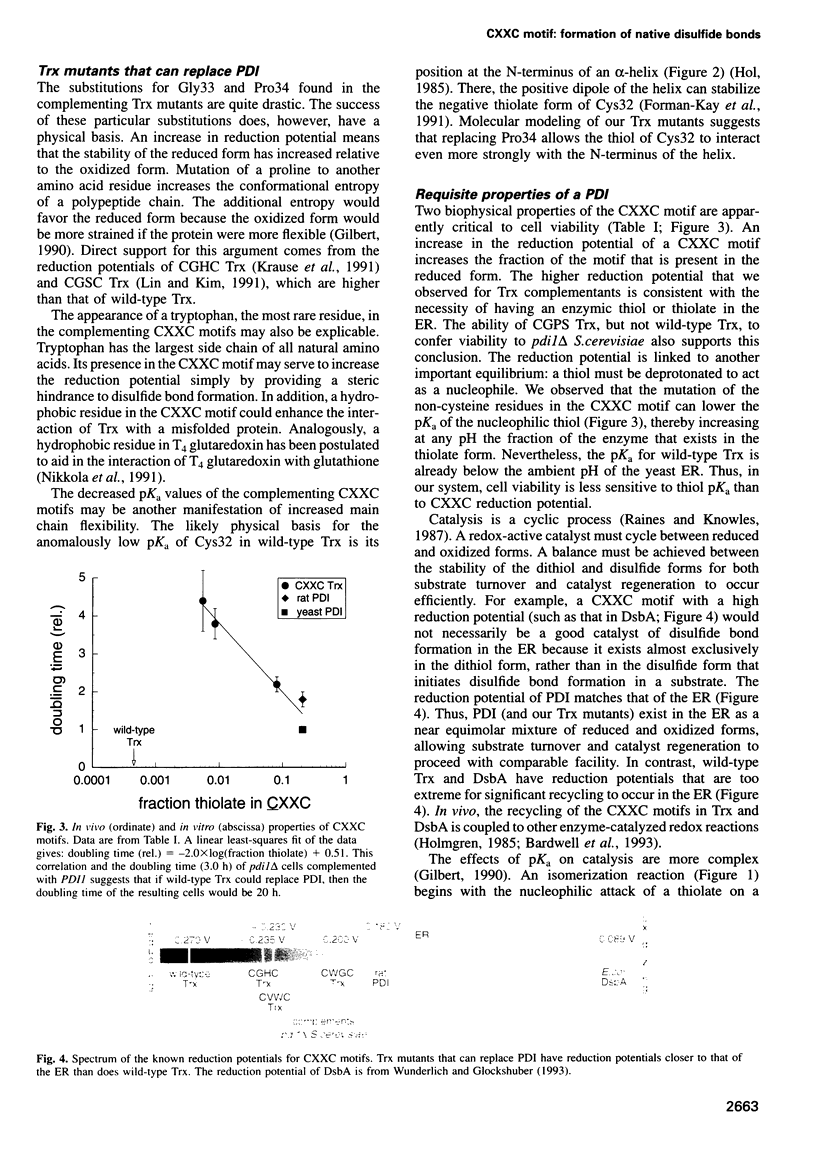




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardwell J. C., Lee J. O., Jander G., Martin N., Belin D., Beckwith J. A pathway for disulfide bond formation in vivo. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1038–1042. doi: 10.1073/pnas.90.3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J. C., McGovern K., Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991 Nov 1;67(3):581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Jayachandran S., Tipper D. J. A glycosylated protoxin in killer yeast: models for its structure and maturation. Cell. 1983 Jan;32(1):169–180. doi: 10.1016/0092-8674(83)90507-x. [DOI] [PubMed] [Google Scholar]
- Brake A. J., Merryweather J. P., Coit D. G., Heberlein U. A., Masiarz F. R., Mullenbach G. T., Urdea M. S., Valenzuela P., Barr P. J. Alpha-factor-directed synthesis and secretion of mature foreign proteins in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4642–4646. doi: 10.1073/pnas.81.15.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Decottignies P., Lozano R. M. Thioredoxin: a multifunctional regulatory protein with a bright future in technology and medicine. Arch Biochem Biophys. 1994 Nov 1;314(2):257–260. doi: 10.1006/abbi.1994.1439. [DOI] [PubMed] [Google Scholar]
- Burbaum J. J., Raines R. T., Albery W. J., Knowles J. R. Evolutionary optimization of the catalytic effectiveness of an enzyme. Biochemistry. 1989 Nov 28;28(24):9293–9305. doi: 10.1021/bi00450a009. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Conformational restrictions on the pathway of folding and unfolding of the pancreatic trypsin inhibitor. J Mol Biol. 1977 Jun 25;113(2):275–293. doi: 10.1016/0022-2836(77)90142-5. [DOI] [PubMed] [Google Scholar]
- Darby N. J., Creighton T. E. Catalytic mechanism of DsbA and its comparison with that of protein disulfide isomerase. Biochemistry. 1995 Mar 21;34(11):3576–3587. doi: 10.1021/bi00011a012. [DOI] [PubMed] [Google Scholar]
- Deutscher M. P. Maintaining protein stability. Methods Enzymol. 1990;182:83–89. doi: 10.1016/0076-6879(90)82010-y. [DOI] [PubMed] [Google Scholar]
- Dyson H. J., Gippert G. P., Case D. A., Holmgren A., Wright P. E. Three-dimensional solution structure of the reduced form of Escherichia coli thioredoxin determined by nuclear magnetic resonance spectroscopy. Biochemistry. 1990 May 1;29(17):4129–4136. doi: 10.1021/bi00469a016. [DOI] [PubMed] [Google Scholar]
- Dyson H. J., Jeng M. F., Model P., Holmgren A. Characterization by 1H NMR of a C32S,C35S double mutant of Escherichia coli thioredoxin confirms its resemblance to the reduced wild-type protein. FEBS Lett. 1994 Feb 14;339(1-2):11–17. doi: 10.1016/0014-5793(94)80375-7. [DOI] [PubMed] [Google Scholar]
- Dyson H. J., Tennant L. L., Holmgren A. Proton-transfer effects in the active-site region of Escherichia coli thioredoxin using two-dimensional 1H NMR. Biochemistry. 1991 Apr 30;30(17):4262–4268. doi: 10.1021/bi00231a023. [DOI] [PubMed] [Google Scholar]
- Edman J. C., Ellis L., Blacher R. W., Roth R. A., Rutter W. J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature. 1985 Sep 19;317(6034):267–270. doi: 10.1038/317267a0. [DOI] [PubMed] [Google Scholar]
- Eklund H., Gleason F. K., Holmgren A. Structural and functional relations among thioredoxins of different species. Proteins. 1991;11(1):13–28. doi: 10.1002/prot.340110103. [DOI] [PubMed] [Google Scholar]
- Elble R. A simple and efficient procedure for transformation of yeasts. Biotechniques. 1992 Jul;13(1):18–20. [PubMed] [Google Scholar]
- Forman-Kay J. D., Clore G. M., Gronenborn A. M. Relationship between electrostatics and redox function in human thioredoxin: characterization of pH titration shifts using two-dimensional homo- and heteronuclear NMR. Biochemistry. 1992 Apr 7;31(13):3442–3452. doi: 10.1021/bi00128a019. [DOI] [PubMed] [Google Scholar]
- Forman-Kay J. D., Clore G. M., Wingfield P. T., Gronenborn A. M. High-resolution three-dimensional structure of reduced recombinant human thioredoxin in solution. Biochemistry. 1991 Mar 12;30(10):2685–2698. doi: 10.1021/bi00224a017. [DOI] [PubMed] [Google Scholar]
- GIVOL D., GOLDBERGER R. F., ANFINSEN C. B. OXIDATION AND DISULFIDE INTERCHANGE IN THE REACTIVATION OF REDUCED RIBONUCLEASE. J Biol Chem. 1964 Sep;239:PC3114–PC3116. [PubMed] [Google Scholar]
- GOLDBERGER R. F., EPSTEIN C. J., ANFINSEN C. B. Acceleration of reactivation of reduced bovine pancreatic ribonuclease by a microsomal system from rat liver. J Biol Chem. 1963 Feb;238:628–635. [PubMed] [Google Scholar]
- Gilbert H. F. Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol. 1990;63:69–172. doi: 10.1002/9780470123096.ch2. [DOI] [PubMed] [Google Scholar]
- Günther R., Srinivasan M., Haugejorden S., Green M., Ehbrecht I. M., Küntzel H. Functional replacement of the Saccharomyces cerevisiae Trg1/Pdi1 protein by members of the mammalian protein disulfide isomerase family. J Biol Chem. 1993 Apr 15;268(11):7728–7732. [PubMed] [Google Scholar]
- Haggren W., Kolodrubetz D. The Saccharomyces cerevisiae ACP2 gene encodes an essential HMG1-like protein. Mol Cell Biol. 1988 Mar;8(3):1282–1289. doi: 10.1128/mcb.8.3.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins H. C., Freedman R. B. The reactivities and ionization properties of the active-site dithiol groups of mammalian protein disulphide-isomerase. Biochem J. 1991 Apr 15;275(Pt 2):335–339. doi: 10.1042/bj2750335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Hol W. G. The role of the alpha-helix dipole in protein function and structure. Prog Biophys Mol Biol. 1985;45(3):149–195. doi: 10.1016/0079-6107(85)90001-x. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2275–2279. doi: 10.1073/pnas.73.7.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Tryptophan fluorescence study of conformational transitions of the oxidized and reduced form of thioredoxin. J Biol Chem. 1972 Apr 10;247(7):1992–1998. [PubMed] [Google Scholar]
- Hwang C., Sinskey A. J., Lodish H. F. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992 Sep 11;257(5076):1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- Hög J. O., von Bahr-Lindström H., Josephson S., Wallace B. J., Kushner S. R., Jörnvall H., Holmgren A. Nucleotide sequence of the thioredoxin gene from Escherichia coli. Biosci Rep. 1984 Nov;4(11):917–923. doi: 10.1007/BF01116889. [DOI] [PubMed] [Google Scholar]
- Jeng M. F., Campbell A. P., Begley T., Holmgren A., Case D. A., Wright P. E., Dyson H. J. High-resolution solution structures of oxidized and reduced Escherichia coli thioredoxin. Structure. 1994 Sep 15;2(9):853–868. doi: 10.1016/s0969-2126(94)00086-7. [DOI] [PubMed] [Google Scholar]
- Jeng M. F., Holmgren A., Dyson H. J. Proton sharing between cysteine thiols in Escherichia coli thioredoxin: implications for the mechanism of protein disulfide reduction. Biochemistry. 1995 Aug 15;34(32):10101–10105. doi: 10.1021/bi00032a001. [DOI] [PubMed] [Google Scholar]
- Katti S. K., LeMaster D. M., Eklund H. Crystal structure of thioredoxin from Escherichia coli at 1.68 A resolution. J Mol Biol. 1990 Mar 5;212(1):167–184. doi: 10.1016/0022-2836(90)90313-B. [DOI] [PubMed] [Google Scholar]
- Krause G., Lundström J., Barea J. L., Pueyo de la Cuesta C., Holmgren A. Mimicking the active site of protein disulfide-isomerase by substitution of proline 34 in Escherichia coli thioredoxin. J Biol Chem. 1991 May 25;266(15):9494–9500. [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMantia M. L., Lennarz W. J. The essential function of yeast protein disulfide isomerase does not reside in its isomerase activity. Cell. 1993 Sep 10;74(5):899–908. doi: 10.1016/0092-8674(93)90469-7. [DOI] [PubMed] [Google Scholar]
- Laboissiere M. C., Sturley S. L., Raines R. T. The essential function of protein-disulfide isomerase is to unscramble non-native disulfide bonds. J Biol Chem. 1995 Nov 24;270(47):28006–28009. doi: 10.1074/jbc.270.47.28006. [DOI] [PubMed] [Google Scholar]
- Laboissière M. C., Chivers P. T., Raines R. T. Production of rat protein disulfide isomerase in Saccharomyces cerevisiae. Protein Expr Purif. 1995 Oct;6(5):700–706. doi: 10.1006/prep.1995.1092. [DOI] [PubMed] [Google Scholar]
- Lim C. J., Geraghty D., Fuchs J. A. Cloning and nucleotide sequence of the trxA gene of Escherichia coli K-12. J Bacteriol. 1985 Jul;163(1):311–316. doi: 10.1128/jb.163.1.311-316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. Y., Kim P. S. Evaluating the effects of a single amino acid substitution on both the native and denatured states of a protein. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10573–10577. doi: 10.1073/pnas.88.23.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. Y., Kim P. S. Urea dependence of thiol-disulfide equilibria in thioredoxin: confirmation of the linkage relationship and a sensitive assay for structure. Biochemistry. 1989 Jun 13;28(12):5282–5287. doi: 10.1021/bi00438a054. [DOI] [PubMed] [Google Scholar]
- Lundström J., Holmgren A. Determination of the reduction-oxidation potential of the thioredoxin-like domains of protein disulfide-isomerase from the equilibrium with glutathione and thioredoxin. Biochemistry. 1993 Jul 6;32(26):6649–6655. doi: 10.1021/bi00077a018. [DOI] [PubMed] [Google Scholar]
- Lundström J., Krause G., Holmgren A. A Pro to His mutation in active site of thioredoxin increases its disulfide-isomerase activity 10-fold. New refolding systems for reduced or randomly oxidized ribonuclease. J Biol Chem. 1992 May 5;267(13):9047–9052. [PubMed] [Google Scholar]
- Luthman M., Holmgren A. Rat liver thioredoxin and thioredoxin reductase: purification and characterization. Biochemistry. 1982 Dec 21;21(26):6628–6633. doi: 10.1021/bi00269a003. [DOI] [PubMed] [Google Scholar]
- Lyles M. M., Gilbert H. F. Catalysis of the oxidative folding of ribonuclease A by protein disulfide isomerase: pre-steady-state kinetics and the utilization of the oxidizing equivalents of the isomerase. Biochemistry. 1991 Jan 22;30(3):619–625. doi: 10.1021/bi00217a005. [DOI] [PubMed] [Google Scholar]
- MOORE E. C., REICHARD P., THELANDER L. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES.V. PURIFICATION AND PROPERTIES OF THIOREDOXIN REDUCTASE FROM ESCHERICHIA COLI B. J Biol Chem. 1964 Oct;239:3445–3452. [PubMed] [Google Scholar]
- Martin J. L., Bardwell J. C., Kuriyan J. Crystal structure of the DsbA protein required for disulphide bond formation in vivo. Nature. 1993 Sep 30;365(6445):464–468. doi: 10.1038/365464a0. [DOI] [PubMed] [Google Scholar]
- Nikkola M., Gleason F. K., Saarinen M., Joelson T., Björnberg O., Eklund H. A putative glutathione-binding site in T4 glutaredoxin investigated by site-directed mutagenesis. J Biol Chem. 1991 Aug 25;266(24):16105–16112. [PubMed] [Google Scholar]
- Noiva R., Freedman R. B., Lennarz W. J. Peptide binding to protein disulfide isomerase occurs at a site distinct from the active sites. J Biol Chem. 1993 Sep 15;268(26):19210–19217. [PubMed] [Google Scholar]
- Pelham H. R., Hardwick K. G., Lewis M. J. Sorting of soluble ER proteins in yeast. EMBO J. 1988 Jun;7(6):1757–1762. doi: 10.1002/j.1460-2075.1988.tb03005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajaniemi T., Helaakoski T., Tasanen K., Myllylä R., Huhtala M. L., Koivu J., Kivirikko K. I. Molecular cloning of the beta-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene. EMBO J. 1987 Mar;6(3):643–649. doi: 10.1002/j.1460-2075.1987.tb04803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polson A., von Wechmar M. B., van Regenmortel M. H. Isolation of viral IgY antibodies from yolks of immunized hens. Immunol Commun. 1980;9(5):475–493. doi: 10.3109/08820138009066010. [DOI] [PubMed] [Google Scholar]
- Puig A., Gilbert H. F. Protein disulfide isomerase exhibits chaperone and anti-chaperone activity in the oxidative refolding of lysozyme. J Biol Chem. 1994 Mar 11;269(10):7764–7771. [PubMed] [Google Scholar]
- Puig A., Lyles M. M., Noiva R., Gilbert H. F. The role of the thiol/disulfide centers and peptide binding site in the chaperone and anti-chaperone activities of protein disulfide isomerase. J Biol Chem. 1994 Jul 22;269(29):19128–19135. [PubMed] [Google Scholar]
- Raines R. T., Knowles J. R. Enzyme relaxation in the reaction catalyzed by triosephosphate isomerase: detection and kinetic characterization of two unliganded forms of the enzyme. Biochemistry. 1987 Nov 3;26(22):7014–7020. doi: 10.1021/bi00396a024. [DOI] [PubMed] [Google Scholar]
- Reutimann H., Straub B., Luisi P. L., Holmgren A. A conformational study of thioredoxin and its tryptic fragments. J Biol Chem. 1981 Jul 10;256(13):6796–6803. [PubMed] [Google Scholar]
- Robinson A. S., Wittrup K. D. Constitutive overexpression of secreted heterologous proteins decreases extractable BiP and protein disulfide isomerase levels in Saccharomyces cerevisiae. Biotechnol Prog. 1995 Mar-Apr;11(2):171–177. doi: 10.1021/bp00032a009. [DOI] [PubMed] [Google Scholar]
- Scherens B., Dubois E., Messenguy F. Determination of the sequence of the yeast YCL313 gene localized on chromosome III. Homology with the protein disulfide isomerase (PDI gene product) of other organisms. Yeast. 1991 Feb;7(2):185–193. doi: 10.1002/yea.320070212. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Boeke J. D. In vitro mutagenesis and plasmid shuffling: from cloned gene to mutant yeast. Methods Enzymol. 1991;194:302–318. doi: 10.1016/0076-6879(91)94023-6. [DOI] [PubMed] [Google Scholar]
- Sinha N. D., Biernat J., McManus J., Köster H. Polymer support oligonucleotide synthesis XVIII: use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984 Jun 11;12(11):4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana C., Stevens T. H. The yeast EUG1 gene encodes an endoplasmic reticulum protein that is functionally related to protein disulfide isomerase. Mol Cell Biol. 1992 Oct;12(10):4601–4611. doi: 10.1128/mcb.12.10.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENETIANER P., STRAUB F. B. The enzymic reactivation of reduced ribonuclease. Biochim Biophys Acta. 1963 Jan 8;67:166–168. doi: 10.1016/0006-3002(63)91812-2. [DOI] [PubMed] [Google Scholar]
- Wang C. C., Tsou C. L. Protein disulfide isomerase is both an enzyme and a chaperone. FASEB J. 1993 Dec;7(15):1515–1517. doi: 10.1096/fasebj.7.15.7903263. [DOI] [PubMed] [Google Scholar]
- Weissman J. S., Kim P. S. Reexamination of the folding of BPTI: predominance of native intermediates. Science. 1991 Sep 20;253(5026):1386–1393. doi: 10.1126/science.1716783. [DOI] [PubMed] [Google Scholar]
- Wetterau J. R., Combs K. A., Spinner S. N., Joiner B. J. Protein disulfide isomerase is a component of the microsomal triglyceride transfer protein complex. J Biol Chem. 1990 Jun 15;265(17):9800–9807. [PubMed] [Google Scholar]
- Wunderlich M., Glockshuber R. Redox properties of protein disulfide isomerase (DsbA) from Escherichia coli. Protein Sci. 1993 May;2(5):717–726. doi: 10.1002/pro.5560020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich M., Otto A., Maskos K., Mücke M., Seckler R., Glockshuber R. Efficient catalysis of disulfide formation during protein folding with a single active-site cysteine. J Mol Biol. 1995 Mar 17;247(1):28–33. doi: 10.1006/jmbi.1995.0119. [DOI] [PubMed] [Google Scholar]
- delCardayré S. B., Ribó M., Yokel E. M., Quirk D. J., Rutter W. J., Raines R. T. Engineering ribonuclease A: production, purification and characterization of wild-type enzyme and mutants at Gln11. Protein Eng. 1995 Mar;8(3):261–273. doi: 10.1093/protein/8.3.261. [DOI] [PubMed] [Google Scholar]